Abstract
Background and objectives
Idiopathic collapsing FSGS has historically been associated with poor renal outcomes. Minimal clinical data exist on the efficacy of immunosuppressive therapy. Our study sought to provide a comprehensive description of renal survival in patients with collapsing and not otherwise specified FSGS after controlling for factors affecting renal prognosis.
Design, setting, participants, & measurements
We performed a retrospective analysis of an inception cohort study of patients diagnosed between 1989 and 2012. All potential patients with collapsing FSGS fulfilling the inclusion criteria were identified and compared with patients with not otherwise specified FSGS (approximately 1:2 ratio) on the basis of biopsy report and record availability. Time to ESRD was analyzed using Cox proportional hazards models.
Results
In total, 187 patients were studied (61 collapsing and 126 not otherwise specified), with a mean follow-up of 96 months. At baseline, patients with collapsing FSGS had higher median proteinuria (12.2 [5.6–14.8] versus 4.4 [2.3–8.1] g/d, respectively; P<0.001), lower median albuminemia (2.4 [1.9–3.0] versus 2.9 [1.8–3.7] g/dl, respectively; P=0.12), and lower median eGFR (48 [26–73] versus 60 [42–92] ml/min per 1.73 m2, respectively; P=0.01) than patients with not otherwise specified FSGS. The proportion of patients with remission of proteinuria was similar in patients with collapsing FSGS and patients with not otherwise specified FSGS (65.7% [23 of 35] versus 63.2% [72 of 114], respectively; P=0.84). The overall renal outcome (ESRD defined as eGFR<15 ml/min per 1.73 m2, dialysis, or transplantation) of patients with collapsing FSGS was not poorer than that of patients with not otherwise specified FSGS in multivariate analyses after adjusting for baseline characteristics and immunotherapy (hazard ratio, 1.78; 95% confidence interval, 0.92 to 3.45).
Conclusions
Compared with not otherwise specified FSGS, idiopathic collapsing FSGS presented with more severe nephrotic syndrome and lower eGFR but had a similar renal survival after controlling for exposure to immunosuppressive treatment. These results highlight the importance of early diagnosis and institution of immunosuppressive therapy in patients with collapsing FSGS.
Keywords: focal segmental glomerulosclerosis; nephrotic syndrome; glomerulonephritis; Follow-Up Studies; glomerular filtration rate; Humans; immunosuppression; Kidney Failure, Chronic; Prognosis; Proportional Hazards Models; proteinuria; renal dialysis
Introduction
Primary FSGS constitutes the most prevalent cause of nephrotic syndrome (1,2). FSGS comprises histologic variants, which diverge in their clinical presentation and response to treatment (3). Idiopathic collapsing FSGS has historically been associated with a rapid decline in renal function (3–5) and a higher frequency of ESRD compared with other FSGS variants. However, most of the studies published to date did not take into account treatment or severity of renal dysfunction at baseline (6–10). The association between immunosuppressive therapy and renal outcome (ESRD) in patients with collapsing FSGS, therefore, remains poorly established.
This study describes and compares the renal survival in patients diagnosed in the contemporary era with collapsing and not otherwise specified (NOS) FSGS after controlling for recognized factors affecting renal prognosis, including immunosuppressive therapy.
Materials and Methods
Study Design and Population
Patients with biopsy-proven FSGS enrolled in the Glomerular Disease Collaborative Network (GDCN) were considered for this inception cohort study. The GDCN FSGS Registry enrolls patients from >250 nephrology clinics primarily in the southeastern United States. Patients with human deficiency virus, hepatitis B and/or C virus infection, intravenous drug use, sickle cell disease, single kidney, reflux nephropathy, or other glomerular disease as well as transplant recipients were excluded. Patients with any level of proteinuria were included in the study to describe the full spectrum of disease and outcomes of patients with collapsing or NOS FSGS. All potential patients with collapsing FSGS diagnosed over the last 20 years fulfilling the inclusion criteria were identified. Approximately two patients with NOS FSGS per each patient with collapsing FSGS were identified on the basis of biopsy report and record availability without any matching criteria. All patients provided a written informed consent. This study was approved by the University of North Carolina’s Institutional Review Board in agreement with the Declaration of Helsinki.
Clinical Data and Definitions
Clinical and laboratory variables were extracted from medical records from the time of renal biopsy to the last available follow-up visit and/or initiation of RRT. Data were collected on patient demographics, disease presentation, comorbidities, laboratory tests, physical examination, and immunosuppressive and antihypertensive therapy. Quantitation of proteinuria was on the basis of either spot urinary protein-to-creatinine ratio (Up/c) or 24-hour urine collection as reported in the medical record. Recorded outcomes were death, ESRD (eGFR<15 ml/min per 1.73 m2, dialysis, or transplantation), and remission of nephrotic syndrome at any time point over the course of the disease. Complete remission was defined as a reduction in proteinuria to ≤0.3 g/24 h (or 0.3 g/g on Up/c) with ≤25% increase in serum creatinine. Partial remission was defined as a reduction in proteinuria by ≥50% to a level of <3.5 g/24 h (or 2.0 g/g on Up/c) with stable renal function. eGFR was calculated using the Modification of Diet in Renal Disease study group formula for adults and the Schwartz formula for children (11,12).
