Abstract
Background
Adherence to antiretroviral therapy is critical to successful treatment of HIV. Few interventions have been demonstrated to improve both adherence and virological outcomes. We sought to determine whether an intervention derived from problem solving theory, Managed Problem Solving (MAPS), would improve antiretroviral outcomes.
Methods
We conducted a randomized investigator blind trial of MAPS compared with usual care in HIV-1 infected individuals at three HIV clinics in Philadelphia, PA. Eligible patients had plasma HIV-1 viral loads >1000 copies/ml and were initiating or changing therapy. MAPS consists of four in-person and 12 telephone-based meetings with a trained interventionist, then monthly follow-up calls for a year. Primary outcome was medication adherence measured using electronic monitors, summarized as fraction of doses taken quarterly over one year. Secondary outcome was undetectable HIV viral load over one year. We assessed 218 for eligibility, with 190 eligible and 180 enrolled, 91 randomized to MAPS and 89 to usual care. 56 participants were lost to follow-up: 33 in MAPS and 23 in usual care.
Results
In primary intention-to-treat analyses, the odds of being in a higher adherence category was 1.78 (95% CI:1.07–2.96) times greater for MAPS than usual care. In secondary analyses, the odds of an undetectable viral load was 1.48 (95% CI: 0.94–2.31) times greater for MAPS than usual care. In as-treated analyses, the effect of MAPS was stronger for both outcomes. There was neither a difference by prior treatment status nor change in effect over time.
Conclusions
MAPS is an effective antiretroviral adherence intervention over the first year with a new regimen. It was equally effective at improving adherence in treatment experienced and naïve patients and did not lose effect over time. Implementation of MAPS should be strongly considered where resources are available.
Trial Registration
ClinicalTrials.gov, NCT00130273
Introduction
Adherence to pharmacotherapy is key to preventing progression of treatable chronic diseases. Unfortunately, clinically significant non-adherence is common in diabetes [1], hypertension [2], hypercholesterolemia [3], and HIV [4], for example. Adherence barriers include depression [5], substance abuse [6], regimen complexity [7], poor social support [8], and low health literacy [9,10], among others. Adherence interventions have had limited effects, which often wane over subsequent months [11–13].
Patients may have multiple adherence barriers and so personalized strategies addressing multiple barriers hold the greatest promise for success. We developed Managed Problem Solving (MAPS) to address barriers to chronic pharmacotherapy adherence. It derives from Problem Solving Therapy (PST), an effective intervention for depression [14]. PST trains individuals in generalizable problem-solving skills including identification of difficulties, generation of solutions, and evaluation of outcomes [15]. It has been adapted to address coping with cancer and schizophrenia, family caregiving, and family relationships [16–18]. MAPS differs from PST; the participant is not trained to become a better generalized problem solver per se. Rather, the interventionist and participant work together to solve specific adherence barriers using the Problem Solving framework. An overlap with PST is starting with small, achievable goals to ensure success and establish credibility. Notably, MAPS does address some problems that patients believe are important, but are only indirectly related to medication adherence. This may reduce stressors that interfere with adherence and encourage continued participation.
We assessed whether MAPS resulted in better adherence to therapy for HIV than usual care. We chose antiretroviral therapy because the relationship between adherence and outcome was well described [19–22], as were the ensuing consequences of disease progression [23], further HIV transmission [24], and emergence of resistance [25].
Methods
Ethics Statement
The study was approved by the Committee on Human Subjects Research of the University of Pennsylvania and the Philadelphia Veterans Affairs Medical Center. All participants provided informed consent and were compensated $30 per data collection visit, but not intervention visits, for a total of $270 if all visits were completed. A Data and Safety Monitoring Committee reviewed severe intervention-related adverse events (e.g., suicidality). There were no early stopping rules. The ClinicalTrials.gov number is NCT00130273.
Study setting and participants
We conducted a randomized investigator-blind trial comparing MAPS to usual care in HIV-1 infected individuals. Recruitment was facilitated by flyers in the clinics and weekly querying of pharmacists and prescribers regarding potential patients at the two HIV clinics in the University of Pennsylvania Health System and the HIV clinic at the Philadelphia Veterans Administration Medical Center. Usual care included meeting with a pharmacist for regimen education and, if desired, provision of pill organizers.
