Abstract
Background and objectives
Because there is substantial biologic intraindividual variation in albumin excretion, randomized trials of albuminuria-reducing therapies may need multiple urine samples to estimate daily urinary albumin excretion. Mailing spot urine samples could offer a convenient and cost-effective method to collect multiple samples, but urine albumin-to-creatinine ratio stability in samples stored at ambient temperatures for several days is unknown.
Design, setting, participants, & measurements
Patients with kidney disease provided fresh urine samples in two tubes (with and without boric acid preservative). Reference aliquots from each participant were analyzed immediately, whereas remaining aliquots were subject to different handling/storage conditions before analysis, including delayed processing for up to 7 days at three different storage temperatures (4°C, 18°C, and 30°C), multiple freeze-thaw cycles, and long–term frozen storage at −80°C, −40°C, and −20°C. We calculated the mean percentage change in urine albumin-to-creatinine ratio for each condition, and we considered samples stable if the 95% confidence interval was within a ±5% threshold.
Results
Ninety-three patients provided samples with detectable albuminuria in the reference aliquot. Median (interquartile range) urine albumin-to-creatinine ratio was 87 (20–499) mg/g. The inclusion of preservative had minimal effect on fresh urine albumin-to-creatinine ratio measurements but reduced the changes in albumin and creatinine in samples subject to processing delay and storage conditions. The urine albumin-to-creatinine ratio was stable for 7 days in samples containing preservative at 4°C and 18°C and 2 days when stored at 30°C. It was also stable in samples with preservative after three freeze-thaw cycles and in frozen storage for 6 months at −80°C or −40°C but not at −20°C.
Conclusions
Mailed urine samples collected with preservative and received within 7 days if ambient temperature is ≤18°C, or within 2 days if the temperature is higher but does not exceed 30°C, are suitable for the measurement of urine albumin-to-creatinine ratio in randomized trials. Preserved samples frozen to −40°C or −80°C for 6 months before analysis also seem suitable.
Keywords: albuminuria, randomized controlled trials, microalbuminuria, proteinuria, Albumins, Boric Acids, Confidence Intervals, creatinine, Freezing, Humans, Kidney Diseases, Randomized Controlled Trials as Topic, Specimen Handling, Temperature, Urinalysis, boric acid
Introduction
Albuminuria is associated with cardiovascular risk and CKD progression (1,2), and some treatments that reduce albuminuria have been shown to slow renal progression (3–5). The effect on albuminuria is, therefore, a commonly used surrogate outcome in early phase trials of novel kidney disease treatments (6–8). Urine albumin-to-creatinine ratio (uACR) measured on a spot sample is more convenient to estimate daily albumin excretion than timed urine collections (the gold standard), and spot first morning void (FMV) samples provide more precise estimates with less within-person variability than random spot samples (9–11).
Despite using FMV samples, within-person variability in uACR remains relatively large (10). Therefore, randomized trials usually need to be large to detect moderate effects on uACR. To reduce sample sizes, collection of three sequential spot urine samples from each participant has been recommended, because the uncertainty in the estimate of an individual’s average 24-hour albuminuria excretion is reduced (12). However, the collection of multiple FMV spot samples in an outpatient clinic is inconvenient for both participants and study staff and increases study costs.
Previous studies have shown that urine can be collected from participants by sending collection tubes packaged with instructions. In a large mailed–based trial of 15,000 patients with diabetes, 70% of requested urine samples were received within 2 days, and 90% of requested urine samples were received within 4 days of collection (13). Although uACR has been reported to be stable for 7 days when cooled to between 4°C and 6°C (14,15), its stability at warmer ambient temperatures is unknown. This study assessed the stability of urine albumin and creatinine across a range of handling and storage conditions.
Materials and Methods
Ethical permission was obtained to collect urine samples from patients with kidney disease attending routine clinic appointments at a tertiary referral renal unit (Oxfordshire REC-A 11/SC/0498). After providing written informed consent, patients provided spot urine samples in a 50-ml collection container. Approximately 20 ml of urine was immediately transferred into a 30-ml universal container containing powdered boric acid preservative (Appleton Woods; selected for its stabilizing effect on red cells and bacteria [16] and because it was available in precoated tubes, which are safe for participants to handle). The remaining urine was transferred into a plain universal container (Fisher Scientific, Waltham, MA). A sample aliquot with and without preservative from each participant was analyzed within 2 hours of collection (the reference aliquots). Additional urine aliquots were subject to different handling and storage conditions before analysis, which included (1) delayed processing (1, 2, 4, and 7 days) with samples held at different storage temperatures (4°C, 18°C, and 30°C), (2) up to three freeze-thaw cycles (from −80°C) on 3 sequential days, and (3) 1- and 6-month frozen storage at different temperatures (−80°C, −40°C, and −20°C).