Patients who were given glucocorticoids alone at a dose ≥30 mg/d (or 0.5 mg/kg for children) were considered treated with glucocorticoids. Length of glucocorticoid therapy was defined as the interval during which the drug was prescribed at high dose (1 mg/kg or ≥30 mg/d). Any exposure to calcineurin inhibitors (CNIs) was considered as treatment, and length of therapy was defined as the interval during which the drug was prescribed, regardless of dosage. Any exposure to angiotensin–converting enzyme inhibitor, angiotensin receptor blocker, or selective aldosterone blocker was defined as renin-angiotensin-aldosterone system (RAAS) inhibition.
Determination of FSGS Variant
All available histologic slides from patients with a previous diagnosis of idiopathic collapsing FSGS were reviewed independently by two nephropathologists (A.M.G. and J.C.J.) who were blinded to the clinical course, treatment, and outcome of patients. FSGS variants were ascertained according to the Columbia FSGS Classification System (13,14). Two FSGS variants were retained for the purpose of this study: NOS and collapsing. Tip lesion and cellular variants were excluded.
The patients with collapsing FSGS were scored for the extent of segmental and global collapsing lesions and segmental and global NOS lesions on the following scale: 0+ (none), 1+ (1%–25%), 2+ (26%–50%), 3+ (51%–75%), or 4+ (>75%) glomeruli with the lesion. Interstitial fibrosis and tubular atrophy (IFTA) was scored for all biopsies on the basis of the estimated percentage of cortex involved with the following scale: 0+ (none), 1+ (1%–25%), 2+ (26%–50%), 3+ (51%–75%), or 4+ (>75%). Vascular disease was similarly scored for patients with NOS FSGS.
Statistical Analyses
Continuous variables with normal distribution were expressed as means±SD and compared using t tests. Not normally distributed variables were expressed as medians (interquartile ranges [IQRs]) and compared with Mann–Whitney U tests. Chi-squared or Fisher exact tests were used to compare categorical variables regarding treatment and ESRD.
Cox proportional hazards models were constructed to assess the association of each potential predictor with renal survival. We tested the Schoenfeld residuals to assess the proportional hazards assumption; there was no evidence of violation of the assumption for all variables in our model. However, log-log plots for immunosuppressive therapy exposure and black race revealed nonparallel curves. Cox survival analyses were then performed using time-dependent variables for exposure to immunosuppression (glucocorticoids and/or CNIs) and stratification on black race. We reported hazard ratios (HRs) with 95% confidence intervals (95% CIs). Potential covariates included in the models were either identified in univariate models or deemed to influence the outcome in prior studies. A change in estimate approach from uni- to multivariate models was also used for variable selection.
Missing values for variables in our Cox model were imputed using an iterative Markov chain Monte Carlo multiple imputation technique (20 imputations); >40% of data for 24-hour proteinuria quantification were missing, because a large proportion of patients had proteinuria assessment with Up/c only. Thus, a model using baseline albuminemia was used in the final survival model to assure construction of reliable models. A sensitivity analysis was also performed to examine the effect of using different conversion factors between Up/c and 24-hour proteinuria on Cox regression model HR estimates (collapsing variant and immunosuppressive therapy); 1.0× and 1.5× conversion factors were studied.
Statistical analyses were performed using SPSS 16.0 (IBM SPSS, Chicago, IL) software and Stata 13 (StataCorp., College Station, TX).
