Abstract
Purinergic signaling is important for many biological processes in humans. Purinoceptors P2Y are widely distributed in human digestive system and different subtypes of P2Y receptors mediate different physiological functions from metabolism, proliferation, differentiation to apoptosis etc. The P2Y receptors are essential in many gastrointestinal functions and also involve in the occurrence of some digestive diseases. Since different subtypes of P2Y receptors are present on the same cell of digestive organs, varying subtypes of P2Y receptors may have opposite or synergetic functions on the same cell. Recently, growing lines of evidence strongly suggest the involvement of P2Y receptors in the pathogenesis of several digestive diseases. In this review, we will focus on their important roles in the development of digestive inflammation and cancer. We anticipate that as the special subtypes of P2Y receptors are studied in depth, specific modulators for them will have good potentials to become promising new drugs to treat human digestive diseases in the near future.
Keywords: P2Y receptors, digestive inflammation, digestive cancer
INTRODUCTION
Purinoceptors are generally divided into P1 receptors which main ligand is adenosine, and P2 receptors which main ligands are nucleotides. Based on their signaling properties, P2 receptors are further subdivided into ionotropic P2X receptors that are nucleotide-gated ion channels and metabotropic P2Y receptors that are G protein-coupled receptors (GPCRs). P2Y receptors are consist of eight subtypes: five Gq/G11-coupled subtypes (P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11), usually activating phospholipase C-IP3 pathway that modulates endoplasmic reticulum calcium release, and three Gi/o-coupled subtypes (P2Y12, P2Y13 and P2Y14), mainly inhibiting adenylyl cyclase to regulate cyclic AMP/protein kinase A (PKA) [1–2]. At present, all eight P2Y receptor subtypes have been cloned in mammalian [3]. Different P2Y receptor subtypes are activated by different nucleotides. ADP has been claimed to be selective agonist of P2Y1, P2Y12 and P2Y13 receptors; however, UTP predominantly binds to P2Y2 and P2Y4 receptors, and to a lesser extent to P2Y6 receptors which preferential agonist is UDP. P2Y14 receptors are mainly activated by UDP-glucose and other UDP-sugars, or by UDP [4]. In recent decades, a growing line of evidence suggests the involvements of P2Y receptors in the pathogenesis of human diseases, and different subtypes of P2Y receptors mediate various pathophysiological processes, ranging from metabolism, proliferation, differentiation to apoptosis. Recent studies also demonstrate that P2Y receptors play important roles in the regulation of physiological functions and pathological processes in the digestive system. In this review, we will focus on the pathophysiological roles of P2Y receptors in digestive inflammation and cancer.
PHYSIOLOGICAL FUNCTIONS OF P2Y RECEPTORS IN THE DIGESTIVE ORGANS
P2Y receptors are widely expressed in digestive organs and their functions vary from neurotransmission, gland secretion, contraction and relaxation of smooth muscle to carbohydrate and lipid metabolism in the digestive system. In accordance with evidence, we first highlight physiological roles of P2Y receptors in the esophagus, stomach, liver, pancreas, and colon (Figure 1).
Figure 1. The physiological functions of P2Y receptors in digestive system.
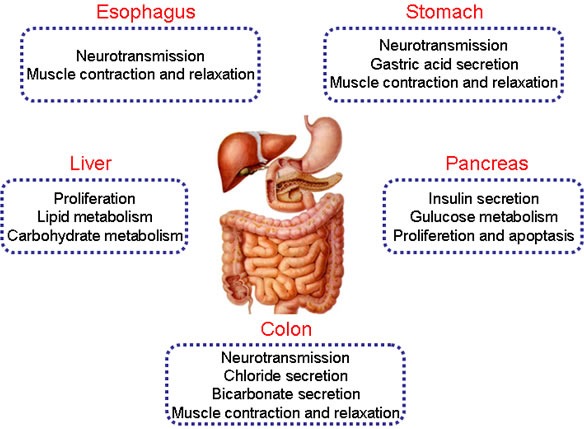
Different subtypes of P2Y receptors are expressed in human esophagus, stomach, liver, pancreas and colon. They play different roles in the regulation of physiological processes, such as neurotransmission, ion transports, metabolism, proliferation and apoptosis, muscle contraction and relaxation in the digestive organs.
Esophagus
The P2Y receptors are functionally expressed in the esophagus to play an important role in the regulation of esophageal motility. In human and porcine esophagus, P2Y1 receptors mediate lower esophageal sphincter (LES) relaxation by regulating neurotransmission [5–6]. P2Y1 receptors also mediate contraction of the circular smooth muscle layer in porcine esophagus [7]. Electrical field stimulation (EFS)-induced contractions is mediated through P2Y receptors in cat esophageal smooth muscle [8]. Feline esophageal contraction is preferentially mediated by P2Y receptors coupled to Gαi3 and Gαq proteins, which activate PLCβ, subsequently increase intracellular Ca2+ and activate PKC [9].
Stomach
Although gastric acid secretion is mainly regulated by P1 adenosine receptors [10], P2Y receptors may also regulate gastric acid secretion, gastric contraction, relaxation and neurotransmission. However, the specific receptor subtypes of P2Y receptors involved and their underlying mechanisms still need further investigation. ATP selectively inhibits histamine-stimulated gastric acid secretion from rabbit parietal cells by acting on P2Y receptors [11]. UTP and UDP can induce contraction of gastric smooth muscle through activation of P2Y receptors [12]. ATP also induces contraction of gastric smooth muscle in guinea pig via activation of P2Y receptors [13]. At least two subtypes of P2Y purinoceptors are involved in gastric contraction in guinea-pig and are related to the elevation of intracellular Ca2+ [14]. Finally, ATP may regulate NANC inhibitory neurotransmission in rat pyloric sphincter through acting on P2Y receptors [15].
Liver
Several P2Y receptor subtypes regulate hepatic physiological functions, such as carbohydrate metabolism, lipid metabolism and proliferation [16–17]. P2Y1 receptors induce glycogen phosphorylase of rat hepatocyte by raising intracellular calcium concentrations but inhibiting cyclic AMP accumulations [18]. P2Y2 receptors in human hepatocytes regulate both glycogen metabolism and proliferation-associated responses through Ca2+ and MAPK pathways [19], and induce ERK phosphorylation, Egr-1 expression, and cyclins and cell cycle progression, which are essential for efficient hepatocyte proliferation [20]. P2Y2 receptors also mediate extracellular ATP-induced c-jun N-terminal kinase signaling and cell cycle progression to promote hepatocellular proliferation [21]. P2Y13 receptors modulate reverse cholesterol transport by increasing hepatic HDL cholesterol uptake, overall hepatocyte cholesterol content, and biliary output [22–24].
Pancreas
P2Y1, 2, 4, 6, 11, 12 and 13 receptor subtypes have been identified in INS-1βcells, mouse, rat and human pancreaticβcells [25–26]. Activation of P2Y receptors inβcells is confirmed to participate in the regulation of insulin secretion, glucose metabolism and an increase in intracellular Ca2+ concentration [27]. Glucose stimulation triggers exocytosis of insulin and ATP through activation of P2Y1 receptors to result in PLC activation and DAG production in MIN6 mouse pancreaticβcells [28]. P2Y1 and P2Y6 receptors in MIN6 cells induce intracellular calcium release and insulin secretion, and prevent TNF-α induced βcells apoptosis [29]. Stimulation of P2Y13 receptors in pancreatic βcells inhibits insulin secretion via calcium influx and inhibition of cyclic AMP production [30]. High glucose and free fatty acids can also induce β cells apoptosis through stimulating P2Y13 to activate apoptotic pathways [31].
