Abstract
Background and objectives
Ethnic differences in outcomes among children with nephrotic syndrome are unknown.
Design, setting, participants, & measurements
We conducted a longitudinal study at a single regional pediatric center comparing ethnic differences in incidence from 2001 to 2011 census data and longitudinal outcomes, including relapse rates, time to first relapse, frequently relapsing disease, and use of cyclophosphamide. Among 711 children, 24% were European, 33% were South Asian, 10% were East/Southeast Asian, and 33% were of other origins.
Results
Over 10 years, the overall incidence increased from 1.99/100,000 to 4.71/100,000 among children ages 1–18 years old. In 2011, South Asians had a higher incidence rate ratio of 6.61 (95% confidence interval, 3.16 to 15.1) compared with Europeans. East/Southeast Asians had a similar incidence rate ratio (0.76; 95% confidence interval, 0.13 to 2.94) to Europeans. We determined outcomes in 455 children from the three largest ethnic groups with steroid-sensitive disease over a median of 4 years. South Asian and East/Southeast Asian children had significantly lower odds of frequently relapsing disease at 12 months (South Asian: adjusted odds ratio; 0.55; 95% confidence interval, 0.39 to 0.77; East/Southeast Asian: adjusted odds ratio; 0.42; 95% confidence interval, 0.34 to 0.51), fewer subsequent relapses (South Asian: adjusted odds ratio; 0.64; 95% confidence interval, 0.50 to 0.81; East/Southeast Asian: adjusted odds ratio; 0.47; 95% confidence interval, 0.24 to 0.91), lower risk of a first relapse (South Asian: adjusted hazard ratio, 0.74; 95% confidence interval, 0.67 to 0.83; East/Southeast Asian: adjusted hazard ratio, 0.65; 95% CI, 0.63 to 0.68), and lower use of cyclophosphamide (South Asian: adjusted hazard ratio, 0.82; 95% confidence interval, 0.53 to 1.28; East/Southeast Asian: adjusted hazard ratio, 0.54; 95% confidence interval, 0.41 to 0.71) compared with European children.
Conclusions
Despite the higher incidence among South Asians, South and East/Southeast Asian children have significantly less complicated clinical outcomes compared with Europeans.
Keywords: nephrotic syndrome, ethnicity, children, steroids, cyclophosphamide, Asian Continental Ancestry Group, Ethnic Groups, European Continental Ancestry Group, Humans, Incidence, Longitudinal Studies, Recurrence
Introduction
Nephrotic syndrome (NS) is the most common childhood kidney disease worldwide, with a reported incidence of 2–7/100,000 children (1). Incidence among certain ethnic groups, specifically South Asians and Africans, is higher, which suggests environmental and/or genetic influences on disease, but there are few large population–based studies that directly assess differences in incidence by ethnicity (2,3). Some reports have included physician-reported surveys, which lack patient-level or follow-up information, and variable treatment regimens that influence outcomes (2,4).
Since prednisone use began in the 1950s, survival has markedly improved but resulted in a clinical course characterized by repeated relapses (5). Initial response to prednisone remains the most significant predictor of long-term outcomes; however, the disease course varies, with some children having frequent relapses, steroid dependence, or steroid resistance requiring steroid-sparing immunosuppression for years. Steroid resistance often indicates progressive kidney disease and is reported in 27.3%–31.1% of South Asian, West Indian/Caribbean, and African children (6,7). Whether the higher burden of steroid resistance and progressive disease is because of referral patterns at tertiary care centers with possible selection bias, greater susceptibility to the development and progression of NS among specific ethnic groups, or physician variability in disease management is unknown (8).
There are few longitudinal studies of childhood NS with a diverse population receiving similar management that address ethnic differences in outcomes of NS (9–12). The aims of our study are to determine ethnic differences in incidence of NS, response to prednisone, and relapse rates using population-based data.
Materials and Methods
Study Population and Design
Children diagnosed with NS between ages 1 and 18 years old managed at The Hospital for Sick Children from January 1, 1993 were included in the cohort and followed until May 21, 2014. These children were living in the Census Metropolitan Area (CMA) of Toronto, Ontario, Canada (confirmed by residential postal code) and referred for management by consultant or affiliated pediatric nephrologists. Those with syndromic disease with multiple organ involvement or congenital or secondary causes of NS were excluded. Ethics approval was obtained from the Research Ethics Board and in adherence with the Declaration of Helsinki.
Initial prednisone treatment prescribed is 60 mg/m2 per day for 6 weeks followed by an alternate day taper, with a similar regimen for relapses using the standard treatment protocol established in 1993 (Supplemental Material). Frequently relapsing or steroid-dependent disease was treated with either cyclophosphamide or calcineurin inhibitors as the second-line. Cyclophosphamide was the primary second–line medication given for a total of 8–12 weeks. Levamisole was used sparingly until 2000 as an alternative to cyclophosphamide. Cyclosporin (1996) and tacrolimus (2003) were also used for steroid-resistant disease.
Data Collection
Data (sex, age at diagnosis, relapse dates, use of second-line medications, and laboratory parameters at diagnosis) were obtained through medical records and an NS database and subsequently validated with nursing care plans and clinic notes.
