The interaction between infection and pediatric acute lymphoblastic leukemia (ALL) has been a focus of epidemiologic study for over 50 years. While a protective effect against ALL has often been reported when surrogates of infection exposure, such as day care attendance, were analyzed,1 studies of documented early infections indicated a subsequent increased risk of ALL.2 A mechanistic explanation for these apparently contradictory findings has yet to be provided. The demonstration that a leukemia-initiating cell (LIC) for pediatric B cell precursor (BCP) ALL frequently arises in utero has defined an extended pre-leukemic phase during which immune modulation could influence subsequent disease progression.3–5 Consistent with this hypothesis, delayed exposure to multiple infections increased leukemia penetrance in Pax5 heterozygous mice.6 A toll-like receptor (TLR) 4-driven up-regulation of intrinsic mutagenic activity was proposed as a potential in vivo driver of this leukemogenesis, based on its contribution to the transformation of ETV6-RUNX1-transduced pre-B cells.7 However, the potentially opposing impact of the induced immune response on the overall outcome of such inflammatory stimulation has not been evaluated. Here, we used the Eμ-ret8,9 and E2A-PBX110 transgenic mouse models of BCP ALL malignancy to investigate the influence of TLR ligation on LIC fate.
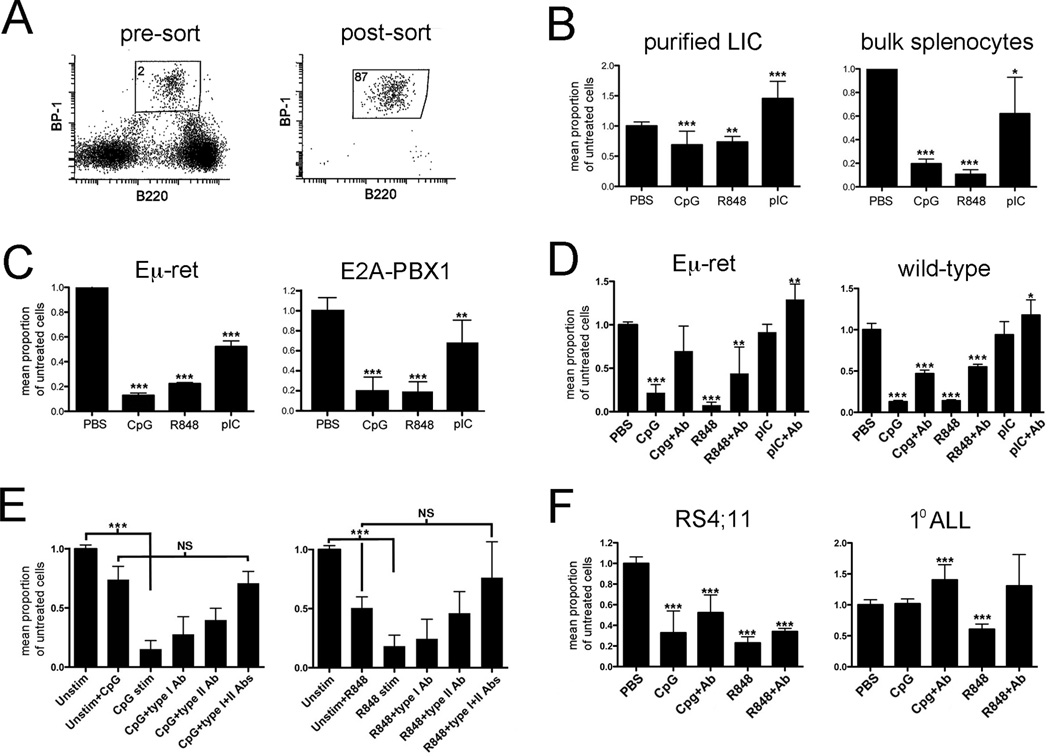
TLRs are important early sensors of infection and induce broad changes in the immune environment.11 We have previously shown that TLR stimulation induces protective T cell responses against fully transformed Eμ-ret leukemia cells in naïve wild-type mice.12 We now extend on that study by evaluating the effect of ligation of endosomal, nucleic acid-sensing TLRs on leukemia progression. An abnormal BCP population is detectable in bone marrow, spleen, and peripheral blood of Eμ-ret mice months prior to disease onset based on its characteristic B220int/BP-1hi phenotype;8,9 this enables both purification of LIC for in vitro studies (Fig. 1A), and correlation of quantitative changes in this cell population with disease progression in vivo. The leukemia-initiating potential of this BCP population is demonstrated by its progression to leukemia in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) recipient mice (data not shown). To evaluate for direct effects, abnormal BCP cells were purified from spleens of 4-week-old Eμ-ret mice and cultured in the presence of TLR ligands (Figure 1B). Both CpG ODN (TLR9 ligand) and R848 (TLR7/8 ligand) caused a significant reduction in viable cell recovery, while polyI:C (TLR3 ligand) induced a significant expansion of the BCP population compared to the PBS-treated control. This finding is consistent with the ability of LPS to stimulate pre-leukemic cells,7 but suggests that the nature of the response may be influenced by the specific TLR receptor that is engaged. To assess the impact of a concurrent immune response on these direct activities, bulk splenocyte cultures from 4–6 week-old Eμ-ret mice were similarly stimulated with TLR ligands (Figure 1B). Notably, the presence of immune effector cells resulted in significant depletion of viable BCP by each TLR ligand (p<0.0001 for each TLR ligand compared to its direct effects).
