Abstract
Objective
Developmental stuttering is characterized by fluent speech punctuated by stuttering events, the frequency of which varies among individuals and contexts. Most stuttering events occur at the beginning of an utterance, suggesting neural dynamics associated with stuttering may be evident during speech preparation.
Methods
This study used EEG to measure cortical activity during speech preparation in men who stutter, and compared the EEG measures to individual differences in stuttering rate as well as to a fluent control group. Each trial contained a cue followed by an acoustic probe at one of two onset times (early or late), and then a picture. There were two conditions: a speech condition where cues induced speech preparation of the picture’s name and a control condition that minimized speech preparation.
Results
Across conditions stuttering frequency correlated to cue-related EEG beta power and auditory ERP slow waves from early onset acoustic probes.
Conclusions
The findings reveal two new cortical markers of stuttering frequency that were present in both conditions, manifest at different times, are elicited by different stimuli (visual cue, auditory probe), and have different EEG responses (beta power, ERP slow wave).
Significance
The cue-target paradigm evoked brain responses that correlated to pre-experimental stuttering rate.
Keywords: stuttering, speech preparation, auditory event-related potentials, EEG, event-related desynchronization
1. Introduction
From the perspective of the brain, speech is not free. Even simple speech utterances require the orchestration of high-level cognition, in the form of the intended message, which is mapped onto the corresponding lexical and phonological representations, which are then expressed by precisely timed muscle contractions of the vocal articulators (Indefrey and Levelt 2004; Indefrey 2011). This complexity provides many opportunities for speech to fail, which is evident in developmental stuttering as well as other speech disorders. Developmental stuttering originates in childhood, and is characterized by the presence of intermittent dysfluencies that disrupt the natural flow of speech (Bloodstein and Bernstein Ranter 2008). Developmental stuttering is considered persistent if stuttering continues into adulthood.
Convergent evidence from lesion (Grant et al. 1999; Fawcett 2005), neuroimaging (Brown et al. 2005), and neurophysiological (Salmelin R, Schnitzler A, Schmitz F, Jancke L, Witte OW et al. 1998; Biermann-Ruben et al. 2005; Salmelin 2007) studies show that coordination within a network of perisylvian sensorimotor systems is vital for fluent speech (Guenther, 2006; Hickok, 2012; Indefrey, 2011). Interactions among motor and sensory (auditory, somatosensory) areas are one aspect of this network’s coordination, and are thought to instantiate feedforward (motor to sensory) and feedback (sensory to motor) control. Many imaging studies have found that adults and children who stutter have anatomical and functional differences throughout sensorimotor speech networks (Fox et al. 1996; Sommer et al. 2002; Foundas et al. 2004, 2013a; Beal et al. 2007, 2013; Watkins et al. 2008; Chang et al. 2008, 2011, 2015; Kikuchi et al. 2011; Mock et al. 2012). Resting-state fMRI studies have shown that adults and children who stutter have functional connectivity deficits between sensorimotor regions even when they are not speaking (Xuan et al. 2012; Chang and Zhu 2013).
Most prior work on the neural mechanisms of stuttering compares people who stutter relative to fluent controls at the level of group analyses. Yet it is well-established that stuttering frequency varies from individual to individual and situation to situation, ranging from being nearly imperceptible to debilitating. Factors such as anticipation of public speaking and message complexity can further impair fluency in people who stutter (Siegel and Haugen 1964; Young 1965; Wells 1979). Conversely, unusual forms of auditory feedback or speech behaviors can improve fluency. Such examples include altered or delayed auditory feedback (Lincoln et al. 2006; Foundas et al. 2013b), sound masking (Barr and Carmel 1969; Conture 1974; Brayton and Conture 1978), and using an unusual/novel speaking style (Azrin et al. 1968; Wingate 1969). Fluency can also be improved by manipulating tactile feedback (Waddell et al. 2012) or by having subjects perform a manual tracking task while speaking (Arends et al. 1988). There is one characteristic of stuttering that is extremely consistent across situations: over 90% of stuttering events occur on the initial sound or syllable of the utterance (Sheehan 1974; Bloodstein and Bernstein Ranter 2008). This observation strongly suggests that neurological markers of stuttering will likely be present before the initial syllable is ever attempted, i.e. during speech preparation.
The current study examined speech preparation in men who stutter (MWS) by recording EEG while participants performed a delayed naming task. In our delayed naming paradigm a one or two syllable cue word is presented first and then followed by a picture that usually corresponds to the cue. The cue is a reliable predictor of the upcoming picture, and thus can be used to prepare a vocal response to the upcoming picture. A control condition used the same stimuli except that the cue did not spell a word, and therefore does not elicit speech preparation of a specific word. Two neuronal responses were examined: event-related potentials (ERPs) to auditory stimuli delivered between the cue and target, and evoked changes in EEG oscillatory power after cue onset. The auditory stimuli probed engagement of the auditory system during speech preparation, which is evident by larger auditory ERP slow waves during speech preparation vs. the control task (Mock et al. 2011). Our previous study using the same subjects and paradigm found the difference in auditory probe slow waves between conditions was smaller in all MWS vs. fluent adults (Mock et al. 2015). The findings supported the hypothesis that MWS showed diminished auditory processing during speech preparation. In contrast, the current paper focused on indexing stuttering severity by using neurophysiological measures, regardless of any relations to motor efference.
The main objective of this study was to determine whether cortical measures (ERP amplitude and EEG power) during speech preparation would correlate to individual differences in stuttering rate. We rationalized that during speech preparation, cortical activity within sensorimotor regions would show differences in relation to an individual’s stuttering rate. Both auditory ERPs and cue-related EEG power reductions in the alpha (8–12 Hz) and beta (12–30 Hz) bands can index sensory and motor activity within speech networks (Crone 1998; Szurhaj et al. 2003; Chevillet et al. 2013; Möttönen et al. 2013). Thus, we hypothesized that EEG power in the alpha and beta bands would show greater desynchronization within sensorimotor regions during speech preparation and would correlate to stuttering rate. We also hypothesized increased auditory processing in the MWS during speech preparation, as indexed by the auditory probe ERP amplitudes, would correlate to an individual’s stuttering rate.
2. Methods
2.1. Participants
The study participants were twenty-four males (MWS = 12, fluent adults = 12, all right handed) that participated in a previous study (Mock et al. 2015). The current study presents new results using EEG measures to examine associations to individual differences in speech fluency. The previous report focused on group differences with respect to motor efference copy during speech preparation. All participants reported normal neurological and psychiatric health, spoke English as their first and primary language, and were in the normal range of hearing thresholds (≤ 25 dB, 0.5–8.0 kHz). Stuttering rate was obtained for all MWS by transcribing two videotaped speech samples (conversation, reading passage). To obtain a stuttering rate for each MWS the percent of dysfluent syllables was quantified by adding the number of stuttering-like dysfluencies (monosyllabic word repetitions, part-word repetitions, blocks and prolongations) from the first 300 syllables of each speech sample and dividing by the total number of spoken syllables. The percent of dysfluent syllables from the conversation and reading passages were averaged to obtain a stuttering rate (mean=9.8 ± 1.3%, range=3.5 to 19.5%). Stuttering rate was independently coded by both the first author and a certified speech-language pathologist, and was reliable (intraclass correlation coefficient = 0.89). Each participant signed a consent form, and all experimental procedures followed a protocol approved by the Tulane University Institutional Review Board that was consistent with the Declaration of Helsinki.
