Abstract
Background and objectives
Patiromer is a nonabsorbed potassium-binding polymer that uses calcium as the counterexchange ion. The calcium released with potassium binding has the potential to be absorbed or bind phosphate. Because binding is not specific for potassium, patiromer can bind other cations. Here, we evaluate the effect of patiromer on urine ion excretion in healthy adults, which reflects gastrointestinal ion absorption.
Design, setting, participants, & measurements
We analyzed the effect of patiromer on urine potassium, sodium, magnesium, calcium, and phosphate in two studies. Healthy adults on controlled diets in a clinical research unit were given patiromer up to 50.4 g/d divided three times a day for 8 days (dose-finding study) or 25.2 g/d in a crossover design as daily or divided (two or three times a day) doses for 18 days (dosing regimen study). On the basis of 24-hour collections, urinary ion excretion during the baseline period (days 5–11) was compared with that during the treatment period (days 13–19; dose-finding study), and the last 4 days of each period were compared across regimens (dosing regimen study).
Results
In the dose-finding study, patiromer induced a dose-dependent decrease in urine potassium, urine magnesium, and urine sodium (P<0.01 for each). Patiromer at 25.2 g/d decreased urine potassium (mean±SD) by 1140±316 mg/d, urine magnesium by 45±1 mg/d, and urine sodium by 225±145 mg/d. Urine calcium increased in a dose-dependent manner, and urine phosphate decreased in parallel (both P<0.01). Patiromer at 25.2 g/d increased urine calcium by 73±23 mg/d and decreased urine phosphate by 64±40 mg/d. Urine potassium, urine sodium, and urine magnesium were unaffected by dosing regimen, whereas the increase in urine calcium was significantly lower with daily compared with three times a day dosing (P=0.01). Urine phosphate also decreased less with daily compared with two or three times a day dosing (P<0.05).
Conclusions
In healthy adults, patiromer reduces urine potassium, urine sodium, urine magnesium, and urine phosphate, while modestly increasing urine calcium. Compared with divided dosing, administration of patiromer once daily provides equivalent reductions in urine potassium, urine sodium, and urine magnesium, with less effect on urine calcium and urine phosphate.
Keywords: patiromer; hyperkalemia; electrolytes; urinary excretion; cation exchange; Animals; Brachyura; calcium; Calcium, Dietary; Cations; Cross-Over Studies; Diet; Gastrointestinal Absorption; Intestinal Absorption; Magnesium; Phosphates; Polymers; Potassium; Sodium
Introduction
Patiromer is a nonabsorbed potassium (K) binder that uses calcium (Ca) as the counterion for K exchange (1). Ca rather than sodium (Na) was chosen to prevent additional Na administration and the potential for Na absorption and volume overload when treating patients who are hyperkalemic, many of whom have heart failure or CKD (2,3). K binding by patiromer may occur in proximal segments of the gastrointestinal (GI) tract but is greatest in the colon, where active K secretion results in high luminal K concentrations, the pH is optimal for ion exchange, and the polymer has the longest residence time (1,4–6). Patiromer encounters a series of different cation environments as it traverses the GI tract (1,7). Its cation-binding properties suggest that it has the potential to bind other cations, including Na and magnesium (Mg) (1,7,8). This potential binding is dependent on the ambient pH as well as the ion concentration along the GI tract (1,5). The Ca ions released during the ion exchange process may be absorbed, rebind to patiromer, remain in solution as a Ca ion, or bind phosphate (P) or other anions, resulting in the formation of relatively insoluble complexes, such as Ca-P salts (9).
The purpose of these studies was to examine the change in steady–state urine ion excretion of K, Na, Ca, Mg, and P in healthy volunteers treated with patiromer as a reflection of intestinal ion absorption.
Materials and Methods
Data from two studies in normal volunteers are reported. Participants on controlled diets in a clinical research unit were given patiromer at varying doses (dose-finding study) or dosing regimens (dosing regimen study), and 24-hour urine samples were collected for assessment of K, Na, Ca, Mg, and P excretion.
