Abstract
Probing of biomolecular complexes by single-molecule force spectroscopy (SMFS) methods including AFM requires proper and suitable coupling methods for immobilization of biomolecules onto the AFM tip and the surface. The use of flexible tethers for the coupling process has dual advantages. First, they allow the specific immobilization of interacting molecules, and second, their flexibility facilitates the proper orientation of the interacting partners. Recently, we developed an approach termed Flexible Nano Array (FNA) in which interacting partners are located on the same polymeric FNA molecule separated by a flexible segment with a defined length. In this paper, we modified the FNA tether approach by incorporating click chemistry with non-metal modification. FNA was synthesized using DNA synthesis chemistry, in which phosphoramidite (PA) spacers containing six ethylene glycol units were used instead of nucleoside triphosphates. During the synthesis, two T modifiers conjugated to two dibenzocyclooctyl (DBCO) residues were incorporated at selected positions within the FNA. The DBCO functionality allows for coupling azide labeled biomolecules via click chemistry. Amyloid peptide Aβ(14–23) terminated with azide was incorporated into the FNA and the reaction was controlled with mass-spectrometry. Assembly of tethered Aβ(14–23) peptides into dimers was characterized by AFM force spectroscopy experiments in which the AFM tip functionalized with FNA terminated with biotin probed a streptavidin-coated mica surface. The formation of the peptide dimer was verified with force spectroscopy that showed the appearance of a specific fingerprint for dimer dissociation followed by a rupture event for the biotin-streptavidin link. The developed approach is capable of multiple probing events to allow the collection of a large set of data for a quantitative analysis of the force spectroscopy events.
Keywords: Amyloid Aggregation, Surface Chemistry, Click Chemistry For Peptides, AFM Force Spectroscopy, Nano Array
INTRODUCTION
Single-molecule techniques including AFM force spectroscopy are widely used for characterizing biomolecular interactions and dynamics of various molecular complexes [1–6]. A key factor in force spectroscopy applications, including AFM force spectroscopy, is the successful immobilization of biomolecules onto the AFM tip and substrate [7]. The interacting partners should be immobilized in such a way to allow them to assemble in the least restrictive fashion. Immobilization can be performed with the use of flexible polymeric tethers, such as a PEG tether [8, 9]. PEG linkers are available in different lengths that allow the separation of specific rupture events from nonspecific short-range adhesion that can occur in SMFS experiments. However, one of major disadvantages of PEG tethers is their heterogeneous lengths, which complicate the quantitative analysis of the contour lengths of force curves [10].
We introduced a new generation of potentially useful phosphoramidite (PA) tethers with PA spacers containing six ethylene glycol units polymerized with homogeneous sizes and predefined lengths. The synthesis of PA tethers is based on a traditional PA chemistry using an automated DNA synthesizer [11]. A well-defined length is one of the primary features of the PA tether. Additionally, PA tethers can be functionalized with different functional groups at their ends, which facilitates the immobilization of various biomolecules. Importantly, specific reactive groups can be introduced into the PA tether that can be used for site-specific immobilization of biomolecules on the tether. This capability was demonstrated in our recent paper in which Aβ(14–23) peptides were coupled with the active groups of the tether to form an array of two Aβ(14–23) peptides separated with a flexible tether of a defined length [12]. To accomplish this, amino T modifier residues were incorporated at selected positions within the FNA during the synthesis step. These residues were converted to maleimide groups capable of covalent bonding with the thiol of the target biomolecule. With this tether, termed a Flexible Nano Array (FNA), we measured interactions between Aβ(14–23) monomers within a transiently formed dimer coupled to the tether via terminal cysteine residues. As a result, it was possible to probe the same pair by performing repeated cycles of approach-retraction during force spectroscopy experiments because the two monomers are immobilized with the same FNA tether prior to the rupture of the tether-surface bond.
Here, we extend this approach further by using metal-free click chemistry to covalently conjugate the target molecules [13, 14]. The high specificity, reliability, and versatility of click chemistry make it an attractive alternative to both the maleimide chemistry of AFM force spectroscopy and the immobilization of molecules within FNA tethers. Two dibenzocyclooctyl (DBCO) residues of the FNA allow us to perform metal-free click chemistry with azide-labeled Aβ(14–23) peptides. The FNA molecule was terminated with a thiol group at one end and a biotin at the opposite end to immobilize FNA and for probing experiments, respectively. FNA containing a thiol group was immobilized to the AFM tip functionalized with maleimide groups. The AFM substrate was covalently functionalized with streptavidin via a PEG linker to enable the formation of biotin-streptavidin during the approach step. Stretching the tether resulted in Aβ(14–23) dimer rupture, and this specific fingerprint was used to identify the spontaneous assembly of Aβ(14–23) monomers in dimers within FNA. Details regarding the FNA assembly process and data analysis are described.
