SUMMARY
The osteoblastic and adipocytic lineages arise from mesenchymal stem cells (MSCs), but few regulators of self-renewal and early cell-fate decisions are known. Here, we show that the Hippo pathway effector YAP1 is a direct target of SOX2 and can compensate for the self-renewal defect caused by SOX2 inactivation in osteoprogenitors and MSCs. Osteogenesis is blocked by high SOX2 or YAP1, accelerated by depletion of either one, and the inhibition of osteogenesis by SOX2 requires YAP1. SOX2 favors adipogenesis and induces PPARγ, but adipogenesis can only occur with moderate levels of YAP1. YAP1 induction by SOX2 is restrained in adipogenesis, and both YAP1 overexpression and depletion inhibit the process. YAP1 binds β-catenin and directly induces the Wnt antagonist Dkk1 to dampen pro-osteogenic Wnt signals. We demonstrate a Hippo-independent regulation of YAP1 by SOX2 that cooperatively antagonizes Wnt/β-catenin signals and regulates PPARγ to determine osteogenic or adipocytic fates.
INTRODUCTION
Bone- and fat-forming cells are both derived from adult multipotent progenitor cells that are often referred to as mesenchymal stem cells (MSCs) or mesenchymal progenitor cells (MPCs). Bone marrow (BM)-derived MSCs (BM-MSCs) in culture have the capacity to self-renew as well as to form differentiated cell types of the mesenchymal lineage, such as osteoblasts, adipocytes, chondrocytes, and myoblasts (Caplan, 1991; Pittenger et al., 1999). Although key transcription factors that specify the different lineages are known, the regulation of self-renewal and cell-fate choice in MSCs and more restricted progenitor cells is not well understood. Several studies have suggested that the osteoblastic and adipocytic lineages are alternate fates, and increased adipogenesis correlates with decreased osteogenesis during development and aging (Takada et al., 2009; Urs et al., 2010; Verma et al., 2002). The transcription factor SOX2 is required to maintain self-renewal and the undifferentiated state in the osteoblastic lineage and MSCs (Basu-Roy et al., 2010; Park et al., 2012b). SOX2 expression is downregulated upon osteoblastic differentiation, and its constitutive expression prevents osteoblastic differentiation by inducing stemness-related genes and inhibiting the Wnt pathway (Holmes et al., 2011; Mansukhani et al., 2005; Park et al., 2012b; Seo et al., 2011), which is pro-osteogenic and inhibits the adipogenic fate (Kang et al., 2007; Prestwich and Macdougald, 2007). SOX2 can bind β-catenin, a key mediator of canonical Wnt signaling, and directly induce expression of the negative regulators APC and GSK3β, which promote β-catenin degradation (Mansukhani et al., 2005; Seo et al., 2011).
SOX2 is a member of the HMG-domain family and is a pluripotency transcription factor that is required to maintain the stemness and self-renewal of embryonic stem cells (ESCs) (Niwa, 2007). It is now evident that SOX2 is required for the homeostasis of several tissues through the maintenance of adult stem cells (Arnold et al., 2011). SOX2 expression is also seen in several undifferentiated cancers, including osteosarcomas (Bass et al., 2009; Basu-Roy et al., 2011; Riggi et al., 2010).
Yes-associated protein 1 (YAP1) is a key downstream effector of the Hippo signaling pathway that controls cell proliferation and organ size (Halder and Johnson, 2011; Pan, 2010; Sudol, 1994; Zhao et al., 2010). YAP1 is a transcriptional coactivator that maintains the pluripotency of ESCs, where it acts as a coactivator of the TEAD transcription factors to regulate several stemness genes (Lian et al., 2010). The transcriptional activity of YAP1 is restrained by phosphorylation via the Hippo (MST/LATS) pathway, a major growth- and tumor-suppressive pathway that is activated by increased cell density and thought to be a mediator of contact inhibition (Zeng and Hong, 2008; Zhao et al., 2007, 2011). When the Hippo pathway is active, YAP1 and its paralog, TAZ (WWTR1), are phosphorylated and sequestered in the cytoplasm, which inhibits their transcriptional activity (Pan, 2007; Zhao et al., 2011). Inactivation of the Hippo pathway leads to increases in the nuclear localization and TEAD-mediated transcriptional activity of YAP1 and TAZ (Ota and Sasaki, 2008; Zhao et al., 2007). TAZ was identified as a fate-determination factor that binds to and activates Runx2, a transcriptional regulator of the osteoblast lineage, while concurrently binding to and inactivating PPARγ, the master regulator of adipogenesis (Hong et al., 2005). Although YAP1 and TAZ are often considered functionally analogous orthologs of Drosophila Yorkie (Yki), here we report that in the osteo-adipo lineage, YAP1’s functions are distinct from those of TAZ.
We demonstrate that YAP1 is a direct transcriptional target of SOX2 in osteoprogenitors and MSCs where SOX2 function is required for self-renewal. Constitutive expression of YAP1 can rescue the lethality caused by SOX2 depletion and restores self-renewal and proliferative capacity. Depletion of either SOX2 or YAP1 enables osteogenesis and prevents adipogenic differentiation. SOX2 favors adipogenesis, which requires physiological levels of YAP1 expression. The SOX2-YAP1 axis is required for blocking osteogenesis, but during adipogenesis, where YAP1 expression is restrained, SOX2 overexpression can compensate for depletion of YAP1. The effect of YAP1 is mostly due to its nuclear transcriptional function because it is mimicked by a transcriptionally active YAP1 mutant or knockdown of hippo pathway components (MST1/2) that restrain nuclear YAP1 transcriptional activity. We show that, like SOX2, YAP1 inhibits Wnt signaling and the depletion of YAP1 induces Wnt signaling. YAP1 binds β-catenin and induces Dkk1, a negative regulator of Wnt signaling, to maintain stemness and prevent osteogenesis.
Our studies identify a functional relation between SOX2 and the Hippo signaling pathway, and indicate that SOX2 and YAP1 act cooperatively as a control switch to regulate self-renewal and mesenchymal cell lineage choice.
RESULTS
Yap1 Is Transcriptionally Regulated by SOX2
SOX2 is required for the self-renewal of osteoprogenitors and affects the expression of numerous genes involved in proliferation, stemness, and intracellular signaling, as revealed by gene-expression analysis of cells in which SOX2 was deleted by CRE virus-mediated excision (Basu-Roy et al., 2010; Seo et al., 2011). To investigate genes directly bound and regulated by SOX2 in osteoprogenitor cells, we performed SOX2 chromatin immunoprecipitation sequencing (ChIP-seq) analysis using both wild-type (WT) osteoprogenitor cells (OBI) and the same cells transduced to express higher amounts (by 5- to 6-fold) of SOX2 (OB1-SOX2). From the overlay of microarray and ChIP-seq data, we found that one of the direct SOX2-regulated targets was YAP1. ChIP-seq analysis showed two “peaks” of SOX2 binding in the Yap1 gene, the first near exon I, overlapping a CpG island, and the second on exon II (Figure 1A). The peaks were detected in WT as well as in SOX2-overexpressing cells, and spanned SOX2 consensus binding sites (red bars in the schematic in Fig. 1B). ChIP-PCR assays further confirmed that SOX2-binding genomic fragments of the Yap1 gene were enriched in SOX2-overexpressing cells (Figure 1B).
Figure 1. YAP1 Is a Target of SOX2.
