Abstract
Background and objectives
Emerging evidence from recently published observational studies and an individual patient data meta–analysis shows that mammalian target of rapamycin inhibitor use in kidney transplantation is associated with increased mortality. Therefore, all-cause mortality and allograft loss were compared between use and nonuse of mammalian target of rapamycin inhibitors in patients from Australia and New Zealand, where mammalian target of rapamycin inhibitor use has been greater because of heightened skin cancer risk.
Design, setting, participants, & measurements
Our longitudinal cohort study included 9353 adult patients who underwent 9558 kidney transplants between January 1, 1996 and December 31, 2012 and had allograft survival ≥1 year. Risk factors for all-cause death and all–cause and death–censored allograft loss were analyzed by multivariable Cox regression using mammalian target of rapamycin inhibitor as a time-varying covariate. Additional analyses evaluated mammalian target of rapamycin inhibitor use at fixed time points of baseline and 1 year.
Results
Patients using mammalian target of rapamycin inhibitors were more likely to be white and have a history of pretransplant cancer. Over a median follow-up of 7 years, 1416 (15%) patients died, and 2268 (24%) allografts were lost. There was a higher risk of all-cause mortality with time–varying mammalian target of rapamycin inhibitor use (hazard ratio, 1.47; 95% confidence interval, 1.23 to 1.76) as well as in the fixed time model analyses comparing mammalian target of rapamycin inhibitor use at baseline (hazard ratio, 1.54; 95% confidence interval, 1.22 to 1.93) and 1 year (hazard ratio, 1.63; 95% confidence interval, 1.32 to 2.01). Time–varying mammalian target of rapamycin inhibitor use was associated with higher risk of death because of malignancy (hazard ratio, 1.37; 95% confidence interval, 1.09 to 1.71). There were no statistically significant differences in the risk of all–cause (hazard ratio, 0.98; 95% confidence interval, 0.85 to 1.12) and death–censored (hazard ratio, 0.85; 95% confidence interval, 0.69 to 1.03) allograft loss between the mammalian target of rapamycin inhibitor use and nonuse groups in the time-varying model as well as the fixed time models.
Conclusions
Mammalian target of rapamycin inhibitor use was associated with a higher risk of all-cause mortality but not allograft loss.
Keywords: mortality; immunosuppression; cohort studies; graft survival; humans; Allografts; kidney transplantation; Longitudinal Studies; risk factors; Skin Neoplasms; Transplant Recipients; Transplantation, Homologous
Introduction
Mammalian target of rapamycin (mTOR) inhibitors sirolimus and everolimus are commonly prescribed maintenance immunosuppressive agents in kidney transplantation. Randomized trials with short–term follow-up have shown that de novo or delayed introduction of mTOR inhibitors with and without calcineurin inhibitors is associated with increased risks of rejection, hyperlipidemia, proteinuria, and delayed wound healing. Compared with calcineurin inhibitors, mTOR inhibitors have been shown to achieve superior allograft function up to 5 years after transplantation and are associated with reduced risks of cytomegalovirus and BK virus infections (1–5). mTOR inhibitors have been shown to reduce the risk of nonmelanoma skin cancers (NMSCs) and nonskin cancer malignancies after kidney transplantation (1,6,7). A Scientific Registry of Renal Transplant Recipients (SRTR) study reported significantly higher risks of death and graft loss with sirolimus versus tacrolimus use (8). Subsequently, a Hungarian study reported a higher mortality risk with mTOR inhibitor use (9). A United Network for Organ Sharing (UNOS) study also reported higher risks of death and graft loss with mTOR inhibitor versus calcineurin inhibitor use (10). More recently, an individual patient data meta–analysis using data from 21 randomized trials showed that sirolimus was associated with a 43% higher risk of all-cause death compared with in controls (7).
Because of heightened skin cancer risk, the use of mTOR inhibitors is greater in Australia than in the United States (11,12). Therefore, the aim of this study was to compare all–cause patient mortality and all–cause and death–censored allograft loss in kidney transplant recipients treated with or without mTOR inhibitors using data from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry.
Materials and Methods
Study Population
The study included a total of 9353 adult patients with ESRD who underwent 9558 living and deceased donor kidney transplants in Australia and New Zealand between January 1, 1996 and December 31, 2012 whose allograft survived at least 1 year. Patients were excluded if they were younger than 18 years old at transplantation, were multiorgan transplant recipients, or had received their first kidney transplant before January 1, 1996.
Data Collection
The ANZDATA Registry collects data in accordance with the Australian Commonwealth Privacy Act and associated state legislation governing health data collection, and individual opt–in patient consent is not required for the registry data. This analysis was performed on an anonymized extract released by the registry to researchers. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. The methods of data collection and validation are described in detail on the ANZDATA Registry website (http://www.anzdata.org.au). The data collected include demographic details; comorbidities; history of pretransplant cancer; donor- and transplant-related characteristics; the number, type, and treatment of rejection episodes; nonskin tumors; melanomas; and skin tumors. The information about immunosuppressive medications and serum creatinine is collected at 1, 2, 3, and 6 months; 1, 2, 3, 5, 7, and 10 years after transplant surgery; and every 5 years thereafter. Exact start and end dates of immunosuppressive medications and blood drug concentrations are not collected. Information on how the causes of death and graft loss are categorized by the ANZDATA Registry is described in Supplemental Table 1.
Study Outcomes
The primary study outcomes were all-cause mortality and all–cause allograft loss. Secondary study outcomes were death with functioning graft, death after graft loss, death–censored allograft loss, cause-specific mortality, and cause–specific allograft loss. All analyses, except death with functioning graft and death after graft loss, included first as well as repeat kidney transplants.
Statistical Analyses
For the purpose of describing the baseline characteristics, the study population was divided into four groups: (1) never treated with mTOR inhibitor (never used group), (2) treated with mTOR inhibitor–based immunosuppressive therapy at baseline (baseline mTOR inhibitor group), (3) commenced mTOR inhibitor within 1 year of transplant surgery (early conversion group), and (4) commenced mTOR inhibitor >1 year after transplant surgery (late conversion group). Use of the commercially available mTOR inhibitors sirolimus and everolimus was included, but they were not analyzed separately because of small numbers. Results for baseline characteristics and clinical outcomes are expressed as frequencies and percentages for categorical variables, means±SD for continuous variables, and medians and interquartile ranges for nonparametric data. Distributions of categorical variables across the four groups were compared by chi-squared test, distributions of continuous variables across the four groups were compared by one-way ANOVA if data were normally distributed, and distributions of continuous variables across the four groups were compared by Kruskal–Wallis test if data were not normally distributed.
