Summary
Sodium and potassium intake are modifiable determinants of hypertension in the general population but have not been studied in patients with systemic lupus erythematosus (SLE). We examined the relationship between urinary excretion of sodium and potassium, as an estimate of intake, and blood pressure in patients with SLE. We studied 178 SLE patients and 86 controls, matched for age, sex, and race. Urine sodium (Na+) and potassium (K+) were measured by flame photometry. Blood pressure was the average of two resting measurements. The associations between systolic (SBP) and diastolic blood pressures (DBP) and estimated 24 hour urinary Na+, K+, and Na+:K+ ratio were tested. The estimated mean 24 hour urinary K+ excretion was lower, and the Na+:K+ ratio was higher in patients with SLE than controls. There were no significant differences in the estimated 24 hour urinary Na+. In patients with SLE, a higher urinary Na+:K+ ratio was associated with higher SBP [β coefficient =4.01, p=0.023] and DBP [β coefficient=4.41, p=0.002] after adjusting for age, sex, and race. SLE patients had significantly lower estimated 24 hour urinary K+ and higher estimated 24 hour urinary Na+: K+ ratio than controls. The urinary Na+:K+ ratio was significantly associated with SBP and DBP.
Keywords: systemic lupus erythematosus, hypertension, sodium, potassium
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that is associated with a 5 fold increased risk of cardiovascular disease (CVD).1 Hypertension represents an important modifiable risk factor for CVD and is more prevalent in patients with SLE than in control subjects.2–5 Traditional risk factors and disease-specific factors, such as increased disease activity, do not fully account for the increased risk of hypertension in SLE,5, 6 and the exact mechanisms are not fully elucidated.7
In the general population, sodium (Na+) and potassium (K+) intake are modifiable determinants of blood pressure. A large, international, epidemiologic study found that for every 1 gram increase in 24 hour urinary Na+ excretion, there is an approximately 1.46 and 0.54 mm Hg increase in systolic blood pressure (SBP) and diastolic blood pressure (DBP), respectively.8 Reduced Na+ intake, defined as less than 2.3 grams per day, reduced SBP by approximately 7–11 mm Hg in the general population.9 Another interventional trial that reduced daily Na+ intake by 1 gram per day lowered the risk of a cardiovascular event by 25%.10 Based on these and other observations, the American Heart Association recommends reduction of daily dietary Na+ intake to 1.5 to 2.4 grams or below per day.11
In addition to Na+, K+ intake also plays an important role in blood pressure and cardiovascular outcomes. Previous studies have found that for every 1 gram increase in 24 hour urinary K+ excretion, there is a reduction in SBP and DBP by 0.65 and 0.42 mm Hg, respectively.8,12 Further, a study with a K+-enriched salt intervention showed a 41% reduction in CVD.13
There are varying methods to estimate the Na+ and K+ intake in patients. First, many studies have utilized food diaries, an approach that is limited by patient self-report14 and is potentially affected by recall bias. Second, other studies have measured 24 hour urine values, which are limited by patient compliance.10,15 Third, spot urine measurements of Na+ and K+ have been used to estimate the 24 hour urinary Na+ and K+ excretion with the validated Kawasaki formula.16,17 This method has been used by large epidemiologic studies.8,18 Recently, a large, international study used spot urine Na+ and K+ values to estimate the 24 hour Na+ and K+ urinary excretion and intake using the Kawasaki formula and found a higher Na+ and a lower K+ were associated with a higher SBP.8
Sodium intake can lead to the generation of pathogenic TH17 cells; thus, sodium has been implicated as a possible factor that exacerbates autoimmune processes.19, 20 Furthermore, systemic inflammation can alter the renal regulation of sodium, potassium, and water.21 Therefore, the relationship of Na+ and K+ intake to hypertension may be stronger in SLE compared with the general population because of increased inflammation in SLE. Na+ and K+ intake and its association with blood pressure have not been studied in SLE. Patients with SLE are at high risk for adverse cardiovascular outcomes. Therefore, identifying modifiable risk factors for hypertension are of interest. We hypothesized that the urinary excretion of Na+ and K+, as an estimate of intake, are related to blood pressure in SLE.
