In the absence of diarrhea, about 90% of ingested potassium (K) is absorbed and excreted into the urine. Renal K excretion is impaired by a marked reduction in kidney function, kidney disorders, or drugs which sharply reduce the distal tubular delivery of sodium and/or reduce the renal tubular aldosterone action. These diseases and drugs increase the serum K concentration. Hyperkalemia has become more prevalent as a result of the increasing use of drugs which block the renin-angiotensin-aldosterone system (RAAS). They include drugs which reduce aldosterone generation (direct renin inhibitors, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers) and others that block aldosterone activity (spironolactone, eplerenone). These drugs are often indicated for patients with already compromised renal function. Therefore, effective and safe means of removing K from the body have been sought.
Until recently, the major way to bind K in the gastrointestinal (GI) tract and thereby enhance excretion in the stool was the administration of sodium polystyrene sulfonate, which is a poorly effective and potentially dangerous medication (1,2). Recently, two new K-binding agents have been developed (patiromer and zirconium cyclosilicate), and one (patiromer) has been approved for use by the Food and Drug Administration (FDA). A safe, oral, nonabsorbable K-binding agent should mitigate the hyperkalemia risk of RAAS blockers and thereby be very beneficial to patients who have been unable to tolerate optimal doses of these agents on that basis. However, while the GI K-binding agents which are currently available or in the developmental pipeline are relatively selective for K, they also bind other ions and substances. Also, these agents all release other cations when K (or another cation) is bound; patiromer releases calcium whereas sodium polystyrene sulfonate and zirconium cyclosilicate each release sodium.
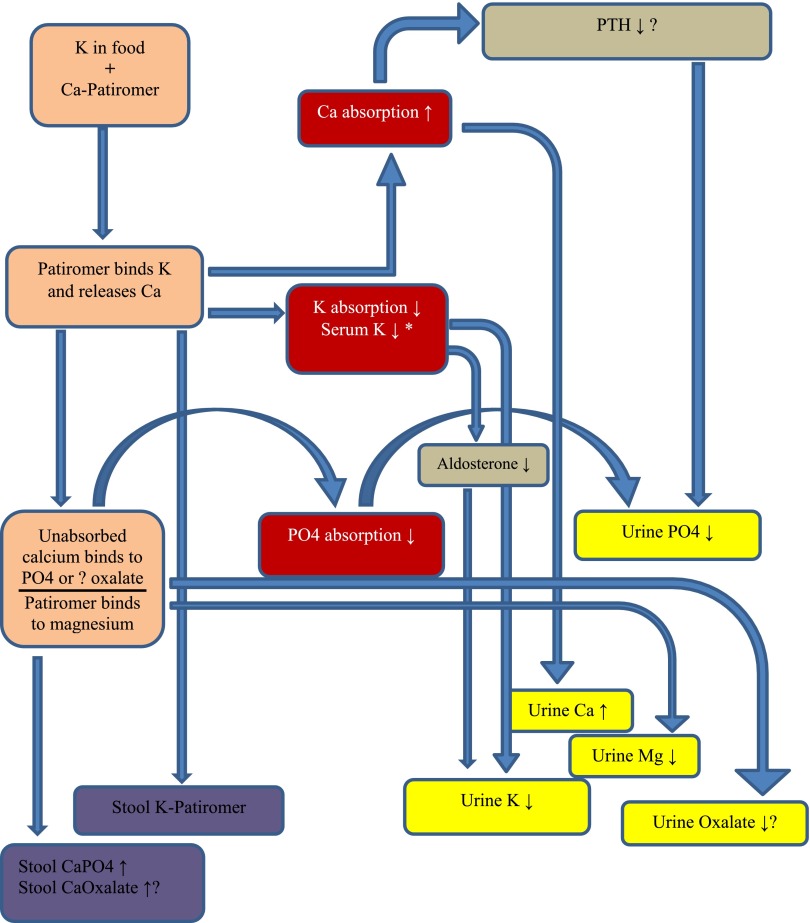
Whereas it is clear that patiromer releases calcium when it binds K (or another cation), the fate of the calcium ions released into the GI lumen is unclear. They may be systemically absorbed by the intestine (and excreted in the urine), bind to anions, such as phosphate or oxalate, in the bowel lumen, or rebind to patiromer. In this issue of the Clinical Journal of American Society of Nephrology, Bushinsky et al. (3) examined these issues by studying the urinary excretion of sodium, K, magnesium, calcium, and phosphate in healthy normal adults given varying doses of patiromer at varying dosing intervals. As expected, urine K excretion fell and urine calcium excretion increased moderately. The urinary excretion of sodium, K, magnesium, and phosphate all decreased, suggesting reduced intestinal absorption, assuming the subjects were all in an equilibrated steady state (see Figure 1).
Figure 1.