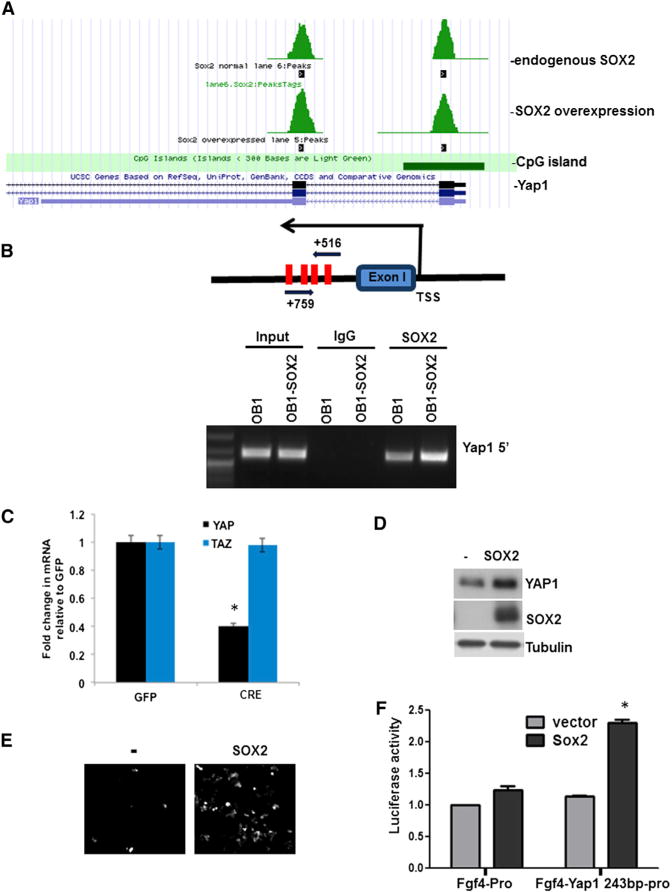
(A and B) SOX2 binds to the Yap1 genomic region in osteoprogenitor cells.
(A) SOX2 CHIP-seq shows two peaks of SOX2-bound genomic sequences around exons I and II of Yap1 in control and SOX2-overexpressing cells.
(B) CHiP-PCR with primers around exon I in SOX2-overexpressing (OB1-SOX2) or control OB1 cells. A schematic of primers and SOX2-binding sites (red bars) in the first peak is shown. TSS, transcription start site.
(C) YAP1 and TAZ mRNA expression analysis in SOX2-depleted osteoprogenitors. SOX2F/F cells were transduced with CRE- or GFP lentivirus, and YAP1 and TAZ expression was analyzed by qRT-PCR. Gene-expression values in CRE-infected cells are normalized to that of GFP-lentivirus-infected cells.
(D) YAP1 expression by western analysis in SOX2F/F osteoprogenitors infected with SOX2 lentivirus. See also Figure S1. (E and F) SOX2 induces expression of a Yap1 reporter.
(E) 293T cells were infected with SOX2 or control (−) lentivirus and transfected with a Yap1 243-bp-region-driven Venus fluorescent reporter.
(F) Activity of the Yap1-luciferase reporter. C3H10T1/2 cells were infected with lentiviral SOX2 or control vector and then transfected with Fgf4 minimal promoter reporter or Fgf4 minimal promoter containing the Yap1 243 bp region. Luciferase activity was normalized to Renilla.
*p < 0.05; error bars represent the average + SD.
We previously described the gene-expression changes in osteoprogenitor cells derived from mice bearing the SOX2 floxed (F) gene (SOX2F/F or SOX2F/−) after in vitro infection with a CRE virus (Seo et al., 2011). SOX2 depletion by CRE-expressing lentivirus in SOX2F/F cells led to reduced expression of YAP1 but not of its paralog, TAZ (Figure 1C). Consistently, overexpression of SOX2 resulted in increased YAP1 expression not only in osteoprogenitors (Figure 1D) but also in MSCs and C3H10T1/2 cells, which serve as a model of multipotent mesenchymal cells (Figure S1A). Using a fibroblast growth factor 4 (FGF4) minimal promoter-driven Venus (enhanced GFP [EGFP]) or firefly luciferase reporter plasmid, we determined whether a 243 bp Yap1 genomic region including the SOX2 binding sites near the first exon could be activated by SOX2. In 293T cells, SOX2 expression induced Venus expression driven by the YAP1 5′ 243 bp region (Figure 1E), and the luciferase activity of a construct with the same genomic region was increased by SOX2 in C3H10T1/2 cells (Figure 1F). Mutagenesis of the SOX2-binding elements in the 243 bp region confirmed that the induction was dependent on SOX2 binding (Figure S1B). These results indicate that YAP1 is a direct downstream target of SOX2.
YAP1 Expression Is Regulated by SOX2 In Vivo and In Vitro
We showed that an osteoblast-specific conditional knockout (CKO) of SOX2 led to a low-bone-density phenotype, and deletion of SOX2 in osteoprogenitor cells caused cell senescence, revealing that SOX2 is important for maintaining osteoprogenitors and for bone formation (Basu-Roy et al., 2010). We determined whether YAP1 or TAZ was regulated in this setting by examining their expression in bone tissue and in calvarial osteoblasts from SOX2 CKO animals. We carried out quantitative RT-PCR (qRT-PCR) using messenger RNA (mRNA) extracted from femurs and calvaria of mice with an Osterix-CRE conditional SOX2 knockout in homozygous and heterozygous configurations. YAP1 expression levels were reduced to 20%–30% of WT in SOX2 CKO femurs and calvaria, whereas the expression of TAZ was not significantly changed (Figures 2A and 2B). Primary osteoblast cultures derived from SOX2 CKO mice also showed decreased YAP1 protein expression, whereas TAZ expression was unaffected (Figure 2C). The reduced YAP1 expression was evident despite the mosaic excision of SOX2 in these mice (Basu-Roy et al., 2010; Seo et al., 2011). To determine whether the decreased YAP1 expression in SOX2 knockout cells affected the expression of known YAP1 target genes, we examined the expression of a YAP1 target gene set (Zhang et al., 2009) in the SOX2 floxed cells 24, 48, and 72 hr after CRE virus infection. Several YAP1 target genes were significantly downregulated upon SOX2 excision, whereas little change occurred in control GFP-virus-infected cells (Figures 2D and S2A). Some of these genes that are known to be important in osteoblast biology were validated by qRT-PCR in SOX2 knockout and YAP1 knockdown cells (Figure S2B). Their downregulation confirmed that these are bona fide YAP1 targets in osteoprogenitor cells.
Figure 2. YAP1 Is Regulated by SOX2 In Vivo and In Vitro, and Can Compensate for SOX2 Depletion in Osteoprogenitors.
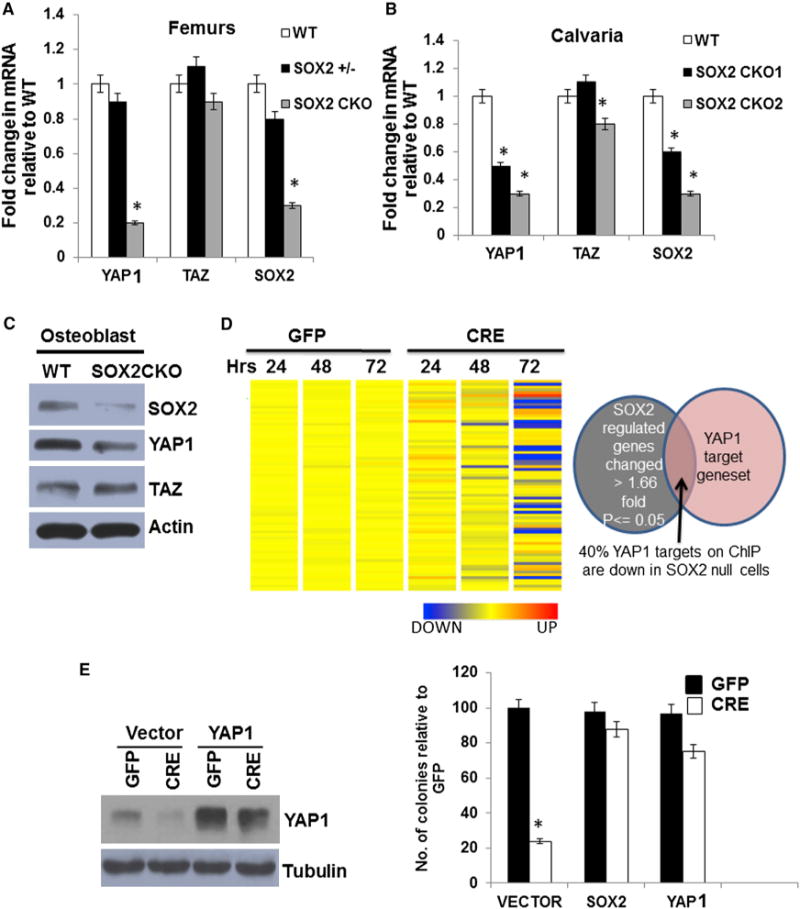
(A and B) SOX2, TAZ, and YAP1 gene expression in vivo. qRT-PCR analysis of mRNA directly from (A) femurs of 8-week-old mice and (B) calvaria of P1 pups. All values are normalized to 18S rRNA and expressed as fold change compared with WT. SOX2 +/−: heterozygote; SOX2 CKO: SOX2 conditional knockout (CKO1 and CKO2 are two independent isolations). *p < 0.05. Error barsrepresent SD.