Materials and Methods
Study Design and Participants
In a cross-sectional study, we evaluated 178 patients with SLE and 86 control subjects frequency-matched for age, sex, and race with available urine specimens. Patients with SLE and control subjects are part of an ongoing cohort study to identify cardiovascular risk factors in SLE; this study has been described previously.22 Briefly, patients with SLE were older than 18 years, met the classification criteria for SLE, 23 and had the disease longer than six months. They were recruited from the practices of local rheumatologists, through a Lupus Foundation newsletter, and by advertisements. Control subjects had no autoimmune disease and were recruited from the patients’ acquaintances, by advertisement, and from a data base of volunteers maintained by the General Clinical Research Center at Vanderbilt University School of Medicine. All participating subjects gave written informed consent prior to enrollment. The study was approved by the institutional review board of Vanderbilt University Medical Center (# 990111).
Patient assessment included a detailed review of medical records, a standardized interview, physical examination, and laboratory testing. Data on demographics, CVD risk factors, and medications were collected. Disease activity and damage were measured using the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) and Systemic Lupus International Collaborating Clinics Damage Index (SDI), respectively.24, 25 The glomerular filtration rate (GFR) was estimated using the Modification of Diet in Renal Disease formula.26
First morning urine specimens were collected and stored at −80°C. The urine samples were centrifuged at 5000 rpm at 4°C for 5 minutes with transfer of 100ul of supernatant urine to a new Eppendorf® tube. The concentrations of urine Na+ and K+ were measured by flame photometry using an EFOX 5053 Electrolyte Analyzer from Eppendorf®. The Kawasaki formula, a method that incorporates age, sex, height, weight, and urinary creatinine, was used to estimate 24 hour urine Na+ and K+ excretion from the spot urine Na+ and K+ concentrations.16 In patients with SLE, serum concentrations of interleukin-6 (IL-6), interleukin-10 (IL-10), and vascular cell adhesion protein-1 (VCAM-1) were measured using a multiplex platform (Lincoplex Inc.).
Blood pressure was determined as the average of two measurements obtained 5 minutes apart after participants had rested quietly in the supine position for 10 minutes. Participants were considered to have hypertension if they were taking antihypertensive agents or had a SBP or DBP of at least 140 mm Hg or 90 mm Hg, respectively. Diabetes mellitus was defined as a fasting blood glucose ≥ 126 mg/dl and/or use of insulin or oral medications for diabetes. Height and weight were measured and body mass index (BMI) calculated. Participants were classified as having metabolic syndrome using the modified World Health Organization definition, which requires the presence of insulin resistance and 2 of the following 3 criteria: central obesity, dyslipidemia, and hypertension.27
Statistical Analysis
Demographic, CVD risk factors, and medication use were compared using Fisher’s exact test while blood pressure and estimated 24 hour urinary Na+, K+, and Na+: K+ ratio were compared using the Wilcoxon rank-sum test in patients with SLE vs. controls. The associations between SBP and DBP and the estimated 24 hour urinary Na+, K+, and Na+: K+ ratio were tested using Spearman correlation and then modeled using linear regression adjusting for age, sex, and race in patients with SLE. All statistical analyses were conducted using IBM SPSS Statistics version 22.0. Two-sided p values < 0.05 were considered significant.
Results
Patients with SLE (n = 178) and controls (n = 86) had a similar mean age and were predominantly female, Caucasian, and well educated (Table 1). Patients with SLE and control subjects had a similar BMI and GFR and rates of diabetes and smoking (Table 1). However, patients with SLE had higher rates of metabolic syndrome compared to control subjects (26% vs. 12%, p = 0.01).
Table 1.