EXPERIMENTAL SECTION
Materials and Methods
All reagents used for the synthesis of the FNA tether were purchased from Glen Research (Sterling, VA): spacer 18 phosphoramidite (18-O Dimethoxytritylhexaethyleneglycol, 1-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite, 10–1918, Glen Research), DBCO-dT-CE phosphoramidite (5′-Dimethoxytrityl-5-[(6-oxo-6-(dibenzo[b,f]azacyclooct-4-yn-1-yl)-capramido-N-hex-6-yl)-3-acrylimido]-2′-deoxy-Uridine, 3′-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite, 10-1539-xx, Glen Research), Thiol-Modifier C6 S-S, (1-O-Dimethoxytrityl-hexyl-disulfide,1′-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite, 10-1936-xx, Glen Research), biotin CPG (3′-Protected Biotin Serinol CPG; #20-2993; GlenResearch). The azide-labeled Aβ(14–23) peptide [K(N3)HQKLVFFVAED] was synthesized and purified by Peptide 2.0 company (VA, USA). Tris (2-carboxyethyl) phosphine (TCEP) hydrochloride was from Hampton Research (Aliso Viejo, CA). Heterobifunctional MAL-PEG3400-SVA was from Laysan Bio (Arab, AL). N-(g-Maleimidobutyryloxysuccinimide ester) GMBS was from Pierce Biotechnology (Grand Island, NY). Streptavidin-thiol was from Protein Mods (Madison, WI) and 1-(3-aminopropyl) silatrane (APS) was synthesized as previously described [15]. All other reagents or solvents were purchased from Sigma–Aldrich.
Synthesis of Aβ(14–23) conjugated FNA
The FNA polymer was synthesized using PA chemistry on the MerMade-12 oligonucleotide synthesizer with the standard 200 nmol DNA protocol in cycles of DMT removal-coupling-cap-oxidation-cap with the coupling time extended to 6 min. The FNA tether was synthesized on a biotin CPG (3′-Protected Biotin Serinol CPG; #20-2993; Glen Research), which is biotin immobilized on a CPG support. During the first cycle, the DMT protective group is removed from a biotin-connected linker to form a free −OH group with 3% dichloroacetic acid in dichloromethane, which is then coupled to the 18 phosphoramidite (S18) spacer unit in the presence of 0.25 M 5-Ethylthio-1H-Tetrazole as a catalyst in acetonitrile. Then, oxidation was performed with 0.02 M iodine in water/pyridine/THF (1:2:7) to produce a stable phosphate linkage. The capping was performed in acetic anhydride/2,6-lutidine/THF (1:1:8) and 16% v/v N-methyl imidazole in THF to protect the unreacted −OH groups from being involved in further reactions. After coupling of four S18 units, the click chemistry reagent dibenzocyclooctyl (DBCO-dT-CE) phosphoramidite was coupled. Then, another six units of S18 were coupled, followed by another DBCO-dT-CE phosphoramidite, and four units of S18. A thiol protected PA group was added at the end, and the final DMT group was removed. Deprotection and removal of FNA was performed by treating with 30% ammonium hydroxide for 16 h. After the removal of ammonia, the FNA was purified using reversed phase high-performance liquid chromatography (acetonitrile gradient in 0.1 M triethylammonium bicarbonate pH 7.5 buffer, Gradient 20–45% in 40 min, Phenomenex Gemini C18 column, 5μ, 250×4.6 mm). The final structure of the linker is represented by the following formula: HO-(CH2)6-S-S-(CH2)6-(S18)4-(DBCO-dT)-(S18)6-(DBCO-dT)-(S18)4-biotin. The purified FNA was then dissolved in water and FNA was coupled with azide containing Aβ(14–23) using metal-free click chemistry for 24 h. A four times molar excess of azide labeled peptide over FNA was used to maximize the conjugation probability of two molecules of peptide with FNA. Finally, the peptide conjugated FNA was purified with RP-HPLC (acetonitrile gradient in 0.1M triethylammonium bicarbonate pH 7.5 buffer, Gradient 20–45% in 40 min, Gemini C18 column, 5μ, 250×4.6 mm) and characterized by ESI (electrospray ionization) mass spectroscopy (Thermo Scientific LTQ LCMS system).