Results
Patient Characteristics
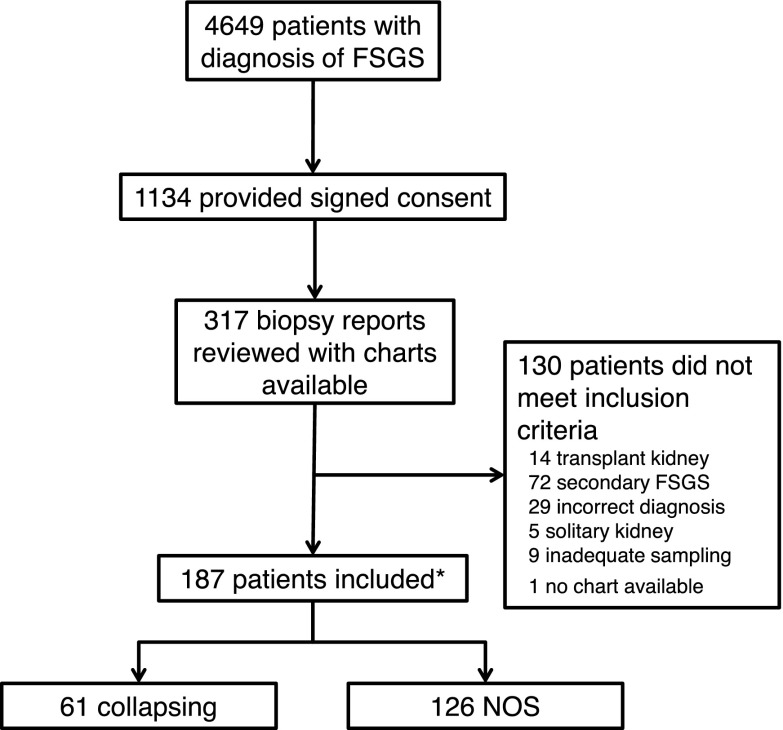
In total, 187 patients (96 women; 106 blacks) were identified (Figure 1), with a mean age at biopsy of 35 years old (IQR, 20–54 years old) (Table 1). None of the children (n=39) in this cohort had a family history of FSGS. At baseline, the median serum creatinine was 1.4 mg/dl (IQR, 0.9–2.1 mg/dl), corresponding to a median eGFR of 55 ml/min per 1.73 m2 (IQR, 36–88 ml/min per 1.73 m2). The median 24-hour proteinuria was 5.6 g/d (IQR, 2.9–12.6 g/d), with 109 (58.3%) patients having proteinuria ≥3.5 g/d. The median serum albumin was 2.6 g/dl (IQR, 1.8–3.7 g/dl).
Figure 1.
Study flow diagram. *All patients with collapsing FSGS diagnosed over the last 20 years were identified. Approximately 2 FSGS not otherwise specified (NOS) cases per each collapsing case were conveniently selected based on record availability without any matching criteria.
Table 1.
Baseline characteristics, follow-up, treatment, and outcomes of 126 patients with not otherwise specified FSGS and 61 patients with collapsing FSGS
| Characteristics | n | NOS | n | Collapsing | P Value | n | All |
|---|---|---|---|---|---|---|---|
| Onset | |||||||
| Age, yr | 126 | 37 (20–57) | 61 | 33 (22–43) | 0.23 | 187 | 35 (20–54) |
| Women | 126 | 56 (44.4) | 61 | 40 (65.6) | <0.01 | 187 | 96 (51.3) |
| Black race | 120 | 58 (48.3) | 58 | 48 (82.8) | <0.001 | 178 | 106 (59.6) |
| Hypertension | 104 | 55 (52.9) | 46 | 24 (52.2) | >0.99 | 150 | 79 (52.7) |
| Serum creatinine, mg/dl | 123 | 1.2 (0.9–1.8) | 59 | 1.5 (1.0–2.8) | 0.002 | 182 | 1.4 (0.9–2.1) |
| eGFR by MDRD equation, ml/min per 1.73 m2 | 122 | 60 (42–92) | 56 | 48 (26–73) | 0.01 | 178 | 55 (36–88) |
| Up/c, g/g | 67 | 6.6 (2.7–10.1) | 26 | 8.9 (6.4–15.5) | 0.02 | 93 | 7.6 (3.5–11.2) |
| 24-h Proteinuria, g/d | 68 | 4.4 (2.3–8.1) | 41 | 12.2 (5.6–14.8) | <0.001 | 109 | 5.6 (2.9–12.6) |
| Serum albumin, g/dl | 104 | 2.9 (1.8–3.7) | 54 | 2.4 (1.9–3.0) | 0.12 | 158 | 2.6 (1.8–3.7) |
| Follow-up | |||||||
| Duration of follow-up, mo | 126 | 73 (24–148) | 61 | 61 (17–117) | 0.19 | 187 | 69 (22–136) |
| Treatment | |||||||
| RAAS inhibition | 125 | 116 (92.1) | 56 | 42 (75.0) | 0.002 | 181 | 158 (87.3) |
| Exposure to glucocorticoids alone | 125 | 35 (28.0) | 56 | 17 (30.4) | 0.86 | 181 | 52 (28.7) |
| Time to glucocorticoids therapy, mo | 34 | 0.4 (0–0.9) | 17 | 0.1 (0.0–0.5) | 0.28 | 51 | 0.3 (0.0–0.9) |
| Length glucocorticoid therapy, mo | 33 | 2.5 (1.9–6.2) | 17 | 3.9 (1.4–6.7) | 0.73 | 50 | 2.7 (1.6–6.7) |
| Exposure to CNIs with or without glucocorticoids | 125 | 46 (36.8) | 56 | 18 (32.1) | 0.73 | 181 | 64 (35.4) |
| Time to CNI therapy,a mo | 46 | 0.5 (0.03–2.4) | 18 | 0.9 (0.4–1.9) | 0.40 | 64 | 0.6 (0.1–2.0) |
| Length of CNI therapy,a mo | 45 | 11.5 (2.9–31.7) | 18 | 18.2 (6.1–26.8) | 0.25 | 63 | 13.5 (4.1–31.7) |
| Outcomes | |||||||
| Complete or partial remission | 114 | 72 (63.2) | 35 | 23 (65.7) | 0.84 | 149 | 95 (63.8) |
| ESRDb | 126 | 33 (26.2) | 61 | 29 (47.5) | <0.01 | 187 | 62 (33.2) |
Continuous variables are expressed as medians (interquartile ranges). Categorical variables are expressed as n (%). NOS, not otherwise specified; MDRD, Modification of Diet in Renal Disease; Up/c, urinary protein-to-creatinine ratio; RAAS, renin-angiotensin-aldosterone system; CNI, calcineurin inhibitor.
