Urine is a rich source of potential biomarkers. It is thought that urine biomarkers, as opposed to blood biomarkers, might more directly reflect ongoing kidney pathology and physiology, because urine is directly produced by the kidney. Urine can also be collected noninvasively and riskfree. However, because the kidneys are continuously filtering, secreting, and reabsorbing, not everything in the final urine was produced there. Nevertheless, many substances in urine can provide important information regarding kidney function and/or dysfunction. One example is albumin. In healthy kidneys, the combined barrier of the glomerular endothelial cells, basement membrane, and podocyte slit diaphragms allow very little albumin into the filtrate. What little that does get by these structures is effectively reabsorbed in the proximal tubule. Elevated urinary albumin excretion is a sensitive and important marker for glomerular disease processes, and the amount of albuminuria carries prognostic significance regarding risk of cardiovascular disease, end stage renal failure, and all-cause mortality (1,2). Even among individuals without an overt renal disease process, increased albuminuria, even within the normal reference range, portends increased risk of cardiovascular events and death. Thus, there is great interest in accurately measuring albumin for both clinical care and clinical research.
The ultimate utility of any biomarker depends on many factors. Can it be reliably and cost effectively measured using standard clinical chemistry platforms? Does the distribution of values differ enough to reliably discriminate between groups with and without disease? And is the biomarker stable enough under standard conditions to allow convenient transport to the laboratory for analysis? For clinical research, it is also important to know the effect of longer–term storage conditions that allow or prohibit use of biobanked samples.
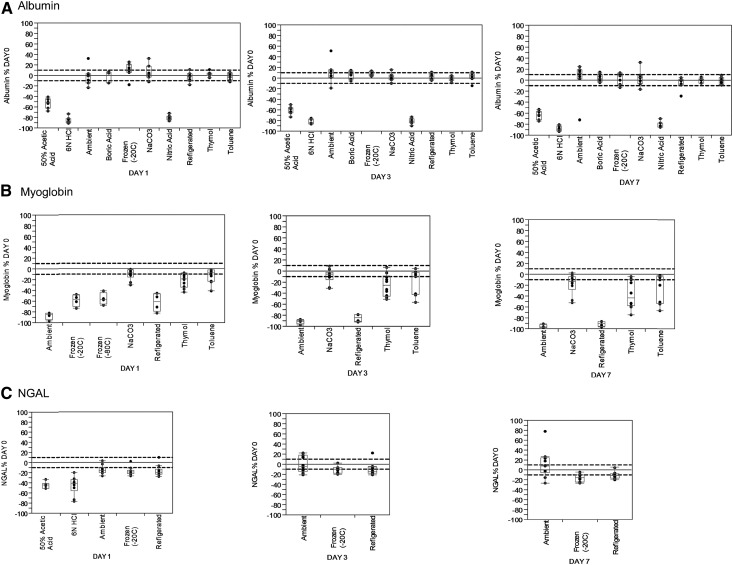
In the clinical laboratory, such as the Mayo Clinic Renal Testing Laboratory, a stability study for 7–14 days using a set of standard conditions tested each urinary analyte as part of the validation process to determine what storage and shipment conditions are acceptable. Variables include storage temperature and use of preservatives designed to acidify, alkalinize, or prevent bacterial growth. Urine albumin is generally stable for up to 7 days under most standard storage conditions (ambient, refrigerated to 4°C, and frozen to −20°C) (Figure 1A). Under these conditions, most measurements are within ±10% of the initial one, which is comparable with the interassay variability for this analyte (coefficient of variation of 3% in the Mayo Clinic Renal Testing Laboratory). As for most (if not all) urine proteins, strong acid preservatives (like hydrochloric or nitric acid) are generally not acceptable for albumin. Urine myoglobin falls on the other end of the spectrum. Even in refrigerated urine, myoglobin stability is well under 24 hours (Figure 1B). Thus, to measure myoglobin, prompt analysis of fresh urine is needed; if not possible, storage under alkaline conditions (e.g., use of sodium carbonate preservative) is mandatory. As a third example, neutrophil gelatinase–associated lipocalin stability data are shown in Figure 1C, and neutrophil gelatinase–associated lipocalin is generally more stable, like albumin. Fortunately, most urine protein biomarkers that have shown clinical utility (and are under current use) have stability more like albumin and less like myoglobin.