Multivariable Cox regression models were used to evaluate risk factors of mortality and allograft loss. Because mTOR inhibitor use varied substantially during the follow-up period, mTOR inhibitor was included in the models as a time-varying covariate to account for post-transplant changes in immunosuppressive regimens. Because the precise dates for start and end of immunosuppressive medications were not collected and the data on medication use were collected less frequently at 3 years after transplant surgery, medication use was carried forward by a prespecified time for the analysis of mTOR inhibitor as a time-varying covariate. These time periods were as follows: carry forward by 1 year for 1–3 post-transplant years, 2 years for 3–5 post-transplant years, 3 years for 5–10 post-transplant years, and 5 years beyond the 10th year post-transplant. Because the ANZDATA Registry does not collect information on immunosuppressive medications after graft loss, the last recorded observation was carried forward for all entries after graft loss. Other covariates in the multivariable regression models were age, sex, ethnicity, cause of ESRD, cardiovascular disease, diabetes mellitus, smoking, pretransplant cancer, body mass index, donor source, peak panel reactive antibody, total HLA mismatches, induction therapy, transplant era, and delayed graft function. Standard errors were calculated using robust variance estimation for the correlated data clustered according to the transplanting hospital. Survival time was calculated from the date of transplant surgery to the date of death or December 31, 2013. To accommodate repeat kidney transplants, data were analyzed using a variance-correction conditional risk set model for ordered allograft loss events, with time for subsequent transplant events reset using the gap time method (Supplemental Figure 1). All models were stratified on allograft number (first, second, or third) (13). Additional patient and allograft survival analyses evaluated mTOR inhibitor use at the fixed time points of baseline and 1 year after the transplant surgery. These analyses were restricted to the first allograft only. For the analysis of risk factors for cause-specific mortality and cause–specific allograft loss, only those outcomes with the total number of events exceeding 100 were analyzed. Subgroup analyses were performed according to the history of pretransplant cancer. The heterogeneity of associations between mortality, allograft loss, and mTOR inhibitor use was assessed by testing the interaction between mTOR inhibitor use and pretransplant cancer status. Statistical analyses were performed using Stata/MP 14 (StataCorp, College Station, TX).
Results
Baseline Characteristics
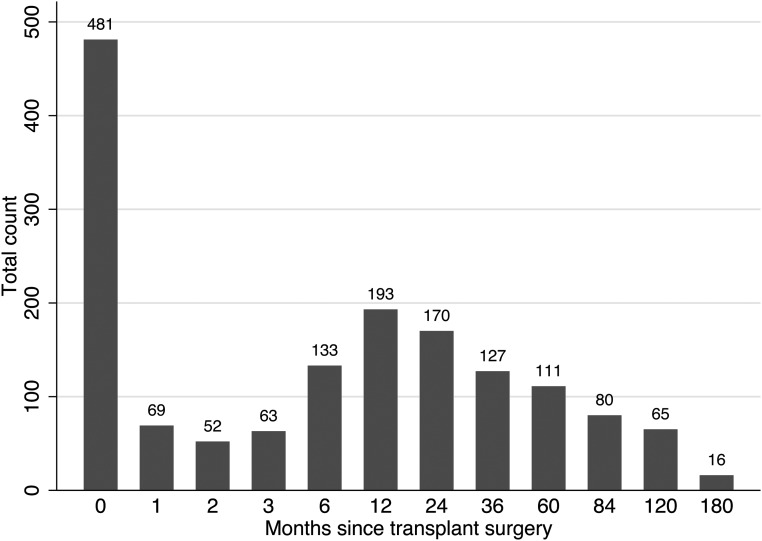
The baseline characteristics of 9353 patients and 9558 transplant operations are described in Tables 1 and 2, respectively. Of the 9558 allografts, mTOR inhibitor use occurred in 1560 (16%) allografts. The timing of initiation of mTOR inhibitor is described in Figure 1.
Table 1.
Baseline patient characteristics by mammalian target of rapamycin inhibitor use
| Variable | Never Used | Baseline Use | Early Conversion | Late Conversion | Total | P Value |
|---|---|---|---|---|---|---|
| N | 7801 | 481 | 504 | 567 | 9353 | |
| Men | 4820 (62) | 315 (65) | 315 (63) | 379 (67) | 5829 (62) | 0.05 |
| Age, yr, mean±SD | 47.1±13.2 | 44.9±12.4 | 48.6±12.8 | 45.9±13.1 | 47±13.1 | <0.001 |
| Ethnicity | <0.001 | |||||
| White | 6295 (81) | 437 (91) | 429 (85) | 486 (86) | 7647 (82) | |
| ATSI | 234 (3) | 5 (1) | 18 (4) | 15 (3) | 272 (3) | |
| Maori/PI | 403 (5) | 1 (<1) | 8 (2) | 9 (2) | 421 (5) | |
| Asian | 451 (6) | 22 (5) | 31 (6) | 33 (6) | 537 (6) | |
| Other | 418 (5) | 16 (3) | 18 (4) | 24 (4) | 476 (5) | |
| Primary cause of ESRDa | 0.004 | |||||
| Chronic glomerulopathy | 3741 (48) | 247 (51) | 265 (53) | 271 (48) | 4524 (48) | |
| Diabetic nephropathy | 758 (10) | 29 (6) | 31 (6) | 34 (6) | 852 (9) | |
| Renovascular disease | 456 (6) | 29 (6) | 25 (5) | 31 (5) | 541 (6) | |
| Polycystic kidney disease | 1203 (15) | 88 (18) | 88 (17) | 89 (16) | 1468 (16) | |
| Reflux or obstructive nephropathy | 863 (11) | 55 (11) | 47 (9) | 74 (13) | 1039 (11) | |
| Other | 441 (6) | 21 (4) | 29 (6) | 41 (7) | 532 (6) | |
| Unknown | 333 (4) | 12 (2) | 19 (4) | 27 (5) | 391 (4) | |
| Comorbid conditiona | ||||||
| Current or former smoker | 3379 (44) | 211 (44) | 229 (46) | 234 (41) | 4053 (44) | 0.50 |
| Chronic lung disease | 355 (5) | 24 (5) | 23 (5) | 24 (4) | 426 (5) | 0.90 |
| Cardiovascular disease | 1148 (15) | 50 (10) | 64 (13) | 62 (11) | 1324 (14) | 0.004 |
| Diabetes mellitus | 1026 (13) | 46 (10) | 58 (12) | 46 (8) | 1176 (13) | 0.001 |
| Cancer | 2298 (29) | 185 (38) | 147 (29) | 261 (46) | 2891 (31) | <0.001 |
All values are the number (percentage) of patients, unless stated otherwise. ATSI, Aboriginal and Torres Strait Islander; PI, Pacific Islander.