Census Data
Population-based data from 2001 to 2011 for children ages 1–18 years old were obtained from the Statistics Canada Census, which is conducted every 5 years, for the Toronto CMA. Estimates of the general pediatric population are on the basis of the Statistics Canada sampling strategy (13). Ethnicity was defined by Statistics Canada through self-report as the following: European, West Central Asian/Middle Eastern (e.g., Afghanistan, Iran, Lebanon, and Syria), South Asian (e.g., India, Bangladesh, and Pakistan), East/Southeast Asian (e.g., China, Philippines, Tibet, and Vietnam), West Indian/Caribbean, African, Latin/Central/South American, Aboriginal, Canadian/American, or multiethnic (Supplemental Material) (14–16). Self-reported ethnicity is highly correlated with genetic ancestry (17). Annual household income and percentage of immigrants were determined on the basis of postal address linked to census data (18).
Ethnicity
Ethnicity was defined by Statistics Canada on the basis of the ethnic or cultural origins of the respondent’s ancestors, where categorization by race was not used (19). Ethnicity was determined through self-report of all four grandparents’ ancestries, health records, or health care providers (Supplemental Material). The remainder was classified using electronic naming programs previously validated in Canada (Nam Pehchan for South Asians or the Chinese Name List) and yielded good agreement in our cohort (Supplemental Material) (2,20–22).
Outcomes
Outcomes were defined by international studies and guidelines and adapted as part of the management plan (23,24). A relapse was defined as protein ≥3.0 g/L on urine dipstick for 3 consecutive days, resulting in prescription of steroids or an increase in dose. Early steroid–resistant NS was defined as starting a second-line medication during the initial steroid course. Steroid-dependent NS was defined as having two or more consecutive relapses during corticosteroid tapering or one relapse within 14 days of completing steroid therapy. Frequently relapsing NS was defined as having two or more relapses within 6 months of diagnosis or four or more relapses in any 12-month period (23). Complete remission was defined as no additional episodes of proteinuria requiring medical intervention.
Statistical Analyses
Incidence of NS (new patients per 100,000 at-risk people) in each year was determined in the at–risk general pediatric population among children ages 1–18 years old.
Relapse rate was calculated as the number of total relapses observed over the entire follow-up period (years) and categorized from initial treatment and after commencement of cyclophosphamide. Analyses focused on the largest ethnic groups (Europeans, South Asians, and East/Southeast Asians). We determined ethnic differences in frequently relapsing NS at 12 months using logistic regression, log–transformed relapse rates by generalized linear modeling (presented as relative risks), and relapse categories using ordinal logistic regression. Time from diagnosis to first relapse and use of cyclophosphamide were assessed using the Kaplan–Meier method. Cox proportional hazard models were used to calculate the relative hazard of time to first relapse and use of cyclophosphamide among ethnic groups. Analyses were clustered by time period (pre- and post-October of 2002) to account for changes in steroid protocol. Parsimonious models were adjusted for age at diagnosis and sex. Statistical analyses were conducted with STATA/SE 12 (StataCorp., College Station, TX), and a significant P value was <0.05.
Results
The largest ethnic groups were Europeans (24%), South Asians (33%), and East/Southeast Asians (10%) (Table 1), with the remainder classified as other (33%) (Supplemental Table 1). Children were discharged after a median of 2.18 years relapse free (interquartile range [IQR], 1.59–3.04 years). East/Southeast Asian children were slightly older at presentation compared with Europeans and South Asians. Among the 479 children from the three largest ethnic groups, 24 were excluded because of initial steroid resistance. The remainder of the cohort was followed until transferred to adult care/another hospital (n=46), discharged from clinic without disease (n=188), lost to follow-up (n=33), dialysis/transplant (n=3), or censored on May 21, 2014 (n=185). Among those lost to follow-up, as confirmed with primary providers, there was no active disease until age 18 years old.
Table 1.
Baseline characteristics of 479 children with nephrotic syndrome from 1993 to 2014
| Patient Characteristics | Mean±SD, Median [Interquartile Range], or n (%) | ||
|---|---|---|---|
| European, n=173 | South Asian, n=237 | East/Southeast Asian, n=69 | |
| Baseline characteristics | |||
| Male | 106 (61.3) | 153 (64.6) | 43 (62.3) |
| Age of diagnosis, yr | 3.70 [2.40–6.81] | 3.42 [2.49–5.38] | 4.20 [2.75–10.82]a |
| Annual household incomeb (Canadian dollars) | 45,987±35,016 | 29,859±8596c | 31,612±13,220c |
| Percentage of immigrants in neighborhoodb | 20.9 [10.0–38.1] | 56.6 [48.1–65.4]c | 56.3 [45.2–71.4]c |
| Laboratory factorsd | |||
| Serum albumin, g/dl | 2.02±0.53 | 1.76±0.48c | 1.97±0.55 |
| Serum creatinine, mg/dl | 0.39 [0.32–0.56] | 0.36 [0.30–0.44]a | 0.53 [0.37–0.71]a |
| Serum cholesterol, mg/dl | 383±114 | 449±125c | 422±100 |
| Clinical outcomese | |||
| Complete remissionf | 26 (15.0) | 52 (21.9) | 19 (27.5) |
| Initial steroid resistance | 13 (7.5) | 6 (2.5)a | 5 (7.2) |
| Steroid dependent at 6 mog | 17 (10.6) | 20 (8.7) | 3 (4.7) |
| Total relapses over entire follow-up,g n | 5 [2–13] | 4 [1–8]a | 2 [0–6]c |
| Time followed, yr | 4.68 [2.66–8.67] | 3.63 [1.84–5.97]c | 3.53 [1.76–5.95]h |
| Relapses per yearg | 1.12 [0.42–1.72] | 1.15 [0.24–1.81] | 0.67 [0–1.53]a |
| Biopsy diagnosis | 59 (34.1) | 50 (21.1)h | 24 (34.8) |
| Minimal change disease | 32 (54.2) | 35 (70.0) | 16 (66.7) |
| FSGS | 23 (39.0) | 14 (28.0) | 5 (20.8) |
| Other | 4 (6.8) | 1 (2.0) | 3 (12.5) |
| Use of second-line medication | 95 (54.9) | 109 (46.0) | 31 (44.9) |
| Cyclophosphamide | 86 (90.5) | 99 (90.8) | 23 (74.2)a |
| Calcineurin inhibitorsi | 9 (9.5) | 7 (6.4) | 8 (25.8)a |
| Complete remission after cyclophosphamide | 29 (33.7) | 33 (33.3) | 9 (39.1) |
Statistical tests were conducted with Europeans as the reference group.