Inclusion criteria were age ≥18 years, HIV-1 plasma viral load>1000 copies/ml, and being treatment naïve and initiating a standard regimen, or treatment experienced and either: 1)restarting their most recent suppressive regimen after >3 months off or 2)initiating a new regimen with three new antiretrovirals with resistance profiles different from their initial regimen or fully susceptible on an HIV resistance assay. After study initiation, we expanded criteria to include regimens with at least two fully active and one partially active drug based on a resistance assay. Treatment responses for this subpopulation were expected to be sufficiently similar to those for fully active regimens to allow for valid conclusions regarding the effect of the intervention on virologic outcome [26]. Exclusion criteria were inability to consent and residence in a setting with automatic medication delivery.
Permuted block randomization with 1:1 allocation was performed in random block sizes of 6–9 individuals. The biostatistician generated a randomization list by computer and placed assignments into sealed security envelopes numbered sequentially and maintained in a locked drawer by the study coordinator. At enrollment, the study coordinator selected the next envelope, which was opened only after consent. The investigators were blinded by prohibiting their access to the data and contact with participants.
Interventionists and Intervention
Interventionists were required to have a college degree and some prior experience working with a patient population. We trained three interventionists during the trial with 15 hours of training covering essentials of HIV management, behavioral science topics including depression and substance abuse, and the Managed Problem Solving treatment manual (available online — XXurlXX).
Managed problem solving consisted of a 5-step process of identifying participants’ barriers to adherence, brainstorming for potential solutions, selecting the best option, monitoring its implementation, and whether the participant considered the implementation useful, and the participant’s adherence. Unsuccessful plans were revised repeating the process. These activities were iterated over the first three months until an effective solution was chosen or no further options could be generated. Baseline screening aided in identifying common potential adherence barriers, using CES-D for depressive symptoms [27], the AUDIT [28] and ASI questionnaires [29] for substance abuse, and a questionnaire regarding HIV knowledge, health, and religious beliefs.
The first session began with education concerning the prescribed regimen, medication adherence expectations, common regimen side-effects, and medication misperceptions (e.g., if you drink alcohol, do not take medications that day). Problem solving addressed 1) daily routines and daily cues, 2) memory and cognitive aids for pill taking and prescription refills, and 3) identifying and utilizing social supports as encouragement. Depression, substance use/abuse, toxicity management, and competing demands were addressed when screening uncovered these potential barriers. In addition, the interventionist asked open-ended questions for participants to identify additional barriers.
MAPS participants met with the interventionist within two weeks of initiating therapy for approximately 60–90 minutes. Three monthly follow-up meetings lasted approximately 20–45 minutes. Follow-ups included displaying a calendar plot of the prior month’s adherence generated by the electronic monitor. During the first three months, weekly telephone calls reinforced the in-person sessions and allowed for problem solving of new issues. Monthly telephone calls for the subsequent nine months reminded patients about obtaining refills and encouraged continuing the adherence strategies.
Prior to working with study patients, mock sessions were repeated until the interventionist was considered proficient by the investigators. Subsequently, trained staff assessed fidelity on 25 randomly selected intervention session audio tapes. Fidelity was scored using a 21-item tool developed for the protocol with scores ranging from 0–100; higher values indicated greater fidelity and >50 was considered adequate. More detail can be found in the MAPS manual at XXurlXX.
Outcomes
The primary outcome was antiretroviral adherence, measured using electronic monitors in all participants (Medication Event Monitoring System, MEMS, AARDEX, Zug, Switzerland), with each bottle opening considered a dose-taking event. Prospective participants planning to use a pill organizer were required to agree to maintain one medication in the MEMS monitored bottle (i.e., outside of the pill box). For patients on multiple antiretrovirals, an algorithm selected the monitored drug in the following order of preference: 1)non-nucleoside analog reverse transcriptase inhibitor, 2)protease inhibitor (ritonavir first), 3)integrase inhibitor, 4)entry inhibitor, or 5) nucleoside analog reverse transcriptase inhibitor.