(C) Western analysis of SOX2, TAZ, and YAP1 expression in WT and SOX2 CKO osteoprogenitors.
(D) Heatmap of YAP1 target genes in SOX2-depleted cells. SOX2F/F osteoprogenitors were infected with a GFP or CRE lentivirus virus for 24, 48, and 72 hr. Expression of the YAP1 target gene set (Zhang et al., 2009) is shown. Venn diagram shows overlap between a subset of genes that were significantly changed by SOX2 knockdown and the YAP1 target gene set. See also Figures S2A and S2B.
(E) Rescue of the colony-forming ability of SOX2-deleted osteoprogenitors by YAP1. A colony assay was conducted on SOX2-deleted cells expressing transgenic SOX2 or YAP1. SOX2F/F cells were infected with a control (vector), SOX2, or YAP1 lentivirus followed by SOX2 deletion with CRE lentivirus, and assayed for colony-forming ability. Western analysis of YAP1 is shown. Each experiment was repeated at least twice. Results from a representative experiment are shown.
*p < 0.05; error bars represent the average + SD.
YAP1 Rescues the Proliferation Defect Caused by SOX2 Depletion
Depletion of SOX2 leads to growth arrest and senescence in primary osteoblasts cultures and osteoprogenitor cell lines. This effect can be clearly measured in a colony-formation assay of SOX2-floxed osteoprogenitor cells infected with a GFP or CRE virus (Basu-Roy et al., 2010). Since YAP1 has a role in self-renewal of ESCs (Lian et al., 2010) and is regulated by SOX2, we tested whether YAP1 is able to replace SOX2 function in the self-renewal of osteoprogenitors and rescue the defect in proliferation caused by SOX2 deletion. SOX2F/F cells were transduced with SOX2 or YAP1 transgenes using lentivirus vectors. The cells were then infected with CRE-expressing lentivirus to excise SOX2, or with a GFP control lentivirus, and the colony-forming ability of GFP- or CRE-expressing cells was measured as previously described (Basu-Roy et al., 2010). In line with our previously published data (Basu-Roy et al., 2010), CRE-mediated deletion in Sox2F/F cells caused a dramatic loss of colony-forming ability (Figure 2E), with the surviving fraction representing cells that had escaped CRE-virus infection. As expected, expression of a SOX2 transgene rescued the defect in colony-forming ability in the SOX2-deleted cells. Rescue was equally efficient in the presence of a YAP1 transgene introduced into Sox2F/F cells. Western analysis confirmed that YAP1 protein expression was reduced in the SOX2-deleted cells and restored in the YAP1 transgene-expressing cells (Figure 2E). These results indicate that YAP1 expression maintained by SOX2 is important for self-renewal of osteoprogenitors.
To determine the specificity of YAP1 for rescuing the proliferation defect caused by SOX2 inactivation, we tested another SOX2 target gene, c-Myc, which was also identified as a direct target in the SOX2 ChIP-seq analysis and is also downregulated upon SOX2 excision. A ChIP-PCR assay confirmed that SOX2 binding to the c-Myc promoter region was enhanced, but c-Myc overexpression failed to restore the defect of colony formation in SOX2 null cells (data not shown). Thus, although c-Myc is also a SOX2 target gene that drives proliferation, in contrast to YAP1, c-Myc expression is not sufficient to compensate for SOX2 function in self-renewal of osteoprogenitors. Interestingly, TAZ (WWTR1), which is not a SOX2 direct target, partially compensated for SOX2 deficiency when overexpressed, although it did so much less efficiently than YAP1 (Figures S2C and S2D), probably due to its high homology to YAP1.
SOX2 and YAP1 Are Expressed in BM and Fat
Since SOX2 is required to maintain self-renewal and the undifferentiated state in the osteoblastic lineage and MSCs (Basu-Roy et al., 2010; Park et al., 2012b), we determined the expression of SOX2 and YAP1 in bone sections, including BM. BM forms a complex stem cell niche that contains cells of the mesenchymal and hematopoietic lineages (Méndez-Ferrer et al., 2010). Immunohistochemistry showed that SOX2 and YAP1 are both expressed in adipocytes adjacent to the femur and tibia (Figure S4A), as well as in adipocytes lining the cortical bone at the bone collar (not shown). SOX2 and YAP1 are undetectable in cortical bone, but positive cells are detected in trabecular bone adjacent to the growth plate, surrounding the areas of bone formation, consistent with the position of immature osteoprogenitor cells (see Figure S4A, inset). Western analysis also confirmed expression of both SOX2 and YAP1 in inguinal fat and compact bone tissue (see Figure S4B). Thus, SOX2 and YAP1 are expressed in immature osteoprogenitor cells and adipocytes.
SOX2 Regulates YAP1 to Maintain Self-Renewal in MSCs
YAP1 expression is induced in MSCs and C3H10T1/2 cells that overexpress SOX2 (Figure S1). To determine whether YAP1 expression was dependent on endogenous SOX2 in MSCs, we isolated MSCs from WT or SOX2 CKO mice and examined the expression of SOX2 and YAP1. Somewhat surprisingly, since the OSX-CRE transgene was not expected to be expressed in MSCs, we found that SOX2 protein was substantially reduced in the SOX2 CKO MSCs and, as in the osteoprogenitors, the expression of YAP1 was reduced, whereas TAZ expression was low and not significantly altered compared with control MSCs (Figure 3A). Consistent with a role for SOX2 in MSC self-renewal, primary MSCs isolated from the SOX2 CKO mice produced fewer colonies than those obtained from heterozygous littermate mice (Figure 3B). A similar reduction in colony formation was seen upon CRE infection of MSCs from SOX2F/F mice (Figure 3C). YAP1 overexpression was able to efficiently rescue this defect in self-renewal in SOX2-depleted MSCs (Figure 3D). As in the osteoprogenitors, although we did not observe any regulation of TAZ by SOX2, TAZ overexpression was able to partially rescue self-renewal in SOX2-depleted MSCs (Figure S3A).
Figure 3. SOX2 Deletion in Primary BM-MSCs Leads to Decreased YAP1 and Reduced Colony Formation that Can Be Rescued by YAP1.
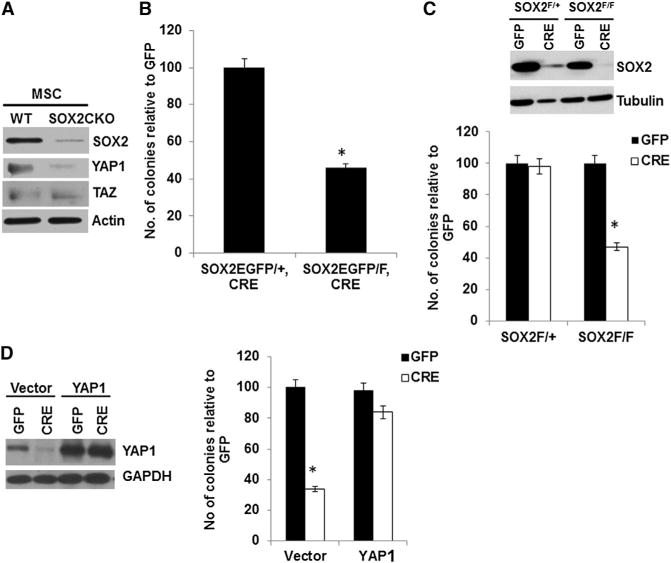
(A) Western analysis of SOX2, YAP1, and TAZ in BM-MSCs isolated from WT or SOX2 CKO mice.