Tip functionalization
Silicon nitride (Si3N4) AFM tips (MSNL10, Bruker AFM probes, Camarillo, CA) were cleaned with ethanol and water, and then dried in air. The tip was then further cleaned by UV treatment for 45 min. The tip was dipped into 1 μM APS in water for 30 min, followed by multiple rinses with water. 1 μM N-(g-Maleimidobutyryloxysuccinimide ester) (GMBS) solution in DMSO was prepared and the tip was dipped into this solution for 2 h. The tip was then rinsed multiple times with water. 50 μL of 10 nM of FNA in sodium phosphate buffer (10 mM, pH 7.0) containing 10 μM Tris (2-carboxyethyl) phosphine (TCEP) was prepared 30 min before functionalization of the tip to ensure that the S-S bond was reduced by TCEP. A droplet of FNA solution was placed on a piece of parafilm and the tip was covered with the FNA droplet (50 μl) and kept at 4°C overnight. The parafilm was placed on a wet tissue paper in a Petri dish to minimize evaporation. After functionalization, the tip was rinsed with water and unreacted maleimide groups were quenched by dipping the tip into a 10 mM solution of 2 mercaptoethanol for 10 min. Finally, the tip was washed in water and stored in 10 mM sodium phosphate buffer (pH 7.0) until it was to be used.
Surface functionalization
Mica sheets (Asheville Schoonmaker Mica, Newport News, VA) were cut into 1.5 cm x 1.5 cm squares and glued onto a microscope glass slide using epoxy glue EPO-TEK-301 (Epoxy Technology, Billerica, MA), and the mica was cleaved with scotch tape. The freshly cleaved mica surface was treated with an aqueous solution of 167 μM APS for 30 min followed by several rinses in deionized water. The surface was then treated with 167 μM MAL-PEG3400-SVA in DMSO for 3 h, followed by washing with water. 10 nM streptavidin-thiol was prepared in 10 μM TCEP in sodium phosphate buffer just 30 min before adding to the surface to ensure that the S-S bond of streptavidin was reduced by TCEP. This solution was added to the surface and kept overnight at 4°C. The substrate was then washed with water and unreacted maleimide groups were quenched by exposing the surface to a 10 mM solution of 2 mercaptoethanol for 15 min. Finally, the surface was washed and covered with buffer until needed. At the end of force measurements, the surface was imaged in liquid to assess streptavidin surface coverage on the substrate.
Force measurement
The Force-Distance (F-D) measurements were performed on an Asylum MFP 3D AFM instrument (MFP-3D, Asylum Research, Santa Barbara, CA). Silicon nitride (Si3N4) AFM tips (MSNL10, Bruker AFM probes, Camarillo, CA) with a nominal spring constant of 0.03 N/m were used for force measurements. The thermal noise analysis method (Igor Pro 6.37) was used to calculate the spring constant of the AFM probes. The ramp size was set to 200 nm throughout the experiment. A low trigger force (100 pN) and dwell time of 1 s was used to maximize the probability of dimer formation before the retraction step. The probe was then retracted at a speed of 500 nm/s in 10 mM sodium phosphate (pH 7.0) buffer at room temperature. Several thousand F-D curves were acquired to obtain a dataset with several hundred rupture events.
Data Analysis
The F-D curves showing fingerprints of Aβ(14–23) dimer dissociations, followed by biotin-streptavidin dissociations, were selected and fitted with the worm-like-chain (WLC) model[16] using the following equation:
where F(x) is the force at the distance of x, kB is the Boltzmann constant, T is the absolute temperature, and Lp and Lc are the persistence length and the contour length, respectively. The F-D curves containing two peaks were then fitted individually with the WLC model. The data for force and contour length were assembled as histograms and fit by Gaussians. The values of the most probable force and contour lengths correspond to maxima on these Gaussians.
