Abstract
Adolescence, broadly defined as the period between childhood and adulthood, is characterized by a variety of neuroanatomical and behavioral changes. In human adolescents, the cerebral cortex, especially the prefrontal cortex, decreases in size while the cortical white matter increases. Puberty appears to be an important factor in both of these changes. However, the white matter continues to grow beyond what is thought to be adolescence, while the grey matter of the cortex stabilizes by young adulthood. The size changes that are the manifestation of cortical reorganization during human adolescence are also seen in cellular reorganization in the rat cortex. The prefrontal cortex loses neurons, dendrites and synapses while myelination in the white matter continues to increase. All of this reorganization is more marked in female rats, and there is evidence both from puberty timing and from removal of the ovaries that puberty plays an important role in initiating these changes in females. The maturation of behavioral functions of the prefrontal cortex, such as inhibitory control, occurs in both humans and rats across adolescence. There is also evidence for puberty as a major factor in decreasing perseveration in rats, but few studies have been done using pubertal status as an experimental variable, and the role of the gonadal steroids in modulating behavior throughout life makes clear effects more difficult to document. In all, puberty appears to be so essential to the changes occurring during adolescence that it should be recorded when possible, especially given the sex difference in pubertal timing.
Keywords: prefrontal cortex, adolescence, puberty, neuron number, inhibitory control
Introduction
Adolescence is the period between childhood and adulthood, characterized by neural maturation and changes in a variety of cognitive behaviors in addition to reproductive behavior. While it is obvious that libido and sexual behavior are set into motion by puberty, it is less apparent that pubertal onset is also involved in the maturation of the cerebral cortex and executive functions that occur during adolescence. It is difficult to establish the role of puberty in these changes in human adolescents where pubertal indices unfold over many years (Marshall and Tanner, 1969; 1970). In addition, specific information regarding pubertal status is often unavailable, especially for autopsy tissue where the cellular underpinnings of the loss of cortical volume can be examined. We will focus on cortical development and work from our laboratory in this review. We will present evidence from both human and animal models that puberty is an important event for many of the neural and behavioral changes that occur during adolescence.
Neural development during adolescence
Humans
Several MRI studies have indicated that the human cerebral cortex decreases in size during adolescence (Jernigan et al., 1991; Giedd et al., 1999; Sowell et al., 1999). While this appears to occur across many cortical areas, the prefrontal cortex (PFC) has the most reliably large decrease (Sowell et al., 2003; Gogtay et al., 2004), and this is especially interesting given that behaviors mediated by the PFC in particular undergo maturation during this time (Durston & Casey, 2006). There was a report that synaptic density in the PFC decreases during adolescence although the number of subjects and ages examined are very small (Huttenlocher, 1979). Still there is some supporting data from macaques (Bourgeois et al, 1994) and through quantification of the synaptic marker, synaptophysin, in the human PFC with Western blots (Glantz et al., 2007). While all of these studies contained both male and female subjects, only Giedd et al. (1999) described different trajectories in cortical maturation between the sexes. Giedd et al. noted the peak of cortical size could be affected by puberty; other investigators did not have the statistical power to draw such inferences.
Rats
The volume of the medial (m) PFC peaks and decreases across adolescence (Van Eden and Uylings, 1985) in rats as it does in humans, and thus rats have been the subjects for more detailed cellular analyses of neuroanatomical changes in the PFC. Adolescence has been previously defined as postnatal days (P) 28-42 (Spear, 2000). Spear admitted that this definition is arbitrary, and we would argue on the basis of the timing of puberty and its importance for adolescence (the topic of this review) that it be extended beyond P42. This is especially true for male rats where puberty occurs between P40 and P48 (Willing and Juraska, 2015; Korenbrot et al., 1977). Work from our laboratory has found several types of pruning between the adolescent period and adults that vary in degree with sex. Markham et al. (2007) found that the number of neurons decreased between P35 and P90, as did the volume of the mPFC in females. It should be noted that this was the total number of neurons, not just neuron density. The calculation of the number of neurons is the density of neurons, obtained with the optical disector, multiplied by the volume of the mPFC from cytoarchitectonic parcellation. Both sexes lost neurons in the mPFC but females had considerably greater losses than males. Interestingly, the adjacent anterior cingulate showed no differences in either sex between P35 and P90, indicating that not every cortical area loses cells during adolescence. The large decrease in the number of neurons after P35 in females was further confirmed in Willing and Juraska (2015) where a significant decrease in the number of neurons in the mPFC occurred between P35 and P45 (Figure 1A).
Figure 1.