When participants supplied <40 ml of urine, the experiments testing the effects of delayed processing and freeze-thaw cycles in samples containing preservative were prioritized. Spot urine samples from 100 participants were estimated to provide 80% power at a 2p=0.05 to confirm equivalent stability to the reference aliquot using a test of equivalence (17) and estimates derived from a previously completed renal progression trial (18).
To reduce albumin aggregation, all samples were subject to 10–30 seconds of vortex mixing and inversion before centrifugation at 3000×g for 10 minutes and analysis. Urine creatinine was measured by a Jaffe rate method, and urine albumin was measured by a turbidimetric method using a Beckman-Coulter DxC800 Clinical Chemistry Analyzer (Beckman Coulter, Inc., Brea CA) and manufacturer's reagents. The urine albumin method combines albumin with specific antibody (polyclonal goat anti–human albumin) to form insoluble antigen-antibody complexes. The change in absorbance at 380 nm because of the formation of these complexes is proportional to the concentration of albumin in the sample. It is a sensitive method, with a lower reportable limit of 2 mg/L. Urine creatinine and albumin were measured sequentially for each participant. Between–batch coefficients of variation (CVs) were determined from two levels of third party quality control material that were measured repeatedly over the time period taken for sample analysis and measured as bracketed quality control (run before and after groups of 50–60 samples). For urine creatinine, CVs were 1.56% at 83.1 mg/dl and 1.23% at 198 mg/dl, and for urine albumin, CVs were 4.88% at 26.2 mg/L and 1.96% at 187 mg/L.
To investigate the effect of preservative on the concentration of urine albumin, creatinine, and uACR, the difference between paired samples with and without preservative was calculated for participants with both measurements. This was expressed as the percentage change relative to the sample without preservative (difference in concentration between the sample with preservative and sample with no preservative divided by the concentration in the sample with no preservative) and compared using the Wilcoxon signed rank test (19).
For the experiments testing the different handling and storage conditions, percentage change in uACR was defined as the difference in concentration between the relevant test and reference aliquot divided by the concentration in the reference aliquot. Analyte stability was defined a priori as a mean percentage change in uACR compared with the reference aliquot with a 95% confidence interval (95% CI) ±5% (δ). For comparison, the acceptable limit of change (20) for uACR (based on assay imprecision) was ±1% (calculated using the following formula: 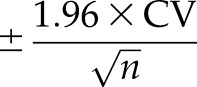 , where the CV for uACR was derived from the between-batch CV for albumin at 26.2 mg/L and creatinine at 198 mg/dl and n was the study sample size).
, where the CV for uACR was derived from the between-batch CV for albumin at 26.2 mg/L and creatinine at 198 mg/dl and n was the study sample size).
Because the distribution of uACR was skewed, log-transformed uACR was used in all models and then, back transformed to allow percentage change on the absolute scale to be reported. For each different handling and storage condition, mean percentage change in albumin, creatinine, or uACR compared with the corresponding reference aliquot (with 95% CI; uncorrected for assay imprecision) was estimated using generalized estimating equations (GEEs), assuming an autoregressive correlation structure (21). The GEE method takes into account any correlation in the data (for example, paired sample comparisons), handles missing values, and allows statistical comparisons between samples with and without preservative subject to different handling and storage conditions. For the overall results, a 95% CI crossing the stability threshold indicated evidence of instability.
The study was not powered to study different types of patients directly, and therefore, it is inappropriate to use the ±5% stability threshold to assess sample stability in subgroups divided by baseline albuminuria or transplant status. Instead, GEEs were used to test for equality of the mean percentage change between these subgroups. Analyses used SAS, version 9.3 (SAS Institute Inc., Cary, NC) and R, version 2.14.2.
Results
Urine samples were collected from 102 patients with kidney disease, of which 93 had detectable albuminuria in at least one of their reference aliquots. Approximately two thirds of these participants had a complete set of measurements available for all handling and storage conditions. The long–term frozen storage experiment had the most missing data, but it still had at least 80 samples with measurements available at each temperature for both types of sample. Mean (SD) age was 56 (13) years old, 60 (65%) were men, 79 (85%) were white, and 20 (22%) had diabetes mellitus (Table 1). In samples with preservative processed immediately, median (interquartile range) uACR was 87 (20–499) mg/g (Table 2), with about one third of participants reporting results in the normoalbuminuric (<30 mg/g), microalbuminuric (30–300 mg/g), and macroalbuminuric (>300 mg/g) ranges.