We summarized adherence as fraction of prescribed number of doses taken over each quarter for a year, ranging from 0 to 1. The secondary outcome was undetectable plasma HIV viral load (UDVL), using a lower limit of 75 copies/ml and measured quarterly for a year (Versant, HIV-1 RNA 3.0, Bayer Corp. Berkeley, CA). These data were collected identically for each group during 9 follow-up visits with the study coordinator over the year.
Analysis
The primary analysis used an “intention to treat” approach. To be most conservative, missing values were assigned a value of zero adherence. Fraction of doses taken was categorized as <0.7, >0.70–0.80, >0.8–0.9, >0.9–0.95, and >0.95. Generalized estimating equations (GEE) with ordinal regression were used to estimate the association between study group and adherence over all four quarters of the year. [30] In secondary analyses, we tested for potential confounding by measured variables by including covariates in the GEE models and inspecting for changes in the point estimate of the relation between study group and outcome.
Analyses of viral suppression were identical those of adherence except using logistic regression [30]. In secondary “as treated” analyses, missing adherence and viral load data were ignored. In further analyses, we assessed whether the effect of the intervention varied over time by including treatment group by time interactions in the models.
Finally, we assessed the relation between adherence (independent variable) and virologic suppression (dependent variable) again using GEE with logistic regression and including missing viral load as detectable.
Sample Size
The planned sample size was 200 individuals to achieve >80% power to detect a <10% difference in adherence between MAPS and usual care, ignoring the contribution of multiple outcome time points per individual. Recruitment was slower than expected and the target was decreased to 180 to achieve 80% power to detect a 10% difference in adherence.
Results
Enrollment and Baseline Characteristics
Between September 2005 and February 2010, 218 individuals were referred for participation, with 190 eligible and 180 (95%) randomized (Figure 1). Of those enrolled, 91 were randomized to MAPS and 89 to usual care. After the target of 180 was reached, enrollment was closed and follow-up extended until February 2011.
Figure 1.
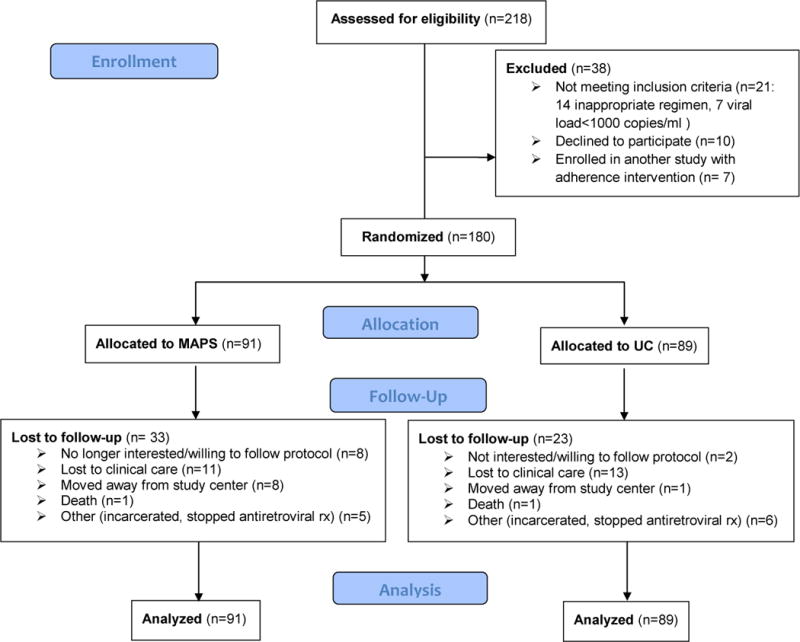
Participant Flow Diagram
Participants’ characteristics are displayed in Table 1. Of the 180 participants, 136 (76%) were on three drug regimens with two nucleoside reverse transcriptase inhibitors (NRTI) and a boosted protease inhibitor (PI) or a non-nucleoside analog reverse transcriptase inhibitor (NNRTI) (see Table 2). The remainder were on various combinations of up to 6 drugs with the most commonly used NRTI combination being tenofovir and emtricitabine in 88 (49%).