(B) Colony assay (in fibroblast colony-forming units [cfu-f]) of BM-MSCs isolated from 4-week-old SOX2EGFP/+, osterix-CRE (heterozygous) or SOX2EGFP/F, osterix-CRE (SOX2 CKO) mice; 105 cells were plated in triplicate and analyzed as in Figure 2E. *p < 0.05.
(C) Western analysis and colony assay of SOX2F/+ or SOX2F/F BM-MSCs following in vitro CRE-mediated deletion of endogenous SOX2. Primary BM-MSCs were isolated from 4-week-old mice infected with control (GFP) or SOX2-deleting (CRE) virus. *p < 0.05.
(D) Rescue of the colony-forming ability of SOX2-deleted BM-MSCs by YAP1. Western analysis and colony assay were conducted on SOX2F/− BM-MSCs overexpressing YAP1 and depleted of endogenous SOX2 by CRE lentivirus infection.
*p < 0.05; error bars represent the average + SD. See also Figure S3.
These experiments indicate that SOX2 is required for self-renewal and maintenance of MSCs, and that YAP1 is a downstream effector of SOX2 in maintaining these stem cells.
SOX2 Is a Lineage-Fate Determinant in MSCs
SOX2 maintains self-renewal in the osteoblast lineage, where its constitutive expression inhibits osteogenic differentiation by maintaining a stemness gene-expression signature and through the downregulation of Wnt/β-catenin signaling (Seo et al., 2011). Wnt signaling is a key switch that drives osteogenesis and inhibits adipogenesis (Kang et al., 2007; Prestwich and Macdougald, 2007). We therefore investigated whether SOX2 affects the choice of MSCs to differentiate into an osteo- or adipogenic lineage. Lentiviral-mediated SOX2 overexpression in primary MSCs or C3H10T1/2 led to inhibition of osteogenesis (Figures 4A and 4B). Consistent with this observation, SOX2 mRNA expression was reduced during osteogenesis (Figure S4C) and SOX2 knockdown enhanced this process (Figure 4C). Thus, in line with its role in inhibiting differentiation in more mature osteogenic cells, SOX2 also prevents osteogenesis in MSCs. Constitutive SOX2 expression enhanced adipogenic differentiation in the same cells, as measured by an increase in adipocytes staining with Oil Red O (Figure 4D). Adipogenesis was substantially increased in SOX2-expressing cells compared with control vector-expressing cells in both MSCs and C3H10T1/2, and strongly decreased in cells expressing SOX2 small hairpin RNA (shRNA). We also confirmed that PPARγ, the master regular of adipogenesis, was induced in SOX2-overexpressing cells and reduced in SOX2-depleted cells (Figure 4E). These results indicate that SOX2 is able to determine MSC lineage fate by favoring the adipogenic state over the osteogenic one. To verify this further, we isolated MSCs from mice in which one of the SOX2 alleles is replaced by an EGFP cassette, driven by the endogenous SOX2 regulatory elements (Ellis et al., 2004), and the other SOX2 allele is WT (Sox2EGFP/+). Initially, MSC cultures contained very few (<0.5%) cells expressing detectable EGFP (not shown). However, upon induction of adipogenic differentiation, the number of GFP-positive cells increased and eventually all cells that were positive for Oil Red staining were also clearly expressing GFP (Figure 4F). Thus, adipogenic differentiation appears to select for cells expressing sustained levels of SOX2 or to induce SOX2 expression.
Figure 4. SOX2 Inhibits Osteogenesis and Enhances Adipogenesis.
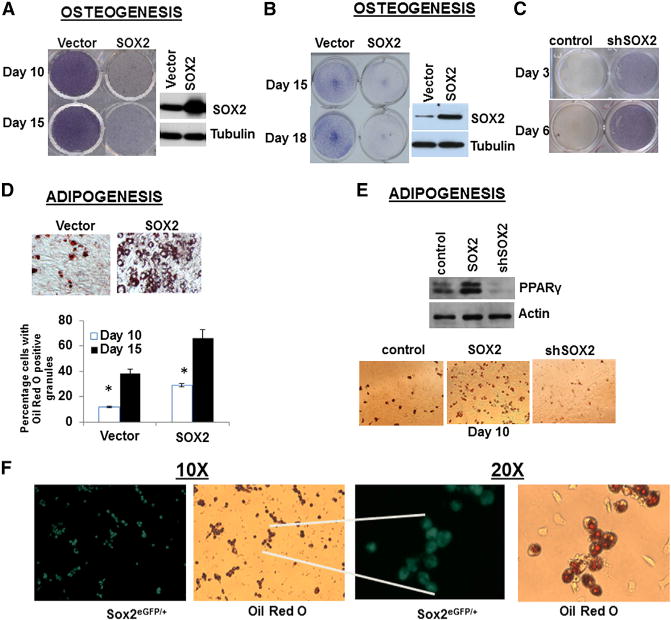
(A and B) Osteogenic differentiation in SOX2-overexpressing BM-MSCs (A) or C3H10T1/2 cells
(B). Cells were infected with SOX2 lentivirus and maintained in osteogenic conditions for the indicated days and stained for alkaline phosphatase (purple color). SOX2 expression by western analysis is shown.
(C) Osteogenic differentiation in C3H10T1/2 cells expressing scrambled (control) or SOX2 shRNA (shSOX2).
(D) Adipogenic differentiation in SOX2-overexpressing BM-MSCs. Cells were infected as in (A) and maintained in adipogenic conditions for 10 days and stained with Oil Red O. Lower panel: quantification of Oil Red O-positive adipocytes.
(E) PPARγ expression and adipogenic differentiation in SOX2-overexpressing or SOX2 knockdown C3H10T1/2 cells. Cells were infected with SOX2 lentivirus (SOX2) or SOX2 shRNA (shSOX2). PPARγ expression by western analysis is shown. Lower panel: Oil Red staining.
(F) Adipogenic differentiation in Sox2EGFP/+ BM-MSCs. Primary cells isolated from Sox2EGFP/+ mice were maintained in adipogenic differentiation medium. SOX2 expression was evaluated after 10 days by fluorescence microscopy and cells stained with Oil Red O. Magnification: 10× and 20×.
*p < 0.05; error bars represent the average + SD. See also Figure S4.
YAP1 Expression or Inactivation of Hippo Signaling Inhibits Osteogenesis and Regulates Adipogenesis
Since YAP1 expression is regulated by SOX2, we sought to determine whether YAP1 can affect osteogenic or adipogenic MSC differentiation. Consistent with it being a SOX2 transcriptional target, YAP1 mRNA is decreased during osteogenesis but increased during adipogenesis (Figure S5A). However, YAP1 protein expression was maintained in osteogenesis compared with undifferentiated cells, but was significantly lower in cells undergoing adipogenesis (Figures 5A and S6). We speculate that posttranscriptional regulatory mechanisms control YAP1 protein levels during osteogenic or adipogenic differentiation (Figures 5A and S6). To determine whether YAP1 regulates osteogenic or adipogenic differentiation of MSCs, we utilized retroviral vectors bearing WT YAP1 or a constitutively active mutant YAP1(2SA) in which serines 127 and 381 were mutated to alanine, or shRNAs for MST1 and MST2, the negative regulators of YAP1 in the Hippo pathway. We found that knockdown of MST1/2 led to increased levels of YAP1 protein (Figures 5D and S5B) and a higher proportion of cells exhibited YAP1 nuclear staining (Figure S5C).
Figure 5. YAP1 Overexpression Inhibits Osteogenesis and Adipogenesis in MSCs.
