To the Editor
Although the role of human regulatory B cells (BREGS) in auto-immunity has been extensively studied, there are limited studies exploring the potential importance of BREGS in allergy.1–7 A subset of peripheral blood BREGS that produces IL-10 (ie, CD19+CD73−CD25+CD71+ BREGS) has recently been demonstrated to inhibit TH2 responses.1–3 BREGS express the cell surface markers CD19 (a B cell marker), CD25 (the alpha chain of the IL-2 receptor also expressed by regulatory T cells or TREGS), and CD71 (a known activation marker of B cells), but express low amounts of CD73 (an ectonucleotidase thought to play a role in T cell suppression).1,2 Murine in vivo studies have demonstrated that antigen specificity is required to develop BREGS capable of secreting IL-10.8 Human BREGS also exhibit allergen specificity as demonstrated in studies of bee venom allergic subjects in whom lower circulating phospholipase A2-specific BREGS were found in venom allergic patients compared to venom tolerant beekeepers and venom immunotherapy patients.1 Importantly, T follicular helper (TFH) cell production of IL-21 is critical for the maturation of BREGS and their production of IL-10.8
In this study we investigated whether subjects with allergic rhinitis had reduced numbers of BREGS, as well as reduced numbers of BREGS expressing CD25hi, as prior studies of TREGS have identified the CD25hi population as the TREG subset most effective in inhibiting T cell responses.9 In addition to examining levels of BREGS and TFH cells in peripheral blood, we ascertained whether BREGS and TFH cells could be detected in human lymph nodes, a potential site of interaction between BREGS, TFH cells, and TH2 cells not previously studied in humans.
To compare circulating levels of BREGS and TFH cells, PBMCs were isolated from 10 allergic rhinitis individuals (mean age: 33.8; gender: 3 males, 7 females) and 7 non-allergic individuals (mean age: 37.9; gender: 2 males, 5 females) in a protocol approved by the University of California San Diego Human Subjects Protection Committee. Allergy status was confirmed by ImmunoCAP specific IgE levels or skin prick testing to cat, dog, cockroach, dust mite, grasses, trees, weeds, and molds (see Table E1 in the Online Repository). Using a previously described gating strategy1, BREGS (CD19+CD73−CD25+CD71+) were identified in isolated PBMCs by FACS using fluorescently labelled CD19, CD73, CD25, and CD71 antibodies as well as their corresponding isotype controls (eBioscience, San Diego, CA)(Fig 1, A). We also examined the percentage of BREGS expressing CD25hi, as prior studies of TREGS have identified the CD25hi population as the TREG subset most effective in inhibiting T cell responses (Fig 1, B).9 TFH-like cells (previously defined in peripheral blood as CD4+PD-1+CXCR5+ T cells)E1,E2 were also detected in PBMCs by FACS using fluorescently labeled CD4, CXCR5, PD-1 antibodies and their corresponding isotype controls (eBioscience)(Fig 1, A). Human lung lymph nodes were obtained from human lungs post-mortem as previously described at the Arkansas Children’s Hospital Research Institute in an IRB exempted protocol.E3 A single cell suspension was prepared by mechanical disruption of lymph nodes for FACS analysis in order to identify BREGS and TFH cells (as described above for PBMCs). Flow cytometry was performed using the BD Accuri C6 (BD Biosciences, San Jose, CA) and Novocyte Flow Cytometer (ACEA Biosciences, Inc., San Diego, CA) and was analyzed using FlowJo software (version 10.0.7, Tree Star, Inc., Ashland, OR). Statistical analyses were performed using one-tailed t tests and results reported as mean ± SEM.
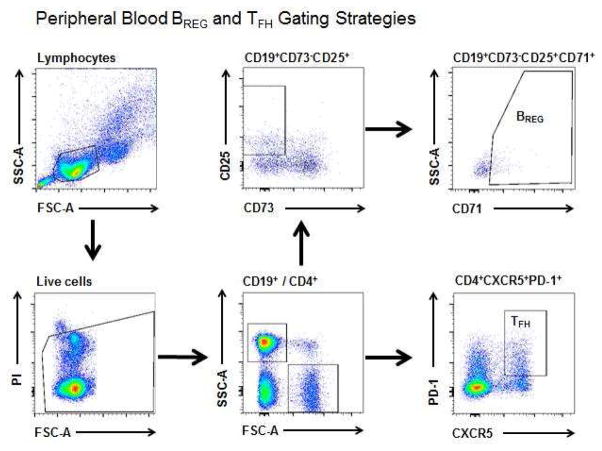
FIG 1. FACS Gating strategy to detect BREGS, CD25hi BREGS and TFH cells in peripheral blood.
