1. Introduction
Temporal lobe epilepsy (TLE) is the most prevalent form of epilepsy in adults, often with high rates of pharmacoresistance [1]. To date, there are no FDA-approved therapies or interventions to cure or prevent TLE. Epilepsy is a paroxysmal disorder, with the defining feature (seizures) occurring with little to no warning. Unpredictable seizures make many normal activities, like driving a car or holding down certain jobs, impossible [2]. Stress is repeatedly reported as one of the most common seizure triggers in patients with an epilepsy diagnosis, including TLE [3–9]. Understanding the molecular mechanisms underlying this association may allow clinicians to predict seizure episodes and/or mitigate their disruptive effect. A second unmet need for TLE patients is a better understanding of, and treatments for, the high incidence of comorbid stress-related psychopathologies, such as depression and anxiety [10–14].
Increased activity of the hypothalamo-pituitary-adrenocortical (HPA) axis is hypothesized to link core epilepsy symptoms and associated stress-related psychopathologies [15–17]. The relationship may be bidirectional [18,19]. Therefore, HPA axis dysfunction in TLE may be a common etiological mechanism underlying stress-evoked seizures and stress-related psychopathologies. The purpose of this review is to 1) summarize the basic functions of the HPA axis, 2) discuss the current evidence that this system is disrupted in TLE, 3) consider potential mechanisms by which the HPA axis is damaged in rodent models of TLE and 4) discuss the implications of HPA axis dysfunction in humans for seizure triggering and psychiatric comorbidities.
2. The HPA axis stress response and the importance of temporal lobe structures in its regulation
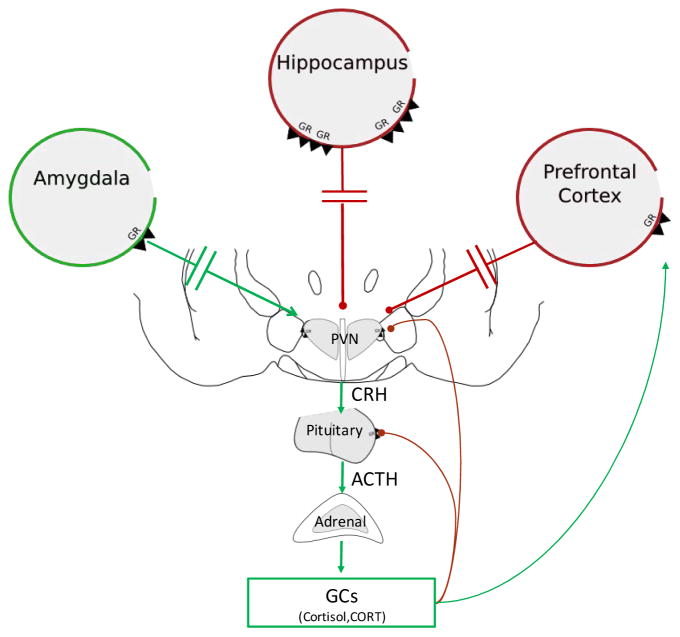
The physiological response to stress is highly conserved throughout vertebrate phylogeny. The HPA axis stress response allows individuals to adapt and cope when faced with real or perceived threats of physical or emotional significance. Upon exposure to stress, neurons in the paraventricular nucleus of the hypothalamus release corticotrophin releasing hormone (CRH), which travels through the hypophyseal portal system to cause release of adrenocorticotrophic hormone (ACTH) from the anterior pituitary. ACTH stimulates the adrenal cortex to synthesize and secrete glucocorticoids; cortisol in primates and corticosterone in rats and mice (Figure 1). Glucocorticoids act in the brain and in the periphery via binding to two major receptor types, the mineralocorticoid receptor (MR) and glucocorticoid receptor (GR). MRs bind glucocorticoids with high affinity in the brain and are thought to be largely saturated at low (non-stress) levels of circulating glucocorticoids. GRs have a lower affinity for glucocorticoids and are responsive over a wide dynamic range. Consequently, GRs are primarily responsible for the physiological effects of stress-induced glucocorticoid secretion. Together, GR/MR binding regulates gene activity to maintain energy homeostasis, control endogenous inflammatory processes and modulate cognition [20]. Glucocorticoids also act to regulate their own secretion via negative feedback pathways.