Baseline characteristics of systemic lupus erythematosus (SLE) patients and matched controls.
| Characteristics | SLE (n = 178) | Controls (n = 86) | P valuea |
|---|---|---|---|
| Mean age (years ± standard deviation) | 40.9 ± 12 | 41.2 ± 12 | 0.76 |
| Female sex (%) | 88% | 86% | 0.70 |
| Race/ethnicity (%) | |||
| Caucasian | 68% | 72% | 0.67b |
| African American | 24% | 23% | |
| Hispanic | 4% | 4% | |
| Asian | 2% | 1% | |
| Other | 2% | 0% | |
| Education (%) | 0.72c | ||
| Less than high school | 3% | 4% | |
| High school graduate | 23% | 15% | |
| Some college | 59% | 57% | |
| College graduate or higher | 15% | 24% | |
| GFR, ml/min per 1.73 m2 | 86.6 ± 26.8 | 89.9 ± 20.1 | 0.26 |
| BMI (kg/m2) | 29.1± 7.2 | 27.2 ± 5.7 | 0.06 |
| Metabolic Syndrome (%) | 26% | 12% | 0.01 |
| Diabetes mellitus (%) | 5% | 1% | 0.28 |
| Currently smoking (%) | 23% | 20% | 0.75 |
Wilcoxon rank-sum and Fisher’s exact test for continuous and dichotomous variables, respectively.
Fisher’s exact test comparing Caucasian vs non-Caucasian.
Fisher’s exact test comparing high school graduate or higher to non-high school graduate.
Of the 178 SLE patients, 28% had renal involvement and 7% neurologic involvement, as defined by the ACR classification criteria.23 The mean (± standard deviation) SLEDAI was 4.2 ± 4.0, and the mean SDI was 1.1 ± 1.5 with 53% having any damage (SDI ≥ 1). For treatment, 69% of SLE patients were currently on hydroxychloroquine and 57% on a systemic corticosteroid.
Compared to control subjects, patients with SLE had significantly higher rates of hypertension (44% vs. 19%, p < 0.001) and anti-hypertensive medication use (36% vs. 12%, p < 0.001), but patients with SLE and control subjects had a similar mean SBP and DBP (Table 2).
Table 2.
Blood pressure and estimated 24 hour urinary Na+, K+, and Na+: K+ ratio in systemic lupus erythematosus (SLE) patients and matched controls.
| Characteristics | SLE (n = 178) | Controls (n = 86) | P valuea |
|---|---|---|---|
| Hypertension (%) | 44% | 19% | <0.001 |
| Mean systolic blood pressure (mm Hg) | 120 ± 17 | 118± 14 | 0.63 |
| Mean diastolic blood pressure (mm Hg) | 73 ± 13 | 71 ± 10 | 0.20 |
| Anti-hypertensive use (%) | 36% | 12% | <0.001 |
| Diuretic use (%) | 18% | 8% | 0.04 |
| ACE inhibitor or ARB use (%) | 21% | 4% | <0.001 |
| Estimated 24 hour urinary sodium (grams) | 4.2 ± 1.8 | 4.5 ± 2.1 | 0.54 |
| Estimated 24 hour urinary potassium (grams) | 2.0 ± 0.7 | 2.4 ± 0.9 | < 0.001 |
| Estimated 24 hour urinary sodium to potassium ratio | 2.2 ± 0.7 | 1.9 ± 0.6 | 0.001 |
Wilcoxon rank-sum
The estimated 24 hour urinary Na+ excretion was similar in patients with SLE and control subjects (4.2 ± 1.8 vs. 4.5 ± 2.1 g, p = 0.54), but the estimated 24 hour urinary K+ excretion was significantly lower in patients with SLE compared to control subjects (2.0 ± 0.7 vs. 2.4 ± 0.9 g, p < 0.001) (Table 2). The estimated 24 hour urinary Na+: K+ ratio was significantly higher in patients with SLE compared to control subjects (2.2 ± 0.7 vs. 1.9 ± 0.6, p = 0.001) (Figure 1).
Figure 1. Estimated 24 hour urinary Na: K ratio in SLE cases versus controls.