RESULTS AND DISCUSSIION
FNA design, assembly and experimental approach
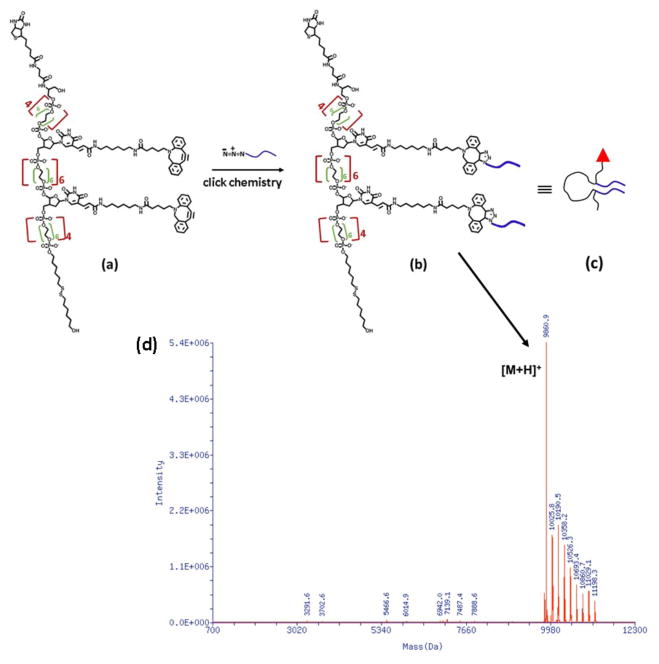
The synthesis and assembly process of FNA is shown schematically in Figure 1. The DNA synthesizer was used to synthesize FNA with PA monomer units. The majority of these PA monomers are PEG-containing S18 units except for two of them, which are DBCO units, as shown in Figure 1a. DBCO units are capable of conjugation with the azide groups of the Aβ(14–23) peptide via click chemistry, as shown in Figure 1b. The final product was analyzed by ESI mass spectroscopy and the results shown in Figure 1d confirm that one FNA contains two peptide molecules.
Figure 1.
(a) The chemical structure of FNA having two dibenzocyclooctyl (DBCO) functionalities for further azide coupling, (b) Conjugation of azide labeled Aβ(14–23) with FNA via metal-free click chemistry, (c) The cartoon showing Aβ(14–23) conjugated FNA tether; the red triangle, black line and blue lines indicate biotin, FNA tether and Aβ(14–23) peptide respectively (d) ESI mass spectra of HPLC pure FNA Aβ(14–23) dimer.
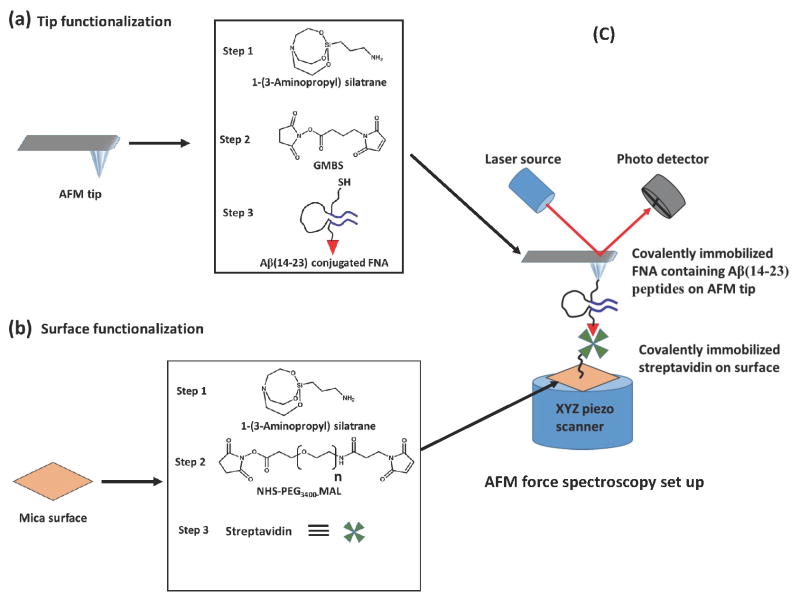
The AFM tip and mica surface were functionalized with the FNA and streptavidin respectively, before force measurements, according to the methods discussed previously and schematically shown in Figure 2. In brief, the AFM tip was treated with APS, followed by treatment with bi-heterofunctional GMBS and finally with the thiol ended FNA (Figure 2a). Similarly, mica substrate was treated with APS, then SVA-PEG-MAL and finally with streptavidin-thiol protein (Figure 2b).
Figure 2.