Figure 1. Hypothalamo-pituitary-adrenocortical axis.
Upon a stressful event (i.e. psychogenic or physical), activation of the paraventricular nucleus of the hypothalamus (PVN) results in the release of corticotrophin releasing hormone (CRH). CRH binds to its receptors in the anterior pituitary to induce the release of adrenocorticotrophic hormone (ACTH) into the circulation. ACTH binds to receptors in the adrenal cortex that result in the synthesis and release of glucocorticoids, cortisol in humans and corticosterone (CORT) in rodents. Glucocorticoids bind to glucocorticoid receptors (GR) in the hypothalamus and pituitary to induce fast negative feedback control over the axis. In addition, glucocorticoids bind to GR located in limbic regions such as the hippocampus, prefrontal cortex, and amygdala to indirectly decrease (red lines) or increase (green lines) HPA axis activity.
While rapid activation of the HPA axis in response to stress is essential for survival, effective termination of this response is critical to avoid potentially deleterious effects of excessive and persistent glucocorticoid secretion [20]. Thus, glucocorticoids also act via negative feedback to constrain activation of the HPA axis [21]. Feedback regulation occurs via two important GR-mediated mechanisms: 1) fast feedback inhibition of CRH-expressing neurons in the paraventricular nucleus of the hypothalamus via non-genomic mechanisms [22] and 2) long-lasting feedback inhibition mediated by genomic actions of GRs on neurons in numerous brain and body compartments, including limbic structures such as the prefrontal cortex, hippocampus and amygdala [21,23–25]. These stress-regulatory limbic structures work in parallel to process stressful stimuli. Their outputs converge into key relay structures (e.g. bed nucleus of the stria terminalis) where information is further processed for the eventual modulation of HPA axis tone and overall reactivity [26].
Prefrontal cortex
The medial prefrontal cortex plays an important role in inhibition of the HPA axis [27–30]. Stimulation of the prefrontal cortex leads to inhibition of the HPA axis response to an acute psychogenic stressor [31]. Lesions of the prefrontal cortex, on the other hand, increase stress-induced secretion of ACTH and corticosterone [28,32,33]. Moreover, GR signaling in the medial prefrontal cortex is involved in negative feedback inhibition of acute as well as chronic stress responses [29].
Hippocampus and subiculum
Lesion studies demonstrate that the hippocampus and subiculum have a predominantly inhibitory role on HPA axis function [34–36]. For instance, surgical removal of the entire hippocampal structure results in a significant increase in expression of CRH mRNA in the paraventricular nucleus of the hypothalamus and an increase in circulating levels of corticosterone [37]. Lesions of the output fibers of the ventral hippocampus produce a similar increase in hypothalamic CRH mRNA expression and an increase in ACTH secretion [38]. Together, these studies indicate a potential role of the ventral hippocampus in regulating basal HPA axis tone. In addition, lesions to the ventral subiculum – the primary target of ventral hippocampal output – result in prolonged corticosterone secretion in response to an acute challenge as well as increased expression of hypothalamic CRH mRNA [34,39,40]. These studies indicate that the ventral subiculum is instrumental to the inhibition of HPA axis stress responses, likely as the primary cortical conduit of hippocampal outflow. Importantly, recent studies suggest that the prefrontal cortex and the ventral subiculum converge at the bed nucleus of the stria terminalis to additively inhibit HPA axis stress responses [41], suggesting interactions between the output of these two limbic HPA inhibitory structures.