Patiromer administration and resultant changes in fecal and urine K, phosphate, and calcium. *Although the serum K did not fall in this study of normal individuals, patiromer has been shown to lower serum K in hyperkalemic patients with chronic kidney disease. Box colors: brown, GI tract; purple, fecal excretion; red, GI absorption changes; gray, hormonal effects; yellow, urinary excretion. Ca, calcium; CaPO4, calcium phosphate; K, potassium; Mg, magnesium; PTH, parathyroid hormone; PO4, phosphate.
What is the potential clinical impact of these findings? The moderate increase in urine calcium excretion, combined with reduced urinary magnesium excretion, may increase the risk of calcium-containing kidney stones. However, urine oxalate excretion was not measured, and, therefore, calcium oxalate supersaturation could not be calculated. If some of the calcium is released from patiromer, especially in the colon, then this may bind oxalate and thereby reduce its absorption. Also, calcium released from patiromer in the small bowel probably functions as a phosphate binder. This would be advantageous for many patients with CKD who require phosphate-binding drugs. However, there is some concern about the potential adverse peripheral vascular and cardiovascular effects of increased oral calcium loads in this population. Some evidence suggests that patients using noncalcium-based phosphate binders have a lower mortality rate than patients using calcium-based phosphate binders (4,5). On the other hand, the amount of calcium released from patiromer and systemically absorbed is modest and may not be problematic. Clarification of these issues will require long-term studies in CKD patients. Although parathyroid hormone levels were not evaluated in this study it is likely they would have fallen in response to the increased calcium absorption from the GI tract.
Patiromer did not reduce serum K in these healthy individuals. Although initially surprising, this is to be expected. In normal patients, excretion of urine K will reduce sharply (to less than 1000 mg/L per day) in response to a major reduction of K intake (or absorption). In this study, the baseline K intake was about 4600–4700 mg/d and baseline urine K excretion about 4400 mg/day. On the highest dose of patiromer the urine K excretion fell by about 1400 mg to about 3000 mg/day. One would not expect a normal patient to measurably reduce their serum K concentration in response to a change in dietary K intake from 4600 mg/day to 3000 mg/day. Therefore, a fall in serum K should not be expected in normal subjects ingesting this dose of patiromer.
Differences between the dose-finding study and the dosing-regimen study are interesting. It is known that calcium-based phosphate binders are most effective when ingested together with meals (6). Consistent with this finding is the observation that patiromer reduces urine phosphate more effectively when ingested twice or three times a day rather than once daily. However, urine calcium excretion was lowest with daily dosing. Furthermore, there was no significant effect of dose timing intervals on reduction of urine K.
Patiromer, when used in patients with diabetic kidney disease, has generated hypomagnesemia (7). In this study of normal patients, urinary magnesium excretion fell, likely reflecting GI binding of magnesium; however, serum magnesium levels did not fall.
Patiromer may also bind oral medications and reduce their bioavailability. This issue has not yet been well studied. Currently, the FDA warns that all other ingested medications be administered 6 hours before or after patiromer administration.
RAAS inhibitors provide major benefits in many common clinical disorders, including congestive heart failure, diabetic nephropathy, and hypertension. Their optimal application may be limited by one of their most common side effects, especially in patients with CKD: hyperkalemia. It is hoped that patiromer will allow clinicians to add/dose optimize angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and aldosterone antagonists while mitigating the hyperkalemia risk.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Effect of Patiromer on Urinary Ion Excretion in Healthy Adults,” on pages 1769–1776.
References
- 1.Gruy-Kapral C, Emmett M, Santa Ana CA, Porter JL, Fordtran JS, Fine KD: Effect of single dose resin-cathartic therapy on serum potassium concentration in patients with end-stage renal disease. J Am Soc Nephrol 9: 1924–1930, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM: Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review. Am J Med 126: 264.e9–264.e24, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Bushinsky DA, Spiegel DM, Gross C, Benton WW, Fogli J, Hill Gallant KM, Du Mond C, Block GA, Weir MR, Pitt B: Effect of Patiromer on Urinary Ion Excretion in Healthy Adults. Clin J Am Soc Nephrol 11: 1769–1776, 2016 [DOI] [PMC free article] [PubMed]
- 4.Patel L, Bernard LM, Elder GJ: Sevelamer Versus Calcium-Based Binders for Treatment of Hyperphosphatemia in CKD: A Meta-Analysis of Randomized Controlled Trials. Clin J Am Soc Nephrol 11: 232–244, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, Lok CE, Fitchett D, Tsuyuki RT: Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet 382: 1268–1277, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Schiller LR, Santa Ana CA, Sheikh MS, Emmett M, Fordtran JS: Effect of the time of administration of calcium acetate on phosphorus binding. N Engl J Med 320: 1110–1113, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, Stasiv Y, Zawadzki R, Berman L, Bushinsky DA; AMETHYST-DN Investigators: Effect of Patiromer on Serum Potassium Level in Patients With Hyperkalemia and Diabetic Kidney Disease: The AMETHYST-DN Randomized Clinical Trial. JAMA 314: 151–161, 2015 [DOI] [PubMed] [Google Scholar]