2.2. Experimental Design and Task
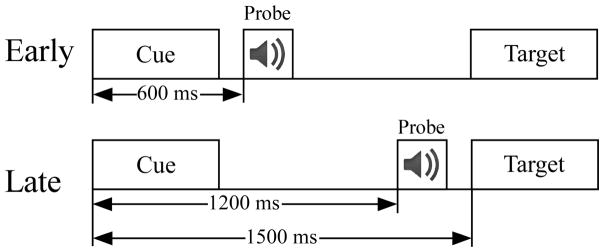
This study used the same cue-target paradigm with two conditions (speech and control) that was used in our previous studies (Mock et al. 2011, 2015) Figure 1). Trials in the speech condition presented a one or two syllable cue word that corresponded to the target picture on 90% of the trials (termed “same trials”). In 10% of the trials the cue did not match the target picture (termed “different trials”). The behavioral results verified that subjects in both groups used the cue to prepare their naming response because reaction times were much slower on the “different” trials (see Results section). The participants were asked to use the cue to prepare a vocal response to the target picture, to be fast but accurate, and to only use one word responses. The interval between the target and cue of the next trial was 3.0 sec.
Figure 1.
A Schematic depicting the cue – target paradigm, separated according to the time of auditory probe onset (early, late).
In the control condition all cues were “XXXX” which provided timing on when the upcoming target would appear but did not allow the subject to prepare a specific vocal response. On most control trials the participants passively viewed the stimuli without naming the picture (90% of trials). To control for attentiveness, on 10% of the control trials a visual prompt was given 1,000 ms after the target that instructed the participant to name the previous target (catch trials). The cues (3 cm height) and targets (picture 7 × 7 cm) were presented in the middle of a computer screen for 500 ms at a distance of 100 cm from each participant.
An auditory probe was presented between the cue and target to measure the impact of speech motor preparation on auditory cortical processing. For each trial one auditory probe was delivered between the cue and target at either an early (600 ms) or late (1200 ms) time point relative to cue onset (p =.50 for early/late time point). The auditory probes (~60 dB nHL, ~200 ms duration) were either a consonant-vowel or pure tone (1,000 Hz, 5 ms rise/fall time). In the speech condition the consonant-vowel probes were subdivided into two classes (match, mismatch). The only difference between the match and mismatch probes was their relationship to the cue on a given trial. The sound of the match probes corresponded to the first consonant-vowel of the cue word, while the mismatch probes did not. Each sound type (match, mismatch, tone) was randomly presented in 20 trials/block (p=.33), yielding 60 different words and pictures per block. Pictures were selected from a standardized set (Snodgrass and Vanderwart 1980). Six blocks were given for each condition (360 trials/condition). All words began with a consonant-vowel and the order of conditions was counterbalanced across participants in each group.
2.3. Electrophysiological recordings
Scalp EEG recordings were conducted in an electrically shielded sound booth using a 64 Ag/AgCl electrode cap (reference between Cz and CPz, impedances ≤ 10 kΩ, 500 Hz digitization rate, DC-100 Hz). Four electrodes were used to monitor eye movements, one above and one below the left eye and one lateral to each eye. Acoustic stimuli were presented binaurally with etymotic headphones. The electrodes were corrected for DC drift and eye blink artifacts (Gratton et al. 1983). Acoustic ERPs were re-referenced offline in a linked mastoid configuration and epoched into 1,200 ms segments (−200 to 1000 ms relative to stimulus onset). Each ERP epoch was visually inspected for additional artifacts before being included in the ERP averages. Each auditory ERP average was digitally filtered (band pass 0.1–16 Hz, 12 dB down) to attenuate slow shifts, and baselined from −100 ms to 0 ms relative to stimulus onset.
Changes in EEG power, termed event-related spectral perturbations (ERSPs), were examined using EEGLAB version 11.054 (Delorme and Makeig 2004) running in Matlab 8.0 (The Mathworks, Inc., Natick, MA U.S.A.). ERSPs are measurements of event-related power increases (synchronization) or decreases (desynchronization) in oscillatory power within a given frequency band relative to a prestimulus baseline period. EEG data were re-referenced to the average of all scalp electrodes and bandpass filtered from 1 to 50 Hz. Continuous data were segmented into epochs lasting from 1000 ms before the cue to 100 ms after the target. ERSP plots were generated by computing the spectral power within equally-spaced frequency bins from 5 to 50 Hz using a short-time Fourier transform with sliding Hanning windows. ERSP plots indicated power (dB) at a given time and frequency by subtracting the trial-averaged pre-stimulus spectral activity (−1000 ms to 0 ms) from post-stimulus activity in each frequency band (Tallon-Baudry et al. 1999; Grandchamp and Delorme 2011). The ratio of power after stimulus onset relative to baseline is expressed in decibels. The cue was presented at 0 ms, onset of the auditory probe was at either 600 ms (early) or 1200 ms (late), and targets were given 1,500 ms after the cue.
Both ERPs and ERSPs index stimulus-induced changes in EEG power relative to a prestimulus baseline. The main difference is that ERPs index changes in EEG power that occur at both a consistent latency and phase across trials. In contrast, ERSPs reflect EEG power changes at a consistent latency across trials, but the phase does not have to be consistent (Makeig 1993). Thus, ERSPs provide an index of change in oscillatory activity that may not be evident in phase-locked responses such as ERPs.
2.4. Data analysis
The current study will focus on ERPs to auditory probes and ERSPs following visual cues, and both measures will be compared to stuttering rate. Activity at frontal midline (FCz), frontal lateral (FC5, FC6) and lateral parietal sites (P5, P6) were quantified. Selection of sites was based on maximum amplitudes in topographic plots of ERP and ERSP activity. ERSPs in the conventional theta (4–8 Hz), alpha (8–12 Hz), and beta (12–30 Hz) bands were examined. For each participant, ERSP power was calculated at a given frequency band and time point, which yielded a 2-dimensional time-frequency plot between presentation of the cue and target stimuli.
2.5. Statistical analysis
The ERP and ERSP analyses used repeated measures analysis of variance (ANOVA; p < .05), with the Greenhouse-Geisser correction for any violations of sphericity. For clarity, when the Greenhouse-Geisser was used the text will retain the original degrees of freedom. Pearson’s correlation coefficient (r) was used to test the association between stuttering rate and ERP or ERSP measures. To better illustrate the associations between stuttering rate and neurophysiological measures within the figures, the 12 MWS were separated into stuttering rate subgroups of mild/moderate MWS (< 10%; avg. = 6.4% ± 0.7%) or severe MWS (≥ 10%; avg. = 13.4% ± 1.4%) (n=6/subgroup, median split). Tukey HSD was used for post hoc comparisons of subgroups (fluent, mild/moderate MWS, severe MWS).
The ERP analyses combined the match, mismatch, and tone probe types because preliminary analyses showed no group differences as a function of probe type. Midline electrode sites were examined first, and then followed by comparison of sites in the left vs. right hemisphere. The ANOVA tests used factors of group (fluent, mild/moderate MWS, severe MWS), condition (speech, control) and probe onset (early, late), and for hemispheric comparisons included the factor of electrode site (left, right). The specific sites depended on the topographic distribution of the specific ERP component, and were chosen on the basis of which sites had the largest amplitudes. Auditory ERPs evoked from the early and late probes were analyzed at frontal (FCz, FC5, FC6) and parietal (Pz, P5, P6) electrodes by calculating the mean voltage within a 100–400 ms window. Due to the onset of the picture stimulus which elicited a visual ERP evident by ~100 ms, the main analysis between auditory probes was restricted to a 100–400 ms time window. To further determine if early auditory probe ERP amplitudes correlate to stuttering rate past 400 ms, 100 ms blocks from 400 to 700 ms were analyzed. Each of the ERP conditions had an average of 160 epochs, with no difference between groups (p = 0.9).