P value ≤0.05 using chi-squared or t test.
Determined using data from Statistics Canada (2006) on the basis of Dissemination Area (19).
P value ≤0.001 using chi-squared or t test.
At onset of nephrotic syndrome, albumin (n=236), creatinine (n=227), and cholesterol (n=205).
Five individuals who never went into remission were excluded from the analyses (n=474) for initial steroid resistance, steroid–dependent nephrotic syndrome, total relapses, and relapse rate.
Complete remission defined as no additional episodes of proteinuria requiring medical intervention after the initial course of therapy.
Twenty-four individuals with initial steroid resistance were excluded from the analysis (n=455).
P value ≤0.01 using chi-squared or t test.
Includes tacrolimus and cyclosporin.
Incidence
Over 10 years, the overall incidence in the Toronto CMA increased from 1.99/100,000 to 4.71/100,000 among children ages 1–18 years old. South Asians had a higher incidence of 15.8/100,000 and an incidence rate ratio of 6.61 compared with Europeans, with increasing rates from 2001 to 2011. By contrast, East/Southeast Asians had a similar incidence of 1.81/100,000 and an incidence rate ratio of 0.76 compared with Europeans in 2011 (Table 2).
Table 2.
Incidence of nephrotic syndrome in Toronto (Census Metropolitan Area), Ontario, Canada per 100,000 children between 1 and 18 years of age
| Incidencea | European | South Asian | East/Southeast Asian | Otherb | Total |
|---|---|---|---|---|---|
| 2001 | |||||
| Population, n | 483,130 | 129,585 | 134,655 | 357,435 | 1,104,805 |
| Patients with new cases of nephrotic syndrome, n | 10 | 2 | 2 | 8 | 22 |
| Incidence | 2.07 | 1.54 | 1.48 | 2.24 | 1.99 |
| Incidence rate ratio | Reference | 0.75 | 0.72 | 1.08 | NA |
| 95% Confidence interval | NA | 0.08 to 3.50 | 0.08 to 3.37 | 0.37 to 3.04 | NA |
| 2006 | |||||
| Population, n | 445,820 | 175,570 | 157,665 | 381,965 | 1,161,020 |
| Patients with new cases of nephrotic syndrome, n | 5 | 7 | 7 | 7 | 26 |
| Incidence | 1.12 | 3.99 | 4.44 | 1.83 | 2.24 |
| Incidence rate ratio | Reference | 3.55 | 3.96 | 1.63 | NA |
| 95% Confidence interval | NA | 0.97 to 14.21 | 1.08 to 15.82 | 0.45 to 6.53 | NA |
| 2011 | |||||
| Population, n | 417,385 | 195,865 | 165,370 | 432,015 | 1,210,635 |
| Patients with new cases of nephrotic syndrome, n | 10 | 31 | 3 | 13 | 57 |
| Incidence | 2.40 | 15.83 | 1.81 | 3.01 | 4.71 |
| Incidence rate ratio | Reference | 6.61 | 0.76 | 1.26 | NA |
| 95% Confidence interval | NA | 3.16 to 15.10 | 0.13 to 2.94 | 0.51 to 3.20 | NA |
NA, not applicable.
Patients with new cases of nephrotic syndrome from April 1 of the preceding year to March 31 of the census year.
Includes individuals classified as West Central Asian/Middle Eastern, West Indian/Caribbean and African, Latin/Central/South American, Aboriginal, multiethnic, and unknown.