Table 1.
Baseline Characteristics by Study Group
| MAPS (n=91) | UC (n=89) | |
|---|---|---|
| Median Age (Range) years | 43 (20–65) | 42 (19–60) |
| Male Sex | 52 (57%) | 56 (63%) |
| Race | ||
| Race Black |
80 (88%) |
73 (82%) |
| White | 9 (10%) | 15 (17%) |
| Other | 2 (2%) | 1 (1%) |
| History of Injection Drug Use | 16 (18%) | 12 (14%) |
| Current Drug Use | 21 (23%) | 27 (30%) |
| Hazardous Alcohol Use | 14 (15%) | 18 (21%) |
| Unemployed | 73 (85%) | 69 (78%) |
| Yearly income<$5000 | 24 (28%) | 33 (36%) |
| Treatment naïve | 40 (44%) | 32 (36%) |
| Once daily regimen | 59 (65%) | 58 (65%) |
| Median # pills per regimen (Q25,75) | 3 (1–5) | 3 (3–5) |
| Baseline viral load (log10 copies/ml)-Q25,75 | 3.24 (2.46–4.32) | 3.47 (2.35–4.40) |
| Baseline CD4 count cells/mm3, Q25,75 | 287 (146–370) | 244 (116–379) |
Table 2.
Index Antiretroviral at Randomization by Study Group
| Index Drug | n | MAPS | Usual Care |
|---|---|---|---|
| Atazanavir/r | 64 | 32 | 32 |
| Efavirenz | 48 | 30 | 18 |
| Darunavir/r | 31 | 15 | 16 |
| Lopinavir/r | 18 | 6 | 12 |
| Fosamprenvir/r | 7 | 4 | 3 |
| Raltegravir | 4 | 2 | 2 |
| Nelfinavir | 2 | 1 | 1 |
| Nevirapine | 2 | 0 | 2 |
| Tipranavir/r | 1 | 1 | 0 |
/r=ritonavir boosted
Dropouts occurred for 33 (36%) in the MAPS group and 23 (26%) in the usual care group (χ2 (df=1) = 2.28, p=0.13). MAPS participants dropped out more commonly for “not wanting to follow the protocol” and moving too far away to follow-up at the site. Other reasons were similar between groups. Fidelity to MAPS was high with a mean score of 82 points (standard deviation=6 points), and ranged from 67 to 90 points.
Adherence Results by Study Group
The proportion of individuals in each adherence category in the MAPS and usual care groups during each quarter is displayed in Figure 2. In the primary analysis, the odds of being in a higher adherence category at any follow-up point was 1.78 (1.07–2.96) times greater for MAPS than usual care. Likewise, in the secondary analysis using an as-treated approach, the odds of being in a higher category of adherence at any follow-up point was 2.33 (1.35–4.05) times greater for MAPS than usual care. In further secondary analyses controlling for age, race, sex, treatment naïve status, use of efavirenz in regimen, baseline viral load and baseline CD4 count, there was no confounding of the relation between study group and adherence outcome. There was no evidence that MAPS’ adherence effect differed over time–the study group by time interaction was neither statistically nor clinically significant (data not shown).
Figure 2.
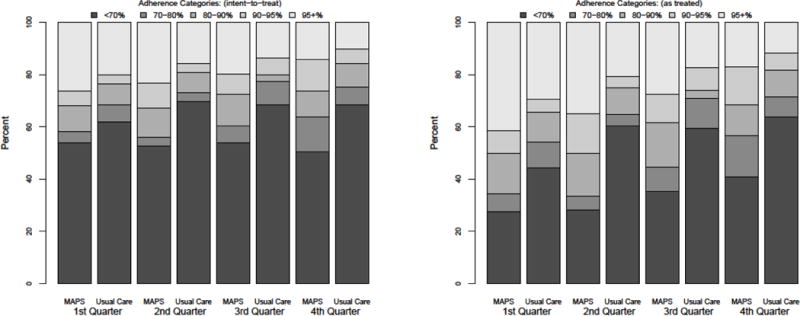
Proportion of participants in each adherence category by arm, per quarter. The left panel displays the intention to treat results with missing values=0% adherence. The right panel displays the as treated results with missing values ignored.