The cue ERSP was separately analyzed in three conventional frequency bands (theta 4–8 Hz, alpha 8–12 Hz, beta 12–30 Hz), according to group, condition, and probe onset. The analysis quantified power from 100–700 ms after cue onset because this time period had the greatest power reductions in both the alpha and beta bands. Increases in theta power are not presented because they largely reflected activity indexed by the ERP measures. Also, when deemed necessary, the alpha band was broken into low (8–10 Hz) and high (10–12 Hz) alpha. As with the ERP analyses, ANOVA tests used factors of group fluent, mild/moderate MWS, severe MWS), condition (speech, control), and probe onset (early, late), but also included a factor of time window (six 100 ms windows: 100–198, 200–298, 300–398, 400–498, 500–598, 600–698 ms). The purpose of the time windows was to quantify the time course of power decreases which then return towards baseline. Anterior and posterior hemisphere analyses were analyzed separately and included within-subject variables of condition, probe onset, window and hemisphere (left, right) with group as the between-subjects factor. Each of the ERSP conditions had an average of 148 epochs with no difference between groups (p = 0.7).
3. Results
3.1. Vocal Reaction Times
In both groups vocal reaction times were much slower on trials where the cue and target differed (same = 483 ± 33 ms, different = 719 ± 37 ms; F(1,22) = 280.8, p < 0.0001), indicating that cue information was used to prepare vocal responses to the target. Vocal reaction times in the MWS were slower than fluent adults only when the cue and target differed (MWS = 787 ± 48 ms, fluent = 651 ± 26 ms; F(1,22) = 11.7, p = 0.002), suggesting the MWS were slower at rapidly updated their speech plan. During the speech trials, the MWS on average produced a stuttering event in 5% of the trials (212 dysfluent responses out of 4,320 trials). Three out of 12 MWS did not register any stuttering events and another 4 out of 12 MWS had < 6 total stuttering events (out of 360 speech trials/participant). Thus, the MWS were more fluent during the experimental task than pre-experiment assessment (9.8%). The above behavioral results were previously reported in (Mock et al. 2015).
3.2. Auditory event-related potentials
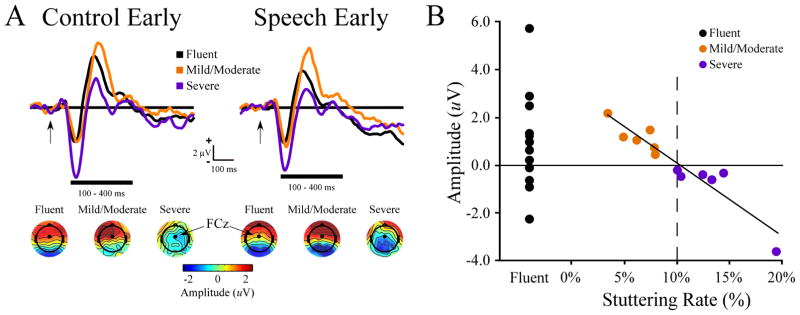
A plot of stuttering rate vs. early probe onset ERP amplitude at the FCz site is shown in Figure 2. Stuttering rate was negatively correlated to amplitude at the early (100–400 ms; r = −.952, p = 0.000002), but not late probe onset time (r = −0.355, p = 0.26). When the most severely dysfluent subject was removed stuttering rate maintained a significant correlation to early probe amplitude (r = −.928, p = 0.00004). The negative correlation between early ERP amplitude and stuttering rate was found in both the control (r = −.926, p = 0.00001) and speech conditions (r = −.880, p = 0.000 2) and for the N100 (r = −.640, p = 0.025) and P200 peak amplitudes (r = −.633, p = 0.027). The correlation extended up to 600 ms after early auditory probe onset (400–500 ms, r = −.723, p = 0.008; 500–600 ms, r = −.663, p = 0.019). Significant correlations to early ERP amplitudes were also seen at the FC5 (r = −.760, p = 0.004) and FC6 sites (r = −.861, p = 0.0003). At FCz there was no group difference in early auditory probe amplitudes when comparing fluent men to all MWS (p = 0.28), fluent men vs. severe (p = 0.052) or fluent men vs. mild/moderate MWS (p = 0.7). There was a group difference when comparing mild/moderate vs. severe MWS (p = 0.046). Also, an effect of probe onset was found were auditory ERP amplitudes were more negative in the late (−.962 ± 0.41 μV) vs. early (0.395 ± 0.36 μV) auditory probes.
Figure 2.
Early auditory probe ERPs and stuttering rate. (A) Early auditory ERP waveform at electrode FCz separated by group (fluent, mild/moderate, severe) and condition (control, speech). Early auditory probe ERP topography averaged from 100–400 ms for each condition and group are shown below the ERP waveforms. (B) Scatterplot of early auditory ERP amplitude (100–400 ms window) showing a negative correlation to stuttering rate in MWS.
Stuttering rate was not correlated to posterior ERP amplitudes for either the early or late auditory probes (averaged across posterior electrodes Pz, P5 and P6; early: r = −0.32, p = 0.33, late: r = −0.05, p = 0.53).
3.3. Event-related spectral perturbations (ERSPs)
Alpha and beta power during the baseline period (−1000 ms to 0 ms) did not differ between MWS and controls in any of the analyses (alpha band: p = 0.21; beta band: p = 0.58). There were also no baseline power differences among the MWS subgroups (mild/moderate vs. severe: alpha: p = 0.19; beta: p = 0.78). Moreover, stuttering rate was not correlated to either baseline alpha (r = 0.462, p =0.13) or beta (r = 0.332, p = 0.29) EEG power. The finding of comparable baseline power for the MWS and fluent controls indicates that any ERSP group differences are due to activity elicited by the stimuli rather than baseline differences.
3.3.1. Anterior sites
Alpha band
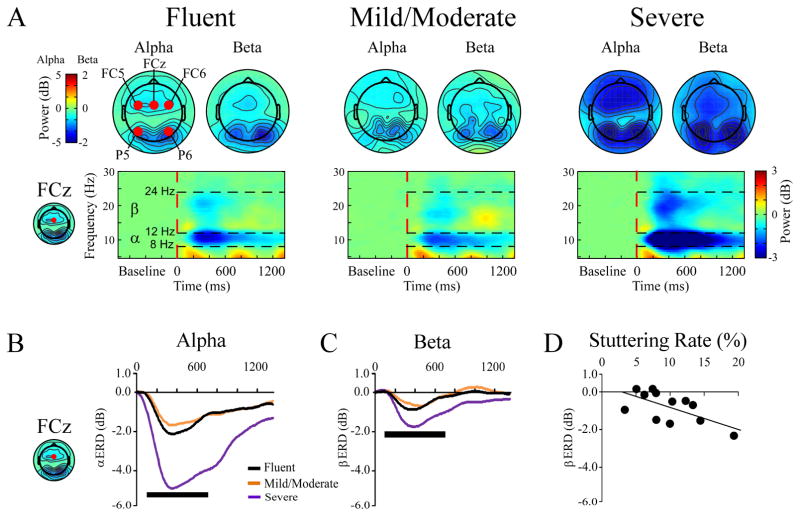
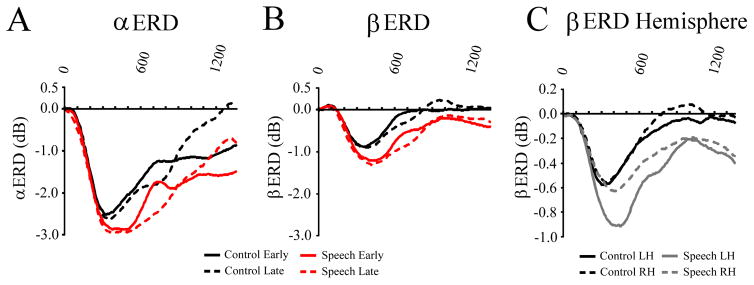
Analysis of the FCz site in the alpha band showed a main effect of group (F(2,21) = 4.4, p = 0.026) Figures 3A,B). Tukey post hoc tests revealed that severe MWS had a larger power decrease relative to both fluent controls as well as mild/moderate MWS (both p-values < 0.05). There were also main effects of condition (F(1,21) = 10.6, p = 0.004; speech > control) and probe onset (F(1,21) = 4.3, p = 0.049; late > early)(Figure 4A). The main effect of window (F(5,17) = 12.6, p < 0.000) indicates that the cue induced a power decrease that gradually returned to baseline. When the analysis included left and right hemisphere sites (F5, F6) there were no significant effects involving hemisphere.