Colon
Large numbers of studies in animals and humans demonstrate that P2Y1 receptors mediate NANC inhibitory transmission of intestinal smooth muscle in mice through release of ATP and NO [10]. Activation of P2Y1 receptors can induce nerve-mediated relaxation via inhibitory neuromuscular transmission in human intestines, guinea pig small intestine and rat colon [32–35]. Endogenous nucleotides acting on P2Y1, 2, 4 receptors evoke intestinal Cl− secretion [36]. The activation of P2Y2 and P2Y4 receptors stimulate Cl− secretion in small and large intestines. Basolateral UTP-induced Cl− secretion in jejunum was partially reduced in P2Y2 knockout (40%) and P2Y4 knockout (60%) null mice [37]. Activation of P2Y2 receptors induces duodenal mucosal bicarbonate secretion via both intracellular Ca2+ release and extracellular Ca2+ entry through store-operated channels [38]. Stimulation of luminal P2Y2 and P2Y4 receptors lead to K+ secretion in mouse distal colonic mucosa [39]; however, activation of basolateral P2Y6 receptors on rat colonic enterocytes induces NaCl secretion via a synergistic increase of [Ca2+]i and cyclic AMP [40].
P2Y RECEPTORS IN DIGESTIVE INFLAMMATION
Over the past decades, many studies have highlighted fundamental roles of P2Y receptors in inflammatory diseases, particularly P2Y2, 6, 12 receptors have been well studied, such as P2Y2 receptor agonists can treat cystic fiborosis, and promote wound healing and leukocyte functions [4, 41–45]. Whereas, P2Y2 receptors have ambivalent functions, such as promoting chronic inflammatory states and fibrotic remodeling [46–48]. However, in inflammation of the digestive system, the studies of P2Y receptors are mainly concentrated on the liver and colon. The involvements of P2Y receptors in the inflammation of other digestive organs, such as esophagitis, gastritis and pancreatitis, are poorly understood and rarely presented in the literatures.
Liver
During inflammatory responses, endogenous release of ATP in the liver can activate purinergic P2 receptors. It was found that large amounts of ATP released from the liver increased the expression of P2Y2 receptors in concanavalin A-induced model of acute hepatitis in C57BL/6 mice. Liver damage and necrosis are largely decreased in C57BL/6 wild-type mice injected with suramin, an inhibitor of P2Y receptors, or in P2Y2 receptors knockout mice, in which acetaminophen-induced liver damage is also alleviated. P2Y2 receptors can promote neutrophil infiltration, regulate cell survival, and promote tumor necrosis factor-mediated cell death, supporting the view that activation of P2Y2 receptors stimulates the recruitment of neutrophils into the liver to cause hepatocyte death [49].
Hepatic stellate cells play an important role in formation of liver fibrosis and liver cirrhosis. ATP increases intracellular Ca2+ in hepatic stellate cells, which is inhibited by suramin. Interestingly, P2Y2 and P2Y4 receptors are expressed in quiescent hepatic stellate cells, whereas P2Y6 receptors are expressed in activated hepatic stellate cells. The activated hepatic stellate cells express the ectonucleotidase nucleoside triphosphate diphosphohydrolase-2 (NTPDase-2) that colocalizes with activated HSC in CCl4-induced cirrhosis. UDP regulates transcription of procollagen-1 in activated HSC via P2Y receptors activation, which is partially inhibited by the P2Y receptors inhibitor suramin, suggesting P2Y receptors may be attractive targets to prevent/treat liver fibrosis [50]. When macrophages in kupffer cells of the liver are activated by various factors, such as lipopolysaccharide (LPS), they produce various cytokines and chemokines to play important roles in hepatitis and liver fibrosis. P2Y2, 5, 6, 12, 13 receptors are strongly expressed in the liver kupffer cells of C57BL/6 mice (KUP5 cells). After stimulation with LPS, KUP5 cells produce IL-6 and TNF-α. Non-selective P2 receptor antagonist, suramin, and P2Y13 receptors selective antagonist, MRS2211, markedly inhibit LPS-induced IL-6 increase in KUP5 cells, whereas both suramin and MRS2211 do not inhibit LPS-induced TNF-α production, suggesting that P2Y13 and other P2Y receptors may be involved in LPS-induced IL-6 production in Kuffer cells and liver inflammation [51].
Colon
Intestinal inflammation can upregulate mRNA expression of P2Y2 and P2Y6 receptors in the colonic mucosa of colitic mice. The mRNA of P2Y2 and P2Y6 receptors are increased in both Crohn's and ulcerative colitis of intestinal human samples compared with noninflamed tissues. The mRNA expression of P2Y2 receptors is also increased in Caco-2 and IEC-6 cells during intestinal inflammation, but it is unknown how inflammation up-regulates expression of P2Y2 receptors. ATP or UTP stimulation of P2Y2 receptors in intestinal epithelial cells increases ICAM-1 expression and promotes transepithelial migration and adhesion of neutrophils and macrophage to the apical surface of IEC. In addition, ATP or UTP facilitates the migration of neutrophil-like PLB-985 cells and macrophage across the Caco-2 monolayer and promotes macrophage-like U-937 cells adhere to IEC monolayers. CD68+ macrophage infiltrates from the colonic epithelium and presents at the apical surface of colonocytes during intestinal inflammation. P2Y2 receptors mediate neutrophil adhesion to the surface of IEC need presence of adherent macrophage. This investigation is helpful to identify potential therapeutic targets to treat inflammatory bowel diseases [52]. Recent studies found that NF-κB p65 could regulate P2Y2 receptors transcription, and activation of P2Y2 receptors by UTP increased both cyclooxygenase-2 (COX-2) expression and PGE2 release in IEC [53]. Further studies showed the effects of CCAAT/enhancer-binding proteinβ (C/EBPβ) and NF-κB p65 on P2Y2 receptor transcription are synergistic during inflammation in IEC [54]. In the enteric nervous system, ATP has long been established as an inhibitory neurotransmitter, 75% of Hirschsprung's disease patients, aganglionosis is confined to the intestine. The expression of P2Y1 and P2Y2 receptors are absent from the submucosal and myenteric plexuses of aganglionic tissue compared to ganglionic tissue and normal controls. The deficiency of P2Y receptors in ganglionic intestine in Hirschsprung's disease suggests the absence of the inhibitory neurotransmitter, ATP. This explains the contracted state of the aganglionic gut in Hirschsprung's disease [55].
The expression of P2Y6 receptors is enhanced by inflammation with TNF-α and IFN-γ both in IEC-6 and Caco-2/15 cells. In Caco-2/15 cells, stimulation P2Y6 receptors by UDP results in an increased expression and release of CXCL8, partially depending on ERK1/2 phosphorylation. UDP also increases ERK1/2 phosphorylation of IEC-6 cells, suggesting the involvement of P2Y6 receptors [56]. Indeed, UDP stimulation of P2Y6 receptors promotes CXCL8 transcription through ERK1/2 activation and the AP-1 complex of transcription factors, aggravating colitis-like disease in mice by stimulating neutrophil recruitment at the site of inflammation. CXLC8 gene expression is regulated at the transcriptional level by mediating ERK1/2-dependent phosphorylation of c-fos in IEC through P2Y6 receptors activation. P2Y6 regulation of CXCL8 expression requires PKCδactivation upstream of the signaling pathway composed of MEK1/2-ERK1/2 and c-fos [57]. Previous reports revealed that T cells play an important role in the pathogenesis of IBD and extracellular nucleotides can regulate colonic epithelial cell damage during inflammation. Interestingly, UDP, P2Y6 receptors selective agonist, activates peripheral T cells and increases mRNA levels of P2Y6 receptors, and raises intracellular calcium concentration. Although P2Y6 receptors are expressed in human T cell infiltrating IBD, the roles of P2Y6 receptors in the pathogenesis of IBD need further studies [58].