With or without glucocorticoids.
At last follow-up.
Sixty-one (32.6%) patients had collapsing FSGS, and 126 (67.4%) had NOS FSGS. A majority of patients in both groups underwent their kidney biopsy between 2000 and 2009 (58.7% for NOS and 54.1% for collapsing). Compared with NOS FSGS, the collapsing group comprised more blacks (82.8% versus 48.3%; P<0.001) and more women (65.6% versus 44.4%; P<0.01). Patients with collapsing FSGS presented with more severe renal dysfunction (median eGFR =48 [IQR, 26–73] ml/min per 1.73 m2 versus 60 [IQR, 42–92] ml/min per 1.73 m2; P=0.01), higher 24-hour proteinuria (12.2 [IQR, 5.6–14.8] g/d versus 4.4 [IQR, 2.3–8.1] g/d; P<0.001), and lower serum albumin level (2.4 [IQR, 1.9–3.0] g/dl versus 2.9 [IQR, 1.8–3.7] g/dl; P=0.12).
At baseline, children had a median age of 12 years old (IQR, 7–15 years old) and presented with median eGFR of 82 ml/min per 1.73 m2 (IQR, 49–98 ml/min per 1.73 m2), median proteinuria of 5.3 g/d (IQR, 2.1–14.8 g/d), and median serum albumin of 2 g/dl (IQR, 1.5–3.1 g/dl). Children were distributed in similar proportions as adults with respect to the FSGS variants (29 with NOS FSGS and 10 with collapsing FSGS).
Exposure to Immunosuppressive Therapy
In the entire FSGS cohort, 116 (62.0%) patients were exposed to immunosuppressive therapy; 52 were treated with glucocorticoids alone, 64 had exposure to CNIs, and seven received other immunosupressives (four received mycophenolate mofetil, two received cyclophosphamide, and one received adalimumab) with or without glucocorticoids. A higher proportion of children (76.9%) than adults (65.5%) was exposed to immunosuppression. Compared with patients on supportive therapy, those receiving immunotherapy presented with significantly greater proteinuria (median Up/c =8.6 [IQR, 4.7–13.0] g/g versus 2.7 [IQR, 1.9–5.5] g/g; P<0.001), lower serum albumin levels (2.2 [IQR, 1.6–3.3] g/dl versus 3.4 [IQR, 2.6–4.0] g/dl; P<0.001), and higher baseline median eGFR (65 [IQR, 43–96] ml/min per 1.73 m2 versus 42 [IQR, 28–65] ml/min per 1.73 m2; P<0.001).
A similar proportion of patients in the collapsing group was treated with glucocorticoids alone (30.4% versus 28.0% for NOS; P=0.86) or had exposure to CNIs (32.1% versus 36.8% for NOS; P=0.73) as in the NOS group. Median time from biopsy to CNI therapy was 0.9 months (IQR, 0.4–1.9 months) in the collapsing group and 0.5 months (IQR, 0.03–2.4 months) in the NOS group (P=0.40). Fewer patients with collapsing FSGS were prescribed RAAS inhibitors (75.0% versus 92.1%; P=0.002).