Table 1.
Baseline characteristics of participants
| Characteristic | All Participants,a n=93 |
|---|---|
| Age, yr | 56 (13) |
| Men | 60 (65%) |
| Race | |
| White | 79 (85%) |
| Black | 5 (5%) |
| Asian | 7 (8%) |
| Not stated | 1 (1%) |
| Diabetes | 20 (22%) |
| Renal status | |
| Predialysis CKD | 25 (27%) |
| Functioning kidney transplant | 67 (72%) |
| Albuminuria | |
| Normoalbuminuria, <30 mg/g | 31 (33%) |
| Microalbuminuria, ≥30 to ≤300 mg/g | 32 (34%) |
| Macroalbuminuria, mg/g | |
| >300 to ≤1000 | 19 (20%) |
| >1000 | 11 (12%) |
Data are mean (SD) or n (%).
There was one participant for whom values of age, sex, race, diabetes status, and renal status were missing.
Table 2.
Median values of urine creatinine concentration, albumin concentration, and albumin-to-creatinine ratio at baseline, overall and by level of albuminuria
| Analyte | Median (IQR) Values of Fresh Samples | Median Percentage Change from Samples with no Preservative (IQR)a | P Value for Difference between Samples Typesa | |
|---|---|---|---|---|
| Preservative | No Preservative | |||
| All participants | ||||
| No. of samplesb | 90 | 88 | ||
| Creatinine, mg/dl | 74.4 (45.6–111.5) | 76.9 (47.2–114.3) | −2.5% (−3.1% to −1.9%) | <0.001 |
| Albumin, mg/L | 65.4 (20.3–328.7) | 62.7 (20.7–289.3) | −2.1% (−4.5% to −0.4%) | <0.001 |
| Albumin-to-creatinine ratio, mg/g | 86.8 (19.5–499.1) | 90.0 (21.4–479.4) | 0.7% (−2.0% to 2.3%) | 0.43 |
| Normo- or microalbuminuria | ||||
| No. of samplesb | 61 | 60 | ||
| Creatinine, mg/dl | 80.7 (53.1–115.6) | 87.0 (58.3–121.3) | −2.6% (−3.0% to −2.0%) | <0.001 |
| Albumin, mg/L | 27.9 (14.9–69.8) | 32.5 (14.8–74.7) | −2.4% (−4.5% to −0.4%) | <0.001 |
| Albumin-to-creatinine ratio, mg/g | 33.3 (13.5–92.4) | 32.9 (13.9–98.0) | 0.5% (−3.0% to 2.3%) | 0.74 |
| Macroalbuminuria | ||||
| No. of samplesb | 29 | 28 | ||
| Creatinine, mg/dl | 53.6 (36.7–85.0) | 54.1 (34.8–85.7) | −2.3% (−3.3% to −1.5%) | <0.001 |
| Albumin, mg/L | 553.1 (328.7–973.5) | 491.0 (289.3–887.1) | −1.6% (−3.7% to −0.4%) | <0.001 |
| Albumin-to-creatinine ratio, mg/g | 857.1 (523.4–1473.4) | 803.2 (508.3–1391.2) | 1.3% (−1.6% to 3.2%) | 0.29 |
IQR, interquartile range.
Among participants with measurements from both preservative and no preservative samples, the percentage change was calculated as the difference in concentration between the sample with preservative and the sample with no preservative divided by the concentration in the sample with no preservative, and it was compared using the Wilcoxon signed rank test (19).
Of the 93 patients in this study with detectable creatinine and albumin, 85 had measurements in both sample types; five only had measurements in samples with preservative, and three only had measurements in samples with no preservative.
The Effect of Boric Acid Preservative on uACR
The addition of preservative reduced urine albumin and creatinine concentration in the fresh reference aliquots with a median percentage change of about 2% but had no effect on uACR (Table 2). In samples subject to delayed processing, mean percentage change in uACR was similar in samples with and without preservative (P values for equality of means for uACR were all >0.05) (Table 3). The benefit of adding preservative to urine samples, however, was to reduce large percentage changes in uACR, an outcome observed in a small proportion of samples without preservative (Supplemental Figure 1).
Table 3.