P2Y RECEPTORS IN DIGESTIVE CANCER
Different subtypes of P2Y receptors are expressed in many cancer cells and tissues to be likely involved in cancer development, such as P2Y receptors in melanoma [59], skin squamous cell carcinoma [60], lung cancer [61–62], prostate cancer [63–66], glioma [67–68], breast cancer [69–75], ovarian cancer [76], and haematological malignancies [77], etc. Recent growing lines of evidence suggest an important role of P2Y receptors in digestive tumorigenesis. Different subtypes of P2Y receptors are present in cancer cells and primary cancer tissues of digestive system; however, the mechanisms by which these receptors play in the devolvement and progression of cancers are still poorly understood. The involvement of P2Y receptors in digestive cancer is mainly investigated in esophageal cancer, hepatocellular carcinoma, biliary cancer, pancreatic cancer and colorectal cancer, which are summarized in Table 1 and Figure 2. Although expression and function of P2Y receptors are well documented in normal human and animal stomach, their involvements in the pathogenesis of gastric cancer have not been explored so far.
Table 1. Involvement of P2Y receptors in various types of digestive cancer.
| Cancer types | Tissue cell line | P2Y receptor subtypes | Signaling pathway | Pathological mechanisms | Reference |
|---|---|---|---|---|---|
| Esophageal cancer | Kyse-140 | P2Y2 | PLC/Ca2+ | anti-proliferative apoptosis-inducing | [78] |
| Hepatic carcinoma | Huh-7 Rat hapatoma cell line | P2Y1,2,13 | Ca2+ | volume-regulatory cell metabolism | [79–81] |
| HePG2 BEL-7404 | P2Y2 | Ca2+ | promoting proliferation migration growth | [82] | |
| Rat hepatoma HTC cells | P2Y2 | Ca2+ MAPK | glucose metabolism | [83] | |
| HePG2 huh-7 | P2Y1,2,4,6 | Ca2+ | unknown | [84] | |
| Biliary cancer | Mz-Cha-1 | P2Y1,2,4,6 | Ca2+ | unknown | [85] |
| Pancreatic cancer | PANC-1 | P2Y1,2, 6 | PLC IP3/PKC | pro-proliferative | [86–87] |
| Colon cancer | HT-29 | P2Y2 | ECAR | tumor cell metabolism | [90] |
| HT-29 calo320MD | P2Y2 | Ca2+ cyclic AMP | anti-proliferative apoptosis-inducing | [91] | |
| HT-29 Primary cancer | P2Y2,4 | Ca2+ | anti-proliferative apoptosis-inducing | [92–93] | |
| HCT8 Caco2 | P2Y1,2,4,6,11,12 | Ca2+ | pro-proliferative apoptosis-inducing | [94] | |
| HT-29 | P2Y2 | ERK P38 MAPK | anti-proliferative apoptosis-inducing | [95–96] | |
| Caco2 | P2Y2,4 | Ca2+ MAPK | proliferative | [97–99] |
Figure 2. P2Y receptors-mediated Ca2+ signaling in proliferation or apoptosis of digestive cancer cells.
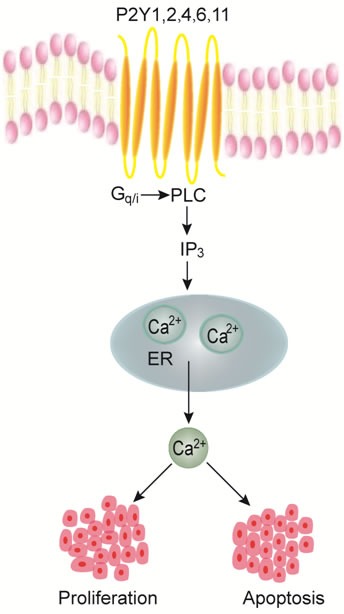
Stimulation of Gq/G11-coupled P2Y receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6 and P2Y11) activates PLC/IP3 pathway to induce intracellular calcium release from the endoplasmic reticulum (ER). An increase in intracellular calcium concentrations would increase the proliferation or apoptosis of different digestive cancer cells.
Esophageal cancer
The squamous esophageal cancer cell line, Kyse-140 cells express mRNA of P2X4, P2X5, and P2Y2 receptors, but not P2X1 and P2X7 receptors that are mainly associated with apoptosis. The mRNA of P2Y2 and P2X4 receptors are also found in biopsies of human squamous esophageal cancer; however, in accordance with the Kyse-140 cells, the mRNA of the P2X1 and P2X7 receptors are not expressed in human squamous esophageal cancer biopsy. Although mRNA of P2Y2 and P2X4 receptors was found in both Kyse-140 and esophageal primary culture cells, only P2Y2 receptors protein specific fluorescence was detected in the membrane of the Kyse-140 and esophageal primary culture cells. ATP, UTP and ATPγS, responding to P2Y2 receptors activation, result in an increase of intracellular Ca2+ levels. Incubation of Kyse-140 cells with the phospholipase C inhibitor U73122 dose-dependently inhibits ATP-induced intracellular Ca2+ level, suggesting that P2Y2 receptors mediate intracellular Ca2+ via phospholipase C (PLC) activation in Kyse-140 cells. Extracellular ATP as well as ATP analogue dose-dependently increase the proportion of cells in the S-phase of the cell cycle in return inhibiting proliferation of primary cell cultures of human esophageal cancer as well as Kyse-140; however, only ATP but not ATP analogue dose-dependently induces caspase-3 activity and increases in apoptosis of Kyse-140 cells through activation of P2Y2 receptors [78]. Together, these findings suggest that purinergic nucleotides may be tumor preventers through P2Y2 receptors/PLC/Ca2+ signaling in squamous esophageal cancer.
Hepatocellular carcinoma
P2Y1 purinergic receptors were found to play a role in the response of hepatocellular carcinoma (HCC) cells to osmotic swelling and involved in the volume-regulatory response [79]. However, in HCC cells, copper inhibits thapsigargin-sensitive Ca2+ stores by acting P2Y2 receptors to lead to inhibition of regulatory volume decrease (RVD) [80]. The mRNA and protein levels of P2Y13 receptors are confirmed in Huh-7 hepatoma cells that can release ATP when exposed to hypotonicity medium. On the other hand, ADP can activate P2Y13 receptors to potentiate volume regulatory decrease (RVD) and then mediate cell metabolism [81].
A recent study showed that both mRNA and protein expression levels of P2Y2 receptors were dramatically higher in native human HCC and human HCC cell lines compared with human normal hepatocytes. Extracellular nucleotides-induced intracellular Ca2+ increase is markedly higher in human HCC cells than normal hepatocytes. Activation of P2Y2 receptors significantly promotes proliferation and migration of HCC cells and volume growth of HCC in nude mice through store-operated calcium channels (SOCs)-mediated Ca2+ signaling [82]. Insulin and ATP induce a dose-dependent increase in p44/42 MAPK phosphorylation in rat HCC cells and chelation of extracellular Ca2+ with EGTA diminishes ATP- and insulin-induced p44/42 MAPK phosphorylation. Patch clamp electrophysiology and fluorescence microscopy showed that insulin and ATP induced monophasic and multiphasic changes in membrane potential and intracellular Ca2+ in HCC cells. Therefore, insulin and ATP effects are synergistic to regulate glucose metabolism of HCC cells [83]. Although the mRNA of P2Y1, P2Y2, P2Y4 and P2Y6 receptors were detected in HepG2 and HuH-7 cells, P2Y1 and P2Y6 receptor agonists, ATP and UDP, did not alter intracellular Ca2+, suggesting that these receptors are not expressed at functional levels. However, UTP through activation of P2Y2 and P2Y4 receptors can mobilize internal Ca2+ via inositol 1,4,5-trisphosphate (IP3) [84]. Therefore, these P2Y receptors may play major roles in the pathogenesis of HCC.