Outcomes
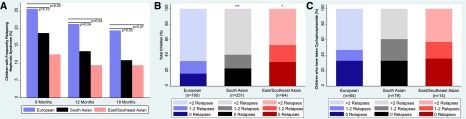
Overall, children of South Asian and East/Southeast Asian descent had a lower absolute number of relapses compared with European children, with more children having complete remission after the initial prednisone course. European children had borderline higher frequency in initial steroid resistance compared with South Asians (P<0.04) and no difference compared with East/Southeast Asians. Frequently relapsing NS at 12 months occurred among 14.5% of children overall. After adjustment, South Asian and East/Southeast Asian children had 45% (adjusted odds ratio [adjOR], 0.55; 95% confidence interval [95% CI], 0.39 to 0.77) and 58% (adjOR, 0.42; 95% CI, 0.34 to 0.51) lower odds, respectively, of developing frequently relapsing NS at 12 months compared with European children (Figure 1A, Table 3). Absolute number of relapses in the entire follow-up period showed that South Asian children had significantly lower odds of having more relapses compared with Europeans (adjOR, 0.64; 95% CI, 0.50 to 0.81). Similarly, compared with European children, East/Southeast Asians had 53% lower odds of having any relapses after disease onset (adjOR, 0.47; 95% CI, 0.24 to 0.91) (Figure 1B). After treatment with cyclophosphamide, the absolute number of relapses did not differ among the three ethnic groups (South Asian: adjOR, 0.68; 95% CI, 0.27 to 1.71; East/Southeast Asian: adjOR, 0.86; 95% CI, 0.07 to 11.29 compared with European children), and on average, 34% of children had no additional relapses (Figure 1C).
Figure 1.
Proportion of frequently relapsing nephrotic syndrome and absolute number of relapses by ethnicity among 455 children with nephrotic syndrome (1993–2014). (A) Data by ethnicity shows frequently relapsing nephrotic syndrome at 6, 12, and 18 months from diagnosis. (B) Absolute total number of relapses by ethnicity. (C) Absolute number of relapses after treatment with cyclophosphamide. *P value ≤0.05; ***P value ≤0.001.
Table 3.
Association of ethnicity with frequently relapsing nephrotic syndrome, relapse rate, time to first relapse, and second–line medication use among 455 children with nephrotic syndrome (1993–2014)
| Outcomes | European, n=160 | South Asian, n=231 | East/Southeast Asian, n=64 |
|---|---|---|---|
| Frequently relapsing nephrotic syndrome at 12 mo | |||
| Events, n | 34 | 31 | 6 |
| Odds ratio | Reference | 0.57 | 0.38 |
| 95% Confidence interval | NA | 0.38 to 0.88 | 0.38 to 0.39 |
| P value | NA | 0.01 | 0.001 |
| Adjusted odds ratioa | Reference | 0.55 | 0.42 |
| 95% Confidence interval | NA | 0.39 to 0.77 | 0.34 to 0.51 |
| P value | NA | 0.001 | 0.001 |
| Relapse rate per person-yearb | |||
| Relative risk | Reference | 0.76 | 0.57 |
| 95% Confidence interval | NA | 0.53 to 1.11 | 0.30 to 1.06 |
| P value | NA | 0.16 | 0.07 |
| Adjusted relative riska | Reference | 0.74 | 0.64 |
| 95% Confidence interval | NA | 0.53 to 1.03 | 0.33 to 1.23 |
| P value | NA | 0.08 | 0.18 |
| Developing first relapsec | |||
| Events, n | 134 | 178 | 44 |
| Hazard ratio | Reference | 0.77 | 0.63 |
| 95% Confidence interval | NA | 0.68 to 0.86 | 0.54 to 0.73 |
| P value | NA | 0.001 | 0.001 |
| Adjusted hazard ratioa | Reference | 0.74 | 0.65 |
| 95% Confidence interval | NA | 0.67 to 0.83 | 0.63 to 0.68 |
| P value | NA | 0.001 | 0.001 |
| Use of cyclophosphamide as second-line medicationd | |||
| Events, n | 60 | 74 | 13 |
| Hazard ratio | Reference | 0.82 | 0.55 |
| 95% Confidence interval | NA | 0.51 to 1.32 | 0.51 to 0.59 |
| P value | NA | 0.42 | 0.001 |
| Adjusted hazard ratioa | Reference | 0.82 | 0.54 |
| 95% Confidence interval | NA | 0.53 to 1.28 | 0.41 to 0.71 |
| P value | NA | 0.39 | 0.001 |
Analysis excluded children with initial steroid resistance during the initial course among these three ethnicities (n=24). NA, not applicable.
Adjusted for age at diagnosis and sex.
By generalized linear model for log–transformed relapse rate.
By Cox proportional hazards.
Truncated at 5 years of follow-up.
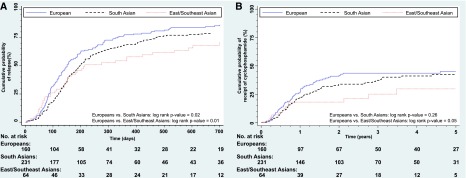
Europeans had a median relapse rate of 1.12/yr (IQR, 0.42–1.72), with a similar absolute rate among South Asians of 1.15/yr (IQR, 0.24–1.81) and significantly lower rate in East/Southeast Asians at 0.67/yr (IQR, 0–1.53; P<0.03). Both South Asians and East/Southeast Asians had lower relapse rates per year compared with Europeans; however, this was not statistically significant (Table 3). Median relapse–free periods were 151, 198, and 214 days among Europeans, South Asians and East/Southeast Asians, respectively. Furthermore, the cumulative incidence rates at 2 years after diagnosis were 85%, 79%, and 70% for Europeans, South Asians, and East/Southeast Asians, respectively. There were significant differences in time to first relapse comparing South Asians (log rank P=0.02) (Figure 2A) and East/Southeast Asians (log rank P=0.01) with Europeans. Children of South Asian origin were 26% (adjusted hazard ratio [adjHR], 0.74; 95% CI, 0.67 to 0.83) less likely to develop the first relapse relative to European children after controlling for age at diagnosis and sex, whereas East/Southeast Asian children had an even lower risk of relapse by 35% (adjHR, 0.65; 95% CI, 0.63 to 0.68). Additional adjustment for albumin, creatinine, and cholesterol did not attenuate the association (Supplemental Material, Supplemental Table 2).