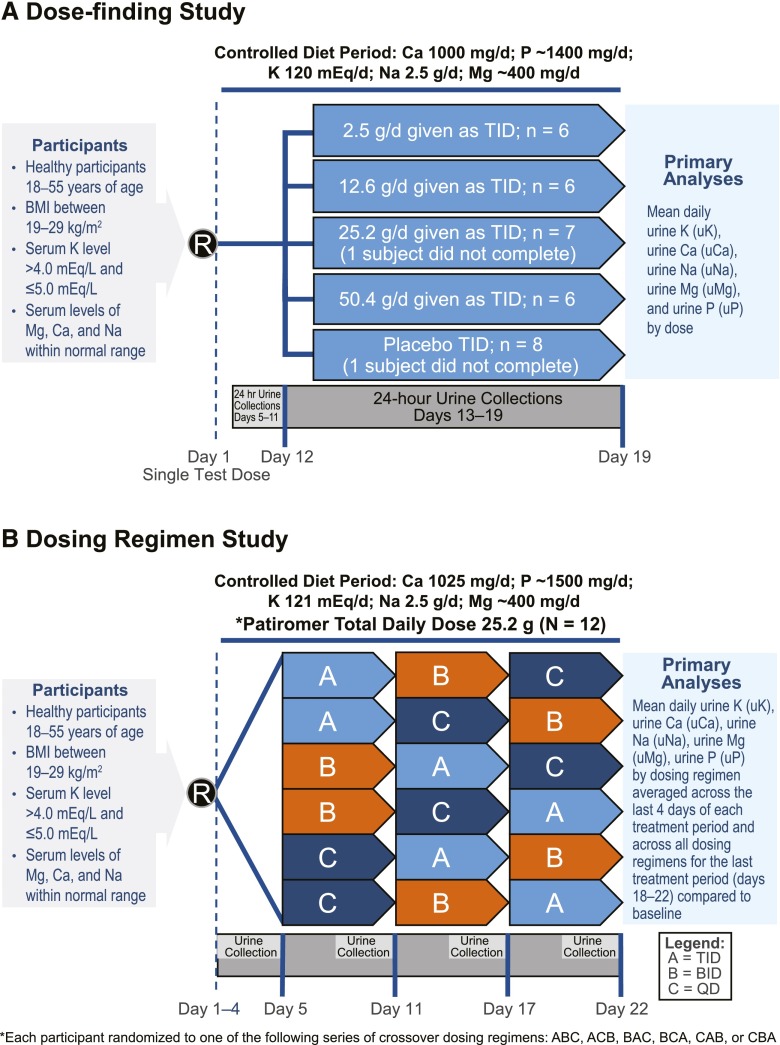
The dose-finding study and the dosing regimen study were both conducted at a single inpatient clinical research unit (Jasper Clinical Research and Development Center, Kalamazoo, MI). Study schema are shown in Figure 1. The administered form of patiromer was modified between studies to increase stability with the addition of a small amount of sorbitol added as a stabilizer, and it is the commercially available form of patiromer.
Figure 1.
Study designs. Study design of healthy volunteers treated with patiromer showing controlled diet and urine collection periods in the (A) dose-finding and (B) dose regimen studies. BID, two times per day; BMI, body mass index; Ca, calcium; K, potassium; Mg, magnesium; Na, sodium; P, phosphate; QD, daily; R, date of randomization; TID, three times per day.
The study protocols were reviewed and approved by an institutional review board (Integreview Ethical Review Board, Austin, TX), and written informed consent was obtained from each participant before enrollment. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and the International Conference on Harmonization/Good Clinical Practice guidelines (10,11).
The dose-finding study was a phase 1 randomized, double–blind, placebo–controlled, parallel group, multiple dose study of patiromer in healthy adult volunteers (Figure 1A). Thirty-three volunteers were enrolled, and 32 were randomly assigned to one of four treatment groups, in which six of eight participants per group received a total daily patiromer dose of 2.5, 12.6, 25.2, or 50.4 g given orally three times a day (with meals). Two of eight participants per group received matching placebo. Participants were admitted to the research unit on day −1 and remained in the unit until discharge on day 20. Participants consumed a controlled diet (see below) from day −1 through the duration of the study. For each treatment group, a single dose of patiromer or placebo was administered on day 1 as a test dose. After acceptable single–dose safety/tolerability was confirmed by the principal investigator and sponsor, an 11-day baseline period was followed by patiromer or placebo administration on days 12–19 (total of 8 days). Twenty-four–hour urine collections were started 4 days after the single test dose of patiromer and collected on days 5–11 and 13–19. The preplanned analyses evaluated urinary ion excretion (K, Ca, Mg, Na, and P) during the baseline period (days 5–11) compared with that during the treatment period (days 13–19; 7-day analysis). To ensure that steady-state conditions were achieved after the institution of the controlled diet and ensure complete clearance of the test dose of patiromer given on day 1, a sensitivity analysis was conducted, in which urine excretion was evaluated on the basis of the last 4 days of the baseline period (days 8–11) and compared with the last 4 days of the treatment period (days 16–19; 4-day analysis).
The dosing regimen study was a phase 1, open label, multiple dose crossover study to evaluate the pharmacology, safety, and tolerability of three patiromer dosing regimens in 12 healthy volunteers. Patiromer was administered orally for a total of 18 days on the basis of one of six random dosing sequences at 25.2 g every day for 6 days, 12.6 g two times per day for 6 days, and 8.4 g three times per day for 6 days (Figure 1B). Each participant was admitted to the research unit on day −1 and remained in the clinic until discharge on day 23. Participants consumed the controlled diet (see below) starting on day −1. Twenty-four–hour urine samples were collected on study days 1–4 (baseline) and the last 4 days of each 6-day period (days 7–10, 13–16, and 19–22). For preplanned comparisons across dosing regimens, the 24-hour urine collections from the last 4 days of each period were examined. For comparisons with baseline, the individual results from all three dosing regimens for the last 4 days (days 19–22) were averaged and compared with the four baseline days (4-day analysis). To validate that the findings were not influenced by the short baseline period, a sensitivity analysis was conducted, in which the last 2 days of the baseline period were also compared with the last 2 days of the final treatment period (days 21 and 22) for urine excretion of K, Na, Ca, Mg, and P (2-day analysis).