Biliary cancer
Although the mRNAs for P2Y1, P2Y2, P2Y4 and P2Y6 purinergic receptors subtypes are found in biliary epithelial cancer cells (Mz-Cha-1), but only P2Y2 receptors are present at the protein level. Not only extracellular ATP dose-dependently results in an intracellular Ca2+ increase, but also UTP produces a similar Ca2+ response and cross-desensitation. ATP induces cytosolic and nuclear Ca2+ transients [85]. To date, only expression of P2Y receptors is observed in biliary epithelial cancer cells, however, the roles of P2Y receptors in biliary cancer need further investigation.
Pancreatic cancer
P2Y receptors, especially P2Y1, P2Y2 and P2Y6 receptors are highly expressed in PANC-1, a duct epithelial cell derived from human primary pancreatic cancer cells. P2Y1 and P2Y6 proteins were also found in PANC-1 cells. ADP activation of P2Y1 receptors and UDP acting P2Y6 receptors increase proliferation of PANC-1 cell through PLC/IP3/PKC pathway. This proliferative action of P2Y receptors may potentially apply to recover pancreatic duct epithelial damage by physiological or pathological processes [86]. UTP or P2Y2 receptor selective agonist MRS2768 can increase proliferation of PANC-1, which is significantly decreased by P2Y receptor antagonist suramin and siRNA against P2Y2 receptors. UTP/P2Y2 receptor regulation of pancreatic cell proliferation depends on PLC/IP3/PKC and phosphorylation of Akt [87]. In INS-1 cells and rat pancreatic islets, ATP at low concentrations increases insulin release via P2Y receptors and PLC; however, ATP at high concentrations inhibits insulin release after metabolizing to adenosine [88]. So far, the roles of P2Y receptors in pancreatic cancer are poorly understood and need lucubrating.
Colorectal cancer
P2Y2 and P2Y4 receptors are overexpressed in human colon cancer compared with normal colon tissues although their functional significance need further studies [89]. Immunocytochemistry and western blot analysis also demonstrate the protein expression of P2Y2 receptors in HT-29 human colon carcinoma cells. ATP or UTP elicits a biphasic effect of extracellular acidification rate by activating P2Y2 receptors in HT-29 cells, but effects of UTP or ATP are resistant to suramin, suggesting that agonists of purinoceptors may affect tumor cell metabolism [90]. The mRNA of P2Y2 receptors is expressed in two colorectal carcinoma cell lines (HT29, Colo320DM) and short-term stimulation of P2Y2 receptors cause both intracellular Ca2+ release and transmembrane Ca2+ influx, and a subsequent increase in cyclic AMP. This effect is inhibited by BAPTA-AM. Prolonged stimulation of P2Y2 receptors induces a time-dependent increase in apoptosis in both cell lines and causes a dose-dependent inhibition of cell proliferation up to 85% (Colo320 DM) or 64% (HT29). Chelating [Ca2+]i with BAPTA-AM almost completely abolishes this effect. Moreover, forskolin or cyclic AMP derivatives cause a rise in intracellular cyclic AMP and lead to synergistic anti-proliferative effect in both cell lines. This finding demonstrates P2Y2 receptors play major roles in anti-proliferative and apoptosis-inducing in colorectal carcinoma cell lines [91]. The primary cell cultures of human colorectal carcinomas and HT29 cell line express functional P2U-receptors (P2Y2 and P2Y4). ATP or UTP at micromolar concentrations leads to a rapid biphasic increase of [Ca2+]i and cross-desensitization between two nucleotides. P2U-receptor agonist ATP derivative ATP-γ-S inhibits proliferation and induces apoptosis of HT 29 cells [92–93].
Two human colorectal carcinoma cell lines (HCT8 and Caco-2) express mRNA of P2Y1, 2, 4, 6, 11, 12 receptors and proteins of P2Y1 and P2Y2 receptors. ATP, at high concentrations, induces apoptosis through P2Y1 receptors; conversely, ATP, at lower concentrations, and UTP stimulates proliferation of human colorectal carcinoma cells, probably acting on P2Y2 receptors. UTP can trigger calcium influxes through either P2Y2 or P2Y4 receptors, which is inhibited by suramin. Therefore, stimulation of purinergic receptors may contribute to the modulation of epithelial carcinoma cell proliferation and apoptosis [94]. Ursolic acid could inhibit proliferation of HT29 and induce apoptosis via P2Y2 receptors-mediated inhibition of ERK phosphorylation and activation of p38 MAPK pathway [95–96]. The mRNA of P2Y2 and P2Y4 receptors is found in Caco-2 cells. ATP, UTP and UDP increase phosphorylation of MAPK by stimulating P2Y receptors, probably through subtypes of P2Y2, P2Y4, P2Y6 and P2Y11 receptors. ATP increases proliferation of Caco-2 cells via activation of P2Y purinergic receptors [97–98]. On the contrary, higher concentrations (1-10 mM) of extracellular ATP or the unhydrolyzed ATP analogue 5′-adenylyimido-diphosphate (AMP-PNP), suppress Caco-2 cell proliferation arresting cells cycling at the S phase by inhibiting PKC, ERK and MAP kinase [99]. The sustained activation of P2 receptors by ATP may lead to IL-8 secretion from human colorectal epithelial cells and may play an important role in tumor progression as well as in the pathology of IBD [100].
CONCLUSIONS
Growing lines of evidence suggest that P2Y receptors are involved in inflammation-associated diseases of liver and colon. P2Y receptors can also regulate metabolism, proliferation, differentiation and apoptosis of digestive cancer cells and tissues. It has been demonstrated that different P2Y receptor subtypes are present on the same cell and that various subtypes of receptors may produce opposite functions. Such as HCT8 and Caco-2 cells express P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, and P2Y12 receptors. Lower concentrations of ATP and UTP stimulate proliferation of these cells via P2Y2 receptors activation, but high concentrations of ATP induce apoptosis and anti-proliferation through P2Y1 and P2X7 receptors. This suggests that the control of cell proliferation by extracellular nucleotides might be regulated by a crucial balance of the activities of the receptor subtypes. The subtypes of P2Y receptors as new therapeutic targets for drug discovery to treat digestive diseases may have extensive clinical significance. We therefore anticipate that as these subtypes of P2Y receptors in digestive organs are further studied, their specific modulators may become promising new drugs to treat digestive diseases, such as inflammation and cancer in the near future.
Acknowledgments
supported by the National Natural Science Foundation of China (No.31371167 and No.81570477 to HD).