Mean percentage change in urine creatinine concentration, albumin concentration, and albumin-to-creatinine ratio after a delay in processing, by preservative use and by storage temperature
| Storage Condition | Mean Percentage Change (95% CI) | Test for Equality of Means by Temperature | |
|---|---|---|---|
| Preservative | No Preservative | ||
| Creatinine | |||
| 4°C, 1 d | −0.1% (−0.4% to 0.2%) | −0.2% (−0.4% to 0.1%) | 0.28 |
| 4°C, 2 d | 0.1% (−0.2% to 0.5%) | 0.2% (−0.0% to 0.4%) | |
| 4°C, 4 d | 0.0% (−0.3% to 0.3%) | 0.3% (0.0% to 0.5%) | |
| 4°C, 7 d | 0.6% (0.3% to 0.9%) | 1.1% (0.7% to 1.4%) | |
| 18°C, 1 d | −0.1% (−0.4% to 0.3%) | 0.1% (−0.2% to 0.4%) | <0.001 |
| 18°C, 2 d | 0.0% (−0.3% to 0.3%) | 0.9% (0.5% to 1.2%) | |
| 18°C, 4 d | −0.1% (−0.5% to 0.4%) | 1.5% (0.8% to 2.2%) | |
| 18°C, 7 d | 0.2% (−0.4% to 0.9%) | 4.7% (3.5% to 5.8%) | |
| 30°C, 1 d | −0.5% (−0.9% to −0.2%) | 0.0% (−0.3% to 0.3%) | <0.001 |
| 30°C, 2 d | −0.7% (−1.0% to −0.3%) | 1.1% (0.5% to 1.6%) | |
| 30°C, 4 d | −1.3% (−2.1% to −0.5%) | 1.4% (−0.3% to 3.2%) | |
| 30°C, 7 d | −2.4% (−3.4% to −1.5%) | 3.0% (−0.3% to 6.3%) | |
| Albumin | |||
| 4°C, 1 d | −0.3% (−1.9% to 1.2%) | 1.4% (−0.8% to 3.6%) | 0.39 |
| 4°C, 2 d | −1.5% (−3.3% to 0.2%) | −0.5% (−3.2% to 2.3%) | |
| 4°C, 4 d | −0.1% (−1.5% to 1.4%) | 0.9% (−1.2% to 3.0%) | |
| 4°C, 7 d | 0.8% (−1.2% to 2.8%) | 1.7% (−1.6% to 5.1%) | |
| 18°C, 1 d | 0.5% (−1.5% to 2.5%) | 1.3% (−1.4% to 4.0%) | 0.06 |
| 18°C, 2 d | −0.1% (−1.6% to 1.3%) | 2.2% (−0.4% to 4.9%) | |
| 18°C, 4 d | 1.0% (−1.0% to 3.0%) | 4.1% (1.2% to 6.9%) | |
| 18°C, 7 d | 2.5% (0.6% to 4.3%) | 7.8% (4.8% to 10.8%) | |
| 30°C, 1 d | 0.9% (−0.7% to 2.4%) | 1.6% (−1.1% to 4.4%) | 0.15 |
| 30°C, 2 d | 1.6% (−0.1% to 3.3%) | 2.3% (−0.3% to 5.0%) | |
| 30°C, 4 d | 3.0% (0.5% to 5.5%) | 5.6% (3.0% to 8.3%) | |
| 30°C, 7 d | 5.1% (2.4% to 7.7%) | 10.3% (6.7% to 13.9%) | |
| Albumin-to-creatinine ratio | |||
| 4°C, 1 d | −0.3% (−1.9% to 1.2%) | 1.5% (−0.7% to 3.7%) | 0.42 |
| 4°C, 2 d | −1.8% (−3.6 to −0.1%) | −0.7% (−3.4% to 2.0%) | |
| 4°C, 4 d | −0.3% (−1.8% to 1.2%) | 0.6% (−1.5% to 2.6%) | |
| 4°C, 7 d | −0.0% (−1.9% to 1.9%) | 0.5% (−2.7% to 3.8%) | |
| 18°C, 1 d | 0.5% (−1.5% to 2.5%) | 1.2% (−1.5% to 3.8%) | 0.48 |
| 18°C, 2 d | −0.3% (−1.7% to 1.2%) | 1.2% (−1.4% to 3.9%) | |
| 18°C, 4 d | 1.0% (−1.0% to 3.0%) | 2.3% (−0.4% to 5.1%) | |
| 18°C, 7 d | 2.1% (0.3% to 3.8%) | 2.7% (−0.0% to 5.4%) | |
| 30°C, 1 d | 1.3% (−0.3% to 2.9%) | 1.5% (−1.3% to 4.2%) | 0.79 |
| 30°C, 2 d | 2.2% (0.4% to 3.9%) | 1.0% (−1.6% to 3.6%) | |
| 30°C, 4 d | 4.2% (1.8% to 6.6%) | 3.9% (0.9% to 6.9%) | |
| 30°C, 7 d | 7.4% (4.9% to 9.9%) | 7.0% (3.0% to 11.1%) | |
95% CI, 95% confidence interval.