Virologic Results by Study Group
In the intention-to-treat analysis of virologic suppression, the odds of having an undetectable viral load at any follow-up point was 1.48 (95% CI: 0.94–2.31) times greater for MAPS than usual care. Likewise, in the as-treated analyses, the odds of having an undetectable viral load at any follow-up point was 1.98 (95% CI: 1.15–3.41) times greater for MAPS than usual care. At all time points, virologic suppression rates for MAPS exceeded those of usual care (see Table 3). Again there was no clear change in the relation between study group and undetectable viral load over time. In further formal analyses, there was no evidence that MAPS’ effect on virologic suppression differed over time–the study group by time interaction was neither statistically nor clinically significant (data not shown).
Table 3.
Virologic Response Rates per Quarter by Study Group
| A. Proportion with UDVL (missing=failure) | MAPS | UC |
|---|---|---|
| Q1 | 53/91 (58%) | 42/89 (47%) |
| Q2 | 53/91 (58%) | 47/89 (53%) |
| Q3 | 52/91 (57%) | 39/89 (44%) |
| Q4 | 54/91 (59%) | 45/89 (51%) |
| Proportion with UDVL (missing=missing) | ||
| Q1 | 53/68 (78%) | 42/67 (63%) |
| Q2 | 53/62 (86%) | 47/70 (67%) |
| Q3 | 52/69 (75%) | 39/59 (66%) |
| Q4 | 54/72 (75%) | 45/76 (59%) |
Effect of Adherence on Virologic Suppression
As expected, there was a strong relation between adherence and treatment response. In the analyses where missing viral loads were imputed as “detectable”, for every 25% increase in proportion of doses taken, the odds of having an undetectable viral load nearly doubled (odds ratio= 1.99 (95% CI: 1.64–2.41). In the analyses where missing viral load was left missing, the odds of undetectable viral load for every 25% increase in doses taken was more than double (odds ratio=2.41 (95% CI: 1.91–3.02).
Other Factors Associated with Adherence and Virologic Response
Treatment experienced individuals were 0.48 (95% CI: 0.29–0.81) times as likely to be in a higher adherence category and 0.48 (95% CI: 0.30–0.76) times as likely to have undetectable viral loads than treatment naïve individuals. Higher baseline viral load was associated with a lower likelihood of virologic suppression. For every 1 log10 higher baseline viral load, the odds of having an undetectable viral load was 0.80 (0.65–0.99). Neither of these relationships differed by study group.
Discussion
This randomized trial of MAPS demonstrated that it is effective at improving adherence over the first year of a new antiretroviral regimen in a population with relatively high rates of non-adherence. We found no evidence that MAPS was less effective in individuals with prior treatment experience despite those individuals being at higher risk of non-adherence. It did not lose effect over time. The impact of this increased adherence was borne out by the higher proportion of individuals with undetectable viral loads. This effect too persisted for the entire treatment period.
Numerous strategies to improve pharmacotherapy adherence have been tested, particularly for antiretroviral therapy [12]. Results have mostly been disappointing, with only a few strategies showing benefits for both adherence and virologic response [31,32]. Directly observed therapy (DOT) has been the most intensively studied, but in unselected populations, has not been associated with important [33] or sustained benefits [34]. However, some evidence suggests that DOT might be useful in select subpopulations, such as active substance abusers [35]. Technological interventions have had mixed results with text messages showing benefit in a developing world setting [32], but not in the developed world [36]. Behavioral strategies have also had mixed success. Simple financial incentives for adherence have had some effect while continued, but not consistently, and typically wane when the financial reward is stopped [37]. Feedback regarding adherence has been used successfully previously, but in a population excluding ethnic minorities, substance abusers, and the mentally ill [31]. Problem solving has been incorporated into larger intervention packages, but not studied on its own [38]. Use of multiple modalities to improve adherence has been suggested for years, but relatively little evidence to date has been generated). Notably, MAPS incorporates both a behavioral strategy and technology– the adherence feedback is generated via electronic monitors.