For both studies, a rotating diet was controlled to be consistent across days for energy, macronutrient, and ion intake. In the dose-finding study, participants were required to consume the entire meal, whereas they were requested to do so in the dosing regimen study. The controlled diet for the dose-finding study rotated meals on an 8-day schedule and provided, on average (range), 2821 (2521–3073) kcal/d consisting of carbohydrates (432 [377–489] g/d), fat (92 [71–128] g/d), and protein (94 [70–114] g/d). The diet was controlled for ions, including K (4582 [4489–4689] mg/d), Na (2508 [2459–2583] mg/d), Mg (394 [323–422] mg/d), and Ca (1000 [966–1053] mg/d). The diet for the dosing regimen study rotated meals on a 6-day schedule and provided, on average, 2677 (2502–2811) kcal/d consisting of carbohydrates (392 [387–439] g/d), fat (88 [82–109] g/d), and protein (104 [80–133] g/d). The diet was also controlled for ions, including K (4718 [4624–4787] mg/d), Na (2495 [2426–2529] mg/d), Mg (409 [351–447] mg/d), and Ca (1025 [951–1098] mg/d).
Laboratory and adverse event (AE) assessments were performed throughout both studies to monitor the safety and tolerability of patiromer. For both studies, all ions were measured on a Roche Modular System (Roche Diagnostics, Indianapolis, IN) and performed by Bronson Methodist Hospital Laboratory (Kalamazoo, MI). The system uses ion-specific electrodes for Na and K, whereas other analytes were measured by spectrophotometric analysis. All urine collected from each participant was measured separately for each 24-hour period. After determination of total volume, an aliquot of urine from each well mixed 24-hour collection was analyzed for K, Ca, Na, Mg, and P. Urine was acidified before obtaining an aliquot for analysis of Ca and Mg.
In both studies, descriptive statistics (n, mean, and SD) were summarized by treatment for mean daily content of urine excretion of K, Na, Mg, Ca, and P separately for the baseline and treatment periods.
In the dose-finding study, mean values at baseline were compared among treatment groups using a one–way ANOVA fixed effects model. Analysis of covariance with the baseline value as the covariate was used for the end point and the change from baseline to end point analyses. If the overall test was significant, pairwise comparisons among treatment groups were made using the least squares means approach.
In the dosing regimen study, mean values on treatment were compared among treatment groups using an ANOVA model that included sequence, period, and treatment as fixed factors and participant within sequence as a random factor. The least squares estimate of the mean of each treatment is presented. Change from baseline was analyzed in the same manner.
All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC); P<0.05 was considered statistically significant.
Results
Demographics
All participants were healthy volunteers with no known medical conditions. In the dose-finding study, the mean±SD age was 33.2±10.1 (range =18–55) years, and the body mass index was 25.0±2.5 (range =19–30) kg/m2. There were 26 men and seven women. Twenty-six were white, five were black, one was Asian, and one was Hispanic. In the dosing regimen study, the age was 34.5±7.2 (range =21–47) years old, and body mass index was 26.2±2.2 (range =22–29) kg/m2. There were nine men and three women. Seven were white, three were black, and two were Hispanic.
In the dose-finding study, 32 participants were initially enrolled. One assigned to the 25.2-g/d patiromer group withdrew from the study, because he could not tolerate the required study diet and was replaced. These 33 participants comprised the safety dataset. Of the 32 participants analyzed for efficacy, one placebo-treated participant was withdrawn from the study before the multidose period because of elevated liver enzymes. During the multiple dose study period, 24 participants received patiromer three times per day (six per dose), and seven received placebo three times per day for 8 days. These 31 participants made up the urine ion analysis set.
In the dosing regimen study, all 12 participants completed all study activities and made up the analysis set.
Serum Chemistries
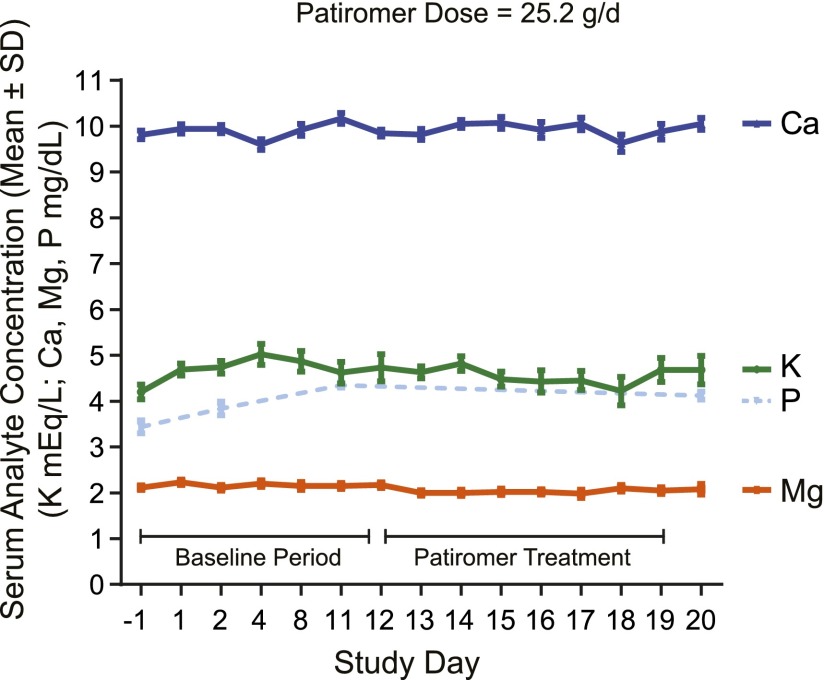
Mean serum K, Na, P, Mg, and Ca values were within the normal range and stable throughout the treatment periods for both studies. Figure 2 shows the serum Ca, K, P, and Mg values over time in the dose-finding study for the maximum recommended patiromer dose of 25.2 g/d.