The Effect of Delayed Processing and Temperature on uACR
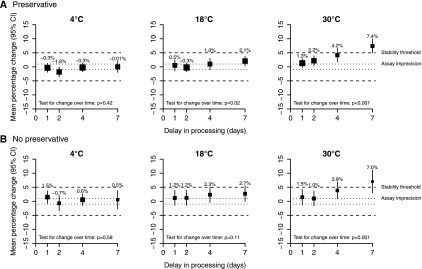
Using the predetermined definition of stability (a change in uACR with a 95% CI within ±5%), both longer processing delay and higher storage temperature led to uACR instability (Figure 1). uACRs in samples held at 30°C before processing were stable at 2 days but not stable at 4 days, regardless of the presence of preservative. The addition of preservative in the sample, however, supported uACR stability in samples stored at 18°C for 7 days.
Figure 1.
Mean percentage change in urine albumin-to-creatinine ratio (uACR) after a delay in processing by storage temperature. Panel (A) samples with preservative, and (B) samples with no preservative. The size of each square is proportional to the quantity of statistical information available. The dashed lines on each plot indicate percentage changes of ±5%, which are the thresholds for stability. If the 95% confidence interval (95% CI) is within this stability threshold, then the uACR samples are considered stable for this storage condition. The dotted lines on each plot indicate percentage changes of ±1%, which are the acceptable limits of change for uACR (based on assay imprecision).
Overall, the mean percentage change in urine albumin was generally larger than that for creatinine (Table 3), and there was some evidence that increases in albumin were slightly larger among those with normo- or microalbuminuria compared with those with macroalbuminuria (Supplemental Table 1) but that these effects were not further influenced by transplant status (data not shown).
The Effect of Freeze-Thaw Cycles
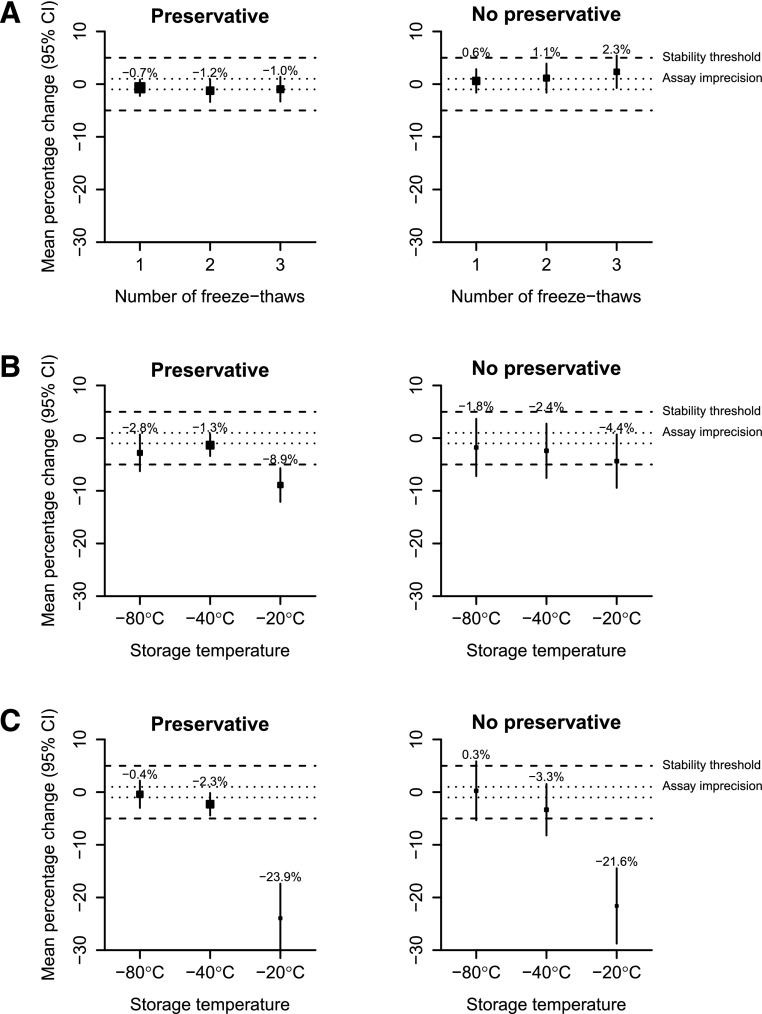
On the basis of the predetermined definition of stability, uACR was stable in spot urine samples with preservative even after three freeze-thaw cycles (Figure 2A, Supplemental Table 2). Among such samples, a small reduction in uACR was evident among those with macroalbuminuria, which was not apparent at lower levels of albuminuria (Supplemental Table 3).