Figure 3.
Event-related spectral perturbation (ERSP) time-frequency results between cues and targets. (A) Time-frequency plots of ERSPs as a function of group (fluent, mild/moderate, severe). Each plot is collapsed across condition (control, speech) and probe onset (early, late). The red circles on the far left topography plot indicate the electrode sites quantified. The time/frequency plots shown are from electrode FCz. (B, C) Waveforms showing the severe MWS had greater ERSP power decreases in the alpha (B) and beta (C) frequency bands. (D) Scatterplot showing beta reductions are correlated to stuttering rate (100–700 ms time window).
 = 100–700 ms time window used to compute ERSP topographic plots and measure ERSP power that was compared to stuttering rate.
= 100–700 ms time window used to compute ERSP topographic plots and measure ERSP power that was compared to stuttering rate.
Figure 4.
Separate ERSP waveforms between cues and targets showing effects of condition, probe onset, and hemisphere. (A, B) Waveforms of ERSPs showing greater ERSP power decrease in the speech vs. control condition in both the alpha (A) and beta (B) frequency bands. (C) Waveform showing beta ERSP as a function of condition and hemisphere (left, FC5; right, FC6). In the speech conditions ERSP reductions were greater in the left hemisphere, while comparable reductions were seen in both hemispheres in the control condition.
Beta band
The group effect did not attain significance (p = 0.13), but there was a group x window interaction (F(10,36) = 2.2, p = 0.042). The interaction was due to beta power taking longer to return to baseline in the severe group, as compared to fluent and mild/moderate MWS groups (Figures 3A, C).
There were main effects of condition (F(1,21) = 9.5, p = 0.006; speech > control), probe onset (F (1,21) = 4.8, p = 0.04; late > early), and window (F(5,17) = 10.4, p < 0.000). There were also interactions for condition x window (F(5,17) = 3.8, p = 0.016) and probe onset x window (F(5,17) = 3.7, p = 0.018)(Figure 4B). The condition x window interaction reflected greater power in the speech condition between ~300–600 ms, while the probe onset x window interaction was due to a power increase shortly after early auditory probe onset.
Comparison of left and right hemisphere sites had an interaction of condition x hemisphere (F(1,21) = 14.0, p = 0.001), which was qualified by a three-way interactions of condition x hemisphere x window (F(5,17) = 4.6, p = 0.008)(Figure 4C). This finding indicates that beta power reductions were larger in the left vs. right hemisphere in the speech condition, particularly ~300–600 ms after cue onset.
Stuttering rate
Stuttering rate was separately compared to alpha and beta ERSP power at FCz between 100–700 ms (Figure 3D). There was a significant negative correlation between stuttering rate and beta band power (r = −0.638, p = 0.026), with a similar trend in the alpha band (r = −0.543, p = 0.068). These same results were seen at FC5 (alpha: r = −0.551, p = 0.063; beta: r = −0.701, p = 0.011) and FC6 (alpha: r = −0.424, p = 0.17; beta: r = −0.624, p = 0.030).
3.3.2. Posterior sites
The analysis of EEG power reductions at posterior locations focused on the lateral sites that had the greatest amount of desynchronization at left (P5) and right (P6) hemisphere sites.
Alpha band
There was a main effect of group (F(2,21) = 4.3, p = 0.027), and post hoc testing showed that the severe group had larger power decreases relative to fluent controls (p < 0.04), and similar trend for mild/moderate MWS (p < 0.06). There were also main effects of condition (F(1,21) = 9.3, p = 0.006; speech > control) and window (F(5,17) = 11.0, p < 0.000), and condition x window interaction (F(1,21) = 3.2, p = 0.034). The condition x window interaction was due to the condition difference being most evident between ~300–600 ms after cue onset.
Beta band
The main effect of group was not significant (p = .15). There were main effects of condition (F(1,21) = 6.2, p = 0.021; speech > control) and window (F(5,17) = 7.0, p = 0.001), along with condition x hemisphere (F(1,21) = 6.0, p = 0.023) and condition x window (F(5,17) = 6.2, p = 0.002) interactions. The condition x hemisphere interaction reflected a larger condition effect in the left hemisphere. The condition x window interaction was due to the condition effect being most evident within later time windows (~300–600 ms).
Stuttering rate
Stuttering rate was compared to alpha and beta ERSP power at P5 (left) and P6 (right) between 100–700 ms. There was a significant negative correlation between stuttering rate and alpha band power at P6 (r = −0.665, p = 0.018), and a trend at P5 (r = −0.551, p = 0.064). Within the beta band there was a significant negative correlation with stuttering rate at both P5 (r = −0.715, p = 0.009) and P6 (r = −0.696, p = 0.012). Follow up analysis found at P5 low alpha (8–10 Hz, r = −0.605, p = 0.037) but not high alpha (10–12 Hz, r = −0.556, p = 0.06) to be negatively correlated to stuttering rate. At P5, both low beta (12–18 Hz, r = −0.671, p = 0.017) and high beta (18–24 Hz, r = −0.743, p = 0.006) were correlated to stuttering rate. At P6, all low and high frequency measures were negatively correlated to stuttering rate (low alpha (8–10 Hz), r = −0.694, p = 0.012; high alpha (10–12 Hz), r = −0.667, p = 0.018; low beta (12–18 Hz), r = −0.697, p = 0.012; high beta (18–24 Hz), r = −0.729, p = 0.007).
4. Discussion
This study tested the hypothesis that cortical activity during speech preparation is associated with individual differences in stuttering rate. The hypothesis was supported by the findings that a clinical measure of stuttering rate correlated to both ERSP power after cue onset and to early auditory probe ERP amplitude. We hypothesized such correlations would be specific to the speech preparation condition, but instead correlations to stuttering rate were seen in both the speech and control conditions. The two measures that correlated to stuttering rate were evident at different times relative to target onset, elicited by stimuli in different modalities, and were manifest as different types of EEG responses (decreased alpha/beta power, early ERP amplitude). See Figure 5. Taken together, the EEG responses that correlate to stuttering rate likely reflect different cortical mechanisms, which can provide insight into specific neuronal processes that may distinguish stuttering severity within individuals who stutter.
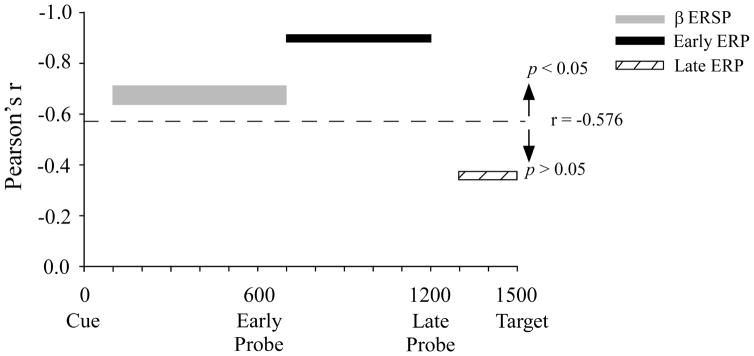
Figure 5.