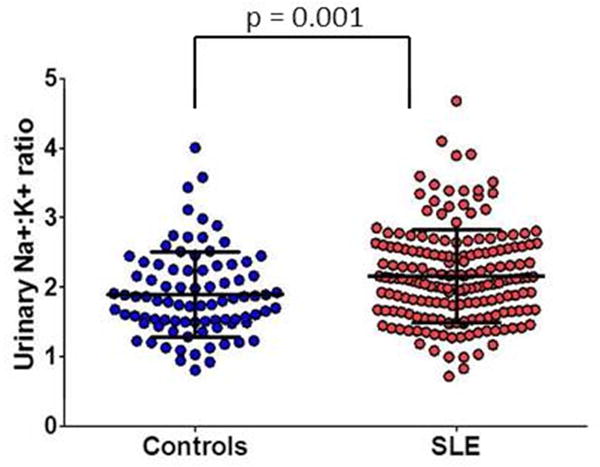
Lines indicate mean ± standard deviation. Differences were compared using the Wilcoxon ranksum test.
When comparing SLE patients with hypertension (n = 79) to controls with hypertension (n = 16), the estimated 24 hour urinary Na+ (4.1 ± 1.9 vs. 3.8 ± 1.5 g, p = 0.59) and K+ (1.9 ± 0.6 vs. 2.2 ± 0.7 g, p = 0.08) were not significantly different but the estimated 24 hour urinary Na+: K+ ratio was significantly higher (2.2 ± 0.7 vs. 1.8 ± 0.8, p = 0.01) in SLE patients with hypertension vs. controls with hypertension.
Sensitivity analyses were performed excluding participants taking anti-hypertensive medications. The estimated 24 hour urinary K+ excretion remained significantly lower and the urinary Na+: K+ ratio remained significantly higher when excluding participants on any anti-hypertensive medication (p = 0.003, p = 0.016, respectively), diuretics (p < 0.001, p = 0.005), and angiotensin-converting-enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) (p < 0.001, p = 0.001). In addition, the estimated 24 hour urinary Na+ (4.1 ± 1.8 vs. 4.2 ± 1.8 g, p = 0.86), K+ (1.9 ± 0.7 vs. 2.0 ± 0.7 g, p = 0.12), and Na+: K+ ratio (2.2 ± 0.7 vs. 2.1 ± 0.6, p = 0.16) did not differ significantly among patients with SLE who were vs. were not currently receiving corticosteroids.
There were 9 patients with SLE that ever had dialysis including 2 patients currently receiving dialysis. Exclusion of these 9 patients did not impact the overall results. Patients who fulfilled (n = 50) vs. did not (n = 128) fulfill the renal criterion of the ACR SLE criteria23 had a similar estimated 24 hour urinary Na+ (3.9 ± 1.7 vs. 4.3 ± 1.8 g, p = 0.33) and Na+: K+ ratio (2.2 ± 0.7 vs. 2.1 ± 0.6, p = 0.33) but a lower estimated 24 hour urinary K+ (1.8 ± 0.5 vs. 2.0 ± 0.8 g, p = 0.03).
In patients with SLE, there were no significant associations in univariate analyses between SBP and the estimated 24 hour urinary Na+, K+, or the Na+: K+ ratio (Table 3). In a model adjusted for age, sex, and race, SBP was not associated with the estimated 24 hour urinary Na+ or K+. However, SBP was associated with the urinary Na+:K+ ratio [β coefficient (95% CI) =4.01 (0.57–7.46), p=0.023].
Table 3.
Association of blood pressure with estimated 24 hour urinary Na+, K+, and Na+: K+ ratio in systemic lupus erythematosus (SLE) patients.