Figure 2.
Serum chemistries with patiromer 25.2 g/d during the dose-finding study. Serum chemistries (means±SD) on maximum recommended patiromer dose (25.2 g/d) over time in the dose-finding study. Ca, calcium; K, potassium; Mg, magnesium; P, phosphate.
Urine Chemistries
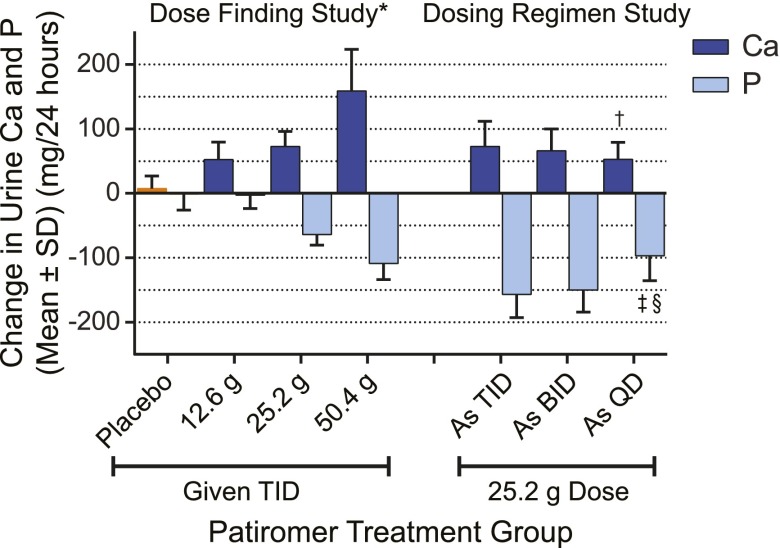
The changes in mean urine ion excretion from baseline for the dose-finding study are shown in Table 1 for both the preplanned (7-day) and sensitivity (4-day) analyses. Patiromer induced a dose-dependent decrease in urine potassium (uK) and urine magnesium (uMg; P<0.01) in both analyses periods. Patiromer also decreased urine sodium (uNa) in a dose-dependent fashion in the preplanned analysis (P<0.01). Urine Ca (uCa) increased and urine phosphate (uP) decreased in a dose-dependent fashion in both analyses (P<0.01) (Figure 3 [preplanned analysis], Table 1).
Table 1.
Mean±SD change in 24-hour urine ion excretion in the dose-finding study by collection analysis (preplanned and sensitivity)
| Analysis Period | Placebo, n=7 | Patiromer Dose, g/d | |||
|---|---|---|---|---|---|
| 2.5, n=6 | 12.6, n=6 | 25.2, n=6 | 50.4, n=6 | ||
| 7-d Preplanned analysis (days 5–11 versus 13–19) | |||||
| uK, mga | −255.0±517.3 | −1.9±136.3 | −531.1±233.7 | −1140.3±316.1b | −1950.9±276.6c |
| uNa, mga | −66.4±177.2 | 106.8±199.1 | 28.5±96.7 | −224.5±144.6 | −618.8±707.9 |
| uMg, mga | −4.7±10.3 | −5.1±16.1 | −33.7±6.9c | −44.9±1.3c | −61.7±10.4c |
| uCa, mga | 7.5±19.3 | 24.3±16.1 | 52.5±27.1b | 72.9±23.4c | 158.9±64.5c |
| uP, mga | −1.0±71.6 | 20.5±41.2 | −2.9±50.3 | −64.0±39.9 | −109.9±60.8d |
| 4-d Sensitivity analysis (days 8–11 versus 16–19) | |||||
| uK, mga | −197.3±617.7 | 8.1±210.2 | −687.4±258.2 | −1274.0±385.6b | −2192.1±292.7c |
| uNa, mg | −84.6±220.7 | 81.5±249.5 | −34.5±149.9 | −152.4±197.4 | −935.3±1332.7 |
| uMg, mga | −9.2±13.1 | 7.2±23.9 | −45.4±4.6c | −48.7±14.3c | −75.5±9.8c |
| uCa, mga | 3.0±17.3 | 22.7±19.7 | 64.3±41.3b | 67.9±26.3c | 141.7±57.0c |
| uP, mga | 10.1±71.1 | 43.0±60.2 | −61.8±45.3 | −60.0±51.1 | −159.2±107.3b |
uK, urine potassium; uNa, urine sodium; uMg, urine magnesium; uCa, urine calcium; uP, urine phosphate.