MAPS is relatively resource intensive, but such strategies can be presumed to be cost effective if they cost less than $1000 per year per participant for a 10% increase in adherence [39]. Although we did not perform a formal cost-effectiveness analysis, we estimate that approximately 20 participants were followed per year by an interventionist committing 15% effort at a salary of ~$50,000/year. Including approximately $150 per electronic monitor per participant, the cost for such an activity is substantially <$1000 per year.
There are several potential limitations to this study. First, although it was a randomized trial, participants could not be blinded to study arm. However, since the treatment was crucial to their health, it is unlikely that their awareness of study arm impacted their behavior outside of the interaction with the interventionist. In fact, it is possible that adherence in the control arm was better than would be expected in this population since this condition provided extra visits with the study coordinator, where more attention was paid to their medication taking. Second, participants were recruited from academic specialty HIV clinics where services for adherence may be greater than in general medical clinics. If so, the effect of MAPS may be even greater in less resourced settings. Third, it is unclear how the dropouts truly affected the results. Although both the intention-to-treat and as-treated analyses favored MAPS, the effect was stronger in the as-treated analyses. It is possible that imputing virologic failure for all missing data was an overly conservative assumption. Yet, in all secondary analyses, changing the assumptions regarding missing data did not change the direction of the effect-that is, MAPS was the favored strategy in all results. Of course, dropouts diminish the cost-effectiveness of the intervention; limiting dropouts from care should be a priority for future refinements of MAPS. Fourth, we used microelectronic monitors to generate the feedback over the initial 3 months. The current cost of these monitors may render them out of reach for most patients and programs. Other objective techniques have been established for monitoring adherence (e.g., pharmacy refill data [4,21,40]), yet it is unclear how they would perform if incorporated into MAPS instead of microelectronic monitors.
This study also has several particular strengths. The randomization, and the evident balance between the groups in most baseline characteristics, minimizes the likelihood that potential confounders biased the observed effect of MAPS. Second, fidelity to the manual of procedures always exceeded minimal standards. Third, the effect was present despite enrolling a population with many life challenges (i.e., poverty, unemployment). Fourth, the effect was apparent in the face of a rigorous and very conservative analytic approach of considering loss to follow-up non-adherence–this stands in contrast to studies analyzing only those who remained in care [31]. Fifth, the follow-up period extended for a full year and there was no attenuation of the effect. Finally, unlike prior multimodal interventions, we have developed an implementation manual and workbook, which were used by the staff in this study. Thus, MAPS has the potential to be implemented rapidly in settings where health behavior change expertise is unavailable.
In conclusion, MAPS improves pharmacotherapy adherence, although the benefit was somewhat diminished by dropouts. Since barriers to adherence and retention in care are similar, MAPS could be expanded to address retention in care as well; however, that remains to be evaluated. Since microelectronic monitors are not widely used in clinical practice, we believe MAPS should be refined to use other objective measures of adherence as the feedback tool. With the availability of the intervention manual, MAPS can be utilized immediately where the resources exist to implement it. MAPS should also be adapted and tested in other treatment settings where adherence to oral pharmacotherapy is critical to health outcomes.
Acknowledgments
Dr. Gross had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The study was supported by R01 MH067498 from the National Institute of Mental Health, UL1 RR024134 from the National Center for Research Resources, core services and support from the Penn Center for AIDS Research (P30 AI 045008), and career support from the Philadelphia Veterans Affairs Medical Center (RG). The results of the study do not necessarily reflect the views of the Department of Veterans Affairs. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. During the course of this study, Dr. Gross received research support via contracts with Bristol-Myers Squibb and Abbott Laboratories for work related to HIV and its treatment, but not for work on this study. Dr. Strom was supported in part by contracts with and received payment for consulting with numerous pharmaceutical companies, none of which was related to HIV or this study.
Footnotes
The authors have declared that no competing interests exist.
This work was presented at the 19th Conference on Retroviruses and Opportunistic Infections in Seattle, WA, March 2012.
This manuscript is dedicated to the memory of Dr. Thomas Ten Have, beloved colleague, teacher, and mentor. We thank the members of the Data and Safety Monitoring Committee, Drs. Harvey M. Friedman and J. Sanford Schwartz. We are most indebted to the volunteer participants and their referring providers.