(a) Immobilization of Aβ(14–23) conjugated FNA with AFM tip; the AFM tip was treated with 1-(3-Aminopropyl) silatrane (APS) (step 1), then treatment with the GMBS (step 2), finally with the thiol ended Aβ(14–23) conjugated FNA (step 3); (b) Immobilization of streptavidin protein on the mica surface; the surface was treated with APS (step 1), followed by treatment with the SVA-PEG3400-MAL (step 2), finally treatment with the streptavidin (step 3); (c) Schematic presentation of AFM force spectroscopy set up.
These amyloid peptides are capable of spontaneous self-assembly into dimers. Dimer rupture was probed in AFM force spectroscopy experiments [17, 18]. According to the synthesis protocol, the two peptides are separated with a PEG polymer with a length of ~ 12 nm [12]. Due to the high flexibility of PEG, the monomers can assemble into a dimer, as shown schematically in Figure 4. The assembly of dimers can be analyzed when the FNA polymer is stretched. The FNA contains thiol groups at one end of the molecule that were used for covalent immobilization onto the AFM tip functionalized with maleimide (Figure 2a). The other end of FNA contains biotin molecules that can form a strong intermolecular complex with streptavidin. These properties allowed FNA to be stretched in the AFM experiment, as shown in Figure 2c and described in the methods section.
Figure 4.
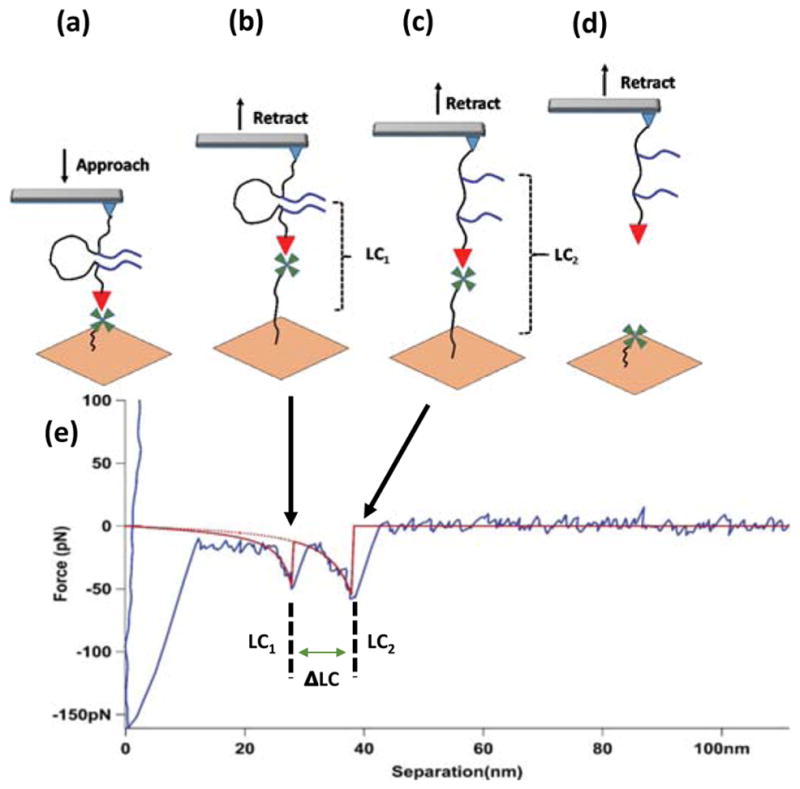
AFM force spectroscopy experiment. (a) The AFM tip functionalized with FNA and terminated with biotin (red triangle) approaches the streptavidin-coated (green) surface; (b) Representation of events during retraction, just before the Aβ(14–23) rupture event; (c) Representation of events before the biotin-streptavidin link rupture; (d) Representation of molecules after two successive complex rupture events; (e) A representative force vs. distance curve (retraction) showing two peaks, the red lines indicate WLC fitting. LC1 and LC2 represent the contour length for the first and second peak, respectively. ΔLC represents (LC2 - LC1), which is equal to the length of the loop between the two strands of the peptide. The two arrows indicate possible situations for two peaks in the force vs. distance curve.