Amygdala
The amygdala receives inputs from both the prefrontal cortex and the hippocampus, acting as a major hub for the integration of emotional information [42]. Unlike the latter structures, principal output nuclei of the amygdala (central and medial nuclei) are involved in HPA axis activation following exposure to stress [24,43–46]. In addition, the amygdala is important for coordinating the expression of fear and anxiety behaviors [47].
Temporal lobe structures (e.g. hippocampus, subiculum, amygdala) are implicated as the primary generators of epileptic activity in TLE [48,49] and are often damaged in the disease. HPA axis dysfunction in epilepsy, therefore, may be linked to structural and functional pathologies caused by TLE. These pathologies may result from the initial epileptogenic insult and/or persistent seizure activity.
3. HPA axis dysfunction in TLE
To date, studies examining the causal relationship between HPA axis dysfunction and epilepsy are limited. To continue exploring this relationship, it is important to consider four complexities that obscure the causal relationship between HPA axis function and epilepsy. Firstly, injuries that precipitate the development of epilepsy can also directly disrupt HPA axis function (3.1). Secondly, single seizures acutely alter HPA function (3.2). Thirdly, chronic recurrent seizures (epilepsy) may alter HPA function in a manner distinct from single seizures (3.3). Finally, chronic seizures occur in a context of brain injury and cellular restructuring, which may alter HPA axis function independent of the seizures themselves (3.4).
3.1 Injuries associated with the development of TLE also result in HPA axis dysfunction
Although the causes of TLE are often unknown, a subset of patients develop epilepsy following a clear precipitating injury, such as head trauma or hypoxic/ischemic injury (for review on TLE pathophysiology and epidemiology [1,50]). Epilepsy can also develop following status epilepticus. Status epilepticus can lead to epilepsy when induced in rodents with chemoconvulsant drugs. It is not known, however, whether unprovoked status epilepticus in humans causes the subsequent epilepsy, or whether the underlying cause of the status epilepticus itself drives epileptogenesis. In either case, these epileptogenic brain injuries all induce rapid dysregulation of the HPA axis, which occurs prior to the clinical manifestations of epilepsy. For instance, while both single seizures and status epilepticus increase cortisol secretion, elevated levels persist for much longer following SE in humans [51]. Similar increases in cortisol following SE have been observed in monkeys [52]. In rodents, increased corticosterone levels are observed in a variety of different SE models, including intra-hippocampal kainic acid [53], systemic pilocarpine injection and systemic kainic acid injection [54]. The degree of corticosterone hyper-secretion has also been shown to correlate with the severity of epileptiform activity [54]. Furthermore, both systemic kainic-acid and pilocarpine induced seizures result in hyper-activation of CRH positive neurons in the paraventricular nucleus of the hypothalamus, leading to significantly higher levels of CRH mRNA and protein following SE among these cells [54,55]. Impairment in GABAergic inhibition of these cells has also been detected [54]. Together, these findings may account for increased ACTH and corticosterone levels evident in the acute aftermath of SE.
Clinical studies also reveal persistent hyper-secretion of cortisol following head trauma [56,57]. The severity of the injury correlates with greater levels of cortisol secretion [56,57]. Rodent studies reveal similar neuroendocrine changes following traumatic brain injury [58,59]. In a study by McCullers and colleagues, a time-course analysis of ACTH and corticosterone levels following brain injury revealed an initial surge at 1.5 hours in rats. At 6 hours post-injury, corticosterone remained elevated despite ACTH normalization. This was followed by a second peak of ACTH 18 hours post-injury [58]. Stress-induced HPA axis hyperactivity, evident as increased corticosterone and ACTH, develops one to two weeks after traumatic brain injury in rats [59]. By contrast, other groups have found depressed stress-induced activity of the HPA axis one week post-TBI, but pronounced hyperactivity 34 and 70 days post-injury [60]. Differences in the severity and type of traumatic brain injury may account for the discordant findings.