Figure 2.
Kaplan–Meier cumulative probability curves for time to first relapse and time to cyclophosphamide by ethnicity. (A) Developing first relapse from diagnosis of nephrotic syndrome in days. (B) Use of cyclophosphamide from diagnosis of nephrotic syndrome in years.
The cumulative incidence rates at 5 years for use of cyclophosphamide among Europeans, South Asians, and East/Southeast Asians were 45%, 42%, and 30%, respectively. The hazard for cyclophosphamide use did not differ significantly between South Asians and Europeans (adjHR, 0.82; 95% CI, 0.53 to 1.28) (Figure 2B) but did differ for East/Southeast Asians and Europeans. East/Southeast Asians were 50% less likely to be prescribed cyclophosphamide compared with Europeans (adjHR, 0.54; 95% CI, 0.41 to 0.71).
Only 133 children in the three major ethnic groups were biopsied (59 Europeans, 50 South Asians, and 24 East/Southeast Asians). Biopsies were typically done a median of 128 (IQR, 42–342) days after initial diagnosis and 128 (IQR, 47–774) days after the first relapse. There was no difference in days to biopsy from either time of diagnosis or first relapse by ethnic group. Eighty-three children were diagnosed with minimal change disease (39% European, 42% South Asian, and 19% East/Southeast Asian), 42 children were diagnosed with FSGS (55% European, 35% South Asian, and 12% East/Southeast Asian), and eight children were diagnosed with nonspecific changes. Because children of South Asian descent had fewer relapses, fewer, consequently, had kidney biopsies. There was no statistical difference in the prevalence of biopsy-confirmed FSGS among the three groups.
Additional analyses considered the variations over time periods, influence of socioeconomic status, immigrant neighborhoods, and outcomes among all smaller ethnic groups (Supplemental Material, Supplemental Tables 3–6). Results remained consistent.
Discussion
We report ethnic differences in incidence and outcomes among a large, diverse cohort of children with NS at a large single regional pediatric center with universal health care access and a common treatment protocol followed over decades. Over the past decade, we show that the incidence of NS in children has increased in the metropolitan region of Toronto from 1.99/100,000 to 4.71/100,000, primarily driven by the higher rates among South Asians over time. In 2011, South Asian children had a greater than six times higher incidence of NS, whereas East/Southeast Asians had a similar rate as Europeans. Notwithstanding this high burden of disease, South Asian and East/Southeast Asian children have better outcomes, including more complete remissions, fewer subsequent relapses, and longer relapse–free periods after initial therapy, compared with European children. East/Southeast Asian children are also significantly less likely to be prescribed second-line agents.
Understanding ethnic differences is important, because it may lead to better insight into reasons for the incidence of disease, the individual variability in response to medication, and the number of relapses. We report an extremely high incidence of NS in contrast to reports from India, where most studies are from tertiary centers treating more patients with complex cases, and there are few data from general pediatricians, which could greatly underestimate the burden of disease (7,25,26). Regional centers in England also report a five to six times higher incidence of NS among South Asians, predominantly those from northern India or Pakistan, compared with Europeans with limited long–term data (2,9). Our results further extend these findings, because we confirm the incidence in a much larger population, with diverse South Asian and East/Southeast Asian populations, over a decade compared with that in the United Kingdom reports. This increase is likely a result of the recent immigration directly from India and China, which accounted for 57% of all newcomers to Canada from 2006 to 2011, with fewer from the diaspora from the West Indies and Africa since the 1970s (1,27). This may point to possible regional differences in incidence within India, but detailed comparisons are not reported or possible in this cohort.
A higher incidence among South Asians indicates susceptibility to the development of NS, but etiologic contributors are unknown. A recent paper, however, reports a novel genetic association of HLA-DQA1 and PLCG2 with steroid-sensitive NS among Sri Lankan children, which was further validated among Europeans (28). In other glomerular diseases, ethnic differences in disease susceptibility and progression have led to novel genetic discoveries, such as APOL1 in those with CKD and FSGS among blacks, along with other susceptibility alleles in IgA nephropathy among East/Southeast Asians (28–33). Moreover, faster progression to ESRD among Asians with IgA nephropathy suggests possible differences in the biology of the underlying disease (30). Studies have also suggested that the frequency of genetic variants identified in the glucocorticoid receptor gene (NR3C1) and glucocorticoid–induced transcript 1 gene may account for ethnic differences (12,34). Ethnic differences could point to potential differences in disease pathology; however, we report only slightly higher rates of steroid resistance in Europeans, which are in contrast to published reports (35). With limited information on environmental and known etiologic factors causing NS, we could not test these hypotheses but are undertaking a prospective study to address possible contributing factors leading to ethnic differences in outcomes (36).