| SLE (n = 178) | |||
|---|---|---|---|
| β (95% CI) p value | Adjusted β (95% CI) p valuea | ||
| Systolic blood pressure | Na+ (grams) | 0.29 (−1.10 – 1.67) p = 0.69 | 0.56 (−0.74 – 1.86) p = 0.40 |
| K+ (grams) | −1.80 (−5.31 – 1.73) p = 0.32 | −2.31 (−5.63 – 1.02) p = 0.17 | |
| Na:K+ | 2.42 (−1.28 – 6.13) p = 0.20 | 4.01 (0.57 – 7.46) p = 0.02 | |
|
|
|||
| Diastolic blood pressure | Na+ (grams) | 0.58 (−0.46 – 1.62) p = 0.28 | 0.75 (−0.28 – 1.78) p = 0.15 |
| K+ (grams) | −1.83 (−4.48 – 0.82) p = 0.17 | −1.84 (−4.48 – 0.80) p = 0.17 | |
| Na:K+ | 3.73 (0.98 – 6.48) p = 0.008 | 4.41 (1.71 – 7.11) p = 0.002 | |
Linear regression, adjusted for age, sex, and race
DBP was not significantly associated with estimated 24 hour urinary Na+ and K+. However, DBP was associated with the urinary Na+: K+ ratio [β coefficient=3.73 (0.98–6.48), p=0.008]. In a model adjusted for age, sex, and race, DBP was not associated with estimated 24 hour urinary Na+ or K+ but remained significantly associated with the urinary Na+: K+ ratio [β coefficient=4.41 (1.71–7.11), p=0.002].
In patients with SLE, SLEDAI was not significantly associated with SBP, (p = 0.64) DBP, (p = 0.21) the estimated 24 hour urinary Na+, (p = 0.64), K+ (p = 0.08), or the Na+: K+ ratio (p = 0.57). SDI was associated with SBP (rho = 0.15, p = 0.04) but not with DBP (rho = 0.10, p = 0.17). SDI was not associated with the estimated 24 hour urinary Na+ (p = 0.91), K+ (p = 0.49), or the Na+: K+ ratio (p = 0.24).
Inflammatory cytokines, IL-10 and IL-6, were not significantly associated with SBP or DBP (Table 4). However, VCAM-1 was associated with SBP (rho = 0.21, p = 0.006) but not DBP. IL-6 and VCAM-1 were not associated with the estimated 24 hour urinary Na+, K+, or the Na+: K+ ratio. However, IL-10 was negatively correlated with the Na+: K+ ratio (rho = −0.196, p = 0.0091).
Table 4.
Association of inflammatory cytokines with blood pressure and estimated 24 hour urinary Na+, K+, and Na+: K+ ratio in systemic lupus erythematosus (SLE) patients.
| Interleukin-6 | Interleukin-10 | Vascular cell adhesion protein-1 | |
|---|---|---|---|
| Systolic blood pressure | rhoa = 0.08 | rho = 0.02 | rho = 0.21 |
| p = 0.33 | p = 0.80 | p = 0.006 | |
| Diastolic blood pressure | rho = 0.06 | rho = −0.02 | rho = 0.09 |
| p = 0.44 | p = 0.81 | p = 0.22 | |
| Na+ (grams) | rho = 0.02 | rho = 0.02 | rho = 0.001 |
| p = 0.81 | p = 0.76 | p = 0.99 | |
| K+ (grams) | rho = −0.08 | rho = −0.14 | rho = 0.04 |
| p = 0.31 | p = 0.07 | p = 0.58 | |
| Na:K+ | rho = 0.13 | rho = −0.20 | rho = −0.03 |
| p = 0.09 | p = 0.009 | p = 0.67 |
Spearman correlation
Discussion
There are two main findings of this study. First, patients with SLE had a significantly lower estimated 24 hour urinary K+ and a higher estimated 24 hour urinary Na+: K+ ratio compared to control subjects. Second, the estimated 24 hour urinary Na+: K+ ratio was significantly associated with SBP and DBP in patients with SLE, even after adjustment for age, sex, and race. Specifically, for every one point increase in the Na+: K+ ratio, there was an approximately 4 mm Hg increase in both SBP and DBP in patients with SLE. Our findings are important because patients with SLE have higher rates of hypertension, and Na+ and K+ intake are potentially modifiable risk factors for hypertension.
Interestingly, patients with SLE had a significantly lower estimated 24 hour urinary K+ compared to control subjects. Causes of low urinary K+ include renal disease and medications such as diuretics. Sensitivity analyses that accounted for anti-hypertensive medications, including diuretics, did not show that these medications were driving the lower K+ in the SLE patients. Further, excluding patients with a previous history of dialysis did not significantly impact the K+. Even though the lower K+ was not statistically associated with a higher SBP and DBP in this study, the lower K+ may be clinically important, as it has been associated with hypertension, CVD, and death in the general population.13–15 Future studies should consider the potential role of K+ in investigating hypertension and CVD outcomes in SLE patients.