Figure 1.
TLR ligands exert direct and immune-mediated effects on leukemia-initiating cells. A) Sorting strategy for purification of abnormal BCP cells from Eμ-ret spleens. There is no counterpart with this phenotype in transgene-negative mouse spleens. The percent of cells in BCP gated region is shown. B) Purified LIC or bulk splenocytes, from spleens of 4 to 6-week-old Eμ-ret mice, were cultured for 48 hours in the presence of the indicated TLR ligands and the number of viable BCP counted. Cumulative results of 4 and 7 independent experiments, respectively, are shown. C) Eμ-ret LIC (CD43+/B220int/BP-1hi) and E2A-PBX1 LIC (GFP+/B220int/BP-1hi) obtained from healthy mice were cultured with NSG bone marrow cells and indicated TLR ligands for 48 hours and then evaluated for viable BCP numbers. Cumulative results from 3 independent experiments are shown. D) Bone marrow cells from 4-week-old mice were stimulated with the indicated TLR ligands for 48 hours in the absence or presence of anti-IFN-α receptor and anti-IFN-γ antibodies (+Ab). Normal (CD43+/B220int/BP-1+/CD24+) and abnormal (CD43+/B220int/BP-1hi) BCP were counted in wild-type and Eμ-ret cultures, respectively. Cumulative results from 7 mice are shown. E) Purified Eμ-ret LIC were cultured for 48 hours in supernatant obtained from 24-hour cultures of BALB/c bone marrow cells stimulated with either TLR9 ligand (CpG, left panel) or TLR 7/8 ligand (R848, right panel). IFN-α receptor (type I) or IFN-γ neutralizing (type II) antibody or both were added at the start of the 48-hour BCP cell culture. Cumulative results from 4 independent cultures are shown. F) Human BCP ALL cell line RS4;11 and a primary BCP ALL sample were cultured in media conditioned with supernatant from 24-hour TLR-stimulated PBMC. The graph depicts pooled results from all donors. In all panels, results are presented as number of viable cells in treated sample as a ratio of number of viable cells in unstimulated control. Unless otherwise shown, significance of difference from control is indicated (* denotes p<0.5, ** p<0.01, *** p<0.001).
The impact of changes in their immune environment on LIC is likely to be influenced by their lineage and stage of differentiation arrest. While, importantly, Eμ-ret mice consistently present with a leukemia arrested at the stage of B cell differentiation observed most often in pediatric ALL, RET is not a recognized driver of clinical disease. This raises the possibility that the observed depletion may be specific for LIC generated by this oncogene. To address this directly, we compared effects on Eμ-ret-derived LIC to those achieved with LIC generated in a multistep ALL model driven by the clinically relevant E2A-PBX1 translocation (Fig. 1C).10 When cultured on NSG bone marrow, LIC from Eμ-ret and Tg(E2A-PBX1) Pax+/− Cd19-Cre mice yielded a similar pattern of TLR-induced depletion. This result confirms the generality of the depletion response to distinct LIC populations and validates the Eμ-ret model for studies of immune influences on LIC survival and ALL progression.