Some missing data.
Table 2.
Baseline transplant characteristics by mammalian target of rapamycin inhibitor use
| Variable | Never Used | Baseline Use | Early Conversion | Late Conversion | Total | P Value |
|---|---|---|---|---|---|---|
| N | 7998 | 481 | 510 | 569 | 9558 | |
| Transplanting state/country | <0.001 | |||||
| New South Wales | 1933 (24) | 166 (35) | 170 (33) | 194 (34) | 2463 (26) | |
| Victoria | 1974 (25) | 97 (20) | 80 (16) | 140 (25) | 2291 (24) | |
| Queensland | 1436 (18) | 71 (15) | 14 (3) | 46 (8) | 1567 (16) | |
| South Australia | 677 (8) | 118 (25) | 133 (26) | 99 (17) | 1027 (11) | |
| Western Australia | 596 (7) | 19 (4) | 92 (18) | 81 (14) | 788 (8) | |
| New Zealand | 1382 (17) | 10 (2) | 21 (4) | 9 (2) | 1422 (15) | |
| Transplant era | <0.001 | |||||
| 1996–2001 | 2182 (27) | 274 (57) | 31 (6) | 183 (32) | 2670 (28) | |
| 2002–2006 | 2033 (25) | 165 (34) | 186 (36) | 251 (44) | 2635 (28) | |
| 2007–2012 | 3783 (47) | 42 (9) | 293 (57) | 135 (24) | 4523 (45) | |
| Graft no. | 0.02 | |||||
| First | 7816 (98) | 481 (100) | 496 (97) | 560 (98) | 9353 (98) | |
| Second | 178 (2) | 0 | 13 (3) | 9 (2) | 200 (2) | |
| Third | 4 (<1) | 0 | 1 (<1) | 0 | 5 (<1) | |
| Donor source | 0.10 | |||||
| Live donor | 3168 (40) | 187 (39) | 206 (40) | 253 (44) | 3814 (40) | |
| Deceased donor | 4830 (60) | 294 (61) | 304 (60) | 316 (56) | 5744 (60) | |
| Donor details | <0.001 | |||||
| DBD-SCDa | 3559 (74) | 250 (85) | 177 (58) | 242 (77) | 4228 (74) | |
| DBD-ECDa | 810 (17) | 40 (14) | 95 (31) | 59 (19) | 1004 (17) | |
| DCD-SCDa | 362 (7) | 3 (1) | 24 (8) | 11 (3) | 400 (7) | |
| DCD-ECDa | 99 (2) | 1 (<1) | 8 (3) | 4 (1) | 112 (2) | |
| Men (donor) | 4128 (52) | 239 (50) | 264 (52) | 289 (51) | 4920 (51) | 0.90 |
| Donor age, yr, mean±SD | 44.8±15.2 | 43.7±15.4 | 51.3±14.3 | 45.2±15.5 | 45.1±15.5 | <0.001 |
| Total ischemia time,b h, median (IQR) | 9 (3–14) | 11 (3–15) | 9 (3–14) | 9 (2–14) | 9 (3–14) | 0.09 |
| Delayed graft function | 1136 (14) | 51 (11) | 89 (18) | 76 (13) | 1352 (14) | 0.02 |
| Immunologic status | ||||||
| HLA mismatches, median (IQR) | 3 (2–5) | 3 (2–4) | 3 (2–5) | 3 (2–4) | 3 (2–5) | 0.10 |
| PRA>50%b | 690 (9) | 48 (10) | 37 (7) | 50 (9) | 825 (9) | 0.50 |
| BMI category at baselineb | 0.03 | |||||
| Healthy weight | 3295 (42) | 202 (42) | 212 (42) | 269 (47) | 3978 (42) | |
| Underweight | 246 (3) | 14 (3) | 17 (4) | 20 (4) | 297 (3) | |
| Overweight | 2801 (35) | 191 (40) | 172 (34) | 195 (34) | 3359 (36) | |
| Obese | 1556 (20) | 72 (15) | 103 (20) | 85 (15) | 1816 (19) | |
| BMI at baseline,b mean±SD | 26.1±4.8 | 25.8±4.4 | 26.1±4.9 | 25.4±4.4 | 26±4.8 | 0.01 |
| Baseline immunosuppressive medicationsb | ||||||
| Calcineurin inhibitor | 7866 (98) | 398 (83) | 493 (97) | 558 (98) | 9315 (97) | <0.001 |
| Antimetabolite | 7857 (98) | 72 (15) | 488 (96) | 554 (97) | 8971 (94) | <0.001 |
| mTORi | 0 | 481 (100) | 0 | 0 | 481 (5) | <0.001 |
| mTORi-CNI combination | 0 | 398 (83) | 0 | 0 | 398 (4) | <0.001 |
| Induction therapy | ||||||
| Anti-CD25 agent | 4391 (55) | 177 (37) | 365 (72) | 264 (46) | 5197 (54) | <0.001 |
| T cell depletion/suppression | 248 (3) | 7 (1) | 23 (5) | 21 (4) | 299 (3) | 0.04 |
| IVIG | 104 (1) | 0 | 5 (<1) | 3 (<1) | 112 (1) | 0.03 |
| B cell depletion | 56 (<1) | 0 | 2 (<1) | 2 (<1) | 60 (<1) | 0.20 |
| Other | 8 (<1) | 0 | 0 | 0 | 8 (<1) | 0.70 |
| None | 3954 (49) | 319 (66) | 170 (33) | 301 (53) | 4744 (50) | <0.001 |
All values are the number (percentage) of patients, unless stated otherwise. DBD, deceased brain death; SCD, standard criteria donor; ECD, expanded criteria donor; DCD, deceased circulatory death; IQR, interquartile range; PRA, panel reactive antibody; BMI, body mass index; mTORi, mammalian target of rapamycin inhibitor; CNI, calcineurin inhibitor; IVIG, intravenous Ig.