In this study, patients with SLE were twice as likely to have hypertension compared to control subjects frequency-matched on age, sex, and race. This finding agrees with another cohort study where patients with SLE were also twice as likely to have hypertension compared to controls.2 While patients with SLE in our study had higher rates of hypertension vs. control subjects, they had a similar mean SBP and DBP compared to control subjects, which indicates well controlled blood pressures on their current anti-hypertensive medications, based on the mean SBP and DBP within the normotensive range.
The pathophysiology of hypertension in SLE is complex and not fully understood. Potential mechanisms that have been proposed are an increased systemic vascular resistance and an altered pressure-natriuresis relationship in the kidneys whereby higher pressures are needed for sodium excretion.7 Recent work highlights the role of inflammation in hypertension, which may be pertinent to understanding the mechanisms of hypertension in SLE. Systemic inflammation can alter the renal handling of Na+ and water, which may implicate Na+ and K+ in the pathophysiology of hypertension.21 There is evidence for both innate and adaptive immune cells infiltrating, releasing cytokines, and causing oxidative stress in the kidney and vasculature to elevate blood pressure and cause organ damage.21 In the vasculature, macrophages and monocytes infiltrate the adventitia to promote oxidative injury, endothelial dysfunction, vascular remodeling, and arterial stiffness, which contribute to increased systemic vascular resistance.21 In the kidney, inflammatory cytokines elicit fibrosis, glomerular damage, and alterations in sodium transporter expression.21 Specifically, interleukin 6 (IL-6) and interferon gamma (IFNγ) in animal models promote angiotensinogen secretion that increases angiotensin II release as well as increases expression of sodium transporters that increases distal sodium reabsorption.28–30 In contrast, the anti-inflammatory cytokine interleukin-10 (IL-10) may have a protective role in hypertension by modulating endothelial dysfunction.31 In summary, inflammatory cytokines can impact both the local renin-angiotensin system and the expression of renal sodium transporters to affect sodium and water balance. Therefore, inflammatory cytokines may affect renal Na+ handling, which could contribute to the increased prevalence of hypertension in SLE.
In our study, IL-10 was negatively correlated with the Na+: K+ ratio but not associated with SBP, DBP, the estimated 24 hour urinary Na+, or K+. The negative correlation could be explained as some studies suggest that IL-10 may have a protective role in hypertension.31 IL-6 was not associated with SBP, DBP, the estimated 24 hour urinary Na, + K+, or the Na+: K+ ratio. While increased IL-6 levels have been associated with hypertension in animal models and humans, it is not clear if there is an association with hypertension in patients with SLE.21 IL-6 levels may fluctuate in patients with SLE as well as be reduced by immunosuppressive medications, which may explain why our cross-sectional study did not detect an association. Future longitudinal studies are needed to study the association between these cytokines and blood pressure in patients with SLE.
Our study has several limitations. First, this is a cross sectional study with a one-time spot urine collection, so causality between urinary Na+ and K+ excretion with blood pressure cannot be inferred. We also do not have longitudinal data on the blood pressure and urinary values that could be helpful in understanding the variability of these measures. As the cohort was initially designed to identify cardiovascular risk factors in SLE, there were half as many controls as cases. Controls were frequency-matched for age, sex, and race and not for other variables such as hypertension so that the contribution of such variables to differences in cardiovascular risk in SLE and control groups could be studied. Second, we used the Kawasaki formula to estimate a 24 hour Na+ and K+ excretion from the spot urine Na+ and K+ measurements. We then used the estimated 24 hour Na+ and K+ excretion as a surrogate for dietary intake. While this method has limitations as an estimation of the dietary intake of Na+ and K+, it does provide some advantages over other collection methods. Namely, using this Kawasaki formula reduces recall bias from patients reporting dietary intake and is more convenient as well as perhaps more accurate than 24 hour urine collections, particularly if patients are not reliable in their collections. Estimated 24 hour urinary Na+ and K+ excretion values obtained from the Kawasaki formula correlate with 24 hour urinary Na+ and K+ values.16, 32 Further, the Kawasaki formula has been validated in both the general population and in patients with hypertension17, 32 and used in large international studies.8, 18, 33–35 Renal disease may have affected the estimated urinary values. SLE patients with the renal criterion of the ACR SLE criteria23 had a lower estimated urinary K+ but similar Na+ and Na+: K+ ratio compared to the SLE patients without the renal criterion. We hypothesize that recommended diet changes in patients with a history of renal disease may have contributed to the lower K+ in the renal criterion group.