Summary of the correlation results. Time plots showing the onset and offset of beta ERSP and auditory probe ERP measures that were significantly correlated to stuttering rate. Width of the beta ERSP bar indicates the range of correlations among three electrode sites (FCz, P5, P6). Early and late ERP correlations are show from electrode FCz.
 = cutoff for Pearson’s r to be significant.
= cutoff for Pearson’s r to be significant.
4.1. Auditory event-related potentials and stuttering severity
One of the best predictors of pre-experimental stuttering rate was the amplitude of fronto-central auditory potentials after early auditory probe onset. The early auditory probes were presented 600 ms after cue onset and the evoked amplitude from 100–600 ms was negatively correlated to stuttering rate. The 100–600 ms window encompassed multiple ERP components (N100, P200) that also independently correlated to an individual’s pre-experimental stuttering rate. The scalp ERP amplitudes to early auditory probes were comparable in MWS and fluent men, but amplitudes among MWS were more negative as an individuals’ pre-experiment stuttering rate increased. Within clinical populations, both neurophysiological (ERP/EEG) and behavioral measures can in tandem be outside of the normal range and correlate to each other (Thatcher et al. 2001; Arciniegas 2011). Also, neurophysiological measures from normal healthy controls can be within normal ranges and still correlate to individual differences in cognitive measures (Polich 2007; Yurgil and Golob 2010; Finnigan and Robertson 2011; Chen et al. 2015; Dong et al. 2015). Given the complex etiology of stuttering, multiple influences on scalp EEG/ERP measures are to be expected, and relatively, small experimental studies are not sufficient to tease apart such complex relations. Even in controls, for example, the auditory N100 amplitude is influenced by a host of stimulus features such as intensity and presentation rate (Naatanen et al. 1987) as well as cognitive factors such as attention (Hugdahl and Nordby 1994; Golob et al. 2002) and short-term memory (Conley et al. 1999; Golob and Starr 2004).
The negative auditory ERP amplitudes in the severe MWS resemble the time course and topography of a negative slow present during auditory attention tasks (Hillyard et al. 1973; Naatanen et al. 1978; Golob et al. 2001). The ERP amplitudes were more negative for the late vs. early auditory probes for all groups, a result also found in our previous study (Mock et al. 2011); suggesting participants increase auditory cortex modulation as target onset approaches. We speculate that individuals with more severe stuttering rates have a greater tendency to modulate auditory attention earlier, right after cue onset. This conclusion fits with previous work that used a similar paradigm and found adults who stutter may differ in how they allocate attentional resources (Maxfield et al. 2010). A related possibility that has barely been explored in the context of stuttering is whether auditory imagery (Hubbard 2010; Tian and Poeppel 2012), which may accompany speech preparation in this task, differs in those who stutter.
Our recent publication using the same subjects and task found auditory ERP conditional differences (speech vs. control) were smaller in all MWS regardless of pre-experimental stuttering rate (Mock et al. 2015). Comparing studies, there is an overlap of time range (100–400 ms) within the scalp auditory ERPs that both correlated to pre-experimental stuttering rate (current study) and was smaller in all MWS (Mock et al. 2015). However, major differences exist. First, stuttering rate was only correlated when auditory probes were presented shortly after cue onset, while Mock et al. (2015) showed that MWS had smaller differences between conditions throughout the delay between cue and target. Second, in Mock et al. (2015) the difference between conditions (control – speech) did not correlate to stuttering rate. Overall, the results from this pair of studies suggest that auditory processing in MWS is involved in both individual stuttering rate differences as well as overall group differences evident during speech preparation.
4.2. Cue-based event-related spectral perturbations (ERSPs) and stuttering rate
In this study the cues elicited transient reductions in alpha and beta band power over fronto-central and posterior lateral electrodes, with larger reductions in the speech vs. control condition. Greater pre-experiment stuttering rates were accompanied by larger power reductions from 100 to 700 ms after cue onset, which was evident in both conditions. Intracortical recordings in humans and animal models show that alpha and beta EEG oscillations originate within reciprocal networks in cortex and thalamus (Steriade and Llinás 1988), and are thought to be critical for coordinated information processing among networks spanning a wide range of spatial scales (Varela et al. 2001). In general, higher frequencies such as the gamma band (> ~30 Hz) reflect smaller networks within cortical regions, while lower frequencies, such as the alpha and beta bands examined here, reflect integration between cortical regions. EEG power is proportional to both the magnitude of membrane potential fluctuations within neurons as well as the synchrony of fluctuations within a neural population (Nunez and Srinivasan 2006; Musall et al. 2014). Alpha and beta power reductions in sensory and motor areas have been related to heightened information processing (Haegens et al., 2011; Pfurtscheller and Lopes da Silva, 1999), and TMS studies directly show that this is accompanied by greater cortical excitability (Romei et al. 2008; Taylor and Thut 2012; Takemi et al. 2013). Thus, the relation between stuttering rate and power reductions following the cue may index activity enhancements within cortical networks that are not conducive to producing fluent speech.
The baseline EEG power used to calculate ERSP power did not differ between groups (MWS, fluent) or within the MWS group (mild/moderate, severe). This directly shows that group differences in ERSPs after cue onset are not an artifact of differences in baseline power. ERSP power changes are not always evident in ERPs because both the timing and phase of oscillatory activity must be consistent across trials in order for ERPs to be present (Pfurtscheller and Lopes da Silva 1999). The initial ERSP power decrease that correlated to stuttering rate occurred before the first probe was delivered, while later ERSP power was not associated with stuttering rate. Thus, the time period when stuttering rate correlated to ERSP measures did not have substantially overlap with the time period when stuttering rate correlated to ERP amplitudes from early auditory probes. See Figure 5.
Beta power reductions were similar to auditory ERP amplitude correlations in that no overall group differences reached significance, yet strong correlations to pre-experimental stuttering rate were still present. We speculate that the association between beta EEG power and stuttering rate reflects increased cortical excitability within sensorimotor regions, which may represent the efficiency of information transfer between sensory and motor regions (Fujioka et al. 2012). This interpretation is consistent with previous work showing the beta frequency is modulated during manual motor preparation (Doyle et al. 2005; Engel and Fries 2010; Tzagarakis et al. 2010). Also, previous manual movement tasks that compared novice vs. expert athletes found larger reductions in EEG power during movement preparation in the novices (Babiloni et al. 2010; Del Percio et al. 2010). Our study found that beta power reductions were left-lateralized only in the speech condition, which further suggests a strong role in left hemisphere sensorimotor networks being vital components during speech preparation and production (Hickok et al. 2011). Left lateralization in the speech condition did not relate to fluency as it was present in both the MWS and control groups (cf. Salmelin et al., 2000). Overall, these findings suggest that modulation of beta power represents the efficiency of coupling between sensory and motor regions, with potentially less efficient coupling as stuttering rate increases.
Greater alpha power reductions in the severe MWS was evident over both anterior and posterior brain regions, but only significantly correlated to stuttering rate at the right posterior electrode site. Alpha power reductions trended towards significant correlation to stuttering rate at frontal electrode sites. Alpha power is known to be influenced by a range of factors such as attentional load (Van Winsum et al. 1984), expectancy (Pfurtscheller 1989), and level of expertise (Del Percio et al. 2009, 2010; Babiloni et al. 2010). Future work is needed to more precisely define the relation of alpha power reductions and stuttering, but attentional control is a likely avenue of work because it relates to the idea of a hypervigilant self-monitoring system in people who stutter (Lickley et al. 2005; Vasic and Wijnen 2005; Bloodstein and Bernstein Ranter 2008).