Data available from 2003 onward.
Some missing data.
Figure 1.
Timing of initiation of mammalian target of rapamycin inhibitor after transplant surgery (months).
Compared with patients in the never used group (Table 1), those in each of the three mTOR inhibitor use groups were more likely to be white and have a history of prior cancer and less likely to have cardiovascular disease and diabetes mellitus. Patients in the baseline mTOR inhibitor and late conversion groups were also younger than those in the never used group. There was significant variation in mTOR inhibitor use across Australian states, and mTOR inhibitor use was less frequent in New Zealand than in Australia (Table 2); 57% of transplants from the baseline mTOR inhibitor group were performed between 1996 and 2001. Patients in the baseline mTOR inhibitor group received calcineurin inhibitors, antimetabolites, and induction therapy less frequently than those in the other groups. However, there were no significant differences between the four groups with respect to donor source (live or decreased), peak panel reactive antibody, HLA mismatch, and total ischemia time.
Clinical Outcomes
Median follow-up was 7 years (range =1–18 years; interquartile range, 3.8–11.3 years). Compared with the never used group, more patients in the baseline mTOR inhibitor and late conversion groups had at least one post–transplant primary NMSC (Table 3). The frequencies of post–transplant primary nonskin cancers were similar across the four groups.
Table 3.
Clinical outcomes by mammalian target of rapamycin inhibitor use
| Clinical Outcome | Never Used | Baseline Use | Early Conversion | Late Conversion | Total | P Value |
|---|---|---|---|---|---|---|
| Follow-up, yr, median (IQR) | 6.6 (3.5–11.1) | 11.3 (7.6–14.6) | 5.8 (3.6–8.2) | 9.6 (6.4–12.8) | 7 (3.8–11.3) | <0.001 |
| Cancers | ||||||
| At least one nonmelanoma skin cancer | 1406 (18) | 103 (23) | 66 (13) | 178 (32) | 1753 (19) | <0.001 |
| At least one SCC | 787 (10) | 50 (11) | 33 (7) | 93 (17) | 963 (10) | <0.001 |
| At least one BCC | 603 (8) | 53 (12) | 33 (7) | 91 (16) | 780 (8) | <0.001 |
| At least one nonskin cancer | 688 (9) | 34 (8) | 33 (7) | 55 (10) | 810 (9) | 0.20 |
| Cause of death | <0.001 | |||||
| All cause | 1159 (15) | 111 (23) | 67 (13) | 79 (14) | 1416 (15) | |
| Malignancy | 330 (28) | 42 (38) | 16 (24) | 25 (32) | 413 (29) | |
| Cardiac | 326 (28) | 31 (28) | 15 (22) | 18 (23) | 390 (28) | |
| Vascular | 88 (8) | 4 (4) | 6 (9) | 7 (9) | 105 (7) | |
| Infection | 182 (16) | 14 (13) | 15 (22) | 14 (18) | 225 (16) | |
| Social | 104 (9) | 10 (9) | 3 (4) | 7 (9) | 124 (9) | |
| Miscellaneous | 129 (11) | 10 (9) | 12 (18) | 8 (10) | 159 (11) | |
| Cause of allograft loss | 0.30 | |||||
| All cause | 1851 (23) | 172 (36) | 103 (20) | 142 (25) | 2268 (24) | |
| Death | 856 (46) | 81 (47) | 54 (52) | 64 (45) | 1055 (47) | |
| Rejection | 705 (38) | 67 (39) | 41 (40) | 65 (46) | 878 (39) | |
| Vascular | 21 (1) | 0 | 0 | 4 (3) | 25 (1) | |
| Technical | 7 (<1) | 0 | 0 | 0 | 7 (<1) | |
| GN | 108 (6) | 11 (6) | 2 (2) | 4 (3) | 125 (6) | |
| Drug withdrawala | 95 (5) | 6 (3) | 4 (3) | 2 (1) | 107 (5) | |
| Miscellaneous | 55 (3) | 7 (4) | 2 (2) | 3 (2) | 67 (3) | |
| Missing data | 4 (<1) | 0 | 0 | 0 | 4 (<1) |
All values are the number (percentage) of patients, unless stated otherwise. IQR, interquartile range; SCC, squamous cell carcinoma; BCC, basal cell carcinoma.
Includes withdrawal of immunosuppressive medications for medical indications as well as nonadherence.
Primary Study Outcomes
All-Cause Mortality.
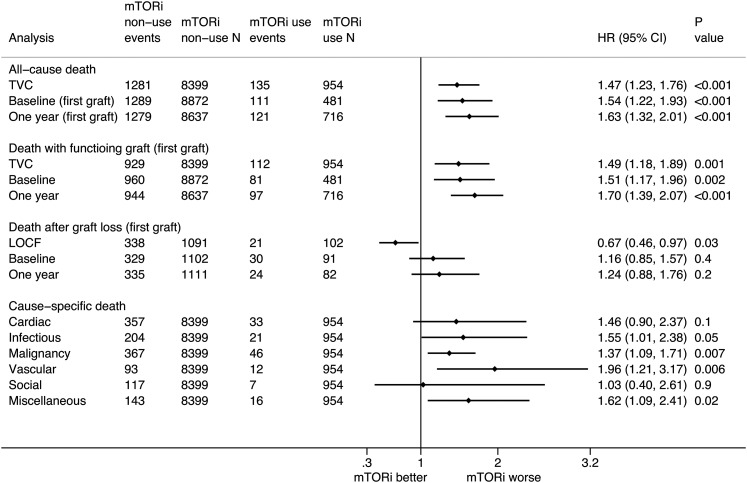
In total, 1416 (15%) patients (Table 3) died at a rate of 2.1 (95% confidence interval [95% CI], 2.0 to 2.2) per 100 person-years (Supplemental Figure 2, Supplemental Table 2). On multivariable Cox regression analysis with mTOR inhibitor use as a time-varying covariate, mTOR inhibitor use was associated with a significantly higher risk of all-cause mortality (adjusted hazard ratio [HR], 1.47; 95% CI, 1.23 to 1.76) (Figure 2). The risk estimates for other variables from the multivariable Cox regression model are presented in Table 4. The mortality risks were significantly higher in the fixed time model analyses comparing mTOR inhibitor use at baseline (HR, 1.54; 95% CI, 1.22 to 1.93) and 1 year (HR, 1.63; 95% CI, 1.32 to 2.01).