Immigration into higher-income countries, such as Canada, with publicly funded health care provides the capacity to study longitudinal outcomes. Prior studies have not shown significant ethnic differences in outcomes, likely because of the use of physician-reported surveys, limited patients from tertiary centers, or a predominance of European children enrolled in studies and trials on the basis of biopsy findings or steroid resistance (2,4,9,37). Studies from Australia and New Zealand on the basis of reports by general pediatricians using variable steroid doses report significantly lower rates of disease, possibly because of reporting bias or limited sample size (4,38). We report that outcomes significantly vary by ethnicity, with South Asians and/or East/Southeast Asians having lower prevalence of initial steroid resistance, lower total relapses, longer first relapse–free period, and longer duration to cyclophosphamide over a median follow-up of almost 4 years. These results show an overall positive prognosis after diagnosis, with only a minority of children have additional relapses, which is consistent with the original International Study of Kidney Disease in Children population (39). Over one quarter of South Asian children and one third of East/Southeast Asian children have complete remission after initial prednisone. This is unexpected given the reported concerns of a rising number of patients with steroid resistance or FSGS (1). Children of South Asian and East/Southeast Asian origin in our cohort have a similar disease pattern, which differs from that in the reports from Australia and New Zealand, having worse outcomes (38,40). Selection or reporting bias, sample size, or underlying pathology may explain these differences in outcomes by ethnicity. The steroid response and lower rates of subsequent relapses among South Asian and East/Southeast Asian children could indicate sensitivity to steroids through inflammatory pathways or genetic factors yet unknown. Interestingly, after cyclophosphamide is given, the response is similar across all groups, with complete remission of 36%. Of note, 58% of children have two or fewer relapses after cyclophosphamide and can expect a good prognosis. Compared with previous studies, there is variability in reported remission rates at 2 years, with cyclophosphamide ranging from 27% to 44%, and sample size, definitions, and follow-up widely differ (39,41–44). Meta-analyses on steroid-sparing agents further highlight the lack of controlled trials with specific dosing regimens, standardized reporting of outcomes, and longer durations of follow-up (45). This is the advantage of a common protocol used in this large cohort, which minimizes treatment bias.
Additional social factors, such as access to care, socioeconomic status, and immigration, may also influence health outcomes. In Ontario, Canada, health is managed in a universal health care system, precluding significant bias in access to care, although the cost of medications is not covered. European children are from neighborhoods with higher household incomes and less immigration but have worse disease outcomes. South Asians and East/Southeast Asians in lower income households are also more likely to be exposed to potential environmental triggers, such as allergies, for relapsing disease, but this is not the case, which was evident by milder clinical outcomes (46). Recent parental immigration could also point to possible factors, such as diet; however, data are not uniformly available to test these hypotheses. Our management has not significantly differed over the years, and cyclophosphamide remains the most common second–line agent. We also did not see any difference in prescribed medications by income.
The major strengths of our study include the large multiethnic cohort using a common protocol with equal health care access and a nurse-managed program for following families and tracking relapses. Additionally, we use population-based data from Statistics Canada to determine incidence for a large census tract in a province that accounts for approximately 40% of the population of Canada (14–16). Our study is strengthened by the inclusion of almost all children managed since diagnosis in a regional health care system and not just those who have more severe disease or biopsies, which provides a long-term understanding of the natural history of childhood NS. Access to medications may be dictated by secondary insurance coverage in Ontario; however, steroids and cyclophosphamide are relatively inexpensive, and social programs can cover medications and urinary albumin dipstick tests. The main limitations of this study include the lack of self-reported ethnicity among some children and the lack of specific details of home management of relapses. In Canada, ethnicity is not routinely collected in administrative data, because it is considered personal information protected under federal law. There are few children of African or Caribbean descent in our cohort reflective of the Toronto population (<4%), which prevents comparison with United States studies. There is also a number of factors that could affect both incidence and outcomes, such as referral patterns, adherence to prescribed treatment, health-seeking behaviors, and relapses that occur after discharge; however, it is unlikely that these differences vary by a specific ethnic group, such that it biases the analyses. The absolute incidence is also conservative, because some children from the periphery of metropolitan region may not be included. However, this should not influence the comparison of outcomes by ethnicity. Additionally, there were insufficient data to elucidate ethnic differences in cholesterol and albumin levels, which could be attributed to environment, disease severity, or genetic predisposition. Moreover, these findings need to be examined in additional cohorts and countries. Regardless of these limitations, our results are consistent with numerous important outcomes over an extended period of follow-up.
Incidence of childhood NS is significantly higher among South Asian children in Ontario compared with other ethnic groups. Outcomes of both South Asian and East/Southeast Asian children are significantly better, with fewer having frequently relapsing disease and more be likely to have complete remission. These results highlight the need to understand potential genetic or environmental factors that may account for these ethnic differences.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the participants and their families for their time and effort as well as the nurses and staff from the Nephrology Clinic at The Hospital for Sick Children and William Osler Health System (Brampton Civic Hospital, Brampton, ON, Canada). We also thank Richard Child for his advice and insight into the electronic patient record.