Figure 1.
Variable stability of 3 representative urine proteins. One-, 3-, and 7-day stabilities of (A) urinary albumin, (B) myoglobin, and (C) neutrophil gelatinase–associated lipocalin (NGAL) by storage condition. Dashed lines indicate ±10% change from the initial day 0 value. Data adapted from clinical validate studies in the Mayo Clinic Renal Testing Laboratory.
A clinical laboratory is generally concerned about stability during the time needed for transport to the laboratory—typically 1 week or so. Studies to validate longer–term storage conditions are more involved, not clinically relevant in most cases, and thus, often not performed. Published studies regarding albumin, the most established urine protein biomarker, suggest that freezing to −20°C is not sufficient (3), and thus, −80°C is required for long-term storage. One reason may be that urine contains protease inhibitors, including uropepsin (4). Because this enzyme functions better at an acidic pH (optimum pH of approximately 2.0) (4), the ambient pH of any urine sample might affect the stability of any proteins in it. The pH dependence of uropepsin activity might also contribute to the negative effect that acidic preservatives tend to have on protein stability and the protection that alkali conditions can confer (Figure 1). For those proteins (like myoglobin) that are not terribly stable, a protease inhibitor might also be beneficial, especially if long-term storage is intended. In this regard, not all may work the same. When the Neptune investigators compared two commercially available protease cocktails, one clearly worked better for a panel of proteins of interest (5). The more effective preparation contained Pepstatin A, a pepsin inhibitor. Although the exact formulation for the other protease cocktail was proprietary and thus, not known, if one is going to the expense and effort to add protease inhibitors to biobanked urine, having a pepsin inhibitor as part of the mixture seems logical on the basis of the available data.
In this issue of the Clinical Journal of the American Society of Nephrology, Herrington et al. (6) provide new data regarding stability of albumin in urine. Fresh urine samples from subjects with normal or increased urinary albumin excretion were stored refrigerated (4°C), at room temperature (18°C), or at slightly elevated temperature (30°C) as well as frozen to three temperatures (−20°C, −40°C, and −80°C) with or without boric acid as a preservative (using commercially precoated tubes designed for this purpose). Samples were stable at 4°C for 1 week with or without boric acid. When boric acid was added, samples were also stable for 1 week at 18°C but only for 4 days without it. At 30°C, samples were stable for 2 days without boric acid and 4 days with it. On average, albumin signals increased over time, with the maximum rise being 10.3% for samples stored at 30°C without preservative for 7 days. Alternatively, albumin levels decreased with freezing. At −20°C, the albumin concentration fell an average of 9.0% without and 3.8% with boric acid. After 6 months at −20°C, albumin concentrations fell over 20%, regardless of the presence or absence of boric acid. Samples were relatively stable at −40°C, with changes on average of 2%–3% at 1 and 6 months. Changes in albumin concentration were even less at −80°C, averaging <1% at 6 months. The presence of boric acid did not have a major effect at either of the lower freezing temperatures.
Thus, the major benefit of boric acid was to stabilize urine albumin before freezing. Although the average change across all samples was relatively modest, especially at 2 or 4 days, there were individual samples with much larger changes. This was evidenced by the much smaller distribution of data shown in figure 1 and supplemental figure 2 in the paper by Herrington et al. (6) at any given time point for samples compared with those without boric acid. How might boric acid exert this beneficial effect? As a weak acid, it would be expected to reduce urine pH (typically to a pH<5, if not slightly lower). Thus, it seems unlikely to inhibit uropepsin activity and might, if anything, enhance it slightly. Conversely, hydrochloric and nitric are strong acids that can easily bring urinary pH in the pH of 2–3 range, which is close to optimum for uropepsinogen activity (4), and this factor might explain why these stronger acids are particularly bad for protein stability. Importantly, however, boric acid has established bacteristatic effects that are pH independent (7). Thus, one might speculate that boric acid could have effects that are sample dependent and related to the bacterial load in any given sample.