Figure 2.
The association between mammalian target of rapamycin inhibitor (mTORi) use and all–cause and cause–specific death in kidney transplant recipients. Analyses using mTORi use at baseline and 1 year are restricted to the first allograft. 95% CI, 95% confidence interval; HR, hazard ratio; LOCF, last observation carried forward; TVC, time-varying covariate.
Table 4.
Hazard ratio (95% confidence interval) for all-cause mortality and all–cause allograft loss associated with individual covariates from the multivariable Cox regression model
| Variable | All-Cause Mortality | All–Cause Graft Loss | ||
|---|---|---|---|---|
| HR, 95% CI | P Value | HR, 95% CI | P Value | |
| Time–varying mTOR inhibitor use | 1.47, 1.23 to 1.76 | <0.001 | 0.98, 0.85 to 1.12 | 0.70 |
| Age per 1-yr increase | 1.05, 1.04 to 1.06 | <0.001 | 1.01, 1.01 to 1.02 | <0.001 |
| Women | 0.97, 0.87 to 1.09 | 0.60 | 0.98, 0.89 to 1.07 | 0.70 |
| Ethnicity | ||||
| White | Reference | Reference | ||
| ATSI | 2.29, 1.88 to 2.79 | <0.001 | 2.15, 1.74 to 2.65 | <0.001 |
| Maori/PI | 1.18, 0.76 to 1.83 | 0.50 | 1.38, 1.14 to 1.68 | 0.001 |
| Asian | 0.66, 0.52 to 0.84 | 0.001 | 0.87, 0.71 to 1.08 | 0.20 |
| Other | 0.72, 0.54 to 0.97 | 0.03 | 0.99, 0.79 to 1.23 | 0.90 |
| Primary cause of ESRD | ||||
| Chronic glomerulopathy | Reference | Reference | ||
| Diabetic nephropathy | 1.54, 1.17 to 2.02 | 0.002 | 1.09, 0.85 to 1.39 | 0.50 |
| Renovascular disease | 1.43, 1.18 to 1.74 | <0.001 | 1.28, 1.08 to 1.52 | <0.01 |
| Polycystic kidney disease | 0.92, 0.81 to 1.04 | 0.20 | 0.76, 0.67 to 0.87 | <0.001 |
| Reflux or obstructive nephropathy | 1.09, 0.89 to 1.33 | 0.40 | 1.02, 0.88 to 1.18 | 0.80 |
| Other | 1.44, 1.16 to 1.78 | 0.001 | 1.16, 0.94 to 1.41 | 0.16 |
| Unknown | 1.08, 0.83 to 1.40 | 0.60 | 1.03, 0.83 to 1.28 | 0.80 |
| Comorbid condition | ||||
| Cardiovascular disease | 1.42, 1.22 to 1.63 | <0.001 | 1.33, 1.18 to 1.49 | <0.001 |
| Diabetes mellitus | 1.41, 1.16 to 1.72 | 0.001 | 1.44, 1.18 to 1.49 | 0.001 |
| Cancer | 1.33, 1.22 to 1.46 | <0.001 | 1.17, 1.07 to 1.29 | 0.001 |
| Current or former smoker | 1.36, 1.23 to 1.51 | <0.001 | 1.33, 1.22 to 1.45 | <0.001 |
| BMI category at baseline | ||||
| Healthy weight | Reference | Reference | ||
| Underweight | 1.51, 1.16 to 1.97 | 0.004 | 1.30, 1.03 to 1.64 | 0.03 |
| Overweight | 0.88, 0.74 to 1.06 | 0.20 | 0.93, 0.84 to 1.02 | 0.10 |
| Obese | 0.98, 0.81 to 1.19 | 0.90 | 1.01, 0.90 to 1.14 | 0.80 |
| Transplant era | ||||
| 1996–2001 | Reference | Reference | ||
| 2002–2006 | 0.81, 0.67 to 0.98 | 0.03 | 0.93, 0.84 to 1.04 | 0.20 |
| 2007–2012 | 0.81, 0.70 to 0.94 | 0.004 | 0.95, 0.80 to 1.13 | 0.60 |
| Donor source | ||||
| Live donor | Reference | Reference | ||
| Deceased donor | 1.21, 1.06 to 1.37 | 0.004 | 1.04, 0.94 to 1.14 | 0.50 |
| Delayed graft function | 1.43, 1.22 to 1.68 | <0.001 | 1.47, 1.31 to 1.66 | <0.001 |
| Immunologic status | ||||
| HLA mismatch per one increase in mismatch | 1.07, 1.04 to 1.11 | <0.001 | 1.08, 1.05 to 1.10 | <0.001 |
| PRA>50% | 1.50, 1.31 to 1.73 | <0.001 | 1.46, 1.28 to 1.68 | <0.001 |
| No induction therapy | 1.08, 0.93 to 1.25 | 0.10 | 1.19, 1.05 to 1.34 | 0.001 |
HR, hazard ratio; 95% CI, 95% confidence interval; mTOR, mammalian target of rapamycin; ATSI, aboriginal and Torres Strait Islander; PI, Pacific Islander; BMI, body mass index; PRA, panel reactive antibody.