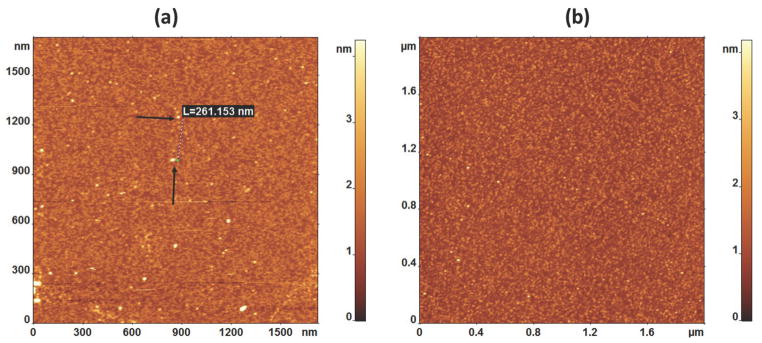
The functionalization was tested by AFM imaging of the mica surface. As seen in Figure 3a, after functionalization, the mica surface is coated with globular features with sizes that are consistent with the size of the streptavidin tetramer. The distance between adjacent streptavidin molecules is more than 100 nm (Figure 3a). This large distance ensures that single molecules can be probed in force spectroscopy experiments [19]. The control experiment in which the surface without streptavidin was imaged is shown in Figure 3b.
Figure 3.
AFM images of (a) The surface with covalently bonded streptavidin (the dotted line showed the distance between two streptavidin blobs shown by black arrows on substrate); and (b) The surface with no streptavidin.
Probing the Aβ (14–23) dimer assembly process with AFM force spectroscopy
The schematics for the AFM force spectroscopy experiments are shown in Figure 4a–d. The AFM tip terminated with FNA (only one molecule is shown for clarity) approaches the mica surface that is functionalized with streptavidin. Biotin on the FNA end (red triangle) captures streptavidin that is attached covalently to the mica surface via a PEG tether (green). The tether stretches during tip retraction (Figure 4b) and an additional force exerted by the tip cantilever ruptures the dimer (Figure 4c). Further retraction of the tip leads to the rupture of the biotin-streptavidin link (Figure 4d).
All of these events are detected in the AFM force curve, as shown in Figure 4e. The two rupture events shown in schemes b and c are indicated with arrows. The rupture events are accompanied with a smooth stretching process and are approximated on the force curve with smooth red curves (WLC approximation [16]). The rupture forces for both events are F1= 50 pN and F2= 62 pN, which correlate well with the data obtained for probing interactions between Aβ (14–23) peptide [12, 17] and biotin-streptavidin [20], respectively. The distances for these events are 41 nm and 52 nm, with a distance between the two rupture events of ~11 nm, which is consistent with the 12 nm distance between the DBCO units in Figure 1a.
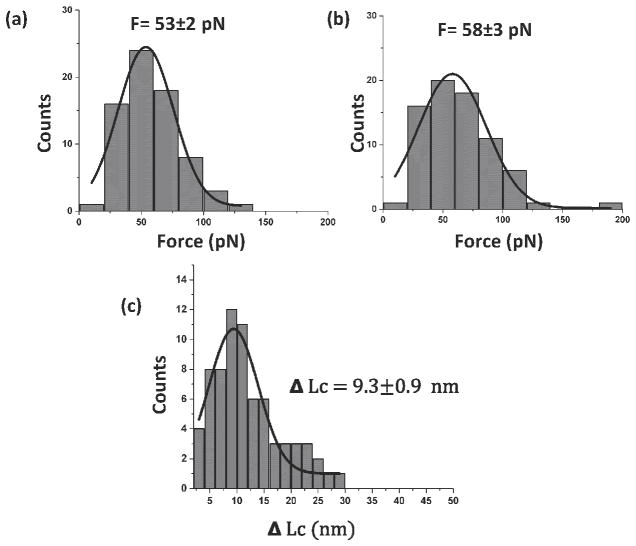
The probing experiments were performed multiple times at different tip locations on the substrate. In each experiment, rupture forces for both events and the distances between them were measured. The data are assembled as histograms in Figure 5, and each dataset was approximated with Gaussians. The rupture forces for the first peak showed that the most probable rupture force for Aβ (14–23) dimer unbinding is 53 ± 2 pN (Figure 5a), which is very close to our previous data (48 ± 6 pN).[12, 17] Similarly, Gaussian fitting of the force histogram for the second rupture event produces a value of 58 ± 3 pN, which is close to the force for biotin-streptavidin dissociation (Figure 5b).[12] The distance between the rupture events (Figure 5c) is 9.3 ± 0.9 nm, which is close to the anticipated distance between the peptide positions in FNA, although slightly less than the expected 12 nm. This difference is due to the peptides’ orientation within the dimer. The scheme in Figure 4a shows the parallel orientation of the monomers and that dissociation of the dimer will lead to unfolding of a 12 nm loop. However, the flexibility of FNA also allows the peptides to be oriented in the antiparallel orientation. Indeed, FNA is flexible as characterized by the persistence length as small as 0.27 nm.[11, 12] Therefore, the FNA spacer between the peptides consists of 36 persistence lengths. According to polymer statistics[21], flexible polymers adopt a random coil configuration, which allows the ends of the polymer to adopt any possible orientation. The antiparallel orientation of the peptides leads to an ~ 2 nm smaller loop size, which is very close to the 9.3 ± 0.9 nm distance obtained in the experiment. Additional evidence supporting the antiparallel orientation of the monomers comes from the force measurements. According to paper[18], the rupture force value 53 ± 2 pN corresponds to the antiparallel orientation of the monomers, while 30 ± 4 pN is anticipated for the parallel orientation of the monomers.