The reduction in number of LIC achieved on NSG bone marrow implicated myeloid cells as an effector population. Endosomal TLR ligation in myeloid cells is a strong inducer of interferons (IFN) that can inhibit IL-7-driven BCP proliferation.13 Thus, we evaluated the contribution of IFN to the BCP depletion activity and investigated whether it affected normal BCP. Bone marrow cells from Eμ-ret or wild-type mice showed similar levels of BCP depletion when stimulated with CpG ODN or R848, and this activity was significantly reduced in the presence of IFN type I- and II-blocking antibodies (Figure 1D). Furthermore, in the absence of IFN activity, an expansion of BCP cells in response to polyI:C was achieved in both settings. The similar responses in wild-type and Eμ-ret cultures indicated that the TLR-induced activity targets BCP in general, rather than only the abnormal LIC. To evaluate if the activity of IFN was exerted directly on BCP, we exposed purified Eμ-ret-derived LIC to supernatant obtained from CpG ODN- or R848-stimulated BALB/c bone marrow cultures (Figure 1E). Such exposure was sufficient to achieve a depletion of similar magnitude to that observed in the presence of immune effector cells. For each TLR ligand, blockade of both type I and II IFN was necessary for maximal inhibition of BCP cell depletion, suggesting overlapping, direct inhibitory effects of IFN-α/β and IFN-γ on BCP cells. The depletion achieved in the presence of IFN-blocking antibodies was similar in magnitude to that resulting from direct activity, evaluated using supernatant from non-stimulated cells to which TLR ligand was added prior to incubation with BCP cells; this indicates that IFN activity accounts for most, if not all, the immune-mediated reduction in BCP cell number. To further evaluate the relevance of this immune pathway to human BCP ALL, we incubated ALL cell line RS4;11 and a primary ALL sample in media conditioned with supernatant from several human PBMC cultures stimulated with R848 or CpG ODN (Fig. 1F). IFN contributed significantly to the reduction in viable ALL cell recovery, though with more variability than was observed for LIC. An unexpected expansion of primary ALL cells was observed with CpG ODN-stimulated supernatant containing blocking antibodies from one donor, further emphasizing the variability in outcomes from such exposures.
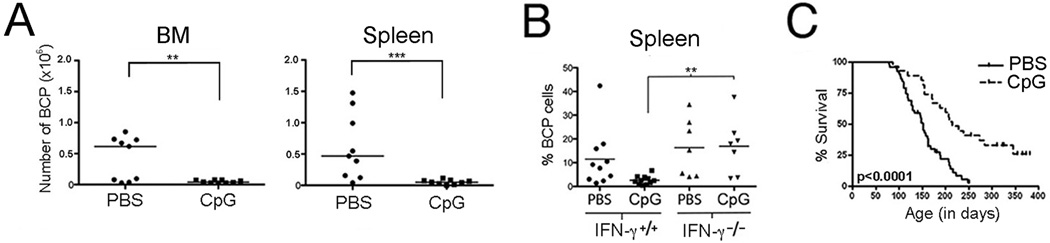
While informative, in vitro studies fail to capture the influence of specific microenvironments on immune activity and outcome. To confirm that a TLR-induced immune response could deplete abnormal BCP cells from an established pre-leukemic setting, we treated 4-week-old Eμ-ret mice with CpG ODN and then assessed LIC burden. At the completion of CpG ODN treatment, there was a significant reduction in the number of LIC in bone marrow and spleen of treated mice (Figure 2A), indicating that neither the presence of intact stroma nor the regulatory pathways that are integral to a functional immune system impart protection from depletion. A dysregulated immune response to a common infection is often invoked in models of BCP ALL etiology.14 Since the specific nature of this dysregulation is currently unknown, here we investigated how the loss of one component of the TLR-induced immune response would affect outcome by comparing BCP cell burden in young IFN-γ+/+ or IFN-γ−/− Eμ-ret mice similarly treated with CpG ODN (Figure 2B). Notably, significantly less BCP cell depletion was achieved in the absence of IFN-γ, suggesting that alterations in downstream pathways can alter TLR-induced depletion activity. Finally, to investigate whether TLR-mediated depletion of early-occurring abnormal BCP cells was capable of altering the course of disease, we treated Eμ-ret mice with CpG ODN between 4 and 8 weeks of age and monitored for leukemia onset. A reduction of BCP cell burden in peripheral blood was achieved at the completion of the CpG ODN treatment (p<0.05, data not shown), which correlated with a significant delay in disease onset in the treated group compared to control (median survival: 215 vs 152 days, p<0.0001; Figure 2C). Several mice remained disease-free beyond one year of age, suggesting that early-life immune response induction could reduce leukemia penetrance in this model.