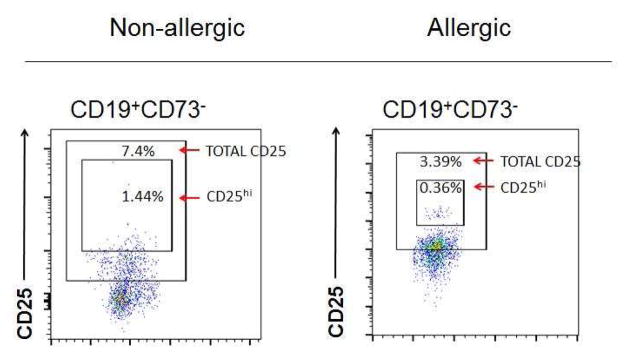
A, Circulating BREGS and TFH-like cells were identified from the circulating lymphocyte population as CD19+CD73−CD25+CD71+ and CD4+PD-1+CXCR5+ respectively using the gating strategy shown. B, Example of FACS of circulating BREGS from a non-allergic individual (left) and an allergic individual (right) including isotype controls were used to quantitate their total CD25+ and CD25hi subsets.
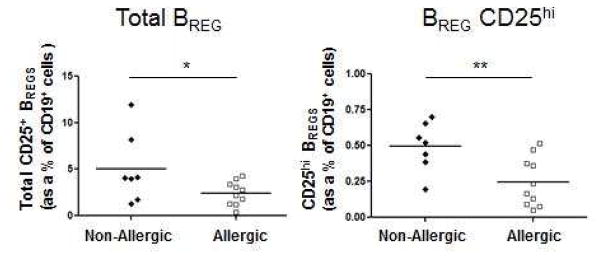
We found that percentages of CD25+ BREGS (2.45 ± 0.41 vs 5.05 ± 1.42)(P < 0.05) and CD25hi BREGS (0.25 ± 0.05 vs 0.49 ± 0.06)(P < 0.01) were lower in allergic rhinitis individuals compared to non-allergic controls (Fig 2, A). The lower levels of BREGS in allergic rhinitis subjects tended to cluster in a similar range, while non-allergic individuals exhibited a wider distribution. Our studies are consistent with reports showing that BREGS are reduced in allergic individuals compared to controls.1,2,4–5,7
FIG 2. FACS Quantitation of BREGS, CD25hi BREGS, and TFH-like cells in allergic rhinitis subjects and controls.
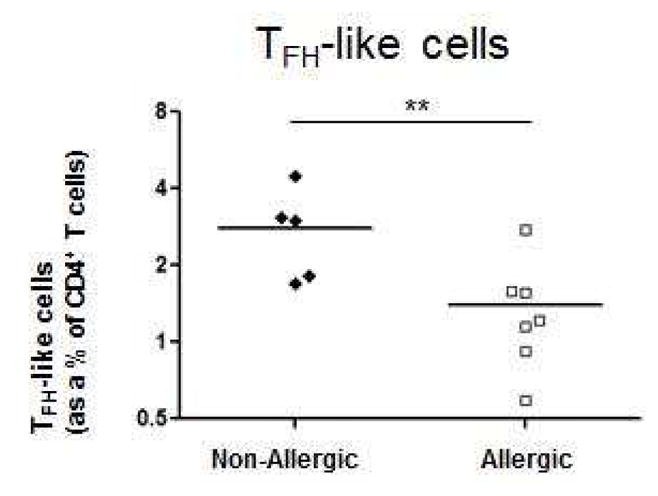
A, Peripheral blood total CD25+ (left) and CD25hi (right) BREGS from allergic rhinitis (n = 10) and non-allergic individuals (n = 7) were quantitated as percentages of CD19+ cells. B, Circulating CD4+PD-1+CXCR5+ TFH-like cells from allergic rhinitis (n = 7) and non-allergic individuals (n = 5) were quantitated as percentages of CD4+ T cells. *P < .05, **P < .01.
Levels of TFH-like cells (CD4+PD-1+CXCR5+) were significantly lower in allergic rhinitis individuals (1.40 ± 0.26) compared to non-allergic individuals (2.81 ± 0.51)(P < 0.01)(Fig 2, B). As BREGS have been recently demonstrated to undergo expansion under the direction of IL-21 produced by CD4+CXCR5+PD-1+ TFH cells in autoimmunityE4, the reduced numbers of TFH-like cells in allergic rhinitis may contribute to the reduced numbers of BREGS observed. We further determined that BREGS and TFH cells are detectable in human lung lymph nodes as has been demonstrated in secondary lymphoid organs in autoimmunity.E1,E2 Thus, BREGS and TFH cells are present in human lung lymph nodes and may regulate adaptive TH2 responses in allergic inflammation in asthma (see Fig E1 and E2 in the Online Repository).