Figure 5.
Statistical analysis of the force spectroscopy experiments. (a) Histogram of the force distribution for the rupture of Aβ(14–23) dimers. The data are approximated with a Gaussian, producing an average force of 53 ± 2 pN; (b) Histogram for biotin-streptavidin unbinding events, with an average force of 58 ± 3 pN; (c) Histogram for the distance between the two rupture events; the average value is 9.3 ± 0.9 nm. All of the histograms were fitted with the Gaussian function and SEM is shown. The number of curves used for each histogram is 74.
CONCLUSION
We demonstrated in this paper that click chemistry can be used for the target specific immobilization of azide-modified peptides within the FNA tether. The DBCO anchoring points for the peptide attachment are incorporated into the FNA during synthesis; therefore, no additional modification of FNA is needed to perform click chemistry coupling. Given the high flexibility of the FNA tether, the two Aβ(14–23) molecules are capable of assembling as a dimer with a structure similar to that formed by free monomers. This finding suggests that the FNA does not restrict monomer orientation during the assembly process into the inherent dimeric configuration. Additionally, FNA is a neutral PEG-based system, allowing it to be compatible for the immobilization of a large number of biomolecules. This feature makes the proposed click chemistry coupling approach suitable to many different biological systems.
Acknowledgments
The work was supported by grants to Y.L.L. from the National Institutes of Health (NIH: GM096039 and GM118006) and the National Science Foundation (MCB 1515346).
Abbreviations
- AFM
Atomic force microscopy
- APS
1-(3-aminopropyl) silatrane
- DBCO
Dibenzocyclooctyl
- DMSO
Dimethyl sulphoxide
- FNA
Flexible nanoarray
- GMBS
N-(g-Maleimidobutyryloxysuccinimide ester)
- PA
phosphoramidite
- PEG
Polyethylene glycol
- SMFS
Single molecule force spectroscopy
- TCEP
Tris (2-carboxyethyl) phosphine
Footnotes
Author’s Contribution
S. M. performed AFM force spectroscopy, imaging, data analysis and the paper preparation. E. V. and A. G. synthesized Aβ(14–23) conjugated with FNA and validated the construct. A. G. and Y. L. designed the experiments and wrote the manuscript. All authors approved the manuscript.
Conflict of Interests
The authors declare no competing interests.