Figure 2.
TLR-mediated immune modulation inhibits leukemia progression. A) A significant decrease in the number of LIC was observed in bone marrow (BM) and spleen of disease-free Eμ-ret mice following a 4-week intraperitoneal treatment with 100µg CpG ODN (CpG). Horizontal bars depict medians. B) The depletion of BCP cells following a 4-week treatment with 100µg CpG ODN was significantly attenuated in IFN-γ−/− mice compared to IFN-γ+/+ Eμ-ret mice (p<0.01). C) Beginning at 4 weeks of age, Eμ-ret mice were treated with either PBS or 100µg CpG ODN every 2 weeks for 8 weeks. CpG ODN administration resulted in significantly improved disease-free survival in CpG ODN (median 215 days, n=27) vs PBS (median 152 days, n=51) treated cohorts (log rank p<0.0001). * denotes p<0.05, ** p<0.01, *** p<0.001.
This study reveals the sensitivity of leukemia-initiating BCP cells from two transgenic mouse models of BCP ALL to changes in immune environment, and is the first to report the impact of these changes on disease development. Our results provide support for the hypothesis that immune modulation during the pre-leukemic phase can significantly alter progression of BCP ALL and provide a feasible mechanistic link for the reported association of early-life infections with protection from ALL. Infections have been associated with both an increase and a decrease in BCP ALL risk; therefore, our study does not capture the full range of potential immune effects. When combined with the emerging evidence of leukemia-promoting immune activity, however, it does expose the complexity of variables that probably underlie these divergent outcomes: variables such as the recognition pathways engaged by the infectious agent, the context and extent of direct stimulation of LIC, and the immune responsiveness of the host, may all contribute to the overall effect. The ability of IFN neutralization to switch the outcome of TLR3 stimulation from pre-leukemic cell depletion to a potentially pro-leukemic proliferative signal, together with the inability to achieve BCP cell depletion in the absence of IFN-γ, underscore the importance of host immune responsiveness in determining outcome. Our study identifies type I and II IFN as in vivo modifiers of LIC fate that may significantly affect the impact of putative leukemia-driving activities such as the ETV6-RUNX1-mediated growth advantage in the presence of TGF-β and TLR4-upregulated mutagenic activity.7,15 Understanding the interactions between these pathways, immune system development, and polymorphisms in immune response genes may reveal a unifying explanation for contrasting influences on BCP ALL risk that have been attributed to early-life infection.
Acknowledgments
This work was supported by grants to GSDR from the Prevent Cancer Foundation, When Everyone Survives Foundation, Michael Cuccione Foundation, and Canadian Institutes of Health Research (CIH; MOP-126122) and a Career Development Award in Prevention from the Canadian Cancer Society. Also supported in part by the American Society of Blood and Marrow Transplantation Robert A. Good Young Investigator Award, Helpful Gifts from the Heart Foundation, and American Cancer Society Mentored Research Scientist Grant in Applied and Clinical Research, MRSG-12-215-01-LIB (AES); a CFRI Graduate Studentship (M.F.); a NCI F31 Pre-doctoral Fellowship CA177092 and Training Grant TG GM-07229 of the University of Pennsylvania (A.D.); a CIHR fellowship grant (N.R.), a Cancer Research Institute Pre-doctoral Emphasis Pathway in Tumor Immunology Training Grant of the University of Pennsylvania (B.Y.); a Leukemia and Lymphoma Society Scholar Award and NIH grant CA125195 (C.H.B.); and NIH grants R01CA102646, R01CA116660, and support from the Weinberg Foundation (SAG); the German Research Foundation (Deutsche Forschungsgemeinschaft, ref DU 1287/2-1) (J.D.