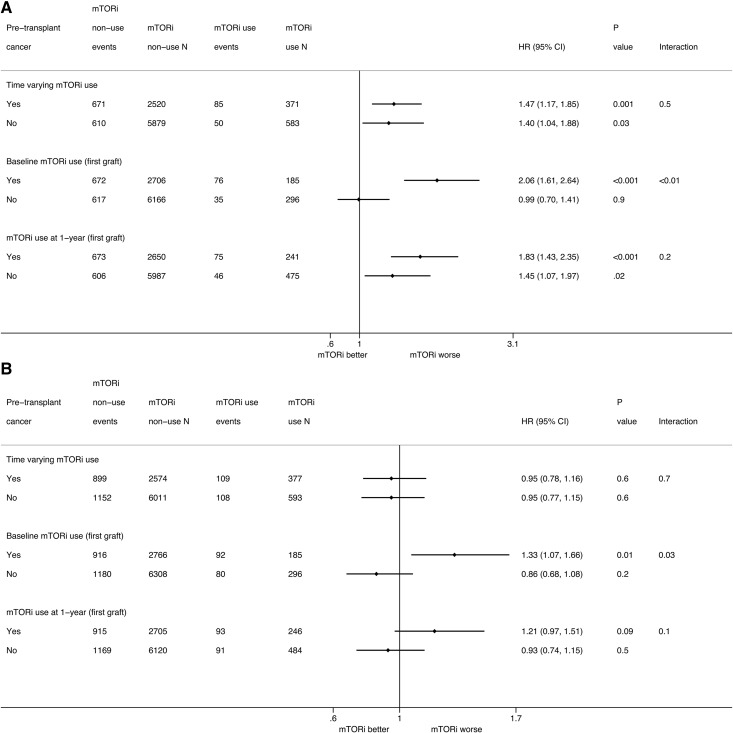
Subgroup analyses according to the history of pretransplant cancer are described in Figure 3A. The interactions between pretransplant cancer status and time–varying and 1-year mTOR inhibitor use were not significant. The interaction between pretransplant cancer status and baseline mTOR inhibitor use was statistically significant (interaction P<0.01). mTOR inhibitor use at baseline was associated with a higher risk of mortality (HR, 2.06; 95% CI, 1.61 to 2.64) in patients with pretransplant cancer but was not associated with a higher risk of mortality in those without pretransplant cancer (HR, 0.99; 95% CI, 0.70 to 1.42).
Figure 3.
The association between mammalian target of rapamycin inhibitor (mTORi) use and outcomes in kidney transplant recipients according to pretransplant cancer status. (A) All-cause death. (B) All–cause allograft loss. Analyses using mTORi use at baseline and 1 year are restricted to the first allograft. 95% CI, 95% confidence interval; HR, hazard ratio.
All–Cause Allograft Loss.
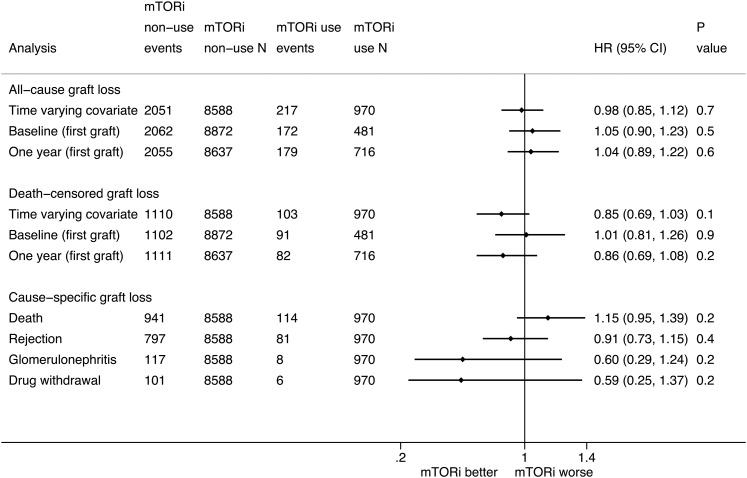
In total, 2268 (24%) allografts were lost at a rate of 3.3 (95% CI, 3.2 to 3.4) per 100 person-years (Table 3). Using multivariable Cox regression analysis with mTOR inhibitor use included as a time-varying covariate, there was no significant difference in the risk of all–cause allograft loss between the mTOR inhibitor use and nonuse groups (HR, 0.98; 95% CI, 0.85 to 1.12) (Figure 4). The risk estimates for other variables from the multivariable Cox regression models are presented in Table 4. There were no significant differences in the risks of allograft loss between the mTOR inhibitor use and nonuse groups in the analyses comparing mTOR inhibitor use at baseline (HR, 1.05; 95% CI, 0.90 to 1.23) and 1 year (HR, 1.04; 95% CI, 0.89 to 1.22).
Figure 4.
The association between mammalian target of rapamycin inhibitor (mTORi) use and all–cause and cause–specific allograft loss in kidney transplant recipients. Analyses using mTORi use at baseline and 1 year are restricted to the first allograft. For repeat transplants, the analyses of cause–specific allograft loss are restricted to the first recorded allograft loss. Drug withdrawal includes withdrawal of immunosuppressive medications for medical indications as well as nonadherence. 95% CI, 95% confidence interval; HR, hazard ratio.
Subgroup analyses comparing time–varying mTOR inhibitor use are depicted in Figure 3B. The interactions between pretransplant cancer status and time–varying and 1-year mTOR inhibitor use were not statistically significant. However, the interaction between pretransplant cancer status and baseline inhibitor use was significant (interaction P value =0.03). mTOR inhibitor use at baseline was associated with a higher risk of allograft loss (HR, 1.33; 95% CI, 1.07 to 1.66) in patients with pretransplant cancer but was not associated with a higher risk of allograft loss (HR, 0.86; 95% CI, 0.68 to 1.08) in those without pretransplant cancer.
Secondary Study Outcomes
Death with Functioning Graft.
In the analysis involving only first grafts, there was a higher risk of death with functioning graft (Figure 2) with time–varying mTOR inhibitor use (HR, 1.49; 95% CI, 1.18 to 1.89) as well as in the fixed time model analyses comparing mTOR inhibitor use at baseline (HR, 1.51; 95% CI, 1.17 to 1.96) and 1 year (HR, 1.70; 95% CI, 1.39 to 2.07). The results of the subgroup analysis according to pretransplant cancer history (Supplemental Figure 3) were similar to those for all-cause mortality.