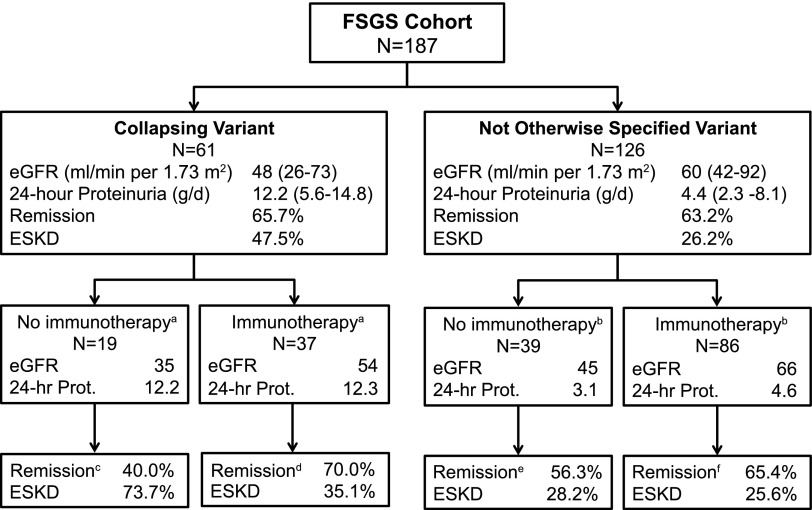
Treatment with any immunosuppressive was associated with different factors at presentation in each variant of FSGS (Figure 2, Table 2). In the NOS group, patients on immunotherapy had significantly more severe proteinuria (4.6 [IQR, 2.7–12.0] g/dl versus 3.1 [IQR, 1.6–6.0] g/dl; P=0.03) and hypoalbuminemia (2.2 [IQR, 1.6–3.5] g/dl versus 3.6 [IQR, 3.3–4.0] g/dl; P<0.001) but higher eGFR (66 [IQR, 46–98] ml/min per 1.73 m2 versus 45 [IQR, 131–70] ml/min per 1.73 m2; P<0.001) at baseline than patients not treated with immunotherapy. In contrast, in the collapsing group, patients on immunotherapy had higher baseline eGFR (54 [IQR, 30–85] ml/min per 1.73 m2 versus 35 [IQR, 27–48] ml/min per 1.73 m2; P=0.04) but similar 24-hour protein excretions and hypoalbuminemia compared with those not treated with immunotherapy.
Figure 2.
Outcomes according to FSGS variant and immunotherapy with glucocorticoids and/or calcineurin inhibitors. Continuous variables are expressed as medians (interquartile ranges). Prot, proteinuria. aData available on a total of 56 patients. bData available on a total of 125 patients. cData were available for five patients. dData were available for 30 patients. eData were available for 32 patients. fData were available for 81 patients.
Table 2.
Baseline characteristics, follow-up, and outcomes of 125 patients with NOS FSGS and 56 patients with collapsing FSGS according to exposure to immunotherapy with glucocorticoids and/or calcineurin inhibitors
| Characteristics | Collapsing, n=56 | NOS, n=125 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Immunotherapy | No Immunotherapy | P Value | Immunotherapy | No Immunotherapy | P Value | |||||
| n | n | n | n | |||||||
| Onset | ||||||||||
| Age, yr | 37 | 29 (19–46) | 19 | 34 (26–42) | 0.63 | 86 | 33 (16–52) | 39 | 48 (23–67) | <0.01 |
| Women, % | 37 | 73.0 | 19 | 52.6 | 0.15 | 86 | 44.2 | 39 | 43.6 | >0.99 |
| Black race, % | 37 | 78.4 | 19 | 78.9 | 0.25 | 84 | 51.2 | 35 | 40.0 | 0.32 |
| Hypertension, % | 37 | 43.2 | 19 | 36.8 | 0.75 | 68 | 52.9 | 35 | 54.3 | 1.00 |
| Serum creatinine, mg/dl | 37 | 1.4 (1.0–2.3) | 19 | 2 (1.5–3) | 0.03 | 84 | 1.1 (0.8–1.6) | 38 | 1.6 (1.1–2.2) | 0.003 |
| eGFR by MDRD equation, ml/min per 1.73 m2 | 36 | 54 (30–85) | 17 | 35 (27–48) | 0.04 | 83 | 66 (46–98) | 38 | 45 (31–70) | <0.001 |
| Up/c, g/g | 20 | 10.5 (8.4–17.8) | 4 | 3.1 (2.0–5.9) | <0.01 | 51 | 7.6 (4.0–11.6) | 16 | 2.7 (1.9–5.5) | 0.002 |
| 24-h Proteinuria, g/d | 24 | 12.3 (6.5–14.7) | 15 | 12.2 (4.4–15.0) | 0.92 | 41 | 4.6 (2.7–12.0) | 26 | 3.1 (1.6–6.0) | 0.03 |
| Serum albumin, g/dl | 33 | 2.3 (1.9–2.9) | 19 | 2.4 (2.0–3.5) | 0.49 | 74 | 2.2 (1.6–3.5) | 29 | 3.6 (3.3–4.0) | <0.001 |
| Follow-up | ||||||||||
| Duration of follow-up, mo | 37 | 74 (40–137) | 19 | 36 (4–71) | 0.003 | 86 | 68 (24–136) | 39 | 76 (21–164) | 0.89 |
| RAAS inhibition, % | 37 | 81.1 | 19 | 63.2 | 0.20 | 86 | 95.3 | 39 | 87.2 | 0.14 |
| Outcomes | ||||||||||
| Remission, % | 30 | 70.0 | 5 | 40.0 | 0.31 | 81 | 65.4 | 32 | 56.3 | 0.39 |
| ESRD,a % | 37 | 35.1 | 19 | 73.7 | 0.01 | 86 | 25.6 | 39 | 28.2 | 0.83 |
Continuous variables are expressed as medians (interquartile ranges). NOS, not otherwise specified; MDRD, Modification of Diet in Renal Disease, Up/c, urinary protein-to-creatinine ratio; RAAS, renin-angiotensin-aldosterone system.