-A.); and the Lucile Packard Foundation for Children’s Health, the Child Health Research Institute and the Stanford NIH-NCATS-CTSA grant #UL1 TR001085 (M.L.C. and J.D.-A.).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Urayama KY, Buffler PA, Gallagher ER, Ayoob JM, Ma X. A meta-analysis of the association between day-care attendance and childhood acute lymphoblastic leukaemia. Int J Epidemiol. 2010;39:718–732. doi: 10.1093/ije/dyp378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crouch S, Lightfoot T, Simpson J, Smith A, Ansell P, Roman E. Infectious illness in children subsequently diagnosed with acute lymphoblastic leukemia: Modeling the trends from birth to diagnosis. Am J Epidemiol. 2012;176:402–408. doi: 10.1093/aje/kws180. [DOI] [PubMed] [Google Scholar]
- 3.Gale KB, Ford AM, Repp R, Borkhardt A, Keller C, Eden OB, et al. Backtracking leukemia to birth: identification of clonotypic gene fusion sequences in neonatal blood spots. Proc Natl Acad Sci U S A. 1997;94:13950–13954. doi: 10.1073/pnas.94.25.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taub JW, Konrad MA, Ge Y, Naber JM, Scott JS, Matherly LH, et al. High frequency of leukemic clones in newborn screening blood samples of children with B-precursor acute lymphoblastic leukemia. Blood. 2002;99:2992–2996. doi: 10.1182/blood.v99.8.2992. [DOI] [PubMed] [Google Scholar]
- 5.Wiemels JL, Ford AM, Van Wering ER, Postma A, Greaves M. Protracted and variable latency of acute lymphoblastic leukemia after TEL-AML1 gene fusion in utero. Blood. 1999;94:1057–1062. [PubMed] [Google Scholar]
- 6.Martin-Lorenzo A, Hauer J, Vicente-Duenas C, Auer F, Gonzalez-Herrero I, Garcia-Ramirez I, et al. Infection exposure is a causal factor in B-precursor acute lymphoblastic leukemia as a result of Pax5 inherited susceptibility. Cancer Discov. 2015:CD–15–0892. doi: 10.1158/2159-8290.CD-15-0892. [DOI] [PubMed] [Google Scholar]
- 7.Swaminathan S, Klemm L, Park E, Papaemmanuil E, Ford A, Kweon S-M, et al. Mechanisms of clonal evolution in childhood acute lymphoblastic leukemia. Nat Immunol. 2015;16:766–774. doi: 10.1038/ni.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasserman R, Zeng XX, Hardy RR. The evolution of B precursor leukemia in the Emu-ret mouse. Blood. 1998;92:273–282. [PubMed] [Google Scholar]
- 9.Zeng XX, Zhang H, Hardy RR, Wasserman R. The fetal origin of B-precursor leukemia in the E-mu-ret mouse. Blood. 1998;92:3529–3536. [PubMed] [Google Scholar]
- 10.Duque-afonso J, Feng J, Scherer F, Lin C, Wong SHK, Wang Z, et al. Comparative genomics reveals multistep pathogenesis of E2A-PBX1 acute lymphoblastic leukemia. J Clin Invest. 2015;125:3667–3680. doi: 10.1172/JCI81158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 12.Seif AE, Barrett DM, Milone M, Brown VI, Grupp SA, Reid GSD. Long-term protection from syngeneic acute lymphoblastic leukemia by CpG ODN-mediated stimulation of innate and adaptive immune responses. Blood. 2009;114:2459–2466. doi: 10.1182/blood-2009-02-203984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Lin Q, Langston H, Cooper MD. Resident bone marrow macrophages produce type 1 interferons that can selectively inhibit interleukin-7-driven growth of B lineage cells. Immunity. 1995;3:475–484. doi: 10.1016/1074-7613(95)90176-0. [DOI] [PubMed] [Google Scholar]
- 14.Greaves M. Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer. 2006;6:193–203. doi: 10.1038/nrc1816. [DOI] [PubMed] [Google Scholar]
- 15.Ford AM, Palmi C, Bueno C, Hong D, Cardus P, Knight D, et al. The TEL-AML1 leukemia fusion gene dysregulates the TGF-β pathway in early B lineage progenitor cells. J Clin Invest. 2009;119:826–836. doi: 10.1172/JCI36428. [DOI] [PMC free article] [PubMed] [Google Scholar]