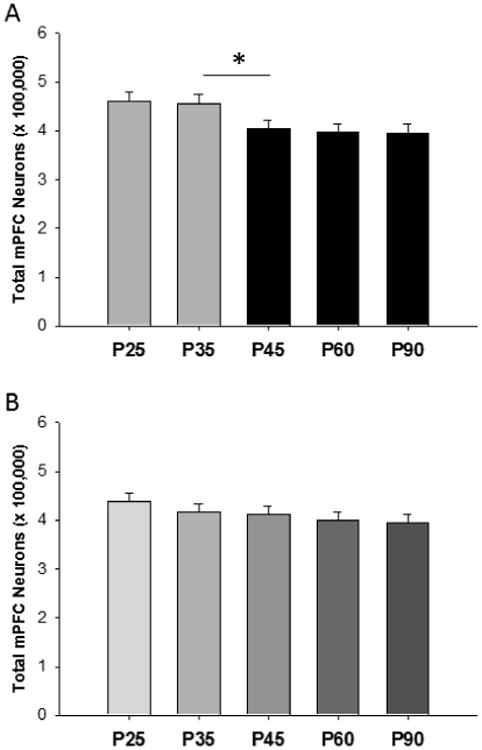
The number of neurons in the rat mPFC at postnatal ages that encompass adolescence. (A) Females lost neurons between P35 and P45, and the average age of pubertal onset was P35. *p<.05. (B) Unlike the females, males only showed a nonsignificant trend (p<.08) for age. From Willing and Juraska, 2015.
Dendritic pruning has also been found between adolescence and adulthood in layer 5 pyramidal neurons in the mPFC (Koss et al., 2014). Both males and females had a decrease in dendritic spine density between P35 and P90 (Figure 2), and females also lost dendritic branches, indicating an even greater decrease in the total number of dendritic spines. Interestingly, both dendritic spines and the total length of the dendritic tree increased in both sexes between P20 (the juvenile period) and P35. Thus the dendritic capacity is at a peak during adolescence in comparison to both juvenile and adult animals. The loss of synapses was confirmed across the whole mPFC in females through quantification of synaptophysin, a synaptic marker, which decreased between P35 and P45 in females (Drzewiecki et al., 2015; submitted). Changes in afferent and efferent projections between the mPFC and basolateral amygdala (BLA) have also been examined during adolescence in males. The number of neurons projecting from the PFC to the basal amygdala decreases from P45 to P90 in male rats, and concomitantly the number of mPFC axons in the basal amygdala decreases (Cressman et al., 2010). It is not clear whether this is due to neuron pruning during adolescence or to pruning of axon collaterals. This occurs while the number of axons from BLA within the mPFC is increasing from the juvenile period into adulthood without a discernable peak in adolescence (Cunningham et al., 2002).
Figure 2.
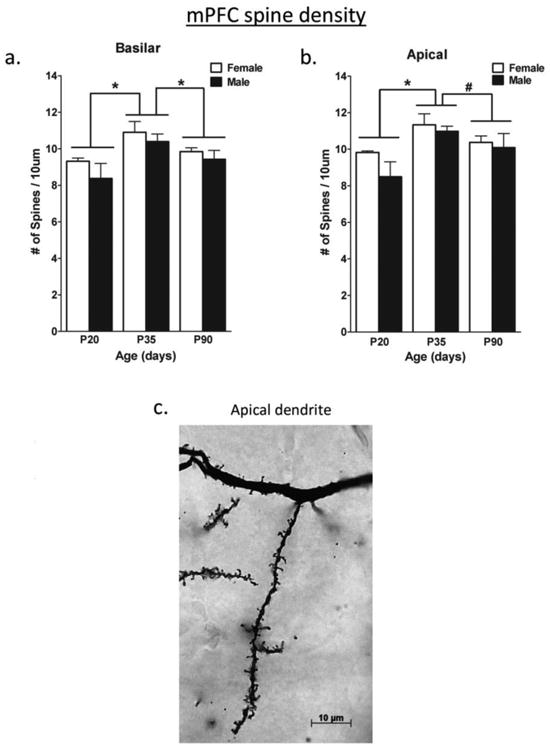
The density of dendritic spines at postnatal ages on layer V pyramidal neurons in the mPFC a) on the basilar dendrites and b) on the apical dendrites. Spines were pruned between P35 and P90. There were no sex differences. c) A photograph of dendritic spines visualized with a Golgi Cox stain. *p<.05; #p<.08 From Koss et al. (2014).
There are also changes in transmitter levels as well as their receptors during adolescence in the mPFC, but the picture is not yet a coherent one. The investigation of transmitter systems has mainly been done in males, and both NMDA receptor binding (from P28 to <P60) and dopamine receptor (D1 and D2) densities (P40 to P80) were found to be higher in the periadolescent period than in adults in the rat PFC (Insel et al, 1990; Andersen et al, 2000). Additionally, the electrophysiological response to dopamine within the male mPFC changes during adolescence (O'Donnell, 2010) with D2 agonists causing mild inhibition prepubertally (P36) but strong excitation after puberty (P50). Lastly, the GABA receptor also alters its composition during adolescence (Smith, 2013), and this topic will be reviewed elsewhere in this issue (Shen at al., 2016 this issue). Thus the adolescent rat mPFC undergoes considerable cellular reorganization much, but not all of which, is pruning.