P<0.01 for overall test of patiromer dose differences from ANOVA.
P<0.01 for pairwise comparison versus placebo from t test.
P<0.001 for pairwise comparison versus placebo from t test.
P<0.02 for pairwise comparison versus placebo from t test.
Figure 3.
Change in urine calcium and phosphate excretion. Mean±SD change in urine calcium (Ca) and phosphate (P) excretion by patiromer dose compared with baseline for both studies (preplanned analyses). Data are from Tables 1 and 3. BID, two times per day; QD, daily; TID, three times per day. *P<0.01 for overall test of patiromer dose differences from analysis of covariance; †P=0.01 versus three times per day for pairwise comparison between regimens from t test; ‡P<0.01 versus three times per day for pairwise comparison between regimens from t test; §P=0.02 versus two times per day for pairwise comparison between regimens from t test.
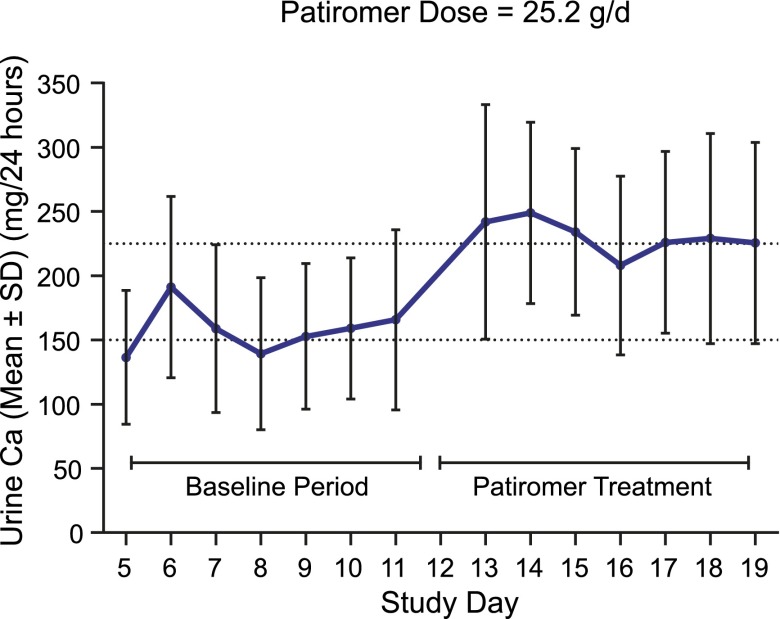
The maximum recommended dose of 25.2 g/d resulted in a net increase in uCa of 72.9±23.4 mg/d in the preplanned analysis (P<0.001 versus placebo) (Table 1). Results were consistent when restricting the analysis to the last 4 days, with a net increase in uCa of 67.9±26.3 mg/d (P<0.001 versus placebo). Analysis of the uCa by study day for the 25.2-g/d dose showed that uCa excretion increased and stabilized rapidly after administration of patiromer (Figure 4).
Figure 4.
Twenty-four-hour urine Ca excretion with patiromer 25.2 g/d during the dose-finding study. Twenty-four–hour urine calcium (Ca) excretion (means±SD) by study day for a 25.2-g/d dose of patiromer in the dose-finding study.
Mean changes in urine ion excretion from defined baseline periods averaged across dosing regimens for the preplanned (4-day) and sensitivity (2-day) analyses for the dosing regimen study are shown in Table 2. All changes were significant in the preplanned analysis. uK decreased 1462.1±274.3 mg/d (37.5±7.0 mEq/d), uNa decreased 463.2±581.7 mg/d (20.1±25.3 mEq/d), uMg decreased 39.1±20.0 mg/d, uP decreased 143.5±138.1 mg/d, and uCa increased 73.5±11.2 mg/d (Figure 3). Restricting the analysis to the last 2 days of the baseline period (to allow a 2-day run in on the controlled diet) versus the last 2 days of the treatment period showed similar results for all ions except uNa, which decreased by 80.5±636.0 mg/d (3.5±27.7 mEq/d; P=0.32).
Table 2.
Mean±SD change in 24-hour urine ion excretion in the dosing regimen study averaged across dosing regimens by collection analysis (preplanned and sensitivity)
| Analysis Period | Patiromer at 25.2 g/d Averaged across Dosing Regimens | P Valuea |
|---|---|---|
| 4-d Preplanned analysis (days 1–4 versus 19–22) | ||
| uK, mg | −1462.1±274.3 | <0.001 |
| uNa, mg | −463.2±581.7 | 0.02 |
| uMg, mg | −39.1±20.0 | <0.001 |
| uCa, mg | 73.5±38.8 | <0.001 |
| uP, mg | −143.5±138.1 | <0.01 |
| 2-d Sensitivity analysis (days 3 and 4 versus 21 and 22) | ||
| uK, mg | −1800.2±312.7 | <0.001 |
| uNa, mg | −80.5±636.0 | 0.32 |
| uMg, mg | −49.5±17.8 | <0.001 |
| uCa, mg | 83.3±40.4 | <0.001 |
| uP, mg | −109.3±134.5 | <0.02 |
uK, urine potassium; uNa, urine sodium; uMg, urine magnesium; uCa, urine calcium; uP, urine phosphate.