References
- 1.Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011;33:74–109. doi: 10.1016/j.clinthera.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Pittman DG, Tao Z, Chen W, Stettin GD. Antihypertensive medication adherence and subsequent healthcare utilization and costs. Am J Manag Care. 2010;16:568–576. [PubMed] [Google Scholar]
- 3.Chan DC, Shrank WH, Cutler D, Jan S, Fischer MA, et al. Patient, physician, and payment predictors of statin adherence. Med Care. 2010;48:196–202. doi: 10.1097/MLR.0b013e3181c132ad. [DOI] [PubMed] [Google Scholar]
- 4.Gross R, Yip B, Re VL, 3rd, Wood E, Alexander CS, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194:1108–1114. doi: 10.1086/507680. [DOI] [PubMed] [Google Scholar]
- 5.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Archives of Internal Medicine. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 6.Lucas GM, Cheever LW, Chaisson RE, Moore RD. Detrimental effects of continued illicit drug use on the treatment of HIV-1 infection. Journal of Acquired Immune Deficiency Syndromes. 2001;27:251–259. doi: 10.1097/00126334-200107010-00006. [DOI] [PubMed] [Google Scholar]
- 7.Bangsberg DR, Ragland K, Monk A, Deeks SG. A single tablet regimen is associated with higher adherence and viral suppression than multiple tablet regimens in HIV+ homeless and marginally housed people. Aids. 2010;24:2835–2840. doi: 10.1097/QAD.0b013e328340a209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris J, Pillinger M, Fromstein D, Gomez B, Garris I, et al. Risk factors for medication non-adherence in an HIV infected population in the Dominican Republic. AIDS Behav. 2011;15:1410–1415. doi: 10.1007/s10461-010-9781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham J, Bennett IM, Holmes WC, Gross R. Medication beliefs as mediators of the health literacy-antiretroviral adherence relationship in HIV-infected individuals. AIDS Behav. 2007;11:385–392. doi: 10.1007/s10461-006-9164-9. [DOI] [PubMed] [Google Scholar]
- 10.Kalichman SC, Ramachandran B, Catz S. Adherence to combination antiretroviral therapies in HIV patients of low health literacy. Journal of General Internal Medicine. 1999;14:267–273. doi: 10.1046/j.1525-1497.1999.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins N, Regan C. A systematic review of the effectiveness of interventions to help older people adhere to medication regimes. Age Ageing. 2004;33:224–229. doi: 10.1093/ageing/afh072. [DOI] [PubMed] [Google Scholar]
- 12.Simoni JM, Amico KR, Pearson CR, Malow R. Strategies for promoting adherence to antiretroviral therapy: a review of the literature. Curr Infect Dis Rep. 2008;10:515–521. doi: 10.1007/s11908-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bain-Brickley D, Butler LM, Kennedy GE, Rutherford GW. Interventions to improve adherence to antiretroviral therapy in children with HIV infection. Cochrane Database Syst Rev. 2011;12:CD009513. doi: 10.1002/14651858.CD009513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nezu AM, Nezu CM, Perri MG. Problem-solving therapy for depression: Theory, research, and clinical guidelines. New York, NY, USA: John Wiley & Sons; 1989. p. 274. [Google Scholar]
- 15.D’Zurilla TJ, Nezu AM. Problem-solving therapies. Dobson, Keith S.; 2001. [Google Scholar]
- 16.Hill-Briggs F, Gemmell L. Problem solving in diabetes self-management and control: a systematic review of the literature. Diabetes Educ. 2007;33:1032–1050. doi: 10.1177/0145721707308412. discussion 1051–1032. [DOI] [PubMed] [Google Scholar]
- 17.Nezu AM, Nezu CM, Felgoise SH, McClure KS, Houts PS. Project Genesis: assessing the efficacy of problem-solving therapy for distressed adult cancer patients. Journal of Consulting & Clinical Psychology. 2003;71:1036–1048. doi: 10.1037/0022-006X.71.6.1036. [DOI] [PubMed] [Google Scholar]
- 18.Nezu CM, Nezu AM, Houts PS. Multiple applications of problem-solving principles in clinical practice. Kuehlwein, Kevin T.; 1993. [Google Scholar]
