Abstract
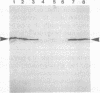
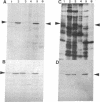
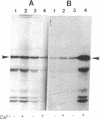
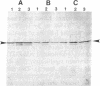
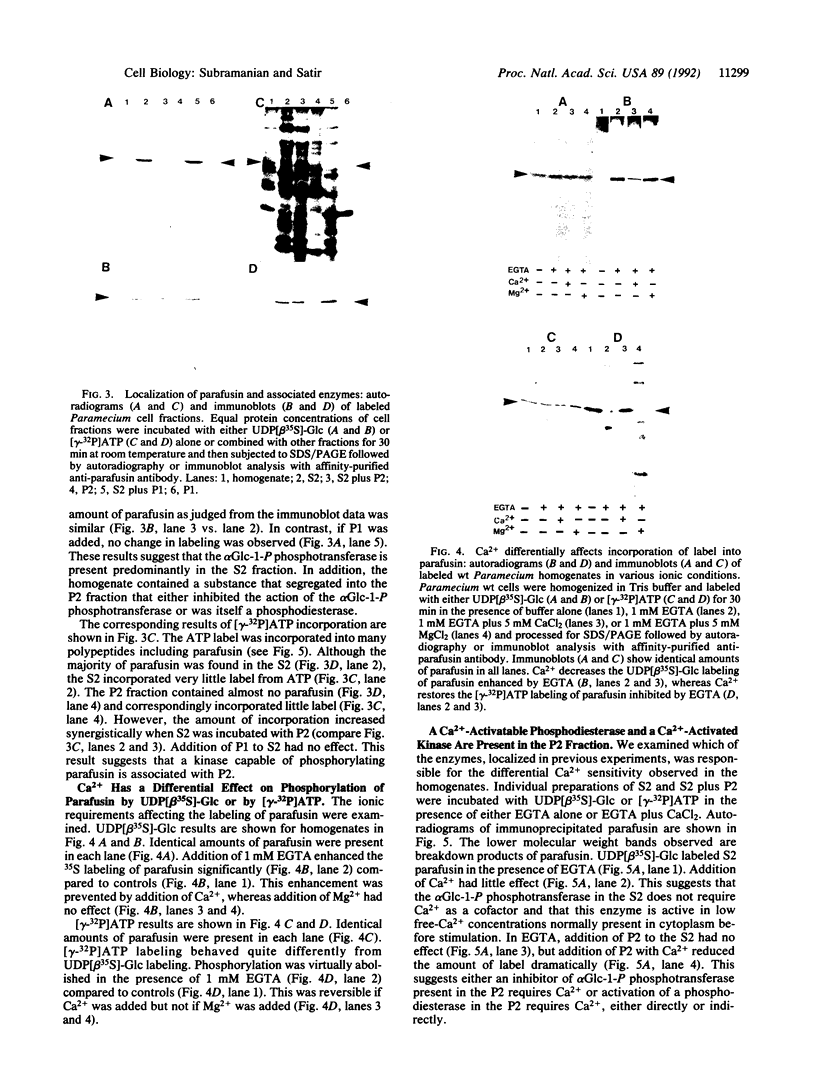
Parafusin, a cytosolic phosphoglycoprotein of M(r) 63,000, is dephosphorylated and rephosphorylated rapidly in a Ca(2+)-dependent manner upon stimulation of exocytosis in vivo in wild-type (wt) Paramecium. In contrast, the temperature-sensitive exocytosis mutant nd9, grown at the nonpermissive temperature (27 degrees C), does not exocytose or dephosphorylate parafusin upon stimulation in the presence of Ca2+; grown at the permissive temperature (18 degrees C), nd9 cells show a wt phenotype. Parafusin contains two types of phosphorylation sites: one where glucose 1-phosphate is added by an alpha-glucose-1-phosphate phosphotransferase and removed by a phosphodiesterase and one where phosphate from ATP is added directly to a serine residue by a protein kinase and removed by a phosphatase. We show here that, in cell fractions from wt Paramecium, both reactions can be carried out in vitro by using uridine(5'-[beta-[35S]thio])diphospho(1)-glucose (UDP[beta 35S]-Glc) and [gamma-32P]ATP, respectively. The characteristics of these pathways are different. Specifically, in the presence of Ca2+, the amount of UDP[beta 35S]-Glc label in parafusin is reduced. In contrast, identical labeling experiments with [gamma-32P]ATP show that Ca2+ enhances labeling of parafusin. Mg2+ had no appreciable effect on either labeling. Removal of the UDP[beta 35S]-Glc label on parafusin in the presence of Ca2+ correlates with the in vivo dephosphorylation seen upon exocytosis. Incubations with UDP[beta 35S]-Glc were then performed with homogenates and nd9 cell fractions grown at 27 degrees C under the ionic conditions used for wt cells. These labelings were not affected by Ca2+, in contrast to results from wt cells but in accord with those obtained earlier with nd9-27 mutant cells in vivo. Factors responsible for both dephosphorylation and Ca2+ sensitivity were found in the high-speed pellet (P2) in wt cells, suggesting that the putative phosphodiesterase is in this fraction and that the defect in the mutant nd9-27 residues in the Ca2+ activation of the phosphodiesterase. We conclude that the in vivo dephosphorylation of parafusin that occurs upon exocytosis is a dephosphoglucosylation due to removal of the alpha-glucose 1-phosphate and more generally that carbohydrates on cytoplasmic glycoproteins may be cyclically added and/or removed in response to extracellular stimuli.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gilligan D. M., Satir B. H. Protein phosphorylation/dephosphorylation and stimulus-secretion coupling in wild type and mutant Paramecium. J Biol Chem. 1982 Dec 10;257(23):13903–13906. [PubMed] [Google Scholar]