Increases in HPA axis activity are also seen acutely in stroke patients [61]. Similar to what is observed following SE, the magnitude of HPA axis hyperactivity following stroke correlates with poor post-stroke prognosis [62]. In animal models, recent evidence suggests that HPA axis dysfunction following global cerebral ischemia may be long lasting [63].This study demonstrated that although baseline corticosterone levels were normal seven days post-injury, stress-induced hyper-secretion of corticosterone was present 27 days post-injury. Furthermore, induction of hypoxia/ischemic injury in rats results in persistent hyper-activation of the HPA axis and spontaneous seizures [64]. Reducing glucocorticoid secretion in response to the injury decreased the frequency of spontaneous seizures [64].
Taken together, these findings indicate that HPA axis dysfunction appears rapidly following epileptogenic brain injuries. Due to a lack of longitudinal data, whether HPA dysfunction results entirely from the initial injury, or whether it becomes more severe in animals that subsequently develop epilepsy, remains an open question.
3.2 Single seizures acutely increase HPA axis function
Single seizure events in humans with epilepsy (generalized tonic clonic and complex partial seizures common in TLE) result in increased activation of the HPA axis [65–70]. These studies, however, cannot dissociate the effects of single seizure events from the effects of chronic epilepsy. Studies in animals, on the other hand, consistently demonstrate that a single, evoked temporal lobe seizure in healthy (non-epileptic) rodents activates the HPA axis, leading to large increases in corticosterone levels. For example, electrical stimulation of the amygdala and dorsal hippocampus increases ACTH and corticosterone levels following both focal and generalized seizure activity [71,72]. These findings indicate that activation of the HPA axis in epilepsy is likely due in part to the direct effects of seizures.
3.3 HPA axis dysfunction in chronic TLE
TLE patients show persistent HPA axis hyperactivity even after prolonged inter-ictal (seizure free) periods. During inter-ictal periods, for example, epileptic patients have elevated morning baseline cortisol [73] and higher ACTH levels in both morning and evening samples [74,75]. TLE patients also fail to suppress cortisol release in response to dexamethasone administration and are hyper-responsive to exogenous administration of CRH. Abnormal responses are evident in patients both on and off anti-seizure medications, implying the abnormalities are not due to drug effects [73]. A recent study found that TLE patients exposed to a psychosocial stress challenge secrete cortisol at levels significantly higher than healthy controls [76]. Despite these changes in response amplitudes, circadian fluctuations of glucocorticoids – albeit with higher levels in the morning – are preserved [77]. Although HPA axis hyperactivity seems to be a consistent pathology found in patients with TLE, the number of studies is relatively small, and more work is required to fully characterize the stress response in these patients.
Studies in rodent models of TLE support the clinical evidence for HPA axis hyperactivity. In the pilocarpine-induced status epilepticus model of TLE, epileptic rats develop persistent HPA axis hyperactivity after two months. Hyperactivity is evident as increased levels of basal corticosterone, impaired glucocorticoid-mediated negative feedback (DEX challenge) and corticosterone hyper-secretion in response to CRH [78,79].
3.4 Damage to limbic structures may result in HPA axis dysfunction
Substantial cell loss and neuronal re-organization (i.e. changes to cell structure and connectivity) are observed in key stress-regulatory regions in TLE patients and rodents exhibiting TLE-like seizures. The hippocampus is primarily affected [80–85], but abnormalities are also seen in other regions, including the subiculum [86], amygdala [84,85,87–89] and frontal cortex (i.e. anterior cingulate, medial prefrontal) [82,85,90]. In humans, mesial temporal sclerosis resulting in shrinkage of the hippocampus is the most common pathological hallmark of TLE [83,91]. Similarly, rodent studies demonstrate substantial hippocampal cell loss, neuronal dispersion, gliosis, sprouting, and aberrant neurogenesis in TLE models [92–100]. These changes occur throughout the epileptogenic process, some arising quickly after the initial precipitating injury [98,101,102], while others take time to develop [93,103,104]. As previously discussed, limbic structures such as the hippocampus, amygdala, and the pre-frontal cortex are important in the proper activation and regulation of the HPA axis [23,24,26] (Figure 1). Because the structural integrity of these key stress-regulatory regions is markedly compromised in TLE, it is possible that impaired HPA axis control may be a consequence of these injuries. Changes in the morphology and structural connectivity of the hippocampus may also contribute to the development of psychiatric comorbidities (for review see [105]).