From one–sample t test to determine if the mean change from baseline is significantly different from zero.
Overall, a patiromer total daily dose of 25.2 g in the dosing regimen study produced similar changes in urine ion excretion as the equivalent dose in the dose-finding study. In the dosing regimen study, uK, uNa, and uMg excretions were unaffected by dosing regimen (one, two, or three times per day) (Table 3). However, the increase in uCa was significantly lower with daily dosing compared with three times per day dosing (P=0.01) (Figure 2, Table 3). uP excretion also decreased less with daily dosing compared with two times per day (P=0.02) or three times per day (P<0.01) dosing (Figure 2, Table 3).
Table 3.
Mean±SD 24-hour urine calcium, phosphate, potassium, sodium, and magnesium excretion in the dosing regimen study (4-day preplanned analysis)
| Urine Analyte | Patiromer Dosing Regimen | ||
|---|---|---|---|
| 8.4 g Three Times per d | 12.6 g Two Times per d | 25.2 g One Time per d | |
| uCa, mg | |||
| Baseline | 143±82 | 143±82 | 143±82 |
| Treatment | 216±97 | 209±97 | 196±90a |
| Change from baseline | 73±39 | 66±34 | 53±26a |
| uP, mg | |||
| Baseline | 890±158 | 890±158 | 890±158 |
| Treatment | 733±99 | 741±102 | 793±103b,c |
| Change from baseline | −157±124 | −150±119 | −97±134b,c |
| uK, mg | |||
| Baseline | 4450±362 | 4450±362 | 4450±362 |
| Treatment | 3010±474 | 2916±327 | 3012±446 |
| Change from baseline | −1440±384 | −1534±295 | −1438±384 |
| uNa, mg | |||
| Baseline | 2869±488 | 2869±488 | 2869±488 |
| Treatment | 2288±268 | 2442±306 | 2323±247 |
| Change from baseline | −581±536 | −428±603 | −546±693 |
| uMg, mg | |||
| Baseline | 134±37 | 134±37 | 134±37 |
| Treatment | 95±23 | 91±23 | 100±23 |
| Change from baseline | −39±22 | −43±18 | −34±21 |
uCa, urine calcium; uP, urine phosphate; uK, urine potassium; uNa, urine sodium; uMg, urine magnesium.
P=0.01 versus three times per day for pairwise comparison between regimens from t test.
P<0.01 versus three times per day for pairwise comparison between regimens from t test.
P=0.02 versus two times per day for pairwise comparison between regimens from t test.
Safety
In both the dose-finding and the dosing regimen studies, patiromer was well tolerated. No serious AEs were reported in participants receiving patiromer. GI events were the most common AEs reported; they were considered mild or moderate in severity, and all resolved by the end of both studies. No participant discontinued patiromer because of an AE in either study.
Discussion
The administration of patiromer to healthy volunteers resulted in decreases in uK, uNa, uMg, and uP and a small increase in uCa. Despite differences in study design (confinement time, baseline period, and patiromer form) and diets, the data across the two studies are consistent. The dose-dependent decrease in uK has been previously reported to reflect the GI binding and fecal elimination of K by patiromer (1).
The decrease in uNa seen with patiromer administration (observed especially at higher doses) suggests that patiromer may bind Na in the GI tract, resulting in decreased Na absorption. Although the magnitude of the reduction was relatively small, especially in the sensitivity analyses (80–150 mg), compared with dietary Na intake, patiromer does not add to dietary Na intake in contrast to other K binders in the United States that use Na as a counterexchange ion (2,12,13).
Administration of patiromer resulted in a dose-dependent increase in uCa. The increase in uCa was approximately 73 mg/d on the maximum Food and Drug Administration–approved patiromer dose of 25.2 g/d (14). The increase in uCa was lower when patiromer was given once daily compared with the same daily total given in divided doses (three times per day). The increase in uCa of approximately 73 mg seen with maximum patiromer administration in these healthy individuals on a Ca-replete diet (1000 mg/d) suggests that, even at the maximum recommended dose of 25.2 g, only a small fraction of the Ca is available for absorption. Despite the total Ca content of patiromer, this limited absorption is consistent with the understanding that the availability of Ca for absorption increases with greater solubility (e.g., Ca citrate) and decreases in the presence of agents that avidly bind Ca, such as patiromer (1), or form relatively insoluble Ca salts, such as Ca P (15). Although patiromer exchanges Ca for K, the increase in uCa does not reflect the total amount of K binding as some of the released Ca may be unavailable for absorption, because it may bind P or other ions, forming relatively insoluble salts that are excreted in the stool. Moreover, the predominant site where the ambient pH is optimal for Ca release and therefore, exchange with K is believed to occur is in the colon, where Ca absorption efficiency is minimal (16). The data from these studies suggest that, by adding daily patiromer to a diet containing a moderate Ca load, the net elemental Ca available for absorption is within the recommended guidelines of the National Academies (17) for the general population and below the total elemental Ca intake recommended for patients with CKD in the Kidney Disease Outcomes Quality Initiative guidelines (18). Whether the small increase in uCa and other changes in urine ion excretion seen in healthy volunteers in this study could increase their propensity for stone formation is not known, because all urine ions necessary for calculation of supersaturation were not measured. However, the vast majority of patients who will require treatment with patiromer for hyperkalemia will have impaired kidney function, which substantially reduces uCa excretion (19). In patients with CKD, clinicians will need to weigh the risk of a potential small increase in Ca absorption compared with the risk of hyperkalemia.