Death after Graft Loss.
In the analysis involving only first grafts, mTOR inhibitor use analyzed as last observation carried forward was associated with a lower risk of death after graft loss (Figure 2). This difference was because of the lower death rate in the mTOR inhibitor use group than in the comparator group (those not receiving mTOR inhibitor at last observation) 1 year after graft loss (Supplemental Table 2).
Death–Censored Graft Loss.
There were no significant differences in the risks of death–censored allograft loss between mTOR inhibitor use and nonuse in the analyses with mTOR inhibitor use as a time-varying covariate (HR, 0.85; 95% CI, 0.69 to 1.03), at baseline (HR, 1.01; 95% CI, 0.81 to 1.26), and at 1 year (HR, 0.86; 95% CI, 0.69 to 1.08).
Cause-Specific Mortality.
Death caused by malignancy (413 deaths; 29%) and cardiac causes (390 deaths; 28%) were the leading causes of death, accounting for 57% of all deaths (Table 3). Time–varying mTOR inhibitor use was associated with higher risks of mortality caused by malignancy (HR, 1.37; 95% CI, 1.09 to 1.71) and infectious causes (HR, 1.55; 95% CI, 1.01 to 2.38) (Figure 2).
Cause–Specific Allograft Loss.
Death with a functioning graft was the most common cause of allograft loss (1055 losses; 47%) followed by rejection (878 losses; 39%) (Table 3). There were no significant differences in specific causes of allograft loss between the mTOR inhibitor use and nonuse groups (Figure 4).
Discussion
This large registry study found that mTOR inhibitor use was associated with a higher risk of all-cause mortality among adult kidney transplant recipients from Australia and New Zealand. mTOR inhibitor use was not significantly associated with the risk of all–cause allograft loss.
This study confirms the findings of higher mortality risk with mTOR inhibitors from three observational studies and an individual patient data meta–analysis (7–10). This study differed from these previous studies in ethnicity, history of pretransplant cancer, and measured exposure of mTOR inhibitor. Nearly one quarter of patients from the SRTR (8) and the UNOS (10) studies were black, whereas the Hungarian study (9) and this study predominantly included white patients. The prevalence of pretransplant cancer was <4% in the UNOS database study, whereas it was 31% in this study. Other studies did not specifically report data on pretransplant cancers. In this study, although data on the type of pretransplant cancer were not available, it is plausible that most patients with a pretransplant cancer history had skin cancer before their transplantation considering the high skin cancer incidence in Australians (14); 19% of patients developed at least one post-transplant NMSC. Thus, this study included patients who were likely to be at an extremely high risk of skin cancers (6,15). Previous studies used mTOR inhibitor exposure at a fixed time (at baseline or study enrolment), and despite high medication discontinuation rates, time-varying changes in the immunosuppressive regimens were not evaluated in survival analyses. Despite these differences in the study population and methodology, this study also found a significant association between mTOR inhibitor use and mortality. In contrast to this study and other studies, an aggregate data meta–analysis (4) and several randomized trials reported no significant association between mTOR inhibitor use and mortality (6,16–19). The durations of follow-up in all of these trials were <2 years, with only one trial reporting clinical outcomes at 3 years (16). This disparity in the risk of patient mortality could be because of small sample size and shorter duration of follow-up.
This study also found that the risk of mortality caused by malignancy was higher with mTOR inhibitor use. It is possible that the result reflects indication bias given that the indication for mTOR inhibitor use (either as primary immunosuppression or after conversion) is not collected by the ANZDATA Registry. For example, the frequency of post-transplant NMSC was higher in the baseline mTOR inhibitor and late conversion groups than in the never used and early conversion groups. Thus, patients with a propensity for skin cancer formation, either in the pre- or post-transplant period, may have been preferentially selected for mTOR inhibitor therapy by clinicians in light of previous randomized trials (6,7,20). The cause-specific analyses should be interpreted with caution because of inadequate statistical power. There was no evidence that the associations between time–varying mTOR inhibitor use and the outcomes of mortality and allograft loss differed according to pretransplant cancer status. However, the associations between baseline mTOR inhibitor use, mortality, and allograft loss were modified by pretransplant cancer status. Given that 38% of patients in the baseline mTOR inhibitor group had a pretransplant cancer history, this finding could be because of indication bias rather than true heterogeneity of treatment associations with the outcomes in this subgroup. Alternatively, mTOR inhibitors may have been used in recipients who were at increased risk of graft loss because of interstitial fibrosis or calcineurin inhibitor toxicity. Nevertheless, these subgroup analyses need to be interpreted with caution, because they were potentially underpowered (21).
This study found that the risk of all–cause allograft loss was comparable between the mTOR inhibitor use and nonuse groups. Previous studies have reported conflicting findings regarding the risk of allograft loss associated with mTOR inhibitor use, with some studies showing greater allograft loss rates (8,10,17,20) and others showing comparable allograft loss rates (4,6,9,18,19). The population (ethnicity and diabetes mellitus), follow-up duration, and design (observational study or randomized trial) of this study were different from those of previous studies (8,10,16,17). These differences may partially explain disparity in findings for all–cause allograft loss between studies.
The strengths of this study are the large cohort size, inclusion of contemporary transplant recipients, long–term follow-up, adjustment for transplant era, and the robustness of the statistical methods. Nevertheless, the study has a number of limitations. The ANZDATA Registry does not collect data on the precise dates of initiation and termination of immunosuppressive medications. Although medication data were carried forward by reasonable durations at different post–transplant periods, this method may have resulted in incorrect classification. Different mTOR inhibitor types, doses, and cumulative exposures and different combinations of immunosuppressive medications were not compared. Adjustments were able to be made for reported pre– and post–transplant comorbidities, immunologic risk actors, and post–transplant allograft outcomes. However, there remains the issue of residual confounding resulting from indication bias (such as preferential prescription of mTOR inhibitors because of a history of skin cancer or calcineurin inhibitor side effects) and physician or center preference.
In conclusion, this registry study found that mTOR inhibitor use in kidney transplant recipients was associated with a higher risk of all-cause mortality. The risk of all–cause graft loss was not altered with mTOR inhibitor use.