Beyond individual structures, effective regulation of the HPA axis is most likely dependent on the coordinated activation and connectivity of a complex network involving limbic cortical and subcortical structures [26]. TLE may lead to impairments in the activation and functional connectivity of such a network [106,107]. Resting fMRI studies in TLE patients suggest substantial reductions in hippocampal, amygdala, and prefrontal cortex functional connectivity [108–111]. Others have found reductions in the functional connectivity between the hippocampus, amygdala and anterior cingulate in patients with TLE [110]. A recent fMRI study measuring BOLD reactivity in response to psychosocial acute stress (negative feedback given for both incorrect and correct answers on a mathematical task) demonstrated reduced activation of the hippocampus and medial prefrontal cortex [76]. It is possible that hypo-activity of these regions may result in reduced inhibitory control over the HPA axis. In the pilocarpine model of TLE, epileptic mice exhibit a substantial decrease in activity-dependent c-Fos expression in the hippocampus during interictal periods, indicative of reduced neuronal firing (~8–19h post-seizure) [112]. Hippocampal hypo-activation during inter-ictal periods may be a protective response following seizures. However, it may also compromise the ability of the hippocampus to regulate HPA axis function, especially when facing a stressful situation. Future studies should determine whether HPA axis dysfunction seen in animal models of TLE correlates with structural and functional changes in limbic activity and connectivity. Since epilepsy may be a disease of neural networks [106], it is possible that prevention or treatment of aberrant limbic networks may have a positive impact on seizure control, psychiatric comorbidities and HPA axis dysfunction.
4. Excess glucocorticoids may compromise the structural and functional integrity of limbic regions
Persistent exposure to excess levels of glucocorticoids can physically change the structure and function of neurons located in key stress-regulatory limbic regions, including the hippocampus and prefrontal cortex [113–116]. These changes have been hypothesized to increase vulnerability to injury [117], which could be particularly relevant in the context of epilepsy.
The hippocampus contains a high density of glucocorticoid receptors (GR and MR) [118] and is thought to be particularly vulnerable to the deleterious effects associated with excess glucocorticoid exposure [119,120]. Because glucocorticoid receptor binding results in the activation of genes known to modulate cellular metabolism, cellular structure and synaptic transmission [121], excess glucocorticoids may underlie structural and functional abnormalities seen following chronic exposure to stress. For instance, exposure to chronic stress and/or excess glucocorticoids induces dendritic atrophy and spine density reductions in the hippocampus [113,122–125]. Similar effects are observed on pyramidal neurons of the medial prefrontal cortex [28,114,126–129], while excess glucocorticoids increase dendritic branching in the amygdala (basolateral nucleus) [130]. Both glucocorticoid synthesis inhibitors and glutamate (NMDA)-receptor antagonists are effective in preventing some of the glucocorticoid-induced structural remodeling in the hippocampus [122]. These data suggest that glucocorticoids and glutamate receptors play an important role in mediating chronic stress-induced dendritic atrophy. In addition, prolonged exposure to excess glucocorticoids reduces hippocampal cell proliferation and adult neurogenesis [131–134]. Both adrenalectomy [135] and treatment with glucocorticoid antagonist (RU486) effectively prevent reductions in neurogenesis associated with chronic corticosterone treatment [136,137] and exposure to chronic stress [138].