- Gilligan D. M., Satir B. H. Stimulation and inhibition of secretion in Paramecium: role of divalent cations. J Cell Biol. 1983 Jul;97(1):224–234. doi: 10.1083/jcb.97.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh T. J., Gilligan D. M., Satir B. H. Purification of and production of an antibody against a 63,000 Mr stimulus-sensitive phosphoprotein in Paramecium. J Biol Chem. 1987 Nov 15;262(32):15734–15739. [PubMed] [Google Scholar]
- Satir B. H., Busch G., Vuoso A., Murtaugh T. J. Aspects of signal transduction in stimulus exocytosis-coupling in Paramecium. J Cell Biochem. 1988 Apr;36(4):429–443. doi: 10.1002/jcb.240360411. [DOI] [PubMed] [Google Scholar]
- Satir B. H., Hamasaki T., Reichman M., Murtaugh T. J. Species distribution of a phosphoprotein (parafusin) involved in exocytosis. Proc Natl Acad Sci U S A. 1989 Feb;86(3):930–932. doi: 10.1073/pnas.86.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir B. H. Signal transduction events associated with exocytosis in ciliates. J Protozool. 1989 Jul-Aug;36(4):382–389. doi: 10.1111/j.1550-7408.1989.tb05531.x. [DOI] [PubMed] [Google Scholar]
- Satir B. H., Srisomsap C., Reichman M., Marchase R. B. Parafusin, an exocytic-sensitive phosphoprotein, is the primary acceptor for the glucosylphosphotransferase in Paramecium tetraurelia and rat liver. J Cell Biol. 1990 Sep;111(3):901–907. doi: 10.1083/jcb.111.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisomsap C., Richardson K. L., Jay J. C., Marchase R. B. An alpha-glucose-1-phosphate phosphodiesterase is present in rat liver cytosol. J Biol Chem. 1989 Dec 5;264(34):20540–20546. [PubMed] [Google Scholar]
- Srisomsap C., Richardson K. L., Jay J. C., Marchase R. B. Localization of the glucose phosphotransferase to a cytoplasmically accessible site on intracellular membranes. J Biol Chem. 1988 Nov 25;263(33):17792–17797. [PubMed] [Google Scholar]
- Wang J. K., Walaas S. I., Sihra T. S., Aderem A., Greengard P. Phosphorylation and associated translocation of the 87-kDa protein, a major protein kinase C substrate, in isolated nerve terminals. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2253–2256. doi: 10.1073/pnas.86.7.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieseniss E., Plattner H. Synchronous exocytosis in Paramecium cells involves very rapid (less than or equal to 1 s), reversible dephosphorylation of a 65-kD phosphoprotein in exocytosis-competent strains. J Cell Biol. 1985 Dec;101(6):2028–2035. doi: 10.1083/jcb.101.6.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]