Abbreviations
- GPCRs
G protein-coupled receptors
- PKA
protein kinase A
- LES
lower esophageal sphincter
- NTPDase-2
nucleoside triphosphate diphosphohydrolase-2
- LPS
lipopolysaccharide
- COX-2
cyclooxygenase-2
- IBD
inflammatory bowel diseases
- RVD
volume regulatory decrease
- SOCs
store-operated calcium channels
- Mz- Cha-1
biliary epithelial cancer cells
- AMP-PNPATP
analogue 5′-adenylyimido-diphosphate
- HCC
hepatocellular carcinoma
- PLC
phospholipase C
- IP3
inositol 1,4,5-trisphosphate
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends in Neurosciences. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29:5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 3.White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27:211–217. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lecea B, Gallego D, Farre R, Opazo A, Auli M, Jimenez M, Clave P. Regional functional specialization and inhibitory nitrergic and nonnitrergic coneurotransmission in the human esophagus. Am J Physiol Gastrointest Liver Physiol. 2011;300:G782–794. doi: 10.1152/ajpgi.00514.2009. [DOI] [PubMed] [Google Scholar]
- 6.Farre R, Auli M, Lecea B, Martinez E, Clave P. Pharmacologic characterization of intrinsic mechanisms controlling tone and relaxation of porcine lower esophageal sphincter. J Pharmacol Exp Ther. 2006;316:1238–1248. doi: 10.1124/jpet.105.094482. [DOI] [PubMed] [Google Scholar]
- 7.Lecea B, Gallego D, Farre R, Clave P. Origin and modulation of circular smooth muscle layer contractions in the porcine esophagus. Neurogastroenterol Motil. 2012;24:779–789. doi: 10.1111/j.1365-2982.2012.01936.x. e355. [DOI] [PubMed] [Google Scholar]
- 8.Cho YR, Jang HS, Kim W, Park SY, Sohn UD. P2X and P2Y Receptors Mediate Contraction Induced by Electrical Field Stimulation in Feline Esophageal Smooth Muscle. Korean J Physiol Pharmacol. 2010;14:311–316. doi: 10.4196/kjpp.2010.14.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon TH, Jung H, Cho EJ, Jeong JH, Sohn UD. The Signaling Mechanism of Contraction Induced by ATP and UTP in Feline Esophageal Smooth Muscle Cells. Mol Cells. 2015;38:616–623. doi: 10.14348/molcells.2015.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signalling. 2014;10:3–50. doi: 10.1007/s11302-013-9397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gil-Rodrigo CE, Bergaretxe I, Carou M, Galdiz B, Salgado C, Ainz LF. Inhibitory action of extracellular adenosine 5′-triphosphate on parietal cells isolated from rabbit gastric mucosa. Gen Physiol Biophys. 1996;15:251–264. [PubMed] [Google Scholar]
- 12.Yuan WS, Wang ZY, Li JJ, Li D, Liu DL, Bai G, Walsh MP, Gui Y, Zheng XL. Uridine adenosine tetraphosphate induces contraction of circular and longitudinal gastric smooth muscle by distinct signaling pathways. Iubmb Life. 2013;65:623–632. doi: 10.1002/iub.1171. [DOI] [PubMed] [Google Scholar]
- 13.Jin Z, Guo HS, Xu DY, Hong MY, Li XL, Xu WX. [Effects of purinergic analogues on spontaneous contraction and electrical activities of gastric antral circular muscle in guinea-pig] Sheng Li Xue Bao. 2004;56:678–684. [PubMed] [Google Scholar]
- 14.Ahn SC, Xu WX, So I, Kim KW, Kang TM. Effects of purinergic agonists on mechanical and electrical activities of gastric smooth muscle of guinea-pig. J Smooth Muscle Res. 1995;31:407–410. [PubMed] [Google Scholar]
- 15.Soediono P, Burnstock G. Contribution of ATP and nitric oxide to NANC inhibitory transmission in rat pyloric sphincter. Br J Pharmacol. 1994;113:681–686. doi: 10.1111/j.1476-5381.1994.tb17046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon CJ, Woods NM, Webb TE, Green AK. Evidence that rat hepatocytes co-express functional P2Y1 and P2Y2 receptors. Br J Pharmacol. 2000;129:764–770. doi: 10.1038/sj.bjp.0703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dixon CJ. Evidence that 2-methylthioATP and 2-methylthioADP are both agonists at the rat hepatocyte P2Y(1) receptor. Br J Pharmacol. 2000;130:664–668. doi: 10.1038/sj.bjp.0703350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon CJ, Hall JF, Webb TE, Boarder MR. Regulation of rat hepatocyte function by P2Y receptors: Focus on control of glycogen phosphorylase and cyclic AMP by 2-methylthioadenosine 5 ‘-diphosphate. Journal of Pharmacology and Experimental Therapeutics. 2004;311:334–341. doi: 10.1124/jpet.104.067744. [DOI] [PubMed] [Google Scholar]
- 19.Dixon CJ, White PJ, Hall JF, Kingston S, Boarder MR. Regulation of human Hepatocytes by P2Y receptors: Control of glycogen phosphorylase, Ca2+, and mitogen-activated protein kinases. Journal of Pharmacology and Experimental Therapeutics. 2005;313:1305–1313. doi: 10.1124/jpet.104.082743. [DOI] [PubMed] [Google Scholar]
- 20.Tackett BC, Sun HD, Mei Y, Maynard JP, Cheruvu S, Mani A, Hernandez-Garcia A, Vigneswaran N, Karpen SJ, Thevananther S. P2Y2 purinergic receptor activation is essential for efficient hepatocyte proliferation in response to partial hepatectomy. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2014;307:G1073–G1087. doi: 10.1152/ajpgi.00092.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thevananther S, Sun HD, Li D, Arjunan V, Awad SS, Wyllie S, Zimmerman TL, Goss JA, Karpen SJ. Extracellular ATP activates c-jun N-terminal kinase signaling and cell cycle progression in hepatocytes. Hepatology. 2004;39:393–402. doi: 10.1002/hep.20075. [DOI] [PubMed] [Google Scholar]
- 22.Vaughn BP, Robson SC, Longhi MS. Purinergic Signaling in Liver Disease. Digestive Diseases. 2014;32:516–524. doi: 10.1159/000360498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fabre AC, Malaval C, Ben Addi A, Verdier C, Pons V, Serhan N, Lichtenstein L, Combes G, Huby T, Briand F, Collet X, Nijstad N, Tietge UJF, Robaye B, Perret B, Boeynaems JM, et al. P2Y13 Receptor is Critical for Reverse Cholesterol Transport. Hepatology. 2010;52:1477–1483. doi: 10.1002/hep.23897. [DOI] [PubMed] [Google Scholar]
- 24.Blom D, Yamin TT, Champy MF, Selloum M, Bedu E, Carballo-Jane E, Gerckens L, Luell S, Meurer R, Chin J, Mudgett J, Puig O. Altered lipoprotein metabolism in P2Y(13) knockout mice. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids. 2010;1801:1349–1360. doi: 10.1016/j.bbalip.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Petit P, Lajoix AD, Gross R. P2 purinergic signalling in the pancreatic beta-cell: control of insulin secretion and pharmacology. Eur J Pharm Sci. 2009;37:67–75. doi: 10.1016/j.ejps.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Lugo-Garcia L, Filhol R, Lajoix AD, Gross R, Petit P, Vignon J. Expression of purinergic P2Y receptor subtypes by INS-1 insulinoma beta-cells: a molecular and binding characterization. Eur J Pharmacol. 2007;568:54–60. doi: 10.1016/j.ejphar.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Farret A, Vignaud M, Dietz S, Vignon J, Petit P, Gross R. P2Y purinergic potentiation of glucose-induced insulin secretion and pancreatic beta-cell metabolism. Diabetes. 2004;53(3):S63–66. doi: 10.2337/diabetes.53.suppl_3.s63. [DOI] [PubMed] [Google Scholar]