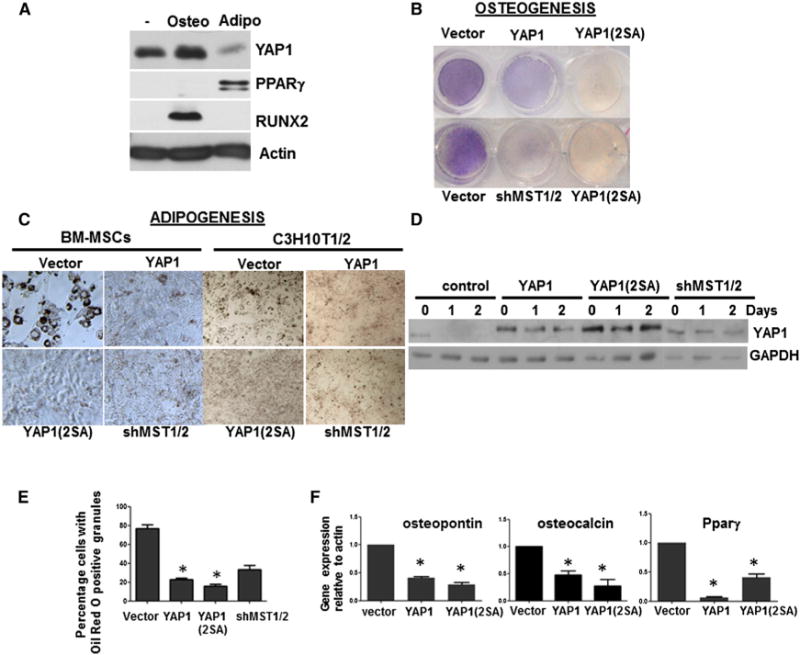
(A) Western analysis of YAP1, RUNX2, and PPARγ in BM-MSCs undergoing osteogenic or adipogenic differentiation for 3 days. See also Figure S5A.
(B) Osteogenic differentiation of primary BM-MSCs infected with control (vector), YAP1, or YAP1(2SA) virus, or with shRNA lentivirus against MST1 and MST2 (shMST1/2).
(C) Adipogenic differentiation of primary BM-MSCs and C3H10T1/2 cells infected as in (B).
(D) Western analysis of YAP1 expression during adipogenesis in C3H10T1/2 cells as in (C). See also Figures S5B and S5C.
(E) Quantification of adipogenesis by Oil Red staining in C3H10T1/2 cells infected as in (C) at 10 days.
(F) qRT-PCR analysis of differentiation markers. mRNA was extracted from C3H10T1/2 cells expressing the indicated constructs. All values are expressed as fold change and normalized to 18S rRNA compared with control.
*p < 0.05; error bars represent the average + SD. See also Figure S6.
Expression of WT YAP1 or YAP1(2SA), or depletion of MST1 and MST2 (shMST1/2) inhibited osteogenic differentiation in primary MSCs (Figure 5B). However, although SOX2 overexpression in MSCs favors adipogenesis, we found that expression of WT YAP1 or mutant YAP1(2SA), or knockdown of MST1/2 inhibited adipogenic differentiation of MSCs and of C310T1/2 cells (Figure 5C). As in the MSCs, adipogenic differentiation of C3H10T1/2 cells led to a decrease of endogenous YAP1 protein (Figure 5D). In contrast, endogenous YAP1 persisted and adipogenesis was inhibited in MST1/2-depleted cells, although not as strongly as in WT YAP1 or mutant YAP1(2SA)-overexpressing cells (Figures 5D and 5E). The inhibition of adipogenesis correlated with increased nuclear localization of YAP1 in YAP1-, YAP1(2SA)-, and MST1/2 shRNA-expressing cells (Figure S5C). Expression of the differentiation-related genes osteopontin, osteocalcin, and PPARγ was also reduced by overexpression of YAP1 and YAP1(2SA) (Figure 5F). Thus, although SOX2 expression in MSCs favors adipogenesis, and YAP1 mRNA is induced by SOX2, YAP1 protein is actually downregulated in this process and its overexpression inhibits adipogenesis.
We reasoned that only a narrow range of YAP1 expression could be compatible with adipogenesis. To test this hypothesis, we sought to determine how different degrees of YAP1 expression influence adipogenesis.
To that end, YAP1 was depleted or transgenic YAP1 was overexpressed or re-expressed in cells depleted of YAP1 (Figure 6A). Knockdown of YAP1 expression by shRNA led to a significant reduction of adipocyte formation, showing that YAP1 is required for adipogenesis (Figure 6B). However, overexpression of YAP1 at much higher levels (~5-fold) also inhibited adipogenesis (Figure 6B). Reflecting this, higher levels of PPARγ were induced under basal conditions, whereas overexpression of YAP1 or knockdown of YAP1 drastically reduced PPARγ induction (Figure 6C). In YAP1 knockdown cells, moderate re-expression of transgenic YAP1 rescued the YAP1 deficiency and enhanced adipogenesis (Figure 6B). Thus, induction of adipogenesis requires moderate levels of YAP1 expression. In sharp contrast to the effect of YAP1 expression levels on adipogenesis, cells with knockdown of YAP1 are enhanced in their ability to undergo osteogenic differentiation, whereas YAP1 overexpression prevents this process (Figure 6D).
Figure 6. Moderate YAP1 Expression Enables Adipogenesis and PPARγ Expression.
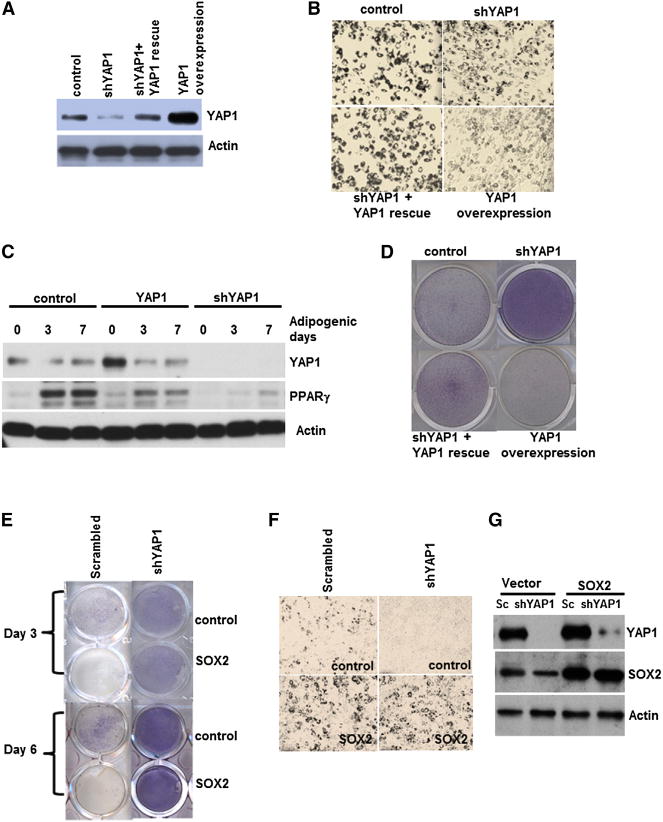
(A) Western analysis of YAP1 expression. C3H10T1/2 cells were infected with YAP1, YAP1shRNA (shYAP1), or shYAP1 and YAP1 (shYAP1 +YAP1 rescue) lentivirus.
(B and D) Adipogenesis and osteogenesis in cells expressing different levels of YAP1. Cells were transduced as in (A), incubated in adipogenic (B) or osteogenic (D) media for 10 days, and stained as in Figures 4A and 4B.
(C) PPARγ expression is regulated by YAP1 during adipogenesis. Western analysis of C3H10T1/2 cells expressing YAP1 or shYAP1 during adipogenic differentiation for the indicated days is shown.
(E) YAP1 is a key downstream target of SOX2 in osteogenesis. Osteogenesis in C3H10T1/2 cells expressing control or SOX2 lentivirus that were infected with either control (scrambled) or shYAP1 is shown.
(F) Adipogenesis assay of cells infected as in (E).
(G) Western analysis of cells used in (E) and (F). See also Figure S6.
To determine whether YAP1 mediates the effects of SOX2 in MSC fate determination, we examined the effect of YAP1 depletion on lineage fate in SOX2-overexpressing cells (Figure 6G). The inhibitory effect of SOX2 overexpression on osteogenic differentiation was blocked in cells expressing shYAP1 (Figure 6E). YAP1 knockdown did not significantly alter adipogenesis in SOX2-overexpressing cells (Figure 6F). Together, these results suggest that YAP1 is induced by SOX2, and that this SOX2-YAP1 axis is important for its inhibitory effect on osteogenic differentiation but is not essential for adipogenesis when SOX2 is overexpressed. YAP1 expression must be restrained, but not abolished, for adipogenic differentiation of MSCs (see Discussion). Thus, we propose that YAP1 acts as a rheostat to regulate the fate determination of MSCs.