We acknowledge funding from The Hospital for Sick Children Research Institute and Physicians’ Services Incorporated.
Some of the data in this article were presented as a poster presentation at the 2014 Pediatric Academic Societies Annual Meeting held May 3–6, 2014 in Vancouver, British Columbia, Canada.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00380116/-/DCSupplemental.
References
- 1.Eddy AA, Symons JM: Nephrotic syndrome in childhood. Lancet 362: 629–639, 2003 [DOI] [PubMed] [Google Scholar]
- 2.McKinney PA, Feltbower RG, Brocklebank JT, Fitzpatrick MM: Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr Nephrol 16: 1040–1044, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Seedat YK: Nephrotic syndrome in the Africans and Indians of South Africa. A ten-year study. Trans R Soc Trop Med Hyg 72: 506–512, 1978 [DOI] [PubMed] [Google Scholar]
- 4.Wong W: Idiopathic nephrotic syndrome in New Zealand children, demographic, clinical features, initial management and outcome after twelve-month follow-up: Results of a three-year national surveillance study. J Paediatr Child Health 43: 337–341, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Greenbaum LA, Benndorf R, Smoyer WE: Childhood nephrotic syndrome--current and future therapies. Nat Rev Nephrol 8: 445–458, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Bhimma R, Adhikari M, Asharam K: Steroid-resistant nephrotic syndrome: The influence of race on cyclophosphamide sensitivity. Pediatr Nephrol 21: 1847–1853, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Mubarak M, Lanewala A, Kazi JI, Akhter F, Sher A, Fayyaz A, Bhatti S: Histopathological spectrum of childhood nephrotic syndrome in Pakistan. Clin Exp Nephrol 13: 589–593, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Samuel S, Morgan CJ, Bitzan M, Mammen C, Dart AB, Manns BJ, Alexander RT, Erickson RL, Grisaru S, Wade AW, Blydt-Hansen T, Feber J, Arora S, Licht C, Zappitelli M: Substantial practice variation exists in the management of childhood nephrotic syndrome. Pediatr Nephrol 28: 2289–2298, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Sharples PM, Poulton J, White RH: Steroid responsive nephrotic syndrome is more common in Asians. Arch Dis Child 60: 1014–1017, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorof JM, Hawkins EP, Brewer ED, Boydstun II, Kale AS, Powell DR: Age and ethnicity affect the risk and outcome of focal segmental glomerulosclerosis. Pediatr Nephrol 12: 764–768, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Yap HK, Han EJ, Heng CK, Gong WK: Risk factors for steroid dependency in children with idiopathic nephrotic syndrome. Pediatr Nephrol 16: 1049–1052, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, Mountain JL, Pérez-Stable EJ, Sheppard D, Risch N: The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med 348: 1170–1175, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Statistics Canada : NHS Profile: 2011–About the Data, Statistics Canada, Ottawa, Ontario, Canada, Government of Canada, 2011 [Google Scholar]
- 14.Statistics Canada : Special Tabulation, Based on 2001 Single and Multiple Ethnic Origin Responses, Statistics Canada, Ottawa, Ontario, Canada, 2014 [Google Scholar]
- 15.Statistics Canada : Special Tabulation, Based on 2006 Single and Multiple Ethnic Origin Responses, Statistics Canada, Ottawa, Ontario, Canada, 2014 [Google Scholar]
- 16.Statistics Canada : Special Tabulation, Based on 2011 Single and Multiple Ethnic Origin Responses, Statistics Canada, Ottawa, Ontario, Canada, 2014 [Google Scholar]
- 17.Tang H, Quertermous T, Rodriguez B, Kardia SL, Zhu X, Brown A, Pankow JS, Province MA, Hunt SC, Boerwinkle E, Schork NJ, Risch NJ: Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am J Hum Genet 76: 268–275, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statistics Canada : Special Tabulation, Based on 2006 Dissemination Area Information, 2011
- 19.Statistics Canada : Ethnic Origin Reference Guide, 2006 Census, 2006 [Google Scholar]
- 20.Cummins C, Winter H, Cheng KK, Maric R, Silcocks P, Varghese C: An assessment of the Nam Pehchan computer program for the identification of names of south Asian ethnic origin. J Public Health Med 21: 401–406, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Quan H, Wang F, Schopflocher D, Norris C, Galbraith PD, Faris P, Graham MM, Knudtson ML, Ghali WA: Development and validation of a surname list to define Chinese ethnicity. Med Care 44: 328–333, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Shah BR, Chiu M, Amin S, Ramani M, Sadry S, Tu JV: Surname lists to identify South Asian and Chinese ethnicity from secondary data in Ontario, Canada: A validation study. BMC Med Res Methodol 10: 42, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group : KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 139–274, 2012 [Google Scholar]
- 24.ISKDC : Early identification of frequent relapsers among children with minimal change nephrotic syndrome. A report of the International Study of Kidney Disease in Children. J Pediatr 101: 514–518, 1982 [DOI] [PubMed] [Google Scholar]