- 28.Wuttke A, Idevall-Hagren O, Tengholm A. P2Y(1) receptor-dependent diacylglycerol signaling microdomains in beta cells promote insulin secretion. Faseb Journal. 2013;27:1610–1620. doi: 10.1096/fj.12-221499. [DOI] [PubMed] [Google Scholar]
- 29.Balasubramanian R, Ruiz de Azua I, Wess J, Jacobson KA. Activation of distinct P2Y receptor subtypes stimulates insulin secretion in MIN6 mouse pancreatic beta cells. Biochem Pharmacol. 2010;79:1317–1326. doi: 10.1016/j.bcp.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amisten S, Meidute-Abaraviciene S, Tan C, Olde B, Lundquist I, Salehi A, Erlinge D. ADP mediates inhibition of insulin secretion by activation of P2Y13 receptors in mice. Diabetologia. 2010;53:1927–1934. doi: 10.1007/s00125-010-1807-8. [DOI] [PubMed] [Google Scholar]
- 31.Tan C, Voss U, Svensson S, Erlinge D, Olde B. High glucose and free fatty acids induce beta cell apoptosis via autocrine effects of ADP acting on the P2Y(13) receptor. Purinergic Signal. 2013;9:67–79. doi: 10.1007/s11302-012-9331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallego D, Gil V, Aleu J, Martinez-Cutillas M, Clave P, Jimenez M. Pharmacological characterization of purinergic inhibitory neuromuscular transmission in the human colon. Neurogastroenterol Motil. 2011;23:792–e338. doi: 10.1111/j.1365-2982.2011.01725.x. [DOI] [PubMed] [Google Scholar]
- 33.Gallego D, Malagelada C, Accarino A, De Giorgio R, Malagelada JR, Azpiroz F, Jimenez M. Nitrergic and purinergic mechanisms evoke inhibitory neuromuscular transmission in the human small intestine. Neurogastroenterology and Motility. 2014;26:419–429. doi: 10.1111/nmo.12293. [DOI] [PubMed] [Google Scholar]
- 34.Wang GD, Wang XY, Hu HZ, Liu S, Gao N, Fang X, Xia Y, Wood JD. Inhibitory neuromuscular transmission mediated by the P2Y1 purinergic receptor in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1483–1489. doi: 10.1152/ajpgi.00450.2006. [DOI] [PubMed] [Google Scholar]
- 35.Grasa L, Gil V, Gallego D, Martin MT, Jimenez M. P2Y(1) receptors mediate inhibitory neuromuscular transmission in the rat colon. British Journal of Pharmacology. 2009;158:1641–1652. doi: 10.1111/j.1476-5381.2009.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christofi FL, Wunderlich J, Yu JG, Wang YZ, Xue JJ, Guzman J, Javed N, Cooke H. Mechanically evoked reflex electrogenic chloride secretion in rat distal colon is triggered by endogenous nucleotides acting at P2Y1, P2Y2, and P2Y4 receptors. Journal of Comparative Neurology. 2004;469:16–36. doi: 10.1002/cne.10961. [DOI] [PubMed] [Google Scholar]
- 37.Ghanem E, Robaye B, Leal T, Leipziger J, Van Driessche W, Beauwens R, Boeynaems JM. The role of epithelial P2Y2 and P2Y4 receptors in the regulation of intestinal chloride secretion. Br J Pharmacol. 2005;146:364–369. doi: 10.1038/sj.bjp.0706353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong X, Smoll EJ, Ko KH, Lee J, Chow JY, Kim HD, Insel PA, Dong H. P2Y receptors mediate Ca2+ signaling in duodenocytes and contribute to duodenal mucosal bicarbonate secretion. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;296:G424–G432. doi: 10.1152/ajpgi.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matos JE, Robaye B, Boeynaems JM, Beauwens R, Leipziger J. K+ secretion activated by luminal P2Y2 and P2Y4 receptors in mouse colon. J Physiol. 2005;564:269–279. doi: 10.1113/jphysiol.2004.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kottgen M, Loffler T, Jacobi C, Nitschke R, Pavenstadt H, Schreiber R, Frische S, Nielsen S, Leipziger J. P2Y6 receptor mediates colonic NaCl secretion via differential activation of cAMP-mediated transport. Journal of Clinical Investigation. 2003;111:371–379. doi: 10.1172/JCI16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gendaszewska-Darmach E, Kucharska M. Nucleotide receptors as targets in the pharmacological enhancement of dermal wound healing. Purinergic Signalling. 2011;7:193–206. doi: 10.1007/s11302-011-9233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boyer JL, Durham T, Barnes M, Navratil T, Schaberg A. Denufosol tetrasodium, a P2Y2 receptor agonist for the treatment of Cystic Fibrosis. Purinergic Signalling. 2010;6:16–16. [Google Scholar]
- 43.Myrtek D, Idzko M. Chemotactic activity of extracellular nucleotideson human immune cells. Purinergic Signal. 2007;3:5–11. doi: 10.1007/s11302-006-9032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrari D, la Sala A, Panther E, Norgauer J, Di Virgilio F, Idzko M. Activation of human eosinophils via P2 receptors: novel findings and future perspectives. Journal of Leukocyte Biology. 2006;79:7–15. doi: 10.1189/jlb.0505286. [DOI] [PubMed] [Google Scholar]
- 45.Knowles MR, Clarke LL, Boucher RC. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N Engl J Med. 1991;325:533–538. doi: 10.1056/NEJM199108223250802. [DOI] [PubMed] [Google Scholar]
- 46.Kunzli BM, Berberat PO, Giese T, Csizmadia E, Kaczmarek E, Baker C, Halaceli I, Buchler MW, Friess H, Robson SC. Upregulation of CD39/NTPDases and P2 receptors in human pancreatic disease. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2007;292:G223–G230. doi: 10.1152/ajpgi.00259.2006. [DOI] [PubMed] [Google Scholar]
- 47.Cicko S, Lucattelli M, Muller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Durk T, Zissel G, Boeynaems JM, Sorichter S, Ferrari D, Di Virgilio F, Virchow JC, et al. Purinergic Receptor Inhibition Prevents the Development of Smoke-Induced Lung Injury and Emphysema. Journal of Immunology. 2010;185:688–697. doi: 10.4049/jimmunol.0904042. [DOI] [PubMed] [Google Scholar]
- 48.Lommatzsch M, Cicko S, Muller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Durk T, Zissel G, Ferrari D, Di Virgilio F, Sorichter S, Lungarella G, Virchow JC, Idzko M. Extracellular Adenosine Triphosphate and Chronic Obstructive Pulmonary Disease. American Journal of Respiratory and Critical Care Medicine. 2010;181:928–934. doi: 10.1164/rccm.200910-1506OC. [DOI] [PubMed] [Google Scholar]
- 49.Ayata CK, Ganal SC, Hockenjos B, Willim K, Vieira RP, Grimm M, Robaye B, Boeynaems JM, Di Virgilio F, Pellegatti P, Diefenbach A, Idzko M, Hasselblatt P. Purinergic P2Y(2) receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology. 2012;143:1620–1629. doi: 10.1053/j.gastro.2012.08.049. e1624. [DOI] [PubMed] [Google Scholar]
- 50.Dranoff JA, Ogawa M, Kruglov EA, Gaca MD, Sevigny J, Robson SC, Wells RG. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G417–424. doi: 10.1152/ajpgi.00294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishimaru M, Yusuke N, Tsukimoto M, Harada H, Takenouchi T, Kitani H, Kojima S. Purinergic signaling via P2Y receptors up-mediates IL-6 production by liver macrophages/Kupffer cells. Journal of Toxicological Sciences. 2014;39:413–423. doi: 10.2131/jts.39.413. [DOI] [PubMed] [Google Scholar]