- 19.Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. Aids. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 20.Gross R, Bilker WB, Friedman HM, Strom BL. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. Aids. 2001;15:2109–2117. doi: 10.1097/00002030-200111090-00006. [DOI] [PubMed] [Google Scholar]
- 21.Nachega JB, Hislop M, Dowdy DW, Lo M, Omer SB, et al. Adherence to highly active antiretroviral therapy assessed by pharmacy claims predicts survival in HIV-infected South African adults. J Acquir Immune Defic Syndr. 2006;43:78–84. doi: 10.1097/01.qai.0000225015.43266.46. [DOI] [PubMed] [Google Scholar]
- 22.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 23.Wood E, Hogg RS, Yip B, Harrigan PR, O’Shaughnessy MV, et al. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 × 10(9) cells/L.[see comment] Annals of Internal Medicine. 2003;139:810–816. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]
- 24.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bangsberg DR, Acosta EP, Gupta R, Guzman D, Riley ED, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. Aids. 2006;20:223–231. doi: 10.1097/01.aids.0000199825.34241.49. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Miller LG, Hays RD, Golin CE, Wu T, et al. Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. J Acquir Immune Defic Syndr. 2006;41:315–322. doi: 10.1097/01.qai.0000197071.77482.6e. [DOI] [PubMed] [Google Scholar]
- 27.Radloff L. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 28.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 29.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 30.Zeger SL, Liang KY. An overview of methods for the analysis of longitudinal data. Stat Med. 1992;11:1825–1839. doi: 10.1002/sim.4780111406. [DOI] [PubMed] [Google Scholar]
- 31.de Bruin M, Hospers HJ, van Breukelen GJ, Kok G, Koevoets WM, et al. Electronic monitoring-based counseling to enhance adherence among HIV-infected patients: a randomized controlled trial. Health Psychol. 2010;29:421–428. doi: 10.1037/a0020335. [DOI] [PubMed] [Google Scholar]
- 32.Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376:1838–1845. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 33.Ford N, Nachega JB, Engel ME, Mills EJ. Directly observed antiretroviral therapy: a systematic review and meta-analysis of randomised clinical trials. Lancet. 2009;374:2064–2071. doi: 10.1016/S0140-6736(09)61671-8. [DOI] [PubMed] [Google Scholar]
- 34.Gross R, Tierney C, Andrade A, Lalama C, Rosenkranz S, et al. Modified directly observed antiretroviral therapy compared with self-administered therapy in treatment-naive HIV-1-infected patients: a randomized trial. Arch Intern Med. 2009;169:1224–1232. doi: 10.1001/archinternmed.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45:770–778. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simoni JM, Huh D, Frick PA, Pearson CR, Andrasik MP, et al. Peer support and pager messaging to promote antiretroviral modifying therapy in Seattle: a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;52:465–473. doi: 10.1097/qai.0b013e3181b9300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088–2097. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- 38.Koenig L, Pals S, Bush T, Pratt Palmore M, Stratford D, et al. Randomized controlled trial of an intervention to prevent adherence failure among HIV-infected patients initiating antiretroviral therapy. Health Psychol. 2008;27:159–169. doi: 10.1037/0278-6133.27.2.159. [DOI] [PubMed] [Google Scholar]
- 39.Goldie SJ, Paltiel AD, Weinstein MC, Losina E, Seage GR, 3rd, et al. Projecting the cost-effectiveness of adherence interventions in persons with human immunodeficiency virus infection. Am J Med. 2003;115:632–641. doi: 10.1016/j.amjmed.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Bisson GP, Rowh A, Weinstein R, Gaolathe T, Frank I, et al. Antiretroviral failure despite high levels of adherence: discordant adherence-response relationship in Botswana. J Acquir Immune Defic Syndr. 2008;49:107–110. doi: 10.1097/QAI.0b013e3181820141. [DOI] [PMC free article] [PubMed] [Google Scholar]