YAP1 Regulates Wnt/β-Catenin Signaling by Inducing Dkk1 and Binding to β-Catenin
SOX2 inhibits Wnt/β-catenin signaling through multiple mechanisms, including binding to β-catenin and inducing negative regulators of Wnt signaling such as APC and GSK3β (Ambrosetti et al., 2008; Holmes et al., 2011; Mansukhani et al., 2005; Park et al., 2012b; Seo et al., 2011). Extensive crosstalk between Hippo signaling and Wnt signaling has been observed in different systems, and both YAP1 and TAZ have been reported to interact with β-catenin (Azzolin et al., 2012; Heallen et al., 2011; Hergovich and Hemmings, 2010; Imajo et al., 2012; Konsavage et al., 2012; Rosenbluh et al., 2012). We examined whether YAP1 affects Wnt/β-catenin signaling in our system. C3H10T1/2 cells stably expressing the Wnt reporter pTOP-luciferase were transduced with control vector or YAP1-expressing lentivirus and then treated with CHIR99021, a potent GSK3β-specific inhibitor that leads to Wnt/β-catenin reporter activation. Luciferase reporter activity in untreated cells was low, reflecting a basal state of Wnt signaling. The increased luciferase activity caused by CHIR99021 treatment was reduced by YAP1 expression (Figure 7A) suggesting that YAP1 inhibits canonical Wnt signaling. We tested whether YAP1 interacts with β-catenin in C3H10T1/2 cells, and found that WT YAP1, but not mutant YAP1(2SA), immunoprecipitates with β-catenin (Figure 7B), suggesting that YAP1 may interfere with β-catenin-dependent Wnt signaling. In line with this hypothesis, we found that chromatin-bound β-catenin was decreased in YAP1-overexpressing cells and increased in shYAP1 cells (Figure S7B). In the same cells, we tested the effect of Wnt3A stimulation on the expression of several genes that we previously identified as Wnt targets in the osteogenic lineage (Ambrosetti et al., 2008). Wnt3A significantly increases the expression of these genes, and this effect was enhanced in shYAP1-expressing cells and reduced in cells overexpressing YAP1 (Figure S7C). Thus, YAP1 inhibits induction of Wnt target genes.
Figure 7. YAP1 Inhibits Wnt/β-catenin Signaling by Binding β-catenin and Inducing Dkk1.
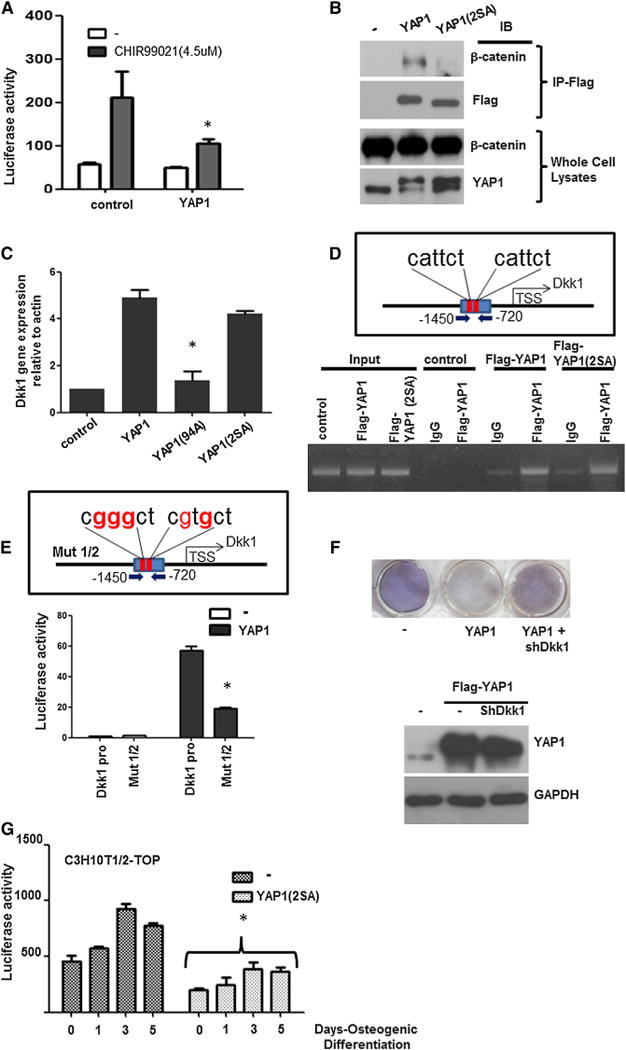
(A) Luciferase assay of Wnt reporter in YAP1-expressing cells. C3H10T1/2 pTOP-FLASH cells were infected with control or YAP1 lentivirus and treated with 4.5 μM of CHIR 99021 for 10 hr. Firefly luciferase activity (Wnt response) was normalized to Renilla.
(B) Immunoprecipitation of β-catenin. C3H10T1/2 cells were infected with flag-YAP1 or flag-YAP1(2SA) virus and whole-cell lysates were immunoprecipitated (IP) with Flag antibody. Immune complexes and whole-cell lysates were immunoblotted (IB) as indicated.
(C) Dkk1 mRNA expression analysis by qRT-PCR. C3H10T1/2 cells were infected with the indicated lentiviral vectors and Dkk1 expression was analyzed.
(D) ChIP assay for the YAP1/TEAD-binding Dkk1 upstream region. C3H10T1/2 cells were infected with control, flag-YAP1, or flag-YAP1(2SA) and ChIP was performed with Flag antibody. Dkk1 genomic fragments were amplified by PCR with specific primers to sequences flanking YAP1/TEAD binding sites (red bars).
(E) Luciferase-reporter assay of Dkk1-promoter (Dkk1-pro) or Dkk1-pro with mutations in TEAD-binding sites (red nucleotides; Mut1/2). A 730 bp region from −1,450 to −720 upstream of the Dkk1 TSS (Dkk1-pro) was subcloned into minimal FGF4 luciferase reporter and transfected into C3H10T1/2 cells expressing YAP1 or control vectors. Luciferase activity was normalized to Renilla.
(F) Osteogenic differentiation. C3H10T1/2 cells were infected by the indicated viral vectors and an osteogenesis assay was performed. Western blot of cells expressing shDkk1 shows that the YAP1 protein level is unaffected.
(G) YAP1 inhibits Wnt during osteogenesis. C3H10T1/2-pTOP-FLASH cells were infected with control (−) or YAP1(2SA) and subjected to osteogenic differentiation for the indicated days. Firefly luciferase activity was normalized to Renilla.
*p < 0.05; error bars represent the average + SD. See also Figure S7.
An analysis to identify potential YAP1 targets (D.L., unpublished data) revealed that Dkk1, the inhibitory ligand of the Wnt pathway, has an upstream promoter region containing YAP1/TEAD consensus sites. qRT-PCR confirmed that Dkk1 mRNA expression was increased by YAP1 or YAP1(2SA) mutant, but not by a TEAD-binding mutant, YAP1(S94A) (Figure 7C). ChIP analysis confirmed the enhanced binding of flag-tagged YAP1 and YAP1(2SA) to the Dkk1 upstream region (Figure 7D). Accordingly, a reporter assay showed that YAP1 enhanced the expression of luciferase from a Dkk1 promoter-reporter plasmid, including two putative YAP1/TEAD-binding consensus sequences, and mutations in these YAP1/TEAD-binding sites led to the reduction of its promoter activity (Figure 7E). These data indicate that YAP1 transcriptionally enhances the expression of Dkk1.
Dkk1 is a potent inhibitor of Wnt signaling and its expression decreases during osteogenic differentiation (Figure S7A). This led us to test whether Dkk1 knockdown could overcome the inhibition of osteogenic differentiation in YAP1-overexpressing MSCs. Primary MSCs constitutively expressing YAP1 were impaired in osteogenic differentiation, but Dkk1 knockdown in these cells was able to overcome the block in osteogenesis (Figure 7F). Furthermore, the expression of a Wnt-luciferase reporter that was induced during osteogenic differentiation was repressed in cells expressing YAP1(2SA) (Figure 7G). These results suggest that together with its binding to β-catenin, the ability of YAP1 to induce Dkk1-mediated inhibition of Wnt signaling plays a significant role in the inhibition of osteogenic differentiation.