- 52.Langlois C, Gendron FP. Promoting M Phi transepithelial migration by stimulating the epithelial cell P2Y(2) receptor. European Journal of Immunology. 2009;39:2895–2905. doi: 10.1002/eji.200939369. [DOI] [PubMed] [Google Scholar]
- 53.Degagne E, Grbic DM, Dupuis AA, Lavoie EG, Langlois C, Jain N, Weisman GA, Sevigny J, Gendron FP. P2Y(2) Receptor Transcription Is Increased by NF-kappa B and Stimulates Cyclooxygenase-2 Expression and PGE(2) Released by Intestinal Epithelial Cells. Journal of Immunology. 2009;183:4521–4529. doi: 10.4049/jimmunol.0803977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Degagne E, Turgeon N, Moore-Gagne J, Asselin C, Gendron FP. P2Y(2) receptor expression is regulated by C/EBP beta during inflammation in intestinal epithelial cells. Febs Journal. 2012;279:2957–2965. doi: 10.1111/j.1742-4658.2012.08676.x. [DOI] [PubMed] [Google Scholar]
- 55.Donnell AMO, Puri P. Deficiency of purinergic P2Y receptors in aganglionic intestine in Hirschsprung's disease. Pediatric Surgery International. 2008;24:77–80. doi: 10.1007/s00383-007-2044-1. [DOI] [PubMed] [Google Scholar]
- 56.Grbic D, Degagne E, Langlois C, Dupuis AA, Gendron FP. Intestinal inflammation increases P2Y6 receptor expression on epithelial cells and the release of CXCL8 by UDP. Purinergic Signalling. 2008;4:S184–S184. doi: 10.4049/jimmunol.180.4.2659. [DOI] [PubMed] [Google Scholar]
- 57.Grbic DM, Degagne E, Larrivee JF, Bilodeau MS, Vinette V, Arguin G, Stankova J, Gendron FP. P2Y6 receptor contributes to neutrophil recruitment to inflamed intestinal mucosa by increasing CXC chemokine ligand 8 expression in an AP-1-dependent manner in epithelial cells. Inflammatory Bowel Diseases. 2012;18:1456–1469. doi: 10.1002/ibd.21931. [DOI] [PubMed] [Google Scholar]
- 58.Somers GR, Hammet FM, Trute L, Southey MC, Venter DJ. Expression of the P2Y6 purinergic receptor in human T cells infiltrating inflammatory bowel disease. Lab Invest. 1998;78:1375–1383. [PubMed] [Google Scholar]
- 59.White N, Ryten M, Clayton E, Butler P, Burnstock G. P2Y purinergic receptors regulate the growth of human melanomas. Cancer Letters. 2005;224:81–91. doi: 10.1016/j.canlet.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 60.Greig AVH, Linge C, Healy V, Lim P, Clayton E, Rustin MHA, McGrouther DA, Burnstock G. Expression of purinergic receptors in non-melanoma skin cancers and their functional roles in A431 cells. Journal of Investigative Dermatology. 2003;121:315–327. doi: 10.1046/j.1523-1747.2003.12379.x. [DOI] [PubMed] [Google Scholar]
- 61.Song S, Jacobson KN, McDermott KM, Reddy SP, Cress AE, Tang H, Dudek SM, Black SM, Garcia JG, Makino A, Yuan JX. ATP Promotes Cell Survival Via Regulation of Cytosolic [Ca2+] and Bcl-2/Bax Ratio in Lung Cancer Cells. Am J Physiol Cell Physiol. 2015 doi: 10.1152/ajpcell.00092.2015. ajpcell 00092 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schafer R, Sedehizade F, Welte T, Reiser G. ATP- and UTP-activated P2Y receptors differently regulate proliferation of human lung epithelial tumor cells. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2003;285:L376–L385. doi: 10.1152/ajplung.00447.2002. [DOI] [PubMed] [Google Scholar]
- 63.Li WH, Qiu Y, Zhang HQ, Liu Y, You JF, Tian XX, Fang WG. P2Y2 receptor promotes cell invasion and metastasis in prostate cancer cells. British Journal of Cancer. 2013;109:1666–1675. doi: 10.1038/bjc.2013.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li WH, Qiu Y, Zhang HQ, Tian XX, Fang WG. P2Y2 Receptor and EGFR Cooperate to Promote Prostate Cancer Cell Invasion via ERK1/2 Pathway. Plos One. 2015:10. doi: 10.1371/journal.pone.0133165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen L, He HY, Li HM, Zheng J, Heng WJ, You JF, Fang WG. ERK1/2 and p38 pathways are required for P2Y receptor-mediated prostate cancer invasion. Cancer Letters. 2004;215:239–247. doi: 10.1016/j.canlet.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 66.Wei Q, Costanzi S, Liu QZ, Gao ZG, Jacobson KA. Activation of the P2Y1 receptor induces apoptosis and inhibits proliferation of prostate cancer cells. Biochem Pharmacol. 2011;82:418–425. doi: 10.1016/j.bcp.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tu MT, Luo SF, Wang CC, Chien CS, Chiu CT, Lin CC, Yang CM. P2Y(2) receptor-mediated proliferation of C(6) glioma cells via activation of Ras/Raf/MEK/MAPK pathway. Br J Pharmacol. 2000;129:1481–1489. doi: 10.1038/sj.bjp.0703182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wypych D, Pomorski P. P2Y1 nucleotide receptor silencing and its effect on glioma C6 calcium signaling. Acta Biochim Pol. 2012;59:711–717. [PubMed] [Google Scholar]
- 69.Joo YN, Jin H, Eun SY, Park SW, Chang KC, Kim HJ. P2Y(2)R activation by nucleotides released from the highly metastatic breast cancer cell contributes to pre-metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment. Oncotarget. 2014;5:9322–9334. doi: 10.18632/oncotarget.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li HJ, Wang LY, Qu HN, Yu LH, Burnstock G, Ni X, Xu MJ, Ma B. P2Y(2) receptor-mediated modulation of estrogen-induced proliferation of breast cancer cells. Molecular and Cellular Endocrinology. 2011;338:28–37. doi: 10.1016/j.mce.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 71.Jin H, Eun SY, Lee JS, Park SW, Lee JH, Chang KC, Kim HJ. P2Y(2) receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Research. 2014:16. doi: 10.1186/bcr3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eun SY, Ko YS, Park SW, Chang KC, Kim HJ. P2Y(2) nucleotide receptor-mediated extracellular signal-regulated kinases and protein kinase C activation induces the invasion of highly metastatic breast cancer cells. Oncology Reports. 2015;34:195–202. doi: 10.3892/or.2015.3972. [DOI] [PubMed] [Google Scholar]
- 73.Sarangi S, Pandey A, Papa AL, Sengupta P, Kopparam J, Dadwal U, Basu S, Sengupta S. P2Y12 receptor inhibition augments cytotoxic effects of cisplatin in breast cancer. Medical Oncology. 2013:30. doi: 10.1007/s12032-013-0567-y. [DOI] [PubMed] [Google Scholar]
- 74.Kim HJ, Jin H, Chang KC, Park SW, Lee JH. Nucleotides released from breast cancer cells MDA-MB-231 increase proliferation and invasion through P2Y(2) receptor activation. Febs Journal. 2012;279:168–169. [Google Scholar]
- 75.Chadet S, Jelassi B, Wannous R, Angoulvant D, Chevalier S, Besson P, Roger S. The activation of P2Y(2) receptors increases MCF-7 breast cancer cells migration through the MEK-ERK1/2 signalling pathway. Carcinogenesis. 2014;35:1238–1247. doi: 10.1093/carcin/bgt493. [DOI] [PubMed] [Google Scholar]
- 76.Schultze-Mosgau A, Katzur AC, Arora KK, Stojilkovic SS, Diedrich K, Ortmann O. Characterization of calcium-mobilizing, purinergic P2Y(2) receptors in human ovarian cancer cells. Mol Hum Reprod. 2000;6:435–442. doi: 10.1093/molehr/6.5.435. [DOI] [PubMed] [Google Scholar]