At last follow-up.
Pathology
In patients with collapsing FSGS, either segmental or global collapsing lesions affected an average of 24% of glomeruli (range of 2.2%–62.5%). The collapsing FSGS specimens had no tip lesions, cellular lesions, or prominent perihilar hyalinosis. None of the specimens had advanced (>75%) IFTA. No significant differences were detected with respect to percentage of glomeruli with collapsing lesions, noncollapsing segmental lesions, or the severity of IFTA between treated and untreated patients or between patients who responded to immunosuppressive therapy and those who did not (Supplemental Table 1).
In patients with NOS FSGS, exposure to immunosuppression was significantly associated with less global sclerosis, a lower IFTA score, and less severe vascular disease (Supplemental Table 2). Severity of IFTA was not associated with response to immunosuppressive therapy (1.0±0.8 versus 1.4±1.1 for the nonremitters; P=0.06).
Remission and ESRD Outcomes
Ninety-five (51%) patients attained a complete or partial remission over the course of their disease in the overall cohort. Twenty-one (61.8%) children reached remission compared with 64.4% of adults. Patients who reached remission had a higher baseline eGFR (66 [IQR, 47–96] ml/min per 1.73 m2 versus 50 [IQR, 35–79] ml/min per 1.73 m2; P=0.01) but a similar degree of proteinuria at baseline compared with patients who did not attain a remission (5.0 [IQR, 2.7–12.2] g/d versus 5.8 [IQR, 2.9–12.0] g/d for remitter; P=0.94). RAAS blockade was not associated with remission (65.4% of remission in exposed versus 46.7% of remission in unexposed; P=0.17). Patients reaching remission had a lower prevalence of ESRD (15.8% versus 42.6%; P=0.001).
Sixty-two (33.2%) patients reached ESRD in the overall cohort. Similarly, 12 of 39 children (30.8%) experienced ESRD. Patients reaching ESRD had a significantly lower eGFR at presentation (median eGFR =38 [IQR, 19–55] ml/min per 1.73 m2 versus 66 [IQR, 46–96] ml/min per 1.73 m2; P<0.001), significantly more severe proteinuria (9.0 [IQR, 3.8–15.0] g/d versus 4.7 [IQR, 2.5–11.6] g/d; P=0.01), and a shorter follow-up time (57.5 [IQR, 20.4–103.8] months versus 73.5 [IQR, 27.4–164.1] months; P=0.03) compared with those who did not reach ESRD. Compared with NOS FSGS, patients with collapsing variant had a higher prevalence of ESRD (47.5% versus 26.2%; P<0.01).
Factors Associated with ESRD
In univariate survival analysis, the collapsing variant was associated with a higher likelihood of ESRD (HR, 2.26; 95% CI, 1.37 to 3.74). Collapsing FSGS was no longer significantly associated with ESRD after controlling for age, sex, race, baseline albuminemia, eGFR, and exposure to any immunosuppressive therapy (HR, 1.78; 95% CI, 0.92 to 3.45) (Table 3). Higher baseline serum albumin (HR, 0.71 by 1 g/dl higher; 95% CI, 0.52 to 0.97) and eGFR (HR, 0.98 by 1 ml/min per 1.73 m2 higher; 95% CI, 0.97 to 0.99) were associated with a better renal survival in this multivariable model. There was no interaction between histologic variant and immunotherapy in our model. Although not statistically significant, exposure to any immunosuppressive therapy was associated with a decreased likelihood of ESRD compared with supportive care without immunosuppression (HR, 0.51; 95% CI, 0.25 to 1.04).
Table 3.
Multivariable correlates of ESRD
| Variables | Univariate Hazard Ratio (95% CI) | Multivariatea Hazard Ratio (95% CI) |
|---|---|---|
| Collapsing variant | 2.26 (1.37 to 3.74) | 1.78 (0.92 to 3.45) |
| Women | 1.56 (0.94 to 2.57) | 1.46 (0.82 to 2.60) |
| Baseline serum albumin by 1 g/dl higher | 0.73 (0.56 to 0.94) | 0.71 (0.52 to 0.97) |
| Baseline eGFR by 1 ml/min per 1.73 m2 higher | 0.98 (0.97 to 0.99) | 0.98 (0.97 to 0.99) |
| Exposure to CNIs/steroids | 0.45 (0.23 to 0.90) | 0.51 (0.25 to 1.04) |
| Age per 1 yr older | 1.01 (1.00 to 1.02) | 1.00 (0.98 to 1.01) |
95% CI, 95% confidence interval; CNI, calcineurin inhibitor.
Cox proportional hazards model with stratification by black race.