Previous EEG studies found a negative correlation between stuttering severity and the topography of the auditory N100 ERP in adults (Liotti et al. 2010), and an earlier auditory component (the M50) to speech feedback in children who stutter (Beal et al. 2011). Functional neuroimaging studies that examined relations to stuttering severity are mixed. Positive correlations to stuttering rate have been found in basal ganglia regions (Giraud et al. 2008; Kell et al. 2009; Ingham et al. 2012), motor cortex and cerebellum (Fox et al. 2000); while negative correlations have been found in the right prefrontal cortex (Preibisch et al. 2003; Kell et al. 2009). The auditory cortex portion of the superior temporal gyrus had particularly complex results, with both positive (Kell et al. 2009; Ingham et al. 2012) and negative correlations (Braun et al. 1997; Fox et al. 2000; Ingham et al. 2012) to stuttering rate. Also, previous studies in children who stutter have found anatomical differences to correlate with stuttering severity in both anterior premotor/motor and posterior sensory areas (Mock et al. 2012; Chang et al. 2015). The present findings complement these imaging studies by revealing a precise time course of auditory processing differences in MWS that strongly correlates to clinically-relevant levels of dysfluency.
Lastly, we note three observations. First, the effect sizes in this small sample were large, and are likely at the limit of what would be expected given the reliability of the underlying measures. Thus, we expect regression to the mean in future work. Nonetheless, the neurophysiological indices of stuttering severity could be useful for the selection and evaluation of personalized therapies to improve fluency in those who stutter. Second, most studies that use a cue-target paradigm to study manual movement preparation examine ERSPs as a function of how much information S1 provides, but do not include a control condition without movement preparation. Thus, our study has also shown that just passive stimulus processing in the context of a cue-target paradigm can yield markers of stuttering severity. Third, these neurophysiological indices of stuttering severity are unlikely to have a 1:1 relationship to the mechanism(s) of developmental stuttering, which may include multiple, non-exclusive factors such as sensorimotor integration, genetic risk, lexical processes, and emotional regulation (Smith 1999; Max et al. 2004; Conture et al. 2006; Civier et al. 2013). Overall, the new findings presented here fit with an emerging literature that shows, in various ways, that neural activity before speaking differs in people who stutter.
Highlights.
Speech preparation in people who stutter was tested by recording EEG in a modified CNV paradigm.
Beta desynchronization and auditory ERPs during speech preparation correlated to stuttering rate.
Similar correlations were seen in a passive CNV task, show generality beyond speech preparation.
Acknowledgments
We thank Edward Conture, Lauren Stowe, and Michael Seay for helpful comments on this paper. This study was supported by NIH grant #P20GM103629.
Footnotes
Conflict of Interest
None of the authors have potential conflicts of interest to be disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arciniegas DB. Clinical electrophysiologic assessments and mild traumatic brain injury: state-of-the-science and implications for clinical practice. Int J Psychophysiol. 2011;82(1):41–52. doi: 10.1016/j.ijpsycho.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Arends N, Povel D, Kolk H. Stuttering as an attentional phenomenon. J Fluency Disord. 1988;13:141–51. [Google Scholar]
- Azrin N, Jones RJ, Flye B. A synchronization effect and its application to stuttering by a portable apparatus. J Appl Behav Anal. 1968;1(4):283–95. doi: 10.1901/jaba.1968.1-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Marzano N, Infarinato F, Iacoboni M, Rizza G, Aschieri P, et al. “Neural efficiency” of experts’ brain during judgment of actions: a high-resolution EEG study in elite and amateur karate athletes. Behav Brain Res. 2010;207(2):466–75. doi: 10.1016/j.bbr.2009.10.034. [DOI] [PubMed] [Google Scholar]
- Barr D, Carmel N. Stuttering inhibition with high frequency narrow-band masking noise. J Aud Res. 1969;9:40–4. [Google Scholar]
- Beal DS, Gracco V, Brettschneider J. A voxel-based morphometry (VBM) analysis of regional grey and white matter volume abnormalities within the speech production network of children who stutter. Cortex. 2013;49(8):2151–61. doi: 10.1016/j.cortex.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal DS, Gracco VL, Lafaille SJ, De Nil LF. Voxel-based morphometry of auditory and speech-related cortex in stutterers. Neuroreport. 2007;18(12):1257–60. doi: 10.1097/WNR.0b013e3282202c4d. [DOI] [PubMed] [Google Scholar]
- Beal DS, Quraan MA, Cheyne DO, Taylor MJ, Gracco VL, De Nil LF. Speech-induced suppression of evoked auditory fields in children who stutter. Neuroimage. 2011;54(4):2994–3003. doi: 10.1016/j.neuroimage.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermann-Ruben K, Salmelin R, Schnitzler A. Right rolandic activation during speech perception in stutterers: a MEG study. Neuroimage. 2005;25(3):793–801. doi: 10.1016/j.neuroimage.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Bloodstein O, Bernstein Ranter N. The Handbook on Stuttering. 6. Clifton Park, NY: Delmar Learning; 2008. [Google Scholar]
- Braun AR, Varga M, Stager S, Schulz G, Selbie S, Maisog JM, et al. Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H2(15)O positron emission tomography study. Brain. 1997;120:761–84. doi: 10.1093/brain/120.5.761. [DOI] [PubMed] [Google Scholar]
- Brayton ER, Conture EG. Effects of noise and rhythmic stimulation on the speech of stutterers. J Speech Hear Res. 1978;21(2):285–94. doi: 10.1044/jshr.2102.285. [DOI] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT. Stuttered and fluent speech production: an ALE meta-analysis of functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):105–17. doi: 10.1002/hbm.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. Neuroimage. 2008;39(3):1333–44. doi: 10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Horwitz B, Ostuni J, Reynolds R, Ludlow CL. Evidence of left inferior frontal-premotor structural and functional connectivity deficits in adults who stutter. Cereb Cortex. 2011;21(11):2507–18. doi: 10.1093/cercor/bhr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Zhu DC. Neural network connectivity differences in children who stutter. Brain. 2013;136:3709–26. doi: 10.1093/brain/awt275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-E, Zhu DC, Choo AL, Angstadt M. White matter neuroanatomical differences in young children who stutter. Brain. 2015;138:694–711. doi: 10.1093/brain/awu400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Chen X, Kuang CW, Huang XT. Neural oscillatory correlates of duration maintenance in working memory. Neuroscience. 2015;290:389–97. doi: 10.1016/j.neuroscience.2015.01.036. [DOI] [PubMed] [Google Scholar]
- Chevillet MA, Jiang X, Rauschecker JP, Riesenhuber M. Automatic phoneme category selectivity in the dorsal auditory stream. J Neurosci. 2013;33(12):5208–15. doi: 10.1523/JNEUROSCI.1870-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civier O, Bullock D, Max L, Guenther FH. Computational modeling of stuttering caused by impairments in a basal ganglia thalamo-cortical circuit involved in syllable selection and initiation. Brain Lang. 2013;126(3):263–78. doi: 10.1016/j.bandl.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley EM, Michalewski HJ, Starr A. The N100 auditory cortical evoked potential indexes scanning of auditory short-term memory. Clin Neurophysiol. 1999;110(12):2086–93. doi: 10.1016/s1388-2457(99)00183-2. [DOI] [PubMed] [Google Scholar]