DISCUSSION
In this report, we show that SOX2 regulates the osteo-adipo lineage fate in MSCs and that it does so, at least in part, by regulating the expression of YAP1, a transcriptional effector that is restrained by the Hippo pathway. SOX2 maintains stemness and inhibits osteogenic differentiation in adult stem cells, whether MSCs or osteoprogenitors, but also is required for adipogenic differentiation. SOX2 directly targets YAP1, and the SOX2-induced YAP1 maintains stemness and inhibits osteogenesis. YAP1 also cooperates with the antagonistic effect of SOX2 on Wnt signaling by binding β-catenin and inducing the expression of the Wnt inhibitor, Dkk1. Depletion of YAP1 derepresses Wnt signaling and enhances osteogenesis. By dampening Wnt signaling, which promotes osteogenesis and inhibits adipogenesis, both SOX2 and YAP1 tip the balance of SOX2-expressing cells toward stemness and allow adipogenic differentiation. Thus, alternate fate choices of osteogenesis or adipogenesis are determined by the levels of SOX2 and YAP1 as well as by the extent of YAP1 phosphorylation and derepression of Wnt signaling.
YAP1 Is a Target of SOX2
YAP1 and TAZ are cofactors for the transcription factors of the TEAD and RUNX family, and are regulated by the conserved Hippo signaling pathway that controls organ size and regeneration (Dong et al., 2007; Halder and Johnson, 2011; Zhao et al., 2010). The Hippo pathway is now being recognized as an integrator of mechanical and cellular-contact-dependent sensory signals with intracellular components that regulate cell-fate decisions (Schroeder and Halder, 2012). The intracellular Hippo pathway consists of a phosphorylation relay by the STE kinases MST1 and MST2, and the NDR kinases LATS1 and LATS2, which phosphorylate YAP1 and TAZ (Zhao et al., 2011), thereby leading to their cytoplasmic retention (Zhao et al., 2009). Thus, active Hippo signals inhibit the transcriptional activity of YAP1 and TAZ, and SOX2 probably counteracts the repressive effect of Hippo signaling on YAP1 transcriptional activity.
We previously reported that loss of self-renewal due to SOX2 deletion in osteoprogenitors can be rescued by the Polycomb factor BMI-1 (Seo et al., 2011). Interestingly, BMI-1-rescued cells have low levels of YAP1, whereas expression of BMI-1 is enhanced in YAP1-rescued cells (not shown), suggesting a SOX2→ YAP1→ BMI-1 axis in these cells.
Interestingly, TAZ can also partially rescue the defect in self-renewal due to SOX2 depletion, although it is not regulated by SOX2. Given their similar function, this is not surprising. However, endogenous TAZ is not sufficient to compensate for SOX2 and YAP1 loss, and rescue by TAZ is less efficient than rescue by YAP, probably because TAZ and YAP have only partially overlapping gene targets (Zhang et al., 2009). The self-renewal function of SOX2 cannot be compensated for by c-MYC, another proproliferative SOX2 target. This finding is in contrast to work by Park et al. (2012b), who reported that SOX2 depletion in human MSCs by shRNA could be rescued by c-MYC. This discrepancy could arise from the fact that CRE-mediated DNA excision in our system results in complete SOX2 ablation, which may not be compensated for by c-MYC. Thus, c-MYC may cooperate with SOX2 in promoting self-renewal, but cannot compensate for complete loss of SOX2 function.
SOX2 and YAP1 Are Determinants of the Adipo-Osteo Lineage
Several lines of evidence suggest a reciprocal relationship between the adipocytic and osteoblastic lineages (Kang et al., 2007; Takada et al., 2009), and our findings indicate that SOX2 and YAP1 regulate this lineage fate choice in MSCs. As with SOX2, YAP1 overexpression blocks osteogenesis, and YAP1 is a key downstream mediator of SOX2 function in this process because the block does not occur if YAP1 is depleted in SOX2-overexpressing cells. Alternately, SOX2 overexpression strongly favors adipogenesis, and we found that depletion of either SOX2 or YAP1 prevents adipogenic differentiation and induction of PPARγ. However, overexpression of SOX2 compensates for depletion of YAP1 and allows adipogenesis to proceed, suggesting that SOX2 and YAP1 could have overlapping functions in this process. Although the overexpression studies suggest that the SOX2-YAP1 axis is not a key mediator of SOX2 function in promoting adipogenesis, we cannot exclude the possibility that basal SOX2 induction of YAP1 transcription is necessary to maintain the discrete levels of YAP1 that are conducive to adipogenesis.
Both YAP1 knockdown by shRNA and YAP1 overexpression impaired adipogenesis, which can readily proceed in cells with moderate levels of YAP1 expression. This finding appears to be somewhat paradoxical and is not reflected in the levels of YAP1 mRNA during adipogenesis. Indeed, although SOX2 mRNA and protein are both induced during adipogenesis, YAP1 mRNA levels increase during adipogenesis, in line with its being a SOX2 target, but the protein is decreased. Thus, it is likely that additional posttranscriptional mechanisms, such as activation of the proadipogenic effect of components of the Hippo pathway (Park et al., 2012a), may restrain YAP1 protein levels during adipogenesis, which appears to be exquisitely sensitive to the concentration of YAP1. Elevated YAP1 strongly induces proliferation in MSCs (U.B.R. and A.M., unpublished data) that could counteract the proadipogenic effect of MST and Sav1 components of the Hippo pathway (Park et al., 2012a). In line with this, we find that YAP1 overexpression inhibits expression of PPARγ, which is reduced upon YAP1 depletion. YAP1 depletion would lead to increased Wnt signaling that inhibits PPARγ and adipogenesis. Thus, SOX2 and YAP1 function in progenitors upstream of PPARγ to regulate cell-fate choice in MSCs.
In contrast to YAP1, TAZ has been described as having a pro-osteogenic and antiadipogenic function. TAZ binds to and promotes RUNX2 activity while blocking PPARγ function, providing an explanation for its effects. Our data indicate that YAP1 actually inhibits osteogenesis. Indeed, YAP1 was reported to inhibit RUNX2 function by sequestering its transcriptional activity (Zaidi et al., 2004), and unlike TAZ, YAP1 did not bind PPARγ (Hong et al., 2005). FGFs are also known to inhibit osteogenesis, and have been reported to be proadipogenic factors (Hutley et al., 2011; Mansukhani et al., 2000). Given that we originally found SOX2 to be an FGF-induced gene in osteoprogenitor cells, we speculate that the FGFs’ effects on fat tissue may also be mediated by SOX2.
Crosstalk among SOX2, Hippo, and Wnt Signaling in the Mesenchymal Lineage
We show that, like SOX2, YAP1 blocks osteogenic differentiation and antagonizes the Wnt signaling pathway by binding β-catenin and inducing the Wnt negative regulator Dkk1. SOX2 can also directly induce Dkk1 expression via a region in the Dkk1 promoter that lies upstream of the YAP1-inducible region that we identified (Park et al., 2012b), suggesting that Dkk1 may be synergistically regulated by SOX2 and YAP1. Wnt signaling is the best-known regulatory “switch” for the osteo-adipo lineage fate choice (Takada et al., 2009). Several genetic and biochemical studies have established that Wnts drive osteogenesis at the expense of adipogenesis (Kang et al., 2007; Song et al., 2012). We previously showed that SOX2 represses the Wnt pathway (Seo et al., 2011). SOX2 can bind β-catenin and also directly induces GSK3β and APC, negative regulators of Wnt signaling. Several Wnt target genes are activated in osteoblast lineage cells in which SOX2 has been deleted (Basu-Roy et al., 2011). Thus, both SOX2 and YAP1 are negative regulators of the Wnt pathway and thereby influence fate choice in the osteo-adipo lineage. Several points of crosstalk between the Hippo and Wnt pathways in both the cytoplasm and nucleus were recently reported (Heallen et al., 2011; Imajo et al., 2012; Varelas et al., 2010). We found that YAP1 was immunoprecipitated with β-catenin, but YAP1(2SA), the transcriptionally active unphosphorylated mutant, was not. This is in line with previous findings that Hippo pathway activation leading to phosphorylated YAP1 prevents Wnt signaling (Imajo et al., 2012). YAP1 not only blocks Wnt signaling and hence osteogenic differentiation, but interestingly is also regulated by mechanotransductive properties that influence cell fate (Dupont et al., 2011).