One solution to the problem of stability would be to run all assays on freshly voided urine or at least promptly refrigerate all samples and assay them as soon as possible (ideally later the same day). However, this is not always feasible, especially if samples are being remotely shipped to the laboratory. In these cases, the data in the work by Herrington et al. (6) suggest that use of a boric acid preservative could be considered. Another important factor to consider is the biologic variability of albumin excretion. Data from many studies suggest the within–subject day to day variability of the urinary albumin-to-creatinine ratio averages about 75% (8). Thus, in clinical practice, changes of 5% or even 10% caused by sample handling may not be as crucial as they at first appear. Alternatively, in a clinical trial in which urine albumin excretion is a key variable, and subjects are submitting samples from remote locations, use of a boric acid preservative might be justified to reduce noise and increase study power. An alternative strategy might be to collect multiple urine samples at each time period to capture some of this intraindividual variability, a strategy recently shown to increase precision and power for studies using albuminuria as an outcome (9). This strategy of multiple repeat collections might also reduce the effect of the rarer single–urine samples with less than average stability (supplemental figure 1 in the work by Herrington et al. [6]). The other important take home message reinforced by Herrington et al. (6) is that −20°C storage is not sufficient for urine proteins, even relatively stable ones like albumin and even for a very short time.
Disclosures
None.
Acknowledgments
The authors thank Nikolay Voskoboev for immense aid analyzing data and helpful discussions.
J.C.L. has research funding from Mayo Clinic O’Brien Research Center grant U54 DK U54DK100227 and Rare Kidney Stone Consortium grant U54KD083908, a part of the Rare Diseases Clinical Research Network, an initiative of the Office of Rare Diseases Research, the National Center for Advancing Translational Sciences (NCATS). This consortium is funded through a collaboration between the NCATS and the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Effect of Processing Delay and Storage Conditions on Urine Albumin-to-Creatinine Ratio,” on pages 1794–1801.
References
- 1.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG; Chronic Kidney Disease Prognosis Consortium: Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 380: 1662–1673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osberg I, Chase HP, Garg SK, DeAndrea A, Harris S, Hamilton R, Marshall G: Effects of storage time and temperature on measurement of small concentrations of albumin in urine. Clin Chem 36: 1428–1430, 1990 [PubMed] [Google Scholar]
- 4.Minamiura N, Ito K, Kobayashi M, Kobayashi O, Yamamoto T: Uropepsinogen in human urine: Its protein nature, activation and enzymatic properties of activated enzyme. J Biochem 96: 1061–1069, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Hogan MC, Lieske JC, Lienczewski CC, Nesbitt LL, Wickman LT, Heyer CM, Harris PC, Ward CJ, Sundsbak JL, Manganelli L, Ju W, Kopp JB, Nelson PJ, Adler SG, Reich HN, Holzmann LB, Kretzler M, Bitzer M: Strategy and rationale for urine collection protocols employed in the NEPTUNE study. BMC Nephrol 16: 190, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrington W, Illingworth N, Staplin N, Kumar A, Storey B, Hrusecka R, Judge P, Mahmood M, Parish S, Landray M, Haynes R, Baigent C, Hill M, Clark S: Effect of processing delay and storage conditions on urine albumin-to-creatinine ratio. Clin J Am Soc Nephrol 11: 1794–1801, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meers PD, Chow CK: Bacteriostatic and bactericidal actions of boric acid against bacteria and fungi commonly found in urine. J Clin Pathol 43: 484–487, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, McQueen MJ, Itoh Y, Lieske JC, Seccombe DW, Jones G, Bunk DM, Curhan GC, Narva AS; National Kidney Disease Education Program-IFCC Working Group on Standardization of Albumin in Urine: Current issues in measurement and reporting of urinary albumin excretion. Clin Chem 55: 24–38, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Kröpelin TF, de Zeeuw D, Andress DL, Bijlsma MJ, Persson F, Parving HH, Heerspink HJ: Number and frequency of albuminuria measurements in clinical trials in diabetic nephropathy. Clin J Am Soc Nephrol 10: 410–416, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]