- Conture EG. Some effects of noise on the speaking behavior of stutterers. J Speech Hear Res. 1974;17(4):714–23. doi: 10.1044/jshr.1704.714. [DOI] [PubMed] [Google Scholar]
- Conture EG, Walden TA, Arnold HS, Graham CG, Hartfield KN, Karrass J. Communication-emotional model of stuttering. In: Ratner NB, Tetnowski J, editors. Stuttering Res Pract Contemp issues approaches. Mahwah, NJ: Lawrence Erlbaum Assoc; 2006. pp. 17–46. [Google Scholar]
- Crone N. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event- related desynchronization. Brain. 1998;121(12):2271–99. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dong S, Reder LM, Yao Y, Liu Y, Chen F. Individual differences in working memory capacity are reflected in different ERP and EEG patterns to task difficulty. Brain Res. 2015;1616:146–56. doi: 10.1016/j.brainres.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Doyle LMF, Yarrow K, Brown P. Lateralization of event-related beta desynchronization in the EEG during pre-cued reaction time tasks. Clin Neurophysiol. 2005;116(8):1879–88. doi: 10.1016/j.clinph.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol. 2010;20(2):156–65. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Fawcett RG. Stroke-associated acquired stuttering. CNS Spectr. 2005;10(2):94–5. doi: 10.1017/s1092852900019416. [DOI] [PubMed] [Google Scholar]
- Finnigan S, Robertson IH. Resting EEG theta power correlates with cognitive performance in healthy older adults. Psychophysiology. 2011;48(8):1083–7. doi: 10.1111/j.1469-8986.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Bollich AM, Feldman J, Corey DM, Hurley M, Lemen LC, et al. Aberrant auditory processing and atypical planum temporale in developmental stuttering. Neurology. 2004;63(9):1640–6. doi: 10.1212/01.wnl.0000142993.33158.2a. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Mock JR, Cindass R, Corey DM. Atypical caudate anatomy in children who stutter. Percept Mot Skills. 2013;116(2):528–43. doi: 10.2466/15.10.PMS.116.2.528-543. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Mock JR, Corey DM, Golob EJ, Conture EG. The SpeechEasy device in stuttering and nonstuttering adults: Fluency effects while speaking and reading. Brain Lang. 2013;126(2):141–50. doi: 10.1016/j.bandl.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Zamarripa F, Xiong JH, Lancaster JL. Brain correlates of stuttering and syllable production. A PET performance-correlation analysis. Brain. 2000;123:1985–2004. doi: 10.1093/brain/123.10.1985. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJJ, Ingham JCC, Hirsch TBB, Downs JHH, Martin C, et al. A PET study of the neural systems of stuttering. Nature. 1996;382:158–62. doi: 10.1038/382158a0. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Trainor LJ, Large EW, Ross B. Internalized timing of isochronous sounds is represented in neuromagnetic β oscillations. J Neurosci. 2012;32(5):1791–802. doi: 10.1523/JNEUROSCI.4107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud A-L, Neumann K, Bachoud-Levi A-C, von Gudenberg AW, Euler HA, Lanfermann H, et al. Severity of dysfluency correlates with basal ganglia activity in persistent developmental stuttering. Brain Lang. 2008;104(2):190–9. doi: 10.1016/j.bandl.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Miranda GG, Johnson JK, Starr A. Sensory cortical interactions in aging, mild cognitive impairment, and Alzheimer ’s disease. Neurobiol Aging. 2001;22:755–63. doi: 10.1016/s0197-4580(01)00244-5. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Pratt H, Starr A. Preparatory slow potentials and event-related potentials in an auditory cued attention task. Clin Neurophysiol. 2002;113(10):1544–57. doi: 10.1016/s1388-2457(02)00220-1. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Starr A. Serial position effects in auditory event-related potentials during working memory retrieval. J Cogn Neurosci. 2004;16(1):40–52. doi: 10.1162/089892904322755548. [DOI] [PubMed] [Google Scholar]
- Grandchamp R, Delorme A. Single-trial normalization for event-related spectral decomposition reduces sensitivity to noisy trials. Front Psychol. 2011;2:236. doi: 10.3389/fpsyg.2011.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AC, Biousse V, Cook AA, Newman NJ. Stroke-associated stuttering. Arch Neurol. 1999;56(5):624–7. doi: 10.1001/archneur.56.5.624. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–84. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Guenther FH. Cortical interactions underlying the production of speech sounds. J Commun Disord. 2006;39(5):350–65. doi: 10.1016/j.jcomdis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Haegens S, Nácher V, Luna R, Romo R, Jensen O. α-Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci U S A. 2011;108(48):19377–82. doi: 10.1073/pnas.1117190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. Computational neuroanatomy of speech production. Nat Rev Neurosci. 2012;13(2):135–45. doi: 10.1038/nrn3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Houde JF, Rong F. Sensorimotor Integration in Speech Processing: Computational Basis and Neural Organization. Neuron. 2011;69(3):407–22. doi: 10.1016/j.neuron.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182(4108):177–80. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hubbard TL. Auditory imagery: empirical findings. Psychol Bull. 2010;136(2):302–29. doi: 10.1037/a0018436. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Nordby H. Electrophysiological correlates to cued attentional shifts in the visual and auditory modalities. Behav Neural Biol. 1994;62(1):21–32. doi: 10.1016/s0163-1047(05)80055-x. [DOI] [PubMed] [Google Scholar]
- Hummel F. Inhibitory control of acquired motor programmes in the human brain. Brain. 2002;125(2):404–20. doi: 10.1093/brain/awf030. [DOI] [PubMed] [Google Scholar]
- Indefrey P. The spatial and temporal signatures of word production components: a critical update. Front Psychol. 2011;2:255. doi: 10.3389/fpsyg.2011.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92(1–2):101–44. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Ingham RJ, Grafton ST, Bothe AK, Ingham JC. Brain activity in adults who stutter: similarities across speaking tasks and correlations with stuttering frequency and speaking rate. Brain Lang. 2012;122(1):11–24. doi: 10.1016/j.bandl.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell CA, Neumann K, von Kriegstein K, Posenenske C, von Gudenberg AW, Euler H, et al. How the brain repairs stuttering. Brain. 2009;132:2747–60. doi: 10.1093/brain/awp185. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Ogata K, Umesaki T, Yoshiura T. Spatiotemporal signatures of an abnormal auditory system in stuttering. Neuroimage. 2011;55(3):891–9. doi: 10.1016/j.neuroimage.2010.12.083. [DOI] [PubMed] [Google Scholar]
- Lickley RJ, Hartsuiker RJ, Corley M, Russell M, Nelson R. Judgment of disfluency in people who stutter and people who do not stutter: results from magnitude estimation. Lang Speech. 2005;48:299–312. doi: 10.1177/00238309050480030301. [DOI] [PubMed] [Google Scholar]
- Lincoln M, Packman A, Onslow M. Altered auditory feedback and the treatment of stuttering: a review. J Fluency Disord. 2006;31(2):71–89. doi: 10.1016/j.jfludis.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Liotti M, Ingham JC, Takai O, Paskos DK, Perez R, Ingham RJ. Spatiotemporal dynamics of speech sound perception in chronic developmental stuttering. Brain Lang. 2010;115(2):141–7. doi: 10.1016/j.bandl.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol. 1993;86(4):283–93. doi: 10.1016/0013-4694(93)90110-h. [DOI] [PubMed] [Google Scholar]
- Max L, Guenther FH, Ghosh SS, Wallace ME. Unstable or insufficiently activated internal models and feedback-baised motor control as sources of dysfluency: a theoretical model of stuttering. Contemp Issues Commun Sci Disord. 2004;31:105–22. [Google Scholar]