In addition to local changes in cortical structure, the white matter carrying cortical afferents and efferents, including the white matter under the PFC, steadily increases during adolescence in humans and in rat models with males showing larger increases than females in both species (Giedd et al., 1999; Perrin et al., 2009; Markham et al., 2007; Willing and Juraska, 2015). The cellular basis for this increase appears to be continuing myelination which is supported by evidence from diffusion tensor imaging of humans (Herting et al., 2012). Electron microscopic quantification of axons in the splenium of the corpus callosum from the visual cortex confirms that the number of myelinated axons increases during adolescence (Figure 3). This occurs in both sexes but more axons are myelinated in males (Kim and Juraska, 1997) while there is no change in axon caliber within a class (myelinated vs unmyelinated). This implies that myelination is the basis for the increases in the size of all of the cortical white matter. Myelination extends to middle age, albeit at a slower rate, in both humans (Bartzokis et al., 2001; Courchesne et al., 2000) and rats (Nunez et al., 2000; Yates and Juraska, 2007) and is not a unique feature of adolescence.
Figure 3.
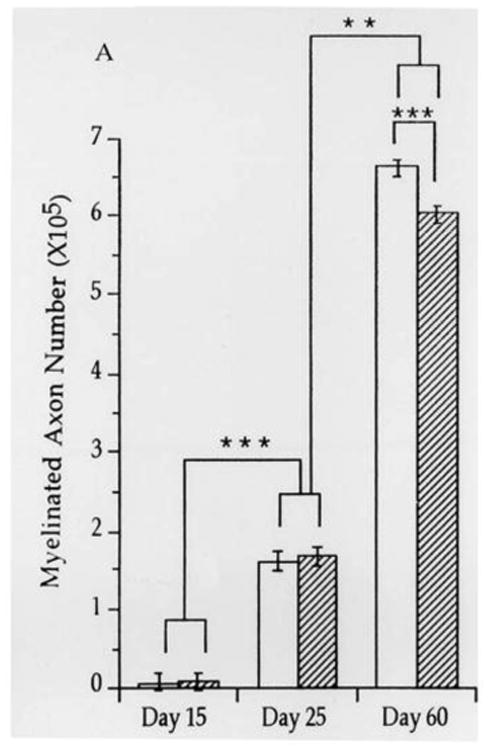
The number of myelinated axons in the splenium of the corpus callosum quantified from electron micrographs. Females are clear bars and males stripped bars. **p<.005; ***p<.0005 From Kim and Juraska, 1997.
Hormonal effects during adolescence
Hormone receptors
One obvious question is which hormone receptors are present peripuberally in the cerebral cortex, particularly within the mPFC. This very relevant topic has not been directly explored in the existing literature. In the adult human temporal cortex, both males and females have moderate levels estrogen receptor (ER) α and ERβ (Gonzalez et al., 2007). It is not known if these receptors are present in the PFC or if they are expressed during the pubertal transition. Like estrogen receptors, androgen receptors (AR) are also expressed in the primate and rat prefrontal cortex in adulthood (Finley & Kritzer, 1999; Aubele & Kritzer, 2012). In adult female rats, Shughrue et al. (1997) found moderate levels of ERβ in the mPFC. Although it is not known if they appear peripubertally, Westberry and Wilson (2012) found that in mice of both sexes, ERα decreases between P4 and P25 in the mPFC while ERβ increases. This indicates that ERβ may play a role in the mPFC during puberty. It also should be noted that progesterone receptors in the mPFC decrease after 10 days of age in rats and reach very low levels by P25 (Willing & Wagner, 2015). Thus while the information is incomplete, ERβ is the most likely candidate for mediating effects in the rodent mPFC.
Hormonal effects in humans
In order to understand the mechanisms behind the numerous cellular changes occurring during adolescence, an essential question is whether these changes are directly due to pubertal hormones or simply chronological age, independent of hormonal activity. There are indications that puberty is necessary for many neuroanatomical changes, although the evidence is circumstantial. Puberty is a protracted event in humans that continues for years. It is assessed through Tanner stage (either physical examination or self-report) and hormone levels, each of which has its limitations (see the discussion in Peper et al., 2011 and Herting et al., 2015). The Tanner stages indicate the long term continuation of rising hormones that may direct prolonged alterations in neural organization. These neural changes are difficult to discern except within an individual in longitudinal studies that include the pre-pubertal period. Additionally, hormone levels are not only generally increasing over time but also vary on a daily basis, in addition to the obvious monthly time frame for females. This makes hormone levels even more unreliable as an indicator of pubertal status.
Nonetheless, the studies that have tracked pubertal status have found that it is often an important factor for several measures. The progression in Tanner stages over a 2 year period marked a thinning of portions of the frontal cortex in both males and females (Herting et al., 2015). Tanner stage also predicted decreases in other cortical regions in a sex-specific manner. There were effects of hormone levels but they were less definitive than pubertal status (Herting et al., 2015). Likewise, both increasing levels of testosterone and dehydroepiandrosterone (DHEA), an adrenal androgen, across adolescence are associated with decreasing cortical thickness in both sexes (Nguyen et al., 2013). Taking a different approach, Raznahan et al. (2010) found that a more efficient androgen receptor allele was associated with more cortical thinning during adolescence in both sexes, even while sex differences persisted in the rate of frontal maturation. The increase in size of both the subcortical white matter in males (Perrin et al., 2009) and the corpus callosum in both males and females (Chavarria et al., 2014) correlated with testosterone levels. Lastly, the rate of cerebral blood flow has been found to vary with self-reported Tanner stage in a sex-dependent fashion (Satterthwaite et al., 2014). In total, these studies indicate that puberty is an important component in the reorganization of the cerebral cortex that can be documented with MRI in humans.