Although the majority of the aforementioned glucocorticoid-induced hippocampal changes are transient and reversible, long-lasting remodeling (i.e. dendritic retraction) may increase vulnerability to neuronal damage following a “second hit” injury [117]. Several studies have shown that glucocorticoids can potentiate neuronal injury in hippocampal cells following exposure to neurotoxins [119] and kainic acid-induced seizures [139–141]. Furthermore, a history of chronic stress is sufficient to induce persistent dendritic retraction in hippocampal neurons and increases vulnerability to neurotoxic challenge [142,143]. These changes were absent in animals exposed to a single acute stressor. This suggests that chronic stress is necessary to induce persistent morphological changes in hippocampal neurons (perhaps by way of cumulative glucocorticoid exposure). Overall, excess exposure to stress hormones may further compromise the structural and functional integrity of limbic regions in TLE. These abnormalities may diminish negative feedback efficacy and/or increase excitatory drive, resulting in HPA axis hyperactivity.
5. Implications of HPA axis dysfunction in TLE
The data discussed in this review suggest that HPA axis hyperactivity is present in TLE. Although more studies are needed to understand the mechanisms by which HPA axis dysfunction develops, we hypothesize that damage to temporal lobe structures, either as a result of an initial epileptogenic injury and/or as a consequence of recurrent ictal activity, may contribute to aberrant top-down regulation of HPA axis activity. Damage would ultimately result in the hypersecretion of stress hormones (CRH, ACTH and glucocorticoids), both at baseline conditions and in response to stress. In this section, we aim to briefly discuss two potential consequences of HPA axis hyperactivity in TLE: 1) Increased seizure susceptibility and 2) Increased vulnerability to stress-related psychopathologies.
5.1 HPA axis hyperactivity may increase seizure susceptibility in TLE
Persistent exposure to excess glucocorticoid increases neuronal excitability [144–146] and decreases seizure thresholds in multiple models of TLE [147–150]. The mechanisms underlying chronic glucocorticoid effects on neuronal excitability and seizure vulnerability are not entirely known. There is some evidence suggesting that both genomic and non-genomic mechanisms following glucocorticoid binding to MR and GR are responsible for the increased neuronal excitability in the hippocampus [146,151]. In addition to glucocorticoids, increased expression of CRH in extra-hypothalamic areas, such as the dentate hilus and the hippocampal CA1 and CA3 regions, occurs acutely in animal models of TLE [152–155]. Due to its reported pro-convulsant effects [156,157], enhanced hippocampal CRH may facilitate the development of seizures in epilepsy [158,159] and/or may contribute to increased hippocampal excitotoxicity [159–161]. In support of this hypothesis, administration of CRH receptor antagonist, astressin, immediately prior to or following kainic acid-induced seizures has shown neuroprotective effects [159]. Additionally, a recent study found that environmental enrichment, which was associated with reductions in CRH and CRH receptor 1 expression in the hippocampus, inhibited kindling epileptogenesis [162].
HPA axis hyperactivity following temporal seizures may contribute to the generation of further ictal discharges. Such action may help explain the presence of seizure clusters that are commonly seen in humans and rodent models of TLE. In a similar manner, under stressful situations, hyper-secretion of glucocorticoids and other stress hormones may contribute to reductions in seizure threshold and increased neuronal excitability in TLE. Enhanced excitability may provide a potential mechanism underlying the propensity for TLE patients to identify stress as a common seizure trigger [9]. In fact, in some patients, evoking an emotional stressful response (i.e. using audio and video recordings) is sufficient to trigger spontaneous seizures [163]. These clinical findings provide evidence that abnormalities in the stress response may facilitate seizure activity in TLE.
5.2 HPA axis hyperactivity may increase susceptibility to stress-psychopathologies in TLE
HPA axis hyperactivity is a clinical feature in a subset of patients with major depression [164–167] and may contribute to the pathogenesis of the disease [168,169]. A natural experiment – Cushing’s disease – provides evidence of a causal link between elevated stress hormones and psychopathology in humans. Cushing’s disease is characterized by the chronic hyper-secretion of stress hormones, including ACTH and cortisol, due to excess growth of the pituitary. Although a causal relationship is not proven, the disease is associated with a high incidence of depression and anxiety [170].