- 77.Conigrave AD, van der Weyden L, Holt L, Jiang L, Wilson P, Christopherson RI, Morris MB. Extracellular ATP-dependent suppression of proliferation and induction of differentiation of human HL-60 leukemia cells by distinct mechanisms. Biochem Pharmacol. 2000;60:1585–1591. doi: 10.1016/s0006-2952(00)00465-2. [DOI] [PubMed] [Google Scholar]
- 78.Maaser K, Hopfner M, Kap H, Sutter AP, Barthel B, von Lampe B, Zeitz M, Scherubl H. Extracellular nucleotides inhibit growth of human oesophageal cancer cells via P2Y(2)-receptors. Br J Cancer. 2002;86:636–644. doi: 10.1038/sj.bjc.6600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Junankar PR, Karjalainen A, Kirk K. The role of P2Y1 purinergic receptors and cytosolic Ca2+ in hypotonically activated osmolyte efflux from a rat hepatoma cell line. J Biol Chem. 2002;277:40324–40334. doi: 10.1074/jbc.M204712200. [DOI] [PubMed] [Google Scholar]
- 80.Dolovcak S, Waldrop SL, Fitz JG, Kilic G. Copper inhibits P2Y(2)-dependent Ca2+ signaling through the effects on thapsigargin-sensitive Ca2+ stores in HTC hepatoma cells. Biochemical and Biophysical Research Communications. 2010;397:493–498. doi: 10.1016/j.bbrc.2010.05.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Espelt MV, Pinto FD, Alvarez CL, Alberti GS, Incicco J, Denis MFL, Davio C, Schwarzbaum PJ. On the role of ATP release, ectoATPase activity, and extracellular ADP in the regulatory volume decrease of Huh-7 human hepatoma cells. American Journal of Physiology-Cell Physiology. 2013;304:C1013–C1026. doi: 10.1152/ajpcell.00254.2012. [DOI] [PubMed] [Google Scholar]
- 82.Xie R, Xu J, Wen G, Jin H, Liu X, Yang Y, Ji B, Jiang Y, Song P, Dong H, Tuo B. The P2Y2 nucleotide receptor mediates the proliferation and migration of human hepatocellular carcinoma cells induced by ATP. J Biol Chem. 2014;289:19137–19149. doi: 10.1074/jbc.M113.540047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haddad PS, Vallerand D, Mathe L, Benzeroual K, Van de Werve G. Synergistic activation of mitogen-activated protein kinase by insulin and adenosine triphosphate in liver cells: permissive role of Ca2+ Metabolism. 2003;52:590–598. doi: 10.1053/meta.2003.50094. [DOI] [PubMed] [Google Scholar]
- 84.Schofl C, Ponczek M, Mader T, Waring M, Benecke H, von zur Muhlen A, Mix H, Cornberg M, Boker KH, Manns MP, Wagner S. Regulation of cytosolic free calcium concentration by extracellular nucleotides in human hepatocytes. Am J Physiol. 1999;276:G164–172. doi: 10.1152/ajpgi.1999.276.1.G164. [DOI] [PubMed] [Google Scholar]
- 85.Elsing C, Georgiev T, Hubner CA, Boger R, Stremmel W, Schlenker T. Extracellular ATP Induces Cytoplasmic and Nuclear Ca2+ Transients via P2Y2 Receptor in Human Biliary Epithelial Cancer Cells (Mz-Cha-1) Anticancer Research. 2012;32:3759–3767. [PubMed] [Google Scholar]
- 86.Ko T, An HJ, Ji YG, Kim OJ, Lee DH. P2Y Receptors Regulate Proliferation of Human Pancreatic Duct Epithelial Cells. Pancreas. 2012;41:797–803. doi: 10.1097/MPA.0b013e31823ba3b3. [DOI] [PubMed] [Google Scholar]
- 87.Choi JH, Ji YG, Lee DH. Uridine triphosphate increases proliferation of human cancerous pancreatic duct epithelial cells by activating P2Y2 receptor. Pancreas. 2013;42:680–686. doi: 10.1097/MPA.0b013e318271bb4b. [DOI] [PubMed] [Google Scholar]
- 88.Verspohl EJ, Johannwille B, Waheed A, Neye H. Effect of purinergic agonists and antagonists on insulin secretion from INS-1 cells (insulinoma cell line) and rat pancreatic islets. Can J Physiol Pharmacol. 2002;80:562–568. doi: 10.1139/y02-079. [DOI] [PubMed] [Google Scholar]
- 89.Nylund G, Hultman L, Nordgren S, Delbro DS. P2Y2- and P2Y4 purinergic receptors are over-expressed in human colon cancer. Auton Autacoid Pharmacol. 2007;27:79–84. doi: 10.1111/j.1474-8673.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 90.Nylund G, Nordgren S, Delbro DS. Expression of P2Y2 purinoceptors in MCG 101 murine sarcoma cells, and HT-29 human colon carcinoma cells. Auton Neurosci. 2004;112:69–79. doi: 10.1016/j.autneu.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 91.Hopfner M, Maaser K, Barthel B, von Lampe B, Hanski C, Riecken EO, Zeitz M, Scherubl H. Growth inhibition and apoptosis induced by P2Y2 receptors in human colorectal carcinoma cells: involvement of intracellular calcium and cyclic adenosine monophosphate. Int J Colorectal Dis. 2001;16:154–166. doi: 10.1007/s003840100302. [DOI] [PubMed] [Google Scholar]
- 92.White N, Burnstock G. P2 receptors and cancer. Trends in Pharmacological Sciences. 2006;27:211–217. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 93.Hopfner M, Lemmer K, Jansen A, Hanski C, Riecken EO, Gavish M, Mann B, Buhr H, Glassmeier G, Scherubl H. Expression of functional P2-purinergic receptors in primary cultures of human colorectal carcinoma cells. Biochem Biophys Res Commun. 1998;251:811–817. doi: 10.1006/bbrc.1998.9555. [DOI] [PubMed] [Google Scholar]
- 94.Coutinho-Silva R, Stahl L, Cheung KK, de Campos NE, de Oliveira Souza C, Ojcius DM, Burnstock G. P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1024–1035. doi: 10.1152/ajpgi.00211.2004. [DOI] [PubMed] [Google Scholar]
- 95.Limami Y, Pinon A, Leger DY, Pinault E, Delage C, Beneytout JL, Simon A, Liagre B. The P2Y2/Src/p38/COX-2 pathway is involved in the resistance to ursolic acid-induced apoptosis in colorectal and prostate cancer cells. Biochimie. 2012;94:1754–1763. doi: 10.1016/j.biochi.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 96.Limami Y, Pinon A, Leger DY, Mousseau Y, Cook-Moreau J, Beneytout JL, Delage C, Liagre B, Simon A. HT-29 colorectal cancer cells undergoing apoptosis overexpress COX-2 to delay ursolic acid-induced cell death. Biochimie. 2011;93:749–757. doi: 10.1016/j.biochi.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 97.Buzzi N, Bilbao PS, Boland R, Boland AR. Extracellular ATP activates MAP kinase cascades through a P2Y purinergic receptor in the human intestinal Caco-2 cell line. Biochimica Et Biophysica Acta-General Subjects. 2009;1790:1651–1659. doi: 10.1016/j.bbagen.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 98.Buzzi N, Boland R, de Boland AR. Signal transduction pathways associated with ATP-induced proliferation of colon adenocarcinoma cells. Biochimica Et Biophysica Acta-General Subjects. 2010;1800:946–955. doi: 10.1016/j.bbagen.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 99.Yaguchi T, Saito M, Yasuda Y, Kanno T, Nakano T, Nishizaki T. Higher concentrations of extracellular ATP suppress proliferation of Caco-2 human colonic cancer cells via an unknown receptor involving PKC inhibition. Cell Physiol Biochem. 2010;26:125–134. doi: 10.1159/000320518. [DOI] [PubMed] [Google Scholar]
- 100.Bahrami F, Kukulski F, Lecka J, Tremblay A, Pelletier J, Rockenbach L, Sevigny J. Purine-Metabolizing Ectoenzymes Control IL-8 Production in Human Colon HT-29 Cells. Mediators of Inflammation. 2014;2014:879895. doi: 10.1155/2014/879895. [DOI] [PMC free article] [PubMed] [Google Scholar]