The decrease in uP of 60–130 mg observed with patiromer suggests decreased GI P absorption, presumably because of binding of P by Ca. The magnitude of the decrease in presumed GI P absorption on the 25.2-g/d dose is within the range reported in patients with CKD given intestinal P binders, and it is similar to that achieved experimentally in vivo with 1000 mg elemental Ca in the form of Ca acetate (approximate Ca acetate dose of 3.8 g) (9) and equivalent to a total dose of almost six Ca acetate tablets (667 mg per tablet) or eight sevelamer tablets (800 mg per tablet) (20). In patients with CKD, reduction in net intestinal P absorption is generally viewed as favorable (18,21).
Patiromer dosed at 25.2 g/d decreased uMg by about 40 mg/d. This decrease presumably reflects Mg binding to patiromer in the GI tract, thereby reducing Mg absorption. Despite this binding, only small decreases in serum Mg were observed in the 12- and 52-week clinical trials of patiromer, and no patient developed Mg concentrations <1.0 mg/dl (12,22). Because many individuals needing treatment for hyperkalemia are also receiving loop diuretics, a known cause for urinary Mg wasting (23,24), attention should be paid to the serum Mg in patiromer-treated patients.
These trials were not designed as formal balance studies, and therefore, balance data are not available. However, the consistency of the findings in urine ion excretion across two independent studies showing identical directional and a similar magnitude of change strongly supports the conclusions. Analyses of preplanned time periods and adjusted time periods to ensure steady-state conditions also show similar results, confirming the reliability of the data. We did not directly study ion absorption in these healthy individuals and instead, used changes in urine ion excretion to reflect intestinal ion absorption. As expected, we did not observe any changes in mean serum K concentration, because normal homeostatic mechanisms in these individuals with intact kidney function over a short period of time are able to closely regulate the level of serum K (25). We did not study patients with CKD, because urine ion excretion in these individuals requires greater time to reach steady state in response to changes in intake and may not reflect changes in GI absorption (19).
In conclusion, we found that, in healthy individuals, patiromer administration not only decreased uK excretion but also, decreased uP and uNa, with only a small decrease in uMg and a small increase in uCa. Because changes in urine ion excretion reflect ion absorption in healthy individuals in the steady state, these data suggest that patiromer is a highly effective K binder, and the fall in uNa and uMg suggests some intestinal binding of these ions as well. The small increase in uCa and the decrease in uP suggest that only a small fraction of the Ca in patiromer is available for absorption and that some of the released Ca is binding to intestinal P.
Disclosures
D.A.B. reports personal fees from Relypsa (Redwood City, CA), Amgen, Inc. (Thousand Oaks, CA), Sanofi Aventis/Genzyme (Cambridge, MA), Tricida (San Francisco, CA), Fresenius Medical Care (Waltham, MA), and OPKO Health (Miami, FL). D.A.B. also has stock options in Relypsa and Tricida. D.M.S., C.G. W.W.B., J.F., and C.D.M. are employees of Relypsa. K.M.H.G. reports personal fees for consulting for Relypsa and Tricida and has received speaker honoraria from Sanofi US (Bridgewater, NJ). G.A.B. reports personal fees from Relypsa. M.R.W. reports personal fees from Relypsa, Janssen Biotech (Horsham, PA), AstraZeneca Pharmaceuticals (Wilmington, DE), Boehringer Ingelheim (Mannheim, Germany), Boston Scientific (Marlborough, MA), Akebia (Cambridge, MA), and MSD (scientific advisor; Kenilworth, NJ). B.P. reports personal fees for consulting with Relypsa, Merck GmbH (Darmstadt, Germany), Bayer HealthCare (Whippany, NJ), AstraZeneca Pharmaceuticals, Boehringer Ingelheim, Forest Laboratories (New York, NY), scPharmaceuticals (Lexington, MA), PharmaIN (Bothell, WA), Tricida, DaVinci Therapeutics (Bohemia, NY), KBP Biosciences (Princeton, NJ), Stealth Peptides (Newton, MA), and AuraSense (Stokie, IL). B.P. has stock options with Relypsa, scPharmaceuticals, PharmaIN, DaVinci Therapeutics, Tricida, KBP Biosciences, and AuraSense. B.P. serves on a data safety monitoring committee for and receives personal fees from Johnson & Johnson (New Brunswick, NJ), Novartis (Basel, Switzerland), and Oxygen Biotherapeutics (Morrisville, NC). B.P. serves on a clinical events committee and receives personal fees from Juventas Therapeutics (Cleveland, OH).