GR antagonists have also shown promise in human clinical trials for the treatment of depressive symptoms [171–174]. Preclinical studies implicate excess glucocorticoid exposure as a potential mechanism for the development of stress-related psychopathologies [175–180]. We and others have shown that GR antagonists have potent anti-depressant effects in rodents [137,181,182] and can in fact reverse some of the physiological and behavioral phenotypes associated with HPA axis hyperactivity [136–138,183]. These data suggest that excess glucocorticoids may influence the development of stress-related psychopathologies, and that glucocorticoid antagonists may be used as effective antidepressant treatment in patients with HPA axis dysfunction.
Evidence that HPA axis hyperactivity is also common to epilepsy does not prove causality. However, it does suggest that excess glucocorticoids may play a role in the expression and/or exacerbation of comorbid psychopathologies. Excess glucocorticoids may contribute to the development of psychopathologies by potentiating changes characteristic of the epileptic brain, such as reduced hippocampal neurogenesis [184,185] and/or structural rearrangements of hippocampal neurons [186]. In addition, impaired connectivity in corticolimbic mood-regulatory circuits occurring as a result of epileptic injury and/or excess glucocorticoid exposure may contribute to psychopathology [187]. Alternatively, increased CRH expression in extra-hypothalamic areas, may play a role in the development of anxiety [188]. Overall, HPA axis hyperactivity is likely one of many factors that influence the high incidence of psychopathology in TLE.
6. Conclusion
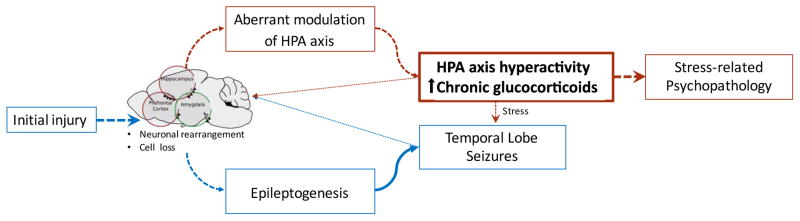
The relationship between stress, seizures and comorbid psychopathology in epilepsy is highly complex. Here, we briefly discuss some evidence that suggests that HPA axis hyperactivity may act as a common physiological mechanism underlying both stress as a precipitant of seizures and the high incidence of comorbid psychiatric illness in TLE. In our proposed model (Figure 2), the initial epileptogenic injury (i.e. the first seizure, brain trauma, hypoxia/ischemia) leads to neuronal damage (structural rearrangements and/or cell death) of limbic structures (i.e. hippocampus, amygdala and prefrontal cortex). Such neuronal damage contributes to a chain of events (i.e. epileptogenesis) that ultimately result in the development of TLE. In addition, damage to key stress-regulatory regions may impair HPA axis function, resulting in ineffective activation/termination of the stress response and overall HPA axis hyperactivity. HPA axis hyperactivity is evident from the chronic elevated secretion of glucocorticoids and other stress hormones. Excess glucocorticoids and repeated seizure activity may further exacerbate limbic damage, feeding into the epileptogenic cycle and further disrupting HPA axis function. Finally, HPA axis hyperactivity, perhaps via glucocorticoid-mediated mechanisms, may provoke seizure activity, particularly in situations of stress, while concomitantly increasing vulnerability for the development of stress-related psychopathologies. Given the widespread effects of stress hormones and their potential to influence seizure activity as well as mood, we believe that this is an important area for further study. Key goals include the development of rational therapies and non-pharmacological interventions aimed at preventing seizures and treating psychiatric comorbidities in epilepsy.
Figure 2. HPA axis dysfunction in TLE: a theoretical model.