- 25.Chandra RK, Manchanda SS, Srivastava RN, Soothill JF: Differential protein clearances in Indian children with the nephrotic syndrome. Arch Dis Child 45: 491–495, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mubarak M, Kazi JI, Shakeel S, Lanewala A, Hashmi S: The spectrum of histopathological lesions in children presenting with steroid-resistant nephrotic syndrome at a single center in Pakistan. ScientificWorldJournal 2012: 681802, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Statistics Canada : Analytical Document—Immigration and Ethnocultural Diversity in Canada, 2011
- 28.Gbadegesin RA, Adeyemo A, Webb NJ, Greenbaum LA, Abeyagunawardena A, Thalgahagoda S, Kale A, Gipson D, Srivastava T, Lin JJ, Chand D, Hunley TE, Brophy PD, Bagga A, Sinha A, Rheault MN, Ghali J, Nicholls K, Abraham E, Janjua HS, Omoloja A, Barletta GM, Cai Y, Milford DD, O’Brien C, Awan A, Belostotsky V, Smoyer WE, Homstad A, Hall G, Wu G, Nagaraj S, Wigfall D, Foreman J, Winn MP; Mid-West Pediatric Nephrology Consortium : HLA-DQA1 and PLCG2 are candidate risk loci for childhood-onset steroid-sensitive nephrotic syndrome. J Am Soc Nephrol 26: 1701–1710, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr., Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ; AASK Study Investigators; CRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbour SJ, Cattran DC, Kim SJ, Levin A, Wald R, Hladunewich MA, Reich HN: Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int 84: 1017–1024, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS; Family Investigation of Nephropathy and Diabetes Research Group : MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 40: 1185–1192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbet SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA: MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 40: 1175–1184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teeninga N, Kist-van Holthe JE, van den Akker EL, Kersten MC, Boersma E, Krabbe HG, Knoers NV, van der Heijden AJ, Koper JW, Nauta J: Genetic and in vivo determinants of glucocorticoid sensitivity in relation to clinical outcome of childhood nephrotic syndrome. Kidney Int 85: 1444–1453, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Ingulli E, Tejani A: Racial differences in the incidence and renal outcome of idiopathic focal segmental glomerulosclerosis in children. Pediatr Nephrol 5: 393–397, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Hussain N, Zello JA, Vasilevska-Ristovska J, Banh TM, Patel VP, Patel P, Battiston CD, Hebert D, Licht CP, Piscione TD, Parekh RS: The rationale and design of Insight into Nephrotic Syndrome: Investigating Genes, Health and Therapeutics (INSIGHT): A prospective cohort study of childhood nephrotic syndrome. BMC Nephrol 14: 25, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trautmann A, Bodria M, Ozaltin F, Gheisari A, Melk A, Azocar M, Anarat A, Caliskan S, Emma F, Gellermann J, Oh J, Baskin E, Ksiazek J, Remuzzi G, Erdogan O, Akman S, Dusek J, Davitaia T, Özkaya O, Papachristou F, Firszt-Adamczyk A, Urasinski T, Testa S, Krmar RT, Hyla-Klekot L, Pasini A, Özcakar ZB, Sallay P, Cakar N, Galanti M, Terzic J, Aoun B, Caldas Afonso A, Szymanik-Grzelak H, Lipska BS, Schnaidt S, Schaefer F; PodoNet Consortium : Spectrum of steroid-resistant and congenital nephrotic syndrome in children: The PodoNet registry cohort. Clin J Am Soc Nephrol 10: 592–600, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sureshkumar P, Hodson EM, Willis NS, Barzi F, Craig JC: Predictors of remission and relapse in idiopathic nephrotic syndrome: A prospective cohort study. Pediatr Nephrol 29: 1039–1046, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr.: Prognostic significance of the early course of minimal change nephrotic syndrome: Report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8: 769–776, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Fletcher J, McDonald S, Alexander SI; Australian and New Zealand Pediatric Nephrology Association (ANZPNA) : Prevalence of genetic renal disease in children. Pediatr Nephrol 28: 251–256, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Kim J, Patnaik N, Chorny N, Frank R, Infante L, Sethna C: Second-line immunosuppressive treatment of childhood nephrotic syndrome: A single-center experience. Nephron Extra 4: 8–17, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kemper MJ, Altrogge H, Ludwig K, Timmermann K, Müller-Wiefel DE: Unfavorable response to cyclophosphamide in steroid-dependent nephrotic syndrome. Pediatr Nephrol 14: 772–775, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Zagury A, de Oliveira AL, de Moraes CA, de Araujo Montalvão JA, Novaes RH, de Sá VM, Monteiro de Carvalho DB, Matuck T: Long-term follow-up after cyclophosphamide therapy in steroid-dependent nephrotic syndrome. Pediatr Nephrol 26: 915–920, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Cammas B, Harambat J, Bertholet-Thomas A, Bouissou F, Morin D, Guigonis V, Bendeddouche S, Afroukh-Hacini N, Cochat P, Llanas B, Decramer S, Ranchin B: Long-term effects of cyclophosphamide therapy in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. Nephrol Dial Transplant 26: 178–184, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Latta K, von Schnakenburg C, Ehrich JH: A meta-analysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr Nephrol 16: 271–282, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Forno E, Celedon JC: Asthma and ethnic minorities: Socioeconomic status and beyond. Curr Opin Allergy Clin Immunol 9: 154–160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.