Hormonal effects in rats
The cellular basis for the effects of puberty can be explored more precisely in rat models since many of the gross size changes found in humans are also seen in rats, such as decreases in cortical volume and increases in white matter volume (Markham et al., 2007). Puberty can be directly prevented by removing the gonads during the juvenile period which preclude puberty, while avoiding the organizational effects of gonadal hormones during early development. Puberty in rats is relatively rapid with clear somatic markers: vaginal opening that coincides with increased luteinizing hormone output in females (Castellano et al., 2011) and preputial separation that marks increased testosterone secretion in males (Korenbrot et al., 1977). Although there are individual and strain differences in the exact day that puberty is reached, we have found that there is virtually no overlap in the range between males and females within a set of litters born in the same season under the same conditions. This helps disentangle the effects of age from those due to puberty and allows for studies in which monitoring of the pre- and post-pubertal period are clearly delineated.
There is considerable evidence that removal of the ovaries before the onset of puberty, usually at P20, stops that pruning within the cortex that occurs in intact females. Ovariectomy (OVX) before puberty resulted in increased numbers of both neurons and glia compared to control females in both the visual cortex and mPFC (Nunez et al., 2002; Koss et al., 2015) (Figure 4). There was no evidence of effects of gonadectomy in males in either of these cortical regions. Pre-pubertal OVX also halted the pruning of dendritic spines in the visual cortex of females (Muñoz-Cueto et al., 1990). Females ovariectomized before puberty had a greater volume of cortical white matter under the frontal cortex than intact controls (Koss et al., 2015), and OVX females had more myelinated axons in the corpus callosum (Yates and Juraska, 2008) which appears to be the basis for the increased volume since there was no change in the number of axons.
Figure 4.
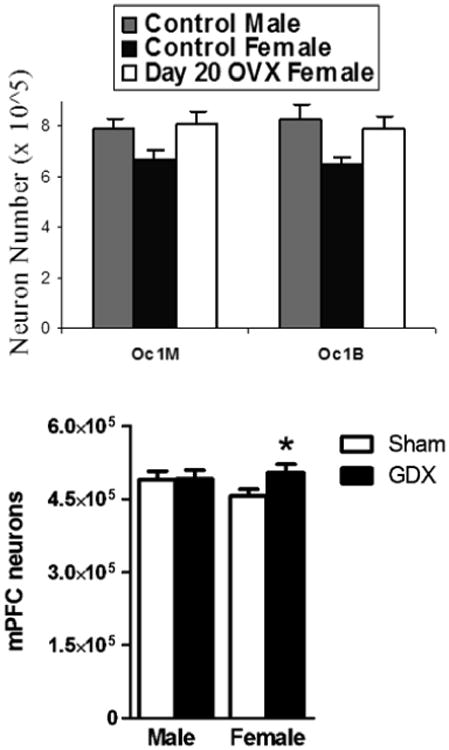
a) The number of neurons in the two subdivisions of the rat primary visual cortex (Oc1M and Oc1B) in adult rats. The control females had significantly (p<.05) fewer neurons in both divisions than control males and day 20 OVX females. Modified from Nunez et al., 2002. b) The number of neurons in the mPFC in adult rats. Gonadectomy (GDX) had occurred at P20. *p<.05 From Koss et al., 2015.
The results from tracking puberty have supported the findings with OVX that the rises in ovarian steroids are the primary cause of many neural changes. The number of neurons in the mPFC of intact females decreases between P35 and 45 (Figure 1A), and the average age of puberty in these females was P35 (range P32-38) (Willing and Juraska, 2015). Counts of GABA immunoreactive interneurons indicated that proportionately more non-GABAergic (pyramidal) neurons were lost than GABAergic were lost in these animals between P35 and P45. Neuron number, in general, was stable before and after the pubertal transition. Consistent with the findings of Koss et al. (2015), males had only statistically marginal decreases across adolescence with no marked change during puberty (average age P45) (Figure 1B).
There were indications of synaptic pruning, visualized with synaptophysin, in both sexes during the pubertal transition in the mPFC, although the data from females was more definitive (Drzewiecki et al., 2015; submitted). Synapse quantification came from the same brains as the neuron counts in Willing and Juraska (2015) and indicates that synapses can be lost without a significant loss of neurons. A different pattern was found in these brains with TH staining of afferent axons. TH is the rate limiting step in dopamine synthesis and the amount of dopamine can covary with TH levels (Masserano and Weiner, 1983). In primates, the upper layers of the PFC show some peripubertal pruning of TH fibers (Rosenberg and Lewis, 1995). This contrast with rats where TH increased in both males and females linearly in the mPFC between P25 and P90 with females showing proportionately more of an increase between P25 and P35 than males (Willing et al., 2015; unpublished data; Susan Andersen, personal communication). This indicates that puberty, although important for cellular reorganization, is not the sole mechanism of neural change during the adolescent period.