References
- 1.Muller DJ, Dufrene YF. Atomic force microscopy as a multifunctional molecular toolbox in nanobiotechnology. Nat Nano. 2008;3:261–269. doi: 10.1038/nnano.2008.100. [DOI] [PubMed] [Google Scholar]
- 2.Neuman KC, Nagy A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat Meth. 2008;5:491–505. doi: 10.1038/nmeth.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyubchenko YL, Kim BH, Krasnoslobodtsev AV, Yu J. Nanoimaging for protein misfolding diseases. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology. 2010;2:526–543. doi: 10.1002/wnan.102. [DOI] [PubMed] [Google Scholar]
- 4.Capitanio M, Pavone FS. Interrogating biology with force: single molecule high-resolution measurements with optical tweezers. Biophysical journal. 2013;105:1293–1303. doi: 10.1016/j.bpj.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulin D, Berghuis BA, Depken M, Dekker NH. Untangling reaction pathways through modern approaches to high-throughput single-molecule force-spectroscopy experiments. Curr Opin Struct Biol. 2015;34:116–122. doi: 10.1016/j.sbi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Ott W, Jobst MA, Schoeler C, Gaub HE, Nash MA. Single-molecule force spectroscopy on polyproteins and receptor-ligand complexes: The current toolbox. J Struct Biol. 2016 doi: 10.1016/j.jsb.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Tong Z, Mikheikin A, Krasnoslobodtsev A, Lv Z, Lyubchenko YL. Novel polymer linkers for single molecule AFM force spectroscopy. Methods. 2013;60:161–8. doi: 10.1016/j.ymeth.2013.02.019. See also more aproaches on the surface chemistry at JKU website: http://www.jku.at/biophysics/content/e257042/e257046/01_simple_introduction_2016_05_06_eng.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim BH, Palermo NY, Lovas S, Zaikova T, Keana JF, Lyubchenko YL. Single-molecule atomic force microscopy force spectroscopy study of Abeta-40 interactions. Biochemistry. 2011;50:5154–5162. doi: 10.1021/bi200147a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maity S, Lyubchenko YL. Probing of Amyloid Aβ (14–23) Trimers by Single-Molecule Force Spectroscopy. J J Mol Transl Med. 2015;1:004. [PMC free article] [PubMed] [Google Scholar]
- 10.Thormann E, Hansen PL, Simonsen AC, Mouritsen OG. Dynamic force spectroscopy on soft molecular systems: Improved analysis of unbinding spectra with varying linker compliance. Colloids and Surfaces B: Biointerfaces. 2006;53:149–156. doi: 10.1016/j.colsurfb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Tong Z, Mikheikin A, Krasnoslobodtsev A, Lv Z, Lyubchenko YL. Novel polymer linkers for single molecule AFM force spectroscopy. Methods. 2013;60:161–168. doi: 10.1016/j.ymeth.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krasnoslobodtsev AV, Zhang Y, Viazovkina E, Gall A, Bertagni C, Lyubchenko YL. A flexible nanoarray approach for the assembly and probing of molecular complexes. Biophysical journal. 2015;108:2333–2339. doi: 10.1016/j.bpj.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eeftens JM, van der Torre J, Burnham DR, Dekker C. Copper-free click chemistry for attachment of biomolecules in magnetic tweezers. BMC Biophys. 2015;8:9. doi: 10.1186/s13628-015-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senapati S, Manna S, Lindsay S, Zhang P. Application of Catalyst-Free Click Reactions in Attaching Affinity Molecules to Tips of Atomic Force Microscopy for Detection of Protein Biomarkers. Langmuir. 2013;29:14622–14630. doi: 10.1021/la4039667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shlyakhtenko LS, Gall AA, Lyubchenko YL. Mica functionalization for imaging of DNA and protein-DNA complexes with atomic force microscopy. Methods Mol Biol. 2013;931:295–312. doi: 10.1007/978-1-62703-056-4_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouchiat C, Wang MD, Allemand J, Strick T, Block SM, Croquette V. Estimating the persistence length of a worm-like chain molecule from force-extension measurements. Biophysical journal. 1999;76:409–413. doi: 10.1016/s0006-3495(99)77207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovas S, Zhang Y, Yu J, Lyubchenko YL. Molecular Mechanism of Misfolding and Aggregation of Aβ(13–23) The Journal of Physical Chemistry B. 2013;117:6175–6186. doi: 10.1021/jp402938p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Lyubchenko Yuri L. The Structure of Misfolded Amyloidogenic Dimers: Computational Analysis of Force Spectroscopy Data. Biophysical journal. 107:2903–2910. doi: 10.1016/j.bpj.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrance OE, Paci E, Radford SE, Brockwell DJ. Extraction of Accurate Biomolecular Parameters from Single-Molecule Force Spectroscopy Experiments. ACS Nano. 2015;9:1315–1324. doi: 10.1021/nn505135d. [DOI] [PubMed] [Google Scholar]
- 20.Taninaka A, Takeuchi O, Shigekawa H. Hidden variety of biotin-streptavidin/avidin local interactions revealed by site-selective dynamic force spectroscopy. Physical Chemistry Chemical Physics. 2010;12:12578–12583. doi: 10.1039/c0cp00259c. [DOI] [PubMed] [Google Scholar]
- 21.Liese S, Netz RR. Influence of length and flexibility of spacers on the binding affinity of divalent ligands. Beilstein Journal of Organic Chemistry. 2015;11:804–816. doi: 10.3762/bjoc.11.90. [DOI] [PMC free article] [PubMed] [Google Scholar]