To determine whether the BREG and TFH cells we quantitated by FACS expressed prototypic cytokines characteristic of BREGS (i.e. IL-10) and TFH cells (i.e. IL-21), we used cell sorting to obtain purified populations of BREGS and TFH cells (>99% pure populations) from one non-allergic blood donor who we had previously noted had increased numbers of these cells (Figures 1 and 2). Using these cell sorted populations, we demonstrated in vitro increased IL-10 protein production by BREGs stimulated with CpG (see Figure E3, A in the Online Repository), as well as increased IL-21 mRNA expression by TFH cells stimulated with anti-CD3 and anti-CD28 antibodies (see Figure E3, B in the Online Repository). A previous study4 in allergic rhinitis and asthma has also noted a reduced % of BREGS and TFH cells which were not phenotyped as in this study to determine whether they expressed prototypic cytokines (IL-10, and IL-21). Another difference between the two studies is the use of different cell surface markers to phenotype BREGS. In this study we used cell surface markers (CD19+CD73−CD25hiCD71+) previously used to characterize BREGS expressing IL-10 in allergic disease1, whereas the BREGS investigated in another study of allergic disease4 used BREG cell surface markers (CD3−CD19+CD24hiCD27+) previously characterized in auto-immune diseases.E5 BREGS identified in auto-immune disease may or may not function the same as BREGS identified in allergic disease and further studies are required to investigate the functional similarities and differences in these BREG subsets. Interestingly, CD38 has recently been reported to identify IL-10 producing BREGS in allergic disease.2 In this study, we did not examine antigen-specific IL-10 production by BREGS. However, previous studies have demonstrated that BREGS (using the same surface markers we used) had antigen-specificity to Phospholipase A2 in bee tolerant beekeepers.1
In summary, our findings show that BREGS (total and CD25hi) are reduced in subjects with allergic rhinitis. We have demonstrated that these BREGS produce IL-10 and as such, could suppress TH2 responses and play an important role in tolerance induction. We also demonstrated a significant reduction in levels of TFH-like cells and their corresponding IL-21 production in allergic subjects, and made the novel observation that these cells are present in human lung lymph nodes. At present, the relative contribution of BREGS compared to the TREGS in inducing tolerance to allergens is unclear. Interestingly, BREGS are able to induce TREGS suggesting that BREGS can have both a direct IL-10 effect on inducing tolerance, as well as an indirect effect through the induction of TREGS.3 Future studies of BREGS and TFH cells in allergic disease may identify their relative importance as compared to TREGS in natural and acquired tolerance induction by allergen immunotherapy.
Supplementary Material
Abbreviations
- BREG
Regulatory B cell
- TREG
Regulatory T cell
- TFH
T follicular helper cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Söllner S, Akdis DG, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131:1204–12. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Stanic B, van de Veen W, Wirz OF, Rückert B, Morita H, Söllner S, et al. IL-10-overexpressing B cells regulate innate and adaptive immune responses. J Allergy Clin Immunol. 2015;135:771–780. doi: 10.1016/j.jaci.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 3.Braza F, Chesne J, Castagnet S, Magnan A, Brouard S. Regulatory functions of B cells in allergic diseases. Allergy. 2014;69:1454–63. doi: 10.1111/all.12490. [DOI] [PubMed] [Google Scholar]
- 4.Kamekura R, Shigehara K, Miyajima S, Jitsukawa S, Kawata K, Yamashita K, et al. Alteration of circulating type 2 follicular helper T cells and regulatory B cells underlies the comorbid association of allergic rhinitis with bronchial asthma. Clin Immunol. 2015;158:204–11. doi: 10.1016/j.clim.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Noh J, Noh G, Kim HS, Kim AR, Choi WS. Allergen-specific responses of CD19(+)CD5(+)Foxp3(+) regulatory B cells (Bregs) and CD4(+)Foxp3(+) regulatory T cell (Tregs) in immune tolerance of cow milk allergy of late eczematous reactions. Cell Immunol. 2012;274:109–14. doi: 10.1016/j.cellimm.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Lee SJ, Noh G, Lee JH. In Vitro Induction of Allergen-Specific Interleukin-10-Producing Regulatory B Cell Responses by Interferon-γ in Non-Immunoglobulin E-Mediated Milk Allergy. Allergy Asthma Immunol Res. 2013;5:48–54. doi: 10.4168/aair.2013.5.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Vlugt LE, Mlejnek E, Ozir-Fazalalikhan A, Janssen Bonas M, Dijksman TR, Labuda LA, et al. CD24(hi)CD27(+) B cells from patients with allergic asthma have impaired regulatory activity in response to lipopolysaccharide. Clin Exp Allergy. 2014;44:517–28. doi: 10.1111/cea.12238. [DOI] [PubMed] [Google Scholar]
- 8.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov El, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491:264–8. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.