Figure 2.
Mean percentage change in urine albumin-to-creatinine ratio concentration after freezing by number of freeze-thaw cycles, duration of frozen storage and storage temperature. Panel (A) number of freeze-thaw cycles, (B) 1 month frozen storage at different temperatures, and (C) 6 months frozen storage at different temperatures. Conventions are the same as for Figure 1. 95% CI, 95% confidence interval.
Long–Term Frozen Storage at Different Temperatures
On the basis of the predetermined definition of stability, uACR was stable in urine samples with preservative when stored for up to 6 months at −80°C or −40°C but not if stored at −20°C, when mean uACR concentration was reduced by about one quarter (Figure 2, B and C). Samples with no preservative showed a similar percentage change to samples with preservative (Supplemental Table 4) but on the basis of the predetermined definition of stability, were unstable at any frozen temperature (Figure 2, B and C). The observed decrease in the change in uACR mainly reflected changes in albumin concentration (Supplemental Table 4). Contrasting the observations from freeze-thaw experiments, there was some evidence that these changes were more pronounced in samples with lower levels of albuminuria compared to those with macroalbuminuric levels (Supplemental Table 5).
Discussion
By quantifying the size of the preanalytic variability in urine albumin and creatinine under different handling and storage conditions, we have shown the suitability of mailed urine for the measurement of uACR in streamlined clinical trials. Using a stringent stability threshold (a change in uACR with a 95% CI within ±5%), urine collected in tubes precoated with boric acid preservative was suitable for uACR analysis if exposed to temperatures up to 18°C and received within 7 days of collection. When preprocessing conditions exceed 18°C, however, uACR in samples with preservative was stable for 2 days but not for 4 days.
The results of this study replicate some of the results from previous studies in which uACR was shown to be stable for 7 days in samples refrigerated at 4°C (14), and unstable if frozen long term at −20°C, leading to significantly lower uACR measurements compared with fresh samples (14,22,23). Our data also showed that storage to −40°C or −80°C (for 6 months) and three freeze-thaw cycles have minimal effect on uACR results, suggesting that mailed urine could also be stored at −40°C and analyzed in batches.
We recognize that a ±5% stability threshold may be considered restrictive, but it is achievable when working with an assay imprecision of ±1% and provides a robust margin to ensure that imprecision introduced by preanalytic handling remains considerably smaller than potentially beneficial proportional treatment effects. We anticipate that future trials might investigate whether reducing albuminuria by 15%–30% on top of current antiproteinuric treatments is clinically important.
Average percentage changes were similar in urine samples with and without preservative. However, large individual percentage changes were seen in some samples, particularly those with no preservative (5% of such samples had changes in uACR>25% after storage for 7 days at 30°C). A possible explanation for the large increase in albumin in a small number of samples (Supplemental Figures 2–4) is sample contamination (i.e., hydrolysis by bacterial proteases increasing the apparent albumin concentration) (24). The level of sample contamination seen in this study may be enough to affect trial results; however, it seemed to be relatively easy to control with the use of a preservative. Individual studies would need to assess the contamination risk, particularly for urine samples being provided in a nonclinical environment.
The decrease in detectable urine albumin in frozen samples may be explained by freezing causing conformational changes in urine proteins, resulting in partial protein precipitation (25,26). This would potentially interfere with the turbidimetric albumin assay, which is reliant on the formation of specific antigen-antibody complexes producing insoluble particles that affect the transmission and scattering of light. Other studies have considered the influence of pH on urine albumin stability, with several reports indicating that higher pH also stabilizes albumin in frozen urine (26–28). Conversely, some reports found no strong evidence for these pH stabilizing effects (14,29,30). Sample handling procedures may, in part, explain the wide range of findings between these studies, because it has been shown that mixing urine samples thoroughly before assay measurement can substantially reduce apparent variation in urine albumin concentration (27,28,31).