Behavioral changes and hormone effects
Adolescence
The adolescent period in humans is known to be associated with changes in performance on a variety of tasks, especially those that are PFC-dependent. Notably, cognitive control, defined as situationally appropriate behavioral responses in the face of conflicting ones, improves considerably between the juvenile period and adulthood (see Durston & Casey, 2006 for review). Included within this definition is behavioral inhibition and cognitive flexibility, which coincide with a decrease in perseverative behavior. In humans, performance on tasks measuring cognitive control improves from childhood to adulthood (reviewed in Lourenco & Casey, 2013; Taylor et al., 2013; Davidson et al., 2006; Casey, 2015). In general, these improvements manifest in laboratory tasks as increases in social cognition, concept formation, task-switching, working memory, and the inhibition of inappropriate responses that were formerly rewarded. It is unclear whether these abilities are improving across adolescence without pubertal influence or are either dependent upon or influenced by the neural reorganization of puberty.
Both humans and mice show attenuated fear extinction during adolescence compared to juveniles and to adults (Pattwell et al., 2012). This task, which involves the PFC, is an illustration of the uniqueness of adolescence that goes across species. There are other examples of increased performance on PFC-dependent tasks between adolescence and adulthood in rats. Compared to adults, adolescent rats display a learning deficit on a delayed alternation task and commit a greater number of perseveration errors (Koss et al., 2011). Additionally, across several tasks, adolescent rats are less sensitive to extinction or reward devaluation compared with adults (Sturman et al., 2010; Andrzejewski et al., 2011; Naneix et al., 2012; Hammerslag & Gulley, 2014), suggesting an impairment in impulse control and behavioral inhibition. These studies collectively suggest that in both humans and rats, PFC maturation during adolescence coincides with decreases in perseverative responding and inhibitory control, functions that are dependent on PFC interactions with other parts of the limbic system such as the basolateral amygdala.
Puberty
Though not typically examined as an experimental variable, there is some evidence that pubertal status plays a role in behaviors that mature during human adolescence (reviewed in Blakemore et al., 2010). Pubertal onset, in particular, seems to trigger an increase in behavioral responsiveness to emotionally salient stimuli, which is correlated with changes in functional connectivity between the PFC and subcortical limbic regions (Ladouceur, 2012; Klapwijk et al., 2013). Additionally, pubertal status has been shown to acutely affect performance on the PFC-dependent match-to-sample task, where pubertal onset actually induced a transient decrease in performance (McGivern et al., 2002), possibly reflecting ongoing functional changes in PFC anatomy or connectivity. A rise in pubertal testosterone in males has been found to be associated with an increase in mental rotation performance (Vuoksima et al., 2012), which also relies on PFC activity. Lastly in male rhesus macaques, pre-pubertal gonadectomy was associated with increases in pre-pulse inhibition responses (Morris et al., 2010), which is dependent on integrated cortical activity including the PFC.
In rats, it has been widely found that steroid hormones in both males and females contribute to performance on learning and memory tasks (reviewed in Juraska & Rubinow, 2008; Luine, 2008; Frick et al., 2010). However, there is little evidence thus far that puberty itself directly results in developmental changes in cognitive behavior. Evidence for a direct role for pubertal onset in cognition has been particularly difficult to elucidate using certain cognitive tasks given that puberty has been shown to alter learning strategies in spatial memory/navigation paradigms (Kanit et al., 2000; Rodriguez et al., 2013). In a recent study from our laboratory (Willing et al., in press), we used pubertal onset as a factor to explore changes in performance in male and female rats on the water maze and also on a reversal task that assessed cognitive flexibility. Pubertal status did not have an effect on short or long-term spatial memory for the location of an escape platform in the initial training. This is consistent with Schenk (1985) who found that adolescent rats performed at the same level as adults in the water maze task. However, when we moved the location of the platform, pre-pubertal males and females had an increased path length compared to post-pubertal animals (Figure 5). Additionally, during these trials, pre-pubertal rats spent more time in the quadrant of the maze where the platform was originally located, which may be indicative of perseverative behavior, than recently post-pubertal and adult animals. No differences were seen for any measure between recently post-pubertal and adult rats. This subtle yet significant effect of pubertal onset suggests that puberty leads to relatively rapid changes in cognitive behavior that may be linked with the neuroanatomical alterations mediated by puberty. Interestingly, with non PFC-dependent tasks, such as basic conditioning and spatial memory, pre-pubertal animals are not different than adults (Stanton & Freeman, 2000; Raineki et al., 2009; Vorhees et al., 2005; Brown et al., 2005; Wojniusz et al., 2013) and in place avoidance pubertal females are impaired (Shen et al., 2010; Shen et al., 2016 this issue). Future studies examining cognitive behavior during adolescence should account for pubertal status and for the sex difference in the age of pubertal onset, since pubertal status may be at least as important as chronological age.