- Maxfield ND, Huffman JL, Frisch SA, Hinckley JJ. Neural correlates of semantic activation spreading on the path to picture naming in adults who stutter. Clin Neurophysiol. 2010;121(9):1447–63. doi: 10.1016/j.clinph.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Mock JR, Foundas AL, Golob EJ. Modulation of sensory and motor cortex activity during speech preparation. Eur J Neurosci. 2011;33(5):1001–11. doi: 10.1111/j.1460-9568.2010.07585.x. [DOI] [PubMed] [Google Scholar]
- Mock JR, Foundas AL, Golob EJ. Speech preparation in adults with persistent developmental stuttering. Brain Lang. 2015;149:97–105. doi: 10.1016/j.bandl.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock JR, Zadina JN, Corey DM, Cohen JD, Lemen LC, Foundas AL. Atypical brain torque in boys with developmental stuttering. Dev Neuropsychol. 2012;37(5):434–52. doi: 10.1080/87565641.2012.661816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möttönen R, Dutton R, Watkins KE. Auditory-motor processing of speech sounds. Cereb Cortex. 2013;23(5):1190–7. doi: 10.1093/cercor/bhs110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musall S, von Pföstl V, Rauch A, Logothetis NK, Whittingstall K. Effects of neural synchrony on surface EEG. Cereb Cortex. 2014;24(4):1045–53. doi: 10.1093/cercor/bhs389. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Gaillard AW, Mantysalo S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. 1978;42(4):313–29. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Picton T, Näätänen R. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24(4):375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Nunez P, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. 2. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Del Percio C, Babiloni C, Marzano N, Iacoboni M, Infarinato F, Vecchio F, et al. “Neural efficiency” of athletes’ brain for upright standing: a high-resolution EEG study. Brain Res Bull. 2009;79(3–4):193–200. doi: 10.1016/j.brainresbull.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Del Percio C, Infarinato F, Iacoboni M, Marzano N, Soricelli A, Aschieri P, et al. Movement-related desynchronization of alpha rhythms is lower in athletes than non-athletes: a high-resolution EEG study. Clin Neurophysiol. 2010;121(4):482–91. doi: 10.1016/j.clinph.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G. Spatiotemporal analysis of alpha frequency components with the ERD technique. Brain Topogr. 1989;2(1–2):3–8. doi: 10.1007/BF01128838. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110(11):1842–57. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preibisch C, Neumann K, Raab P, Euler HA, von Gudenberg AW, Lanfermann H, et al. Evidence for compensation for stuttering by the right frontal operculum. Neuroimage. 2003;20(2):1356–64. doi: 10.1016/S1053-8119(03)00376-8. [DOI] [PubMed] [Google Scholar]
- Romei V, Brodbeck V, Michel C, Amedi A, Pascual-Leone A, Thut G. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex. 2008;18(9):2010–8. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmelin R. Clinical neurophysiology of language: the MEG approach. Clin Neurophysiol. 2007;118(2):237–54. doi: 10.1016/j.clinph.2006.07.316. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Schnitzler A, Schmitz F, Freund HJ. Single word reading in developmental stutterers and fluent speakers. Brain. 2000;123:1184–202. doi: 10.1093/brain/123.6.1184. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Schnitzler A, Schmitz F, Jancke L, Witte OW, Freund HJ. Functional organization of auditory cortex is different in stutters and fluent speakers. Neuroreport. 1998;9(10):2225–9. doi: 10.1097/00001756-199807130-00014. [DOI] [PubMed] [Google Scholar]
- Sheehan JG. Stuttering behavior: A phonetic analysis. J Commun Disord. 1974;7(3):193–212. doi: 10.1016/0021-9924(74)90031-8. [DOI] [PubMed] [Google Scholar]
- Siegel GM, Haugen D. Audience size and variations in stuttering behavior. J Speech Hear Res. 1964;7:381–8. doi: 10.1044/jshr.0704.381. [DOI] [PubMed] [Google Scholar]
- Smith A. Stuttering: A unified approach to a multifactorial, dynamic disorder. In: Ranter Bernstein, Healey EC., editors. Stuttering Res Pract Bridg gap. Mahwah, NJ: Lawrence Erlbaum Assoc; 1999. pp. 27–44. [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn. 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, Büchel C. Mechanisms of disease Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet. 2002;360:380–3. doi: 10.1016/S0140-6736(02)09610-1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Szurhaj W, Derambure P, Labyt E, Cassim F, Bourriez J-L, Isnard J, et al. Basic mechanisms of central rhythms reactivity to preparation and execution of a voluntary movement: a stereoelectroencephalographic study. Clin Neurophysiol. 2003;114(1):107–19. doi: 10.1016/s1388-2457(02)00333-4. [DOI] [PubMed] [Google Scholar]
- Takemi M, Masakado Y, Liu M, Ushiba J. Event-related desynchronization reflects downregulation of intracortical inhibition in human primary motor cortex. J Neurophysiol. 2013;110(5):1158–66. doi: 10.1152/jn.01092.2012. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Kreiter A, Bertrand O. Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans. Vis Neurosci. 1999;16(3):449–59. doi: 10.1017/s0952523899163065. [DOI] [PubMed] [Google Scholar]
- Taylor PCJ, Thut G. Brain activity underlying visual perception and attention as inferred from TMS-EEG: a review. Brain Stimul. 2012;5(2):124–9. doi: 10.1016/j.brs.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, North DM, Curtin RT, Walker RA, Biver CJ, Gomez JF, et al. An EEG Severity Index of Traumatic Brain Injury. J Neuropsychiatry Clin Neurosci. 2001;13(1):77–87. doi: 10.1176/jnp.13.1.77. [DOI] [PubMed] [Google Scholar]
- Tian X, Poeppel D. Mental imagery of speech: linking motor and perceptual systems through internal simulation and estimation. Front Hum Neurosci. 2012;6:314. doi: 10.3389/fnhum.2012.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G. Beta-band activity during motor planning reflects response uncertainty. J Neurosci. 2010;30(34):11270–7. doi: 10.1523/JNEUROSCI.6026-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2(4):229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vasic N, Wijnen F. Stuttering as a monitoring deficit. In: Hartsuiker RJ, Bastiaanse R, Postma A, Wijnen F, editors. Phonol encoding Monit Norm Pathol speech. Hove: Psychology Press; 2005. pp. 226–47. [Google Scholar]
- Waddell DE, Goggans PM, Snyder GJ. Novel tactile feedback to reduce overt stuttering. Neuroreport. 2012;23(12):727–30. doi: 10.1097/WNR.0b013e328356b108. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2008;131:50–9. doi: 10.1093/brain/awm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G. Effect of sentence structure on stuttering. J Fluency Disord. 1979;4(2):123–9. [Google Scholar]
- Wingate ME. Sound and Pattern in “Artificial” Fluency. J Speech Lang Hear Res. 1969;12(4):677. doi: 10.1044/jshr.1204.677. [DOI] [PubMed] [Google Scholar]
- Van Winsum W, Sergeant J, Geuze R. The functional significance of event-related desynchronization of alpha rhythm in attentional and activating tasks. Electroencephalogr Clin Neurophysiol. 1984;58(6):519–24. doi: 10.1016/0013-4694(84)90042-7. [DOI] [PubMed] [Google Scholar]
- Xuan Y, Meng C, Yang Y, Zhu C, Wang L, Yan Q, et al. Resting-state brain activity in adult males who stutter. PLoS One. 2012;7(1):e30570. doi: 10.1371/journal.pone.0030570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MA. Audience size, perceived situational difficulty, and stuttering frequency. J Speech Hear Res. 1965;8(4):401–7. doi: 10.1044/jshr.0804.401. [DOI] [PubMed] [Google Scholar]
- Yurgil KA, Golob EJ. Neural activity before and after conscious perception in dichotic listening. Neuropsychologia. 2010;48(10):2952–8. doi: 10.1016/j.neuropsychologia.2010.06.004. [DOI] [PubMed] [Google Scholar]