Variability introduced by delayed processing is undesirable, because it introduces random error (noise) into statistical comparisons, reducing a trial’s statistical power to detect any treatment effect (signal). There are several sources of noise in trials of albuminuria-reducing therapies, which include uACR instability during preanalytic handling and storage (which this study has quantified), error in uACR measurement caused by analyzer imprecision (which this study has also quantified for the assays used), and biologic within–individual variability in uACR (which other studies have quantified [12,18]). For comparison, the CVs for each of these sources of variation are presented in Supplemental Table 6, which shows that the noise introduced by a 4-day delay in processing of a sample stored at 18°C is only slightly larger than the noise introduced by analyzer measurement error and an order of magnitude smaller than the biologic variation observed in a group of patients with kidney disease and albuminuria typical of those studied in trials. All of these sources of variation need to be considered together to estimate the effect of introducing mail-based design on trial sample sizes. After aggregation, it can be shown that the sample size for a hypothetical albuminuria trial would be effectively unchanged if mail-based design was used (Supplemental Table 6).
In this study, urine was collected in an outpatient clinic environment where contamination was uncommon. The potential benefits of preservative use for mailed urine samples collected at home (where contamination risk could be higher) may, therefore, have been underestimated. Another limitation is that, despite being a comparatively large stability study, two thirds of the population did not have macroalbuminuria (a population often selected for randomized trials of treatments for albuminuria), and a large proportion of recruits had a functioning renal transplant. There was some evidence that the overall results may be modified by levels of albuminuria (Supplemental Tables 1, 3, and 5) but results were not further modified by transplant status (data not shown). Increases in albumin that resulted from delayed processing and decreases in albumin that resulted from long–term frozen storage were perhaps slightly larger among those with lower levels of albuminuria (these frozen storage findings are consistent with other reports [14,32,33]), but freeze-thaw changes were potentially larger among those with macroalbuminuric levels. The sizes of these possible differences were, however, small.
In conclusion, urine collected in preservative-coated tubes, mailed, and received within 7 days if ambient temperature is ≤18°C or within 2 days if the temperature is higher but does not exceed 30°C is suitable for the precise measurement of uACR. Samples with preservative frozen to −40°C or −80°C for 6 months and/or thawed up to three times before analysis also seem suitable. The implication of these results is that studies can use urine mailed to a central laboratory to make studies lower cost and more convenient for participants without any necessity to increase study sample size.
Disclosures
The Clinical Trial Service Unit and Epidemiological Studies Unit, which is part of the Nuffield Department of Population Health, University of Oxford, has a staff policy of not accepting honoraria or consultancy fees.
Supplementary Material
Acknowledgments
This study was supported by the Medical Research Council United Kingdom (MRC; reference A310), which also provided support for the Clinical Trial Service Unit and Epidemiological Studies Unit as an MRC Hub for Trials Methodology Research.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “The Influence of Processing and Storage Conditions on Renal Protein Biomarkers,” on pages 1726–1728.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.13341215/-/DCSupplemental.
References
- 1.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG; Chronic Kidney Disease Prognosis Consortium : Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 380: 1662–1673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I; Collaborative Study Group : Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, Maschio G, Brenner BM, Kamper A, Zucchelli P, Becker G, Himmelmann A, Bannister K, Landais P, Shahinfar S, de Jong PE, de Zeeuw D, Lau J, Levey AS: Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med 135: 73–87, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD; Collaborative Study Group : The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Lambers Heerspink HJ, Gansevoort RT, Brenner BM, Cooper ME, Parving HH, Shahinfar S, de Zeeuw D: Comparison of different measures of urinary protein excretion for prediction of renal events. J Am Soc Nephrol 21: 1355–1360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heerspink HJ, Kröpelin TF, Hoekman J, de Zeeuw D; Reducing Albuminuria as Surrogate Endpoint (REASSURE) Consortium : Drug-induced reduction in albuminuria is associated with subsequent renoprotection: A meta-analysis. J Am Soc Nephrol 26: 2055–2064, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inker LA, Levey AS, Pandya K, Stoycheff N, Okparavero A, Greene T; Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) : Early change in proteinuria as a surrogate end point for kidney disease progression: An individual patient meta-analysis. Am J Kidney Dis 64: 74–85, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidney Disease Improving Global Outcomes (KDIGO): KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R: First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol 20: 436–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginsberg JM, Chang BS, Matarese RA, Garella S: Use of single voided urine samples to estimate quantitative proteinuria. N Engl J Med 309: 1543–1546, 1983 [DOI] [PubMed] [Google Scholar]