Disclosures
S.B.C. and W.H.L. have served on the advisory boards for Novartis (North Ryde, NSW, Australia) and Pfizer (West Ryde, NSW, Australia). S.V.B., E.M.P., M.B., P.A.C., C.M.H., S.P.M., G.W., and D.W.J. have no conflicts of interest relevant to the contents of this paper to disclose.
Supplementary Material
Acknowledgments
We acknowledge the substantial contributions of the entire Australian and New Zealand nephrology community (physicians, surgeons, database managers, nurses, renal operators, and patients) in providing information for and maintaining the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry database.
D.W.J. is supported by the Queensland Government Health Research Fellowship. The ANZDATA Registry is funded by the Australian Organ and Tissue Donation and Transplantation Authority, the New Zealand Ministry of Health, Kidney Health Australia, and the Australia and New Zealand Society of Nephrology.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00190116/-/DCSupplemental.
References
- 1.Lim WH, Eris J, Kanellis J, Pussell B, Wiid Z, Witcombe D, Russ GR: A systematic review of conversion from calcineurin inhibitor to mammalian target of rapamycin inhibitors for maintenance immunosuppression in kidney transplant recipients. Am J Transplant 14: 2106–2119, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Mulay AV, Cockfield S, Stryker R, Fergusson D, Knoll GA: Conversion from calcineurin inhibitors to sirolimus for chronic renal allograft dysfunction: A systematic review of the evidence. Transplantation 82: 1153–1162, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Murakami N, Riella LV, Funakoshi T: Risk of metabolic complications in kidney transplantation after conversion to mTOR inhibitor: A systematic review and meta-analysis. Am J Transplant 14: 2317–2327, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Webster AC, Lee VW, Chapman JR, Craig JC: Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: A systematic review and meta-analysis of randomized trials. Transplantation 81: 1234–1248, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Moscarelli L, Caroti L, Antognoli G, Zanazzi M, Di Maria L, Carta P, Minetti E: Everolimus leads to a lower risk of BKV viremia than mycophenolic acid in de novo renal transplantation patients: A single-center experience. Clin Transplant 27: 546–554, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Campbell SB, Walker R, Tai SS, Jiang Q, Russ GR: Randomized controlled trial of sirolimus for renal transplant recipients at high risk for nonmelanoma skin cancer. Am J Transplant 12: 1146–1156, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Knoll GA, Kokolo MB, Mallick R, Beck A, Buenaventura CD, Ducharme R, Barsoum R, Bernasconi C, Blydt-Hansen TD, Ekberg H, Felipe CR, Firth J, Gallon L, Gelens M, Glotz D, Gossmann J, Guba M, Morsy AA, Salgo R, Scheuermann EH, Tedesco-Silva H, Vitko S, Watson C, Fergusson DA: Effect of sirolimus on malignancy and survival after kidney transplantation: Systematic review and meta-analysis of individual patient data. BMJ 349: g6679, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivas TR, Schold JD, Guerra G, Eagan A, Bucci CM, Meier-Kriesche HU: Mycophenolate mofetil/sirolimus compared to other common immunosuppressive regimens in kidney transplantation. Am J Transplant 7: 586–594, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Cortazar F, Molnar MZ, Isakova T, Czira ME, Kovesdy CP, Roth D, Mucsi I, Wolf M: Clinical outcomes in kidney transplant recipients receiving long-term therapy with inhibitors of the mammalian target of rapamycin. Am J Transplant 12: 379–387, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isakova T, Xie H, Messinger S, Cortazar F, Scialla JJ, Guerra G, Contreras G, Roth D, Burke GW 3rd, Molnar MZ, Mucsi I, Wolf M: Inhibitors of mTOR and risks of allograft failure and mortality in kidney transplantation. Am J Transplant 13: 100–110, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Boyle G, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2013 Annual Data Report: Kidney. Am J Transplant 15[Suppl 2]: 1–34, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Lim WH: ANZDATA Registry: Transplantation. In: 37th Report, edited by Clayton P, Adelaide, South Australia, Australia, Australia and New Zealand Dialysis and Transplant Registry, 2015, pp 8-1–8-29 [Google Scholar]
- 13.Box-Steffensmeier JM, De Boef S: Repeated events survival models: The conditional frailty model. Stat Med 25: 3518–3533, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Perera E, Gnaneswaran N, Staines C, Win AK, Sinclair R: Incidence and prevalence of non-melanoma skin cancer in Australia: A systematic review. Australas J Dermatol 56: 258–267, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Bouwes Bavinck JN, Hardie DR, Green A, Cutmore S, MacNaught A, O’Sullivan B, Siskind V, Van Der Woude FJ, Hardie IR: The risk of skin cancer in renal transplant recipients in Queensland, Australia. A follow-up study. Transplantation 61: 715–721, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Ekberg H, Bernasconi C, Tedesco-Silva H, Vítko S, Hugo C, Demirbas A, Acevedo RR, Grinyó J, Frei U, Vanrenterghem Y, Daloze P, Halloran P: Calcineurin inhibitor minimization in the Symphony study: Observational results 3 years after transplantation. Am J Transplant 9: 1876–1885, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, Margreiter R, Hugo C, Grinyó JM, Frei U, Vanrenterghem Y, Daloze P, Halloran PF; ELITE-Symphony Study : Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 357: 2562–2575, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Flechner SM, Glyda M, Cockfield S, Grinyó J, Legendre C, Russ G, Steinberg S, Wissing KM, Tai SS: The ORION study: Comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant 11: 1633–1644, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Holdaas H, Rostaing L, Serón D, Cole E, Chapman J, Fellstrøm B, Strom EH, Jardine A, Midtvedt K, Machein U, Ulbricht B, Karpov A, O’Connell PJ; ASCERTAIN Investigators : Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: A randomized, multicenter, 24-month study. Transplantation 92: 410–418, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, Broeders N, del Marmol V, Chatelet V, Dompmartin A, Kessler M, Serra AL, Hofbauer GF, Pouteil-Noble C, Campistol JM, Kanitakis J, Roux AS, Decullier E, Dantal J; TUMORAPA Study Group : Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med 367: 329–339, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Rothwell PM: Treating individuals 2. Subgroup analysis in randomised controlled trials: Importance, indications, and interpretation. Lancet 365: 176–186, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.