Figure 5.
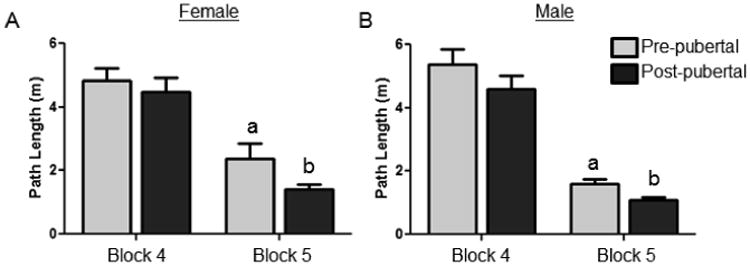
Path length to reach the novel platform location in the Morris water maze. Path length was significantly lower in post-pubertal males and females in comparison to prepubertal after the location of an escape platform was moved. a>b p<03 Modified from Willing et al., in press.
Conclusions
There is considerable evidence that puberty is a central event in the reorganization of the cortex, especially the prefrontal cortex, during adolescence in both humans and rats. The evidence for the role of pubertal onset in the maturation of the functions of the cortex is less definitive, given the paucity of studies done where puberty was used as an experimental variable. Still, many types of behavior are influenced by gonadal steroids throughout life, suggesting that puberty could affect neural reorganization as well as the onset of hormonal modulation of behavior. Because these are not easily separable in humans, we suggest more work in rodents is needed to investigate the effects of pubertal onset on the development of higher order behavioral functioning.
Highlights.
Pruning occurs in the prefrontal cortex of adolescent humans and rats
White matter increases throughout adolescence
Evidence indicates that pubertal onset affects reorganization in the cortex
Puberty may also impact the development of inhibitory control
Documentation of pubertal status is valuable when studying adolescence
Acknowledgments
The recent unpublished work was supported by NIH MH099625 to JMJ and NIH Training Grant T32 ES007326 currently supports JW.
Footnotes
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Andrzejewski ME, Schochet TL, Feit EC, Harris R, McKee BL, Kelley AE. A comparison of adult and adolescent rat behavior in operant learning, extinction, and behavioral inhibition paradigms. Behav Neurosci. 2011;125(1):93–105. doi: 10.1037/a0022038. [DOI] [PubMed] [Google Scholar]
- Aubele T, Kritzer MF. Androgen influence on prefrontal dopamine systems in adult male rats: localization of cognate intracellular receptors in medial prefrontal projections to the ventral tegmental area and effects of gonadectomy and hormone replacement on glutamate-stimulated extracellular dopamine level. Cereb Cortex. 2012;22(8):1799–1812. doi: 10.1093/cercor/bhr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58(5):461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31(6):926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4(1):78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15(3):275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Bentsen AH, Sanchez-Garrido MA, RuizPino F, Romero M, GarciaGaliano D, Aguilar E, Pinilla L, Dieguez C, Mikkelsen JD, Tena-Sempere M. Early metabolic programming of puberty onset: Impact of changes in postnatal feeding and rearing conditions on the timing of puberty and the development of the hypothalamic kisspeptin system. Endocrinology. 2011;152(9):3396–3408. doi: 10.1210/en.2010-1415. [DOI] [PubMed] [Google Scholar]
- Chavarria MC, Sanchez FJ, Chou YY, Thompson PM, Luders E. Puberty in the corpus callosum. Neuroscience. 2014;265:1–8. doi: 10.1016/j.neuroscience.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216(3):672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol. 2010;518(14):2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition and task switching. Neuropsychologia. 2006;44(11):2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Casey BJ. What have we learned about cognitive development from neuroimaging? Neuropsychologia. 2006;44(11):2149–2157. doi: 10.1016/j.neuropsychologia.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Drzewiecki CM, Willing J, Juraska JM. Changes in the number of synapses in the medial prefrontal cortex across adolescence. Society for Neuroscience Meeting; Chicago. 2015. Abstract 569.24. [Google Scholar]
- Finley SK, Kritzer MF. Immunoreactivity for intracellular androgen receptors in identified subpopulations of neurons, astrocytes and oligodendrocytes in primate prefrontal cortex. J Neurobiol. 1999;40(4):446–457. [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Harburger LL. A new approach to understanding the molecular mechanisms through which estrogens affect cognition. Biochim Biophys Acta. 2010;1800(10):1045–1055. doi: 10.1016/j.bbagen.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149(3):582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerslag LR, Gulley JM. Age and sex differences in reward behavior in adolescent and adult rats. Dev Psychobiol. 2014;56(4):611–621. doi: 10.1002/dev.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex. 2012;22(9):1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Gautam P, Spielberg JM, Dahl RE, Sowell ER. A longitudinal study: changes in cortical thickness and surface area during pubertal maturation. PLoS One. 2015;10(3):e0119774. doi: 10.1371/journal.pone.0119774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex- developmental changes and effects of aging. Brain Res. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Insel TR, Miller LP, Gelhard RE. The ontogeny of excitatory amino acid receptors in rat forebrain- I. N-methyl-D-aspartate and quisqualate receptors. Neuroscience. 1990;35(1):31–43. doi: 10.1016/0306-4522(90)90117-m. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;115(Pt 5):2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Rubinow MJ. Hormones and Memory. In: Eichenbaum H, editor. Memory Systems Vol [3] of Learning and Memory: A Comprehensive Reference, (4 vols, JByrne, Editor) Academic Press: Elsevier; 2008. pp. 503–520. [Google Scholar]