In conclusion, we have described a Hippo-independent regulation of YAP1 by SOX2 that influences self-renewal and lineage-fate determination in the osteo-adipo lineage.
EXPERIMENTAL PROCEDURES
Cell Culture
The immortalized osteoprogenitor cells, OB1, Sox2F/F, and Sox2F/−, have been previously described (Basu-Roy et al., 2010; Mansukhani et al., 2000). C3H10T1/2 was obtained from ATCC. C3H10T1/2-TOP cells (Seo et al., 2011) were grown in 400 μg/ml G418. Primary BM-MSCs from WT, Sox2F/F or Sox2F/F Osx-CRE, and SOX2EGFP/+ (Ellis et al., 2004) mice were isolated from 4- to 6-week-old femurs. MSC isolation was carried out according to the protocol of the media manufacturer (StemCell Technologies) and grown in MesenCult (No. 05511). BM-MSC or C3H10T1/2 cells were infected with YAP1 or YAP1 (2SA) retrovirus and selected with 2 μg/ml puromycin. To obtain MST1/2-depleted cells, cells were cotransduced with shRNA against MST1 and MST2 in a lentiviral vector in vitro, and selected with 500 μg/ml of hygromycin and 2 μg/ml of puromycin.
ChIP-Seq and Data analysis
For SOX2-bound regions in osteoprogenitors, chromatin for immunoprecipitation was prepared as previously described (Seo et al., 2011). See Extended Experimental Procedures for further details.
Differentiation Assay
In vitro osteogenic and adipogenic differentiation was carried out as previously described (Basu-Roy et al., 2011).
Colony Assay
Complementation of SOX2 deletion in the SOX2F/F and SOX2F/− osteoprogenitor cell lines was carried out by colony-formation assay as described previously (Basu-Roy et al., 2010; Seo et al., 2011).
Gene-Expression Analysis by Real-Time qRT-PCR and Western Blotting
mRNA was prepared with the use of Trizol Reagent (Invitrogen). Real-time qRT-PCR analysis was carried out as previously described (Basu-Roy et al., 2010). For the specific primers and antibodies used, see Extended Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Jeremy Onuma and Jeffrey Kraynak for technical assistance and Dr. Larysa Pevny for the SOX2 (EGFP) mice. This research was supported by grants from the St. Baldrick’s Foundation (to A.M.), the National Creative Research Program (20120001228 to D.L.), and the Cullen Foundation (to P.H.G.). E.S. was funded by NRF, Korea (2013R1A1A2009701). U.B.R. was supported by a Vilcek Foundation fellowship.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures and seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2013.05.029.
References
- Ambrosetti D, Holmes G, Mansukhani A, Basilico C. Fibroblast growth factor signaling uses multiple mechanisms to inhibit Wnt-induced transcription in osteoblasts. Mol Cell Biol. 2008;28:4759–4771. doi: 10.1128/MCB.01849-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K, Sarkar A, Yram MA, Polo JM, Bronson R, Sengupta S, Seandel M, Geijsen N, Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu-Roy U, Ambrosetti D, Favaro R, Nicolis SK, Mansukhani A, Basilico C. The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ. 2010;17:1345–1353. doi: 10.1038/cdd.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu-Roy U, Seo E, Ramanathapuram L, Rapp TB, Perry JA, Orkin SH, Mansukhani A, Basilico C. Sox2 maintains self renewal of tumor-initiating cells in osteosarcomas. Oncogene. 2011;31:2270–2282. doi: 10.1038/onc.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, Hutton S, Taranova O, Hayashi S, McMahon A, Rao M, Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A, Hemmings BA. TAZ-mediated crosstalk between Wnt and Hippo signaling. Dev Cell. 2010;18:508–509. doi: 10.1016/j.devcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Holmes G, Bromage TG, Basilico C. The Sox2 high mobility group transcription factor inhibits mature osteoblast function in transgenic mice. Bone. 2011;49:653–661. doi: 10.1016/j.bone.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science. 2005;309:1074–1078. doi: 10.1126/science.1110955. [DOI] [PubMed] [Google Scholar]
- Hutley LJ, Newell FS, Kim YH, Luo X, Widberg CH, Shurety W, Prins JB, Whitehead JP. A putative role for endogenous FGF-2 in FGF-1 mediated differentiation of human preadipocytes. Mol Cell Endocrinol. 2011;339:165–171. doi: 10.1016/j.mce.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β-catenin signalling. EMBO J. 2012;31:1109–1122. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- Konsavage WM, Jr, Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/β-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730–11739. doi: 10.1074/jbc.M111.327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansukhani A, Bellosta P, Sahni M, Basilico C. Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J Cell Biol. 2000;149:1297–1308. doi: 10.1083/jcb.149.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansukhani A, Ambrosetti D, Holmes G, Cornivelli L, Basilico C. Sox2 induction by FGF and FGFR2 activating mutations inhibits Wnt signaling and osteoblast differentiation. J Cell Biol. 2005;168:1065–1076. doi: 10.1083/jcb.200409182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H. How is pluripotency determined and maintained? Development. 2007;134:635–646. doi: 10.1242/dev.02787. [DOI] [PubMed] [Google Scholar]
- Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135:4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BH, Kim DS, Won GW, Jeon HJ, Oh BC, Lee Y, Kim EG, Lee YH. Mammalian ste20-like kinase and SAV1 promote 3T3-L1 adipocyte differentiation by activation of PPARγ. PLoS ONE. 2012a;7:e30983. doi: 10.1371/journal.pone.0030983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SB, Seo KW, So AY, Seo MS, Yu KR, Kang SK, Kang KS. SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through Dickkopf-1 and c-MYC. Cell Death Differ. 2012b;19:534–545. doi: 10.1038/cdd.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Prestwich TC, Macdougald OA. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol. 2007;19:612–617. doi: 10.1016/j.ceb.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggi N, Suvà ML, De Vito C, Provero P, Stehle JC, Baumer K, Cironi L, Janiszewska M, Petricevic T, Suvà D, et al. EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes Dev. 2010;24:916–932. doi: 10.1101/gad.1899710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, et al. β-catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder MC, Halder G. Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin Cell Dev Biol. 2012;23:803–811. doi: 10.1016/j.semcdb.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Seo E, Basu-Roy U, Zavadil J, Basilico C, Mansukhani A. Distinct functions of Sox2 control self-renewal and differentiation in the osteoblast lineage. Mol Cell Biol. 2011;31:4593–4608. doi: 10.1128/MCB.05798-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Liu M, Ono N, Bringhurst FR, Kronenberg HM, Guo J. Loss of wnt/beta-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J Bone Miner Res. 2012;27:2344–2358. doi: 10.1002/jbmr.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9:2145–2152. [PubMed] [Google Scholar]
- Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- Urs S, Venkatesh D, Tang Y, Henderson T, Yang X, Friesel RE, Rosen CJ, Liaw L. Sprouty1 is a critical regulatory switch of mesenchymal stem cell lineage allocation. FASEB J. 2010;24:3264–3273. doi: 10.1096/fj.10-155127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H, et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol. 2002;55:693–698. doi: 10.1136/jcp.55.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi SK, Sullivan AJ, Medina R, Ito Y, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J. 2004;23:790–799. doi: 10.1038/sj.emboj.7600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–192. doi: 10.1016/j.ccr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S, Xiong Y, Lei QY, Guan KL. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem. 2009;284:13355–13362. doi: 10.1074/jbc.M900843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Lei QY, Guan KL. Harness the power: new insights into the inhibition of YAP/Yorkie. Dev Cell. 2009;16:321–322. doi: 10.1016/j.devcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862–874. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