Discussion
Since its first description (4,15), idiopathic collapsing FSGS has been associated with more severe nephrotic syndrome and worse renal outcome than other variants of FSGS. Reports are scarce on the response of idiopathic collapsing FSGS to immunosuppressive therapy, perhaps because of a perceived treatment futility (16). Cohort studies have reported a very low rate of CNI therapy (<14%) in patients with collapsing FSGS (8,9). Importantly, few studies evaluated the response to treatment after adjusting for baseline characteristics and selection of immunosuppressive treatment. In this large cohort study, the ESRD outcomes of patients with collapsing and NOS FSGS were compared, adjusting for treatment and baseline measures of severity of disease. Our data confirm the previously reported association of collapsing FSGS with black ancestry, more severe proteinuria, and lower eGFR at presentation as well as the overall worse prognosis compared with NOS FSGS. More importantly, our findings show that, after controlling for exposure to immunosuppressive therapy, histologic variant (collapsing versus NOS) was not associated with a significantly worse renal survival.
This study contributes new information on idiopathic collapsing and NOS FSGS, notably with respect to response to treatment. Whenever an analysis of response to therapy is undertaken, it is important to first evaluate factors associated with the decision to treat and the choice of therapy. Interestingly, we detected a difference in the factors associated with the decision to treat with immunosuppressive agents between the two variants of FSGS. Patients with NOS FSGS and mild nephrotic syndrome did not receive immunotherapy. For both FSGS variants, patients treated with glucocorticoids and/or CNI had better preserved eGFR at baseline. However, the decision to use immunotherapy seems to have been influenced by the severity of proteinuria, hypoalbuminemia and IFTA in the NOS group only. GFR seems to be the main factor associated with treatment in patients with collapsing FSGS, suggesting a perception of futility in treating patients with collapsing FSGS and severely decreased renal function. One can also see the reluctance of using CNIs in patients with low eGFR or AKI at presentation. Of note, there were no significant differences in histologic characteristics between patients with collapsing FSGS treated and untreated with CNI and/or glucocorticoids.
The differences in baseline severity of disease among patients on supportive therapy may partly explain the large overall unadjusted difference in ESRD among the two variants (73.7% for collapsing versus 28.2% for NOS FSGS). At baseline, patients with collapsing FSGS treated with immunotherapy had statistically significantly lower eGFR and more severe proteinuria than treated patients with NOS FSGS. Despite these baseline differences in disease severity, there were similar crude proportions of patients with collapsing and NOS FSGS treated with CNIs or glucocorticoids who attained a partial or complete remission (remission rate of 70.0% versus 65.4%, respectively; P=0.82) and renal failure (ESRD; 35.1% versus 25.6%, respectively; P=0.29) (Figure 2). Collapsing variant alone was not a significant correlate of poor renal outcome in our multivariate survival model (HR, 1.78; 95% CI, 0.92 to 3.45). In the recent prospective FSGS clinical trial (10), the histologic variant was also not an independent predictor of renal survival after adjusting for baseline eGFR, proteinuria, and age, although the total number of patients with collapsing FSGS was very small (n=16).
There were no significant differences in the length of CNI therapy and time of follow-up between the collapsing and NOS variants. Patients with complete or partial remission and those who did not reach ESRD received longer courses of CNIs than patients with no remission. These results are likely explained by treatment discontinuation when lack of response or inexorable progression to ESRD is perceived. However, the possibility that longer treatment may favor better response to treatment and outcome cannot be excluded from this retrospective analysis.
Fewer patients with collapsing FSGS received RAAS inhibitors compared with those with NOS FSGS. This difference may be attributable to a greater frequency of patients with very low eGFR among patients with collapsing FSGS and the heightened risk of hyperkalemia. Of note is that, given the high proportion of patients receiving RAAS inhibitors (>80%), there was no association found between exposure to RAAS inhibitors and ESRD.
The study cohort of patients with collapsing FSGS included a greater proportion of women than men. Although similar proportions of men and women were enrolled in the registry, a greater number of men with collapsing FSGS was excluded from analysis because of findings consistent with secondary disease. In the multivariate models, the effect of sex on renal survival was no longer apparent after correcting for exposure to immunotherapy.
Although the proportion of patients with spontaneous remission (Table 1) may seem somewhat higher than in previous observational cohort studies (6,7,17), this is most likely attributable to the very small number of patients with collapsing FSGS who did not receive immunotherapy (n=19) and the very small number of patients with enough available clinical data (n=5). In fact, the numbers of spontaneous remission are very similar to those reported by Chun et al. (7). Among patients with NOS FSGS and spontaneous remission, many had relatively mild disease.
The strengths of our study are the comparison between uniformly diagnosed patients with collapsing and NOS FSGS, the similar exposure to contemporary immunosuppressive therapy (including 87% treatment with RAAS inhibitors), and the generalizability of our findings, because they are on the basis of a diverse patient population followed in community and academic nephrology practices.