- 12.Kröpelin TF, de Zeeuw D, Andress DL, Bijlsma MJ, Persson F, Parving HH, Heerspink HJ: Number and frequency of albuminuria measurements in clinical trials in diabetic nephropathy. Clin J Am Soc Nephrol 10: 410–416, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aung T, Haynes R, Barton J, Cox J, Murawska A, Murphy K, Lay M, Armitage J, Bowman L; ASCEND Study Collaborative Group: Cost-effective recruitment methods for a large randomised trial in people with diabetes: A Study of Cardiovascular Events in Diabetes (ASCEND). Trials 17: 286, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osberg I, Chase HP, Garg SK, DeAndrea A, Harris S, Hamilton R, Marshall G: Effects of storage time and temperature on measurement of small concentrations of albumin in urine. Clin Chem 36: 1428–1430, 1990 [PubMed] [Google Scholar]
- 15.Hofmann W, Guder WG: A diagnostic programme for quantitative analysis of proteinuria. J Clin Chem Clin Biochem 27: 589–600, 1989 [DOI] [PubMed] [Google Scholar]
- 16.Delanghe J, Speeckaert M: Preanalytical requirements of urinalysis. Biochem Med (Zagreb) 24: 89–104, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker E, Nowacki AS: Understanding equivalence and noninferiority testing. J Gen Intern Med 26: 192–196, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R; SHARP Investigators : The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 377: 2181–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilcoxon F: Individual comparisons by ranking methods. Biometrics 1: 80–83, 1945 [Google Scholar]
- 20.Zhang DJ, Elswick RK, Miller WG, Bailey JL: Effect of serum-clot contact time on clinical chemistry laboratory results. Clin Chem 44: 1325–1333, 1998 [PubMed] [Google Scholar]
- 21.Liang KY, Zeger SL: Longitudinal data analysis using generalized linear models. Biometrika 73: 13–22, 1986 [Google Scholar]
- 22.Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, McQueen MJ, Itoh Y, Lieske JC, Seccombe DW, Jones G, Bunk DM, Curhan GC, Narva AS; National Kidney Disease Education Program-IFCC Working Group on Standardization of Albumin in Urine : Current issues in measurement and reporting of urinary albumin excretion. Clin Chem 55: 24–38, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Brinkman JW, de Zeeuw D, Lambers Heerspink HJ, Gansevoort RT, Kema IP, de Jong PE, Bakker SJ: Apparent loss of urinary albumin during long-term frozen storage: HPLC vs immunonephelometry. Clin Chem 53: 1520–1526, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kania K, Byrnes EA, Beilby JP, Webb SAR, Strong KJ: Urinary proteases degrade albumin: Implications for measurement of albuminuria in stored samples. Ann Clin Biochem 47: 151–157, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Ikeda K, Ichihara K, Hashiguchi T, Hidaka Y, Kang D, Maekawa M, Matsumoto H, Matsushita K, Okubo S, Tsuchiya T, Furuta K; Committee for Standardization, The Japanese Society of Laboratory Medicine (JSLM) : Evaluation of the short-term stability of specimens for clinical laboratory testing. Biopreserv Biobank 13: 135–143, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Lambers Heerspink HJ, Nauta FL, van der Zee CP, Brinkman JW, Gansevoort RT, de Zeeuw D, Bakker SJ: Alkalinization of urine samples preserves albumin concentrations during prolonged frozen storage in patients with diabetes mellitus. Diabet Med 26: 556–559, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Townsend JC, Sadler WA, Shanks GM: The effect of storage pH on the precipitation of proteins in deep frozen urine samples. Ann Clin Biochem 24: 111–112, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Brinkman JW, Heerspink HL, de Zeeuw D, Gansevoort RT, Bakker SJ: Urinary pH affects albumin concentrations after prolonged frozen storage. Nephrol Dial Transplant 22: 3670, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Vermes I, Spooren PF: Influence of biological variations and sample handling on measured microalbuminuria in diabetic patients. J Clin Lab Anal 6: 368–374, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Elving LD, Bakkeren JA, Jansen MJ, de Kat Angelino CM, de Nobel E, van Munster PJ: Screening for microalbuminuria in patients with diabetes mellitus: Frozen storage of urine samples decreases their albumin content. Clin Chem 35: 308–310, 1989 [PubMed] [Google Scholar]
- 31.Innanen VT, Groom BM, de Campos FM: Microalbumin and freezing. Clin Chem 43: 1093–1094, 1997 [PubMed] [Google Scholar]
- 32.Brinkman JW, de Zeeuw D, Duker JJ, Gansevoort RT, Kema IP, Hillege HL, de Jong PE, Bakker SJ: Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 51: 2181–2183, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Shield JP, Hunt LP, Morgan JE, Pennock CA: Are frozen urine samples acceptable for estimating albumin excretion in research? Diabet Med 12: 713–716, 1995 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.