Acknowledgments
Julie Obeid, an employee of Relypsa and Narender Dhingra of AlphaBioCom (funded by Relypsa), provided scientific editorial support.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Patiromer–an Oral Calcium-Loaded Potassium Binder: Kalirrhea with Calciuresis,” on pages 1723–1725.
References
- 1.Li L, Harrison SD, Cope MJ, Park C, Lee L, Salaymeh F, Madsen D, Benton WW, Berman L, Buysse J: Mechanism of action and pharmacology of patiromer, a nonabsorbed cross-linked polymer that lowers serum potassium concentration in patients with hyperkalemia [published online ahead of print February 7, 2016]. J Cardiovasc Pharmacol Ther [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanofi-Aventis Canada, Inc. : Kayexalate (Sodium Polystyrene Sulfonate) Package Insert, Laval, Quebec, Canada, Sanofi-Aventis Canada, Inc., 2014 [Google Scholar]
- 3.Xiao S: Division Memorandum: FDA Center for Drug Evaluation and Research. Medical Review for Application Number 205739Orig205731s205000, Silver Spring, MD, FDA Center for Drug Evaluation and Research, 2015
- 4.Agarwal R, Afzalpurkar R, Fordtran JS: Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 107: 548–571, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Fordtran JS, Locklear TW: Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis 11: 503–521, 1966 [DOI] [PubMed] [Google Scholar]
- 6.Wrong O, Metcalfe-Gibson A: The electrolyte content faeces. Proc R Soc Med 58: 1007–1009, 1965 [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips SF, Giller J: The contribution of the colon to electrolyte and water conservation in man. J Lab Clin Med 81: 733–746, 1973 [PubMed] [Google Scholar]
- 8.Buysse JM, Huang IZ, Pitt B: PEARL-HF: Prevention of hyperkalemia in patients with heart failure using a novel polymeric potassium binder, RLY5016. Future Cardiol 8: 17–28, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Mai ML, Emmett M, Sheikh MS, Santa Ana CA, Schiller L, Fordtran JS: Calcium acetate, an effective phosphorus binder in patients with renal failure. Kidney Int 36: 690–695, 1989 [DOI] [PubMed] [Google Scholar]
- 10.World Medical Association : World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310: 2191–2194, 2013 [DOI] [PubMed] [Google Scholar]
- 11.ICH: ICH Harmonised Tripartite Guideline: Guideline for good clinical practice E6. Presented at the International Conference on Harmonisation, Geneva, Switzerland, 1996 [Google Scholar]
- 12.Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, Stasiv Y, Zawadzki R, Berman L, Bushinsky DA; AMETHYST-DN Investigators : Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: The AMETHYST-DN Randomized Clinical Trial. JAMA 314: 151–161, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Kosiborod M, Rasmussen HS, Lavin P, Qunibi WY, Spinowitz B, Packham D, Roger SD, Yang A, Lerma E, Singh B: Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: The HARMONIZE randomized clinical trial. JAMA 312: 2223–2233, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Relypsa : Veltassa (Patiromer Sorbitex Calcium) Package Insert, Redwood City, CA, Relypsa, 2015 [Google Scholar]
- 15.Sheikh MS, Maguire JA, Emmett M, Santa Ana CA, Nicar MJ, Schiller LR, Fordtran JS: Reduction of dietary phosphorus absorption by phosphorus binders. A theoretical, in vitro, and in vivo study. J Clin Invest 83: 66–73, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bronner F, Pansu D: Nutritional aspects of calcium absorption. J Nutr 129: 9–12, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA: The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J Clin Endocrinol Metab 96: 53–58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 19.Craver L, Marco MP, Martínez I, Rue M, Borràs M, Martín ML, Sarró F, Valdivielso JM, Fernández E: Mineral metabolism parameters throughout chronic kidney disease stages 1-5--achievement of K/DOQI target ranges. Nephrol Dial Transplant 22: 1171–1176, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Daugirdas JT, Finn WF, Emmett M, Chertow GM; Frequent Hemodialysis Network Trial Group : The phosphate binder equivalent dose. Semin Dial 24: 41–49, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Cupisti A, Gallieni M, Rizzo MA, Caria S, Meola M, Bolasco P: Phosphate control in dialysis. Int J Nephrol Renovasc Dis 6: 193–205, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, Wittes J, Christ-Schmidt H, Berman L, Pitt B; OPAL-HK Investigators : Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 372: 211–221, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Quamme GA: Renal magnesium handling: New insights in understanding old problems. Kidney Int 52: 1180–1195, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Ryan MP, Devane J, Ryan MF, Counihan TB: Effects of diuretics on the renal handling of magnesium. Drugs 28[Suppl 1]: 167–181, 1984 [DOI] [PubMed] [Google Scholar]
- 25.Gumz ML, Rabinowitz L, Wingo CS: An integrated view of potassium homeostasis. N Engl J Med 373: 60–72, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]