- Kanit L, Taskiran D, Yilmaz OA, Balkan B, Demirgören S, Furedy JJ, Pögün S. Sexually dimorphic cognitive style in rats emerges after puberty. Brain Res Bull. 2000;52(4):243–248. doi: 10.1016/s0361-9230(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Kim JH, Juraska JM. Sex differences in the development of axon number in the splenium of the rat corpus callosum from postnatal day 15 through 60. Brain Res Dev Brain Res. 1997;102(1):77–85. doi: 10.1016/s0165-3806(97)00080-1. [DOI] [PubMed] [Google Scholar]
- Klapwijk ET, Goddings AL, Burnett Heyes S, Bird G, Viner RM, Blakemore SJ. Increased functional connectivity with puberty in the mentalising network involved in social emotion processing. Horm Behav. 2013;64(2):314–322. doi: 10.1016/j.yhbeh.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial separation as an external sign of pubertal development in the male rat. Biol Reprod. 1977;17(2):298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- Koss WA, Franklin AD, Juraska JM. Delayed alternation in adolescent and adult male and female rats. Dev Psychobiol. 2011;53(7):724–731. doi: 10.1002/dev.20543. [DOI] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats. Synapse. 2014;68(2):61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- Koss WA, Lloyd MM, Sadowski RN, Wise LM, Juraska JM. Gonadectomy before puberty increases the number of neurons and glia in the medial prefrontal cortex of female, but not male, rats. Dev Psychobiol. 2015;57(3):305–312. doi: 10.1002/dev.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD. Neural systems supporting cognitive-affective interactions in adolescence: the role of puberty and implications for affective disorders. Front Integr Neurosci. 2012;6:65. doi: 10.3389/fnint.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco F, Casey BJ. Adjusting behavior to changing environmental demands with development. Neurosci Biobehav Rev. 2013;37(9 Pt B):2233–2242. doi: 10.1016/j.neubiorev.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. Sex steroids and cognitive function. J Neuroendocrinol. 2008;20(6):866–872. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144(3):961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masserano JM, Weiner N. Tyrosine hydroxylase regulation in the central nervous system. Mol Cell Biochem. 1983;53-54(1-2):129–152. doi: 10.1007/BF00225250. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Andersen J, Byrd D, Mutter KL, Reilly J. Cognitive efficiency on a match to sample task decreases at the onset of puberty in children. Brain Cogn. 2002;50(1):73–89. doi: 10.1016/s0278-2626(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Morris RW, Fung SJ, Rothmond DA, Richards B, Ward S, Noble PL, Woodward RA, Weickert CS, Winslow JT. The effect of gonadectomy on prepulse inhibition and fear-potentiated startle in adolescent rhesus macaques. Psychoneuroendicrinology. 2010;35(6):896–905. doi: 10.1016/j.psyneuen.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cueto JA, García-Segura LM, Ruiz-Marcos A. Developmental sex differences and effect of ovariectomy on the number of cortical pyramidal cell dendritic spines. Brain Res. 1990;515(1-2):64–68. doi: 10.1016/0006-8993(90)90577-x. [DOI] [PubMed] [Google Scholar]
- Naneix F, Marchand AR, Di Scala G, Pape JR, Coutureau E. Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence. J Neurosci. 2012;32(46):16223–16232. doi: 10.1523/JNEUROSCI.3080-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, McCracken JT, Ducharme S, Cropp BF, Botteron KN, Evans AC, Karama S. Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. J Neurosci. 2013;33(26):10840–10848. doi: 10.1523/JNEUROSCI.5747-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez JL, Nelson J, Pych JC, Kim JH, Juraska JM. Myelination in the splenium of the corpus callosum in adult male and female rats. Brain Res Dev Brain Res. 2000;120(1):87–90. doi: 10.1016/s0165-3806(99)00193-5. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol. 2002;52(4):312–321. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. Adolescent maturation of cortical dopamine. Neurotox Res. 2010;18(3-4):306–312. doi: 10.1007/s12640-010-9157-3. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, Soliman F, Glatt CE, Casey BJ, Ninan I, Lee FS. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci. 2012;109(40):16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Sex differences in the growth of white matter during adolescence. Neuroimage. 2009;45(4):1055–1066. doi: 10.1016/j.neuroimage.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Raineki C, Shionoya K, Sander K, Sullivan RM. Ontogeny of odor-LiCl vs. odorshock learning: similar behaviors but divergent ages of functional amygdala emergence. Learn Mem. 2009;16(2):114–121. doi: 10.1101/lm.977909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Lee Y, Stidd R, Long R, Greenstein D, Clasen L, Addington A, Gogtay N, Rapoport JL, Giedd JN. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci. 107(39):16988–16993. doi: 10.1073/pnas.1006025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez CA, Chamizo VD, Mackintosh NJ. Do hormonal changes that appear at the onset of puberty determine the strategies used by female rats when solving a navigation task? Horm Behav. 2013;64(1):122–135. doi: 10.1016/j.yhbeh.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: A tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358(3):383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Shinohara RT, Wolf DH, Hopson RD, Elliot MA, Vanderskar SN, Ruparel K, Calkins ME, Roalf DR, Gennatas ED, Jackson C, Erus G, Prabhakaran K, Davatzikos C, Detre JA, Hakonarson H, Gur RC, Gur RE. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc Natl Acad Sci. 2014;111(23):8643–8648. doi: 10.1073/pnas.1400178111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk F. Development of place navigation in rats from weaning to puberty. Behav Neural Biol. 1985;43(1):69–85. doi: 10.1016/s0163-1047(85)91510-9. [DOI] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for α4βδ GABAA receptors in shaping learning deficits at puberty in mice. Sci. 2010;327(5972):1515–8. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Smith SS. α4βδ GABAA receptors and tonic inhibitory current during adolescence: effects on mood and synaptic plasticity. Front Neural Circuits. 2013;7:135. doi: 10.3389/fncir.2013.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2(10):859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH., Jr . Developmental studies of eyeblink conditioning in the rat. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink Classical Conditioning Vol [2], Animal Models. Springer Science & Business Media; 2000. pp. 105–134. [Google Scholar]
- Sturman DA, Mandell DR, Moghaddam B. Adolescents exhibit behavioral differences from adults during instrumental learning and extinction. Behav Neurosci. 2010;124(1):16–25. doi: 10.1037/a0018463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SJ, Barker LA, Heavey L, McHale S. The typical developmental trajectory of social and executive functions in late adolescence and early adulthood. Dev Psychol. 2013;49(7):1253–1265. doi: 10.1037/a0029871. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HB. Postnatal volumetric development of the prefrontal cortex in the rat. J Comp Neurol. 1985;241(3):268–264. doi: 10.1002/cne.902410303. [DOI] [PubMed] [Google Scholar]
- Voorhees CV, Reed TM, Morford LL, Fukumura M, Wood SL, Brown CA, Skelton MR, McCrea AE, Rock SL, Williams MT. Periadolescent rats (P41-50) exhibit increased susceptibility to D-methamphetamine-induced long-term spatial and sequential learning deficits compared to juvenile (P21-30 or P31-40) or adult rats (P51- 60) Neurotoxicol Teratol. 2005;27(1):117–134. doi: 10.1016/j.ntt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Vuoksima E, Kaprio J, Eriksson CJ, Rose RJ. Pubertal testosterone predicts mental rotation performance of young adult males. Psychoneuroendocrinology. 2012;37(11):1791–1800. doi: 10.1016/j.psyneuen.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westberry JM, Wilson ME. Regulation of estrogen receptor alpha gene expression in the mouse prefrontal cortex during early postnatal development. Neurogenetics. 2012;13(2):159–167. doi: 10.1007/s10048-012-0323-z. [DOI] [PubMed] [Google Scholar]
- Willing J, Brodsky JM, Cortes LR, Kim T, Juraska JM. Development of dopaminergic fibers in the medial prefrontal cortex of male and female rats during adolescence. Society for Neuroscience Meeting; Chicago. 2015. Abstract 569.12. [Google Scholar]
- Willing J, Drzewiecki CM, Cuenod BA, Cortes LR, Juraska JM. A role for puberty in water maze performance in male and female rats. Behavioral Neuroscience. doi: 10.1037/bne0000145. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Juraska JM. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience. 2015;301:268–275. doi: 10.1016/j.neuroscience.2015.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing J, Wagner CK. Progesterone receptor expression in the developing mesocortical dopamine pathway: importance for complex cognitive behavior in adulthood. Neuroendocrinology. 2015 doi: 10.1159/000434725. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojniusz S, Ropstad E, Evans N, Robinson J, Solbakk AK, Endestad T, Haraldsen IR. Sex-specific development of spatial orientation is independent of peripubertal gonadal steroids. Psychoneuroendicrinology. 2013;38(9):1709–1716. doi: 10.1016/j.psyneuen.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Yates MA, Juraska JM. Increases in size and myelination of the rat corpus callosum during adulthood are maintained into old age. Brain Res. 2007;1142:13–18. doi: 10.1016/j.brainres.2007.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MA, Juraska JM. Pubertal ovarian hormone exposure reduces the number of myelinated axons in the splenium of the rat corpus callosum. Exp Neurol. 2008;209(1):284–287. doi: 10.1016/j.expneurol.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