In conclusion, patients with SLE had a significantly lower estimated 24 hour urinary K+ and higher estimated 24 hour urinary Na+: K+ ratio than control subjects. The estimated 24 hour urinary Na+: K+ ratio was significantly associated with SBP and DBP in patients with SLE. This study highlights the potential role of Na+ and K+ intake to hypertension in SLE and represents an initial step to exploring the mechanisms of hypertension in SLE. Future prospective and longitudinal studies will be helpful to characterize the effect of modifications in the Na+ and K+ intake on hypertension in patients with SLE. As hypertension is a major contributor to adverse cardiovascular outcomes in SLE, identifying modifiable risk factors for hypertension is important in reducing cardiovascular morbidity and mortality.
Acknowledgments
Financial Support: Supported by grants NIH/NIAMS 5 T32 AR059039-05 (Barnado), NIH/NICHD 5 K12HD043483-12 (Barnado), K23AR064768 (Chung), Rheumatology Research Foundation (Chung), NIH/NIAMS P60AR056116 (Stein), NIH/NIAMS T32 GM07569 (Okafor), UL1 RR024975-01 (Stein), UL1 TR000445-06 (Stein), Alpha Omicron Pi (Stein), NCATS/NIH UL1 TR000445
Footnotes
Conflict of Interest: none
The Authors declare that there is no conflict of interest.
References
- 1.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: Comparison with the Framingham study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 2.Bruce IN, Urowitz MB, Gladman DD, Ibanez D, Steiner G. Risk factors for coronary heart disease in women with systemic lupus erythematosus: the Toronto Risk Factor Study. Arthritis Rheum. 2003;48:3159–3167. doi: 10.1002/art.11296. [DOI] [PubMed] [Google Scholar]
- 3.Sabio JM, Mediavilla JD, Fernandez-Torres C, Aliaga L, Jimenez-Alonso J. Risk factors related to hypertension in a Spanish systemic lupus erythematosus cohort. Lupus. 2001;10:451–452. doi: 10.1191/096120301678646227. [DOI] [PubMed] [Google Scholar]
- 4.Sabio JM, Zamora-Pasadas M, Jimenez-Jaimez J, et al. Metabolic syndrome in patients with systemic lupus erythematosus from Southern Spain. Lupus. 2008;17:849–859. doi: 10.1177/0961203308093554. [DOI] [PubMed] [Google Scholar]
- 5.Sabio JM, Vargas-Hitos JA, Navarrete-Navarrete N, et al. Prevalence of and factors associated with hypertension in young and old women with systemic lupus erythematosus. J Rheumatol. 2011;38:1026–1032. doi: 10.3899/jrheum.101132. [DOI] [PubMed] [Google Scholar]
- 6.Petri M. Detection of coronary artery disease and the role of traditional risk factors in the Hopkins Lupus Cohort. Lupus. 2000;9:170–175. doi: 10.1191/096120300678828226. [DOI] [PubMed] [Google Scholar]
- 7.Ryan MJ. The pathophysiology of hypertension in systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1258–1267. doi: 10.1152/ajpregu.90864.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mente A, O’Donnell MJ, Rangarajan S, et al. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med. 2014;371:601–611. doi: 10.1056/NEJMoa1311989. [DOI] [PubMed] [Google Scholar]
- 9.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 10.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP) BMJ. 2007;334:885–888. doi: 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2960–2984. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297:319–328. doi: 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang HY, Hu YW, Yue CS, et al. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr. 2006;83:1289–1296. doi: 10.1093/ajcn/83.6.1289. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Liu T, Kuklina EV, et al. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011;171:1183–1191. doi: 10.1001/archinternmed.2011.257. [DOI] [PubMed] [Google Scholar]