The limitations of this study are inherent to its retrospective nature. These include a lack of uniform treatment protocol, follow-up intervals, or laboratory tests. As a result, accurate times to therapeutic response, relapse, or other end points were difficult to ascertain. Given our dataset, it was not possible to explore other cutpoints to define a remission. Combinations of immunosuppressive agents prevented an ability to distinguish between benefits of CNIs alone or in combination with glucocorticoids. This study is unable to determine whether the greater severity of disease and decline in eGFR at presentation in the collapsing FSGS group are because of a more fulminant course of disease compared with NOS FSGS or a greater delay in referral or diagnosis because of socioeconomic factors or access to care hurdles, notably among black patients. A higher frequency of the two APOL1 risk alleles has been previously described among patients with collapsing FSGS (18). Our study did not have APOL1 genotyping on all patients. An analysis of the association between APOL1 risk alleles and either FSGS variant or disease severity and outcomes could, therefore, not be performed.
In summary, patients with idiopathic collapsing FSGS present with more severe proteinuria and renal dysfunction and have an overall worse renal outcome compared with patients with NOS FSGS. However, our data show that the difference in outcomes may be attributable to the baseline severity of disease and possible differences in the decision to treat with immunosuppressive therapy. Indeed, after adjusting for baseline characteristics and immunotherapy, we show a similar long–term renal survival between the two FSGS variants. These results highlight the importance of early diagnosis and institution of immunosuppressive therapy in patients with idiopathic collapsing FSGS. Although exposure to CNI therapy may be of benefit in preventing ESRD in patients with idiopathic collapsing FSGS, optimal therapy remains to be formally determined in a prospective trial.
Disclosures
None.
Supplementary Material
Acknowledgments
L.-P.L. received salary support from the Hôpital Maisonneuve-Rosemont Scholarship of Improvement Program, the Société Québécoise de Néphrologie, and the Department of Medicine, Université de Montréal. V.K.D was supported by NephCure Foundation Nephrotic Syndrome Study Network Career Development Award grant U-54-DK-083912.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13091215/-/DCSupplemental.
References
- 1.Haas M, Meehan SM, Karrison TG, Spargo BH: Changing etiologies of unexplained adult nephrotic syndrome: A comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis 30: 621–631, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Kitiyakara C, Eggers P, Kopp JB: Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis 44: 815–825, 2004 [PubMed] [Google Scholar]
- 3.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Detwiler RK, Falk RJ, Hogan SL, Jennette JC: Collapsing glomerulopathy: A clinically and pathologically distinct variant of focal segmental glomerulosclerosis. Kidney Int 45: 1416–1424, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Korbet SM: Treatment of primary FSGS in adults. J Am Soc Nephrol 23: 1769–1776, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Valeri A, Barisoni L, Appel GB, Seigle R, D’Agati V: Idiopathic collapsing focal segmental glomerulosclerosis: A clinicopathologic study. Kidney Int 50: 1734–1746, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Chun MJ, Korbet SM, Schwartz MM, Lewis EJ: Focal segmental glomerulosclerosis in nephrotic adults: Presentation, prognosis, and response to therapy of the histologic variants. J Am Soc Nephrol 15: 2169–2177, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Thomas DB, Franceschini N, Hogan SL, Ten Holder S, Jennette CE, Falk RJ, Jennette JC: Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int 69: 920–926, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Stokes MB, Valeri AM, Markowitz GS, D’Agati VD: Cellular focal segmental glomerulosclerosis: Clinical and pathologic features. Kidney Int 70: 1783–1792, 2006 [DOI] [PubMed] [Google Scholar]
- 10.D’Agati VD, Alster JM, Jennette JC, Thomas DB, Pullman J, Savino DA, Cohen AH, Gipson DS, Gassman JJ, Radeva MK, Moxey-Mims MM, Friedman AL, Kaskel FJ, Trachtman H, Alpers CE, Fogo AB, Greene TH, Nast CC: Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol 8: 399–406, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Agati V: Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol 23: 117–134, 2003 [DOI] [PubMed] [Google Scholar]
- 14.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43: 368–382, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Weiss MA, Daquioag E, Margolin EG, Pollak VE: Nephrotic syndrome, progressive irreversible renal failure, and glomerular “collapse”: A new clinicopathologic entity? Am J Kidney Dis 7: 20–28, 1986 [DOI] [PubMed] [Google Scholar]
- 16.Choi MJ: Histologic classification of FSGS: Does form delineate function? Clin J Am Soc Nephrol 8: 344–346, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Schwartz MM, Evans J, Bain R, Korbet SM: Focal segmental glomerulosclerosis: Prognostic implications of the cellular lesion. J Am Soc Nephrol 10: 1900–1907, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Kopp JB, Winkler CA, Zhao X, Radeva MK, Gassman JJ, D’Agati VD, Nast CC, Wei C, Reiser J, Guay-Woodford LM, Pollak MR, Hildebrandt F, Moxey-Mims M, Gipson DS, Trachtman H, Friedman AL, Kaskel FJ; FSGS-CT Study Consortium: Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS Clinical Trial. J Am Soc Nephrol 26: 1443–1448, 2015 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.