- 15.Cook NR, Obarzanek E, Cutler JA, et al. Trials of Hypertension Prevention Collaborative Research G: Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med. 2009;169:32–40. doi: 10.1001/archinternmed.2008.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol. 1993;20:7–14. doi: 10.1111/j.1440-1681.1993.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura M, Kusano Y, Takahashi T, Owada M, Sugawara T. Effectiveness of a spot urine method in evaluating daily salt intake in hypertensive patients taking oral antihypertensive drugs. Hypertens Res. 2006;29:397–402. doi: 10.1291/hypres.29.397. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371:612–623. doi: 10.1056/NEJMoa1311889. [DOI] [PubMed] [Google Scholar]
- 19.Wu C, Yosef N, Thalhamer T, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Shea JJ, Jones RG. Autoimmunity: Rubbing salt in the wound. Nature. 2013;496:437–439. doi: 10.1038/nature11959. [DOI] [PubMed] [Google Scholar]
- 21.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116:1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 23.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 24.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 25.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 26.Botev R, Mallie JP, Couchoud C, et al. Estimating glomerular filtration rate: Cockcroft-Gault and Modification of Diet in Renal Disease formulas compared to renal inulin clearance. Clin J Am Soc Nephrol. 2009;4:899–906. doi: 10.2215/CJN.05371008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reilly MP, Wolfe ML, Rhodes T, Girman C, Mehta N, Rader DJ. Measures of insulin resistance add incremental value to the clinical diagnosis of metabolic syndrome in association with coronary atherosclerosis. Circulation. 2004;110:803–809. doi: 10.1161/01.CIR.0000138740.84883.9C. [DOI] [PubMed] [Google Scholar]
- 28.Jain S, Shah M, Li Y, Vinukonda G, Sehgal PB, Kumar A. Upregulation of human angiotensinogen (AGT) gene transcription by interferon-gamma: involvement of the STAT1-binding motif in the AGT promoter. Biochim Biophys Acta. 2006;1759:340–347. doi: 10.1016/j.bbaexp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Satou R, Miyata K, Gonzalez-Villalobos RA, Ingelfinger JR, Navar LG, Kobori H. Interferon-gamma biphasically regulates angiotensinogen expression via a JAK-STAT pathway and suppressor of cytokine signaling 1 (SOCS1) in renal proximal tubular cells. FASEB J. 2012;26:1821–1830. doi: 10.1096/fj.11-195198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamat NV, Thabet SR, Xiao L, et al. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-gamma−/− and interleukin-17A−/− mice. Hypertension. 2015;65:569–576. doi: 10.1161/HYPERTENSIONAHA.114.04975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM. Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension. 2009;54:619–624. doi: 10.1161/HYPERTENSIONAHA.109.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mente A, O’Donnell MJ, Dagenais G, et al. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single morning fasting urine compared to 24-h measures in 11 countries. J Hypertens. 2014;32:1005–1014. doi: 10.1097/HJH.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 33.O’Donnell MJ, Yusuf S, Mente A, et al. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306:2229–2238. doi: 10.1001/jama.2011.1729. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama M, Tomiyama H, Kuwajima I, et al. Low salt intake and changes in serum sodium levels in the combination therapy of low-dose hydrochlorothiazide and angiotensin II receptor blocker. Circ J. 2013;77:2567–2572. doi: 10.1253/circj.cj-13-0287. [DOI] [PubMed] [Google Scholar]
- 35.Poss J, Ewen S, Schmieder RE, et al. Effects of renal sympathetic denervation on urinary sodium excretion in patients with resistant hypertension. Clin Res Cardiol. 2015;104:672–678. doi: 10.1007/s00392-015-0832-5. [DOI] [PubMed] [Google Scholar]