An initial precipitating injury (i.e. the first seizure, brain trauma, hypoxia/ischemia) compromises the structural integrity of limbic circuits leading to neuronal rearrangements and cell loss in stress-regulatory regions such as the hippocampus, prefrontal cortex, and amygdala. These changes give rise to epileptogenesis and the subsequent development of TLE, while simultaneously compromising the functional integrity of stress regulatory mechanisms resulting in HPA axis hyperactivity and increased vulnerability for the development of psychopathologies (depression and anxiety). Both epileptic seizures and chronic exposure to excess glucocorticoids may potentiate the neuronal damage on limbic stress-regulatory regions. Furthermore, HPA axis hyperactivity results in exaggerated responses to stress that may facilitate epileptic discharges.
Highlights.
Stress is one of the most commonly reported seizure-triggers, and stress-related psychopathologies are highly comorbid with TLE
The HPA axis is hyperactive in TLE
The functionality and structural integrity of limbic regions important in stress-regulation are compromised in TLE
HPA axis dysfunction in epilepsy may result from aberrant connectivity of limbic structures
Acknowledgments
We would like to thank Katja Jylkka M.A. for helpful editing of the manuscript.
Footnotes
Contributions. ACW wrote the manuscript and created all tables and figures. All other authors participated in the editing process.
Conflict of interest. ACW is supported by NINDS F30-NS-095578 and T32-GM-063483. MBS has funding from K12-HD-051953. MDP receives research support from Eisai, Neuren, UCB, Epilepsy Foundation, American Epilepsy Society and FDA. He has served on data safety monitoring boards for Upsher Smith and Astellas. SCD receives funding from NINDS grants NS-062806 and NS-065020. JPH is supported by MH-049698 and MH-101729. NIH and other funding agencies had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Téllez-Zenteno JF, Hernández-Ronquillo L. A Review of the Epidemiology of Temporal Lobe Epilepsy. Epilepsy Res Treat. 2012;2012:1–5. doi: 10.1155/2012/630853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopes da Silva F, Blanes W, Kalitzin SN, Parra J, Suffczynski P, Velis DN. Epilepsies as dynamical diseases of brain systems: basic models of the transition between normal and epileptic activity. Epilepsia. 2003;44(Suppl 1):72–83. doi: 10.1111/j.0013-9580.2003.12005.x. 12005 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Fisher RS. Epilepsy from the Patient’s Perspective: Review of Results of a Community-Based Survey. Epilepsy Behav. 2000;1:S9–S14. doi: 10.1006/ebeh.2000.0107. [DOI] [PubMed] [Google Scholar]
- 4.Spector S, Cull C, Goldstein LH. Seizure precipitants and perceived self-control of seizures in adults with poorly-controlled epilepsy. Epilepsy Res. 2000;38:207–16. doi: 10.1016/s0920-1211(99)00093-5. [DOI] [PubMed] [Google Scholar]
- 5.Nakken KO, Refsland G, Lillestølen KM, Solaas MH. Seizure-precipitating factors in epilepsy--what do patients report? Tidsskr Den Nor Lægeforening Tidsskr Prakt Med Ny Række. 2005;125:2172–4. [PubMed] [Google Scholar]
- 6.Haut SR, Vouyiouklis M, Shinnar S. Stress and epilepsy: a patient perception survey. Epilepsy Behav. 2003;4:511–4. doi: 10.1016/s1525-5050(03)00182-3. [DOI] [PubMed] [Google Scholar]
- 7.Haut SR, Hall CB, Masur J, Lipton RB. Seizure occurrence: precipitants and prediction. Neurology. 2007;69:1905–10. doi: 10.1212/01.wnl.0000278112.48285.84. [DOI] [PubMed] [Google Scholar]
- 8.Novakova B, Harris PR, Ponnusamy A, Reuber M. The role of stress as a trigger for epileptic seizures: A narrative review of evidence from human and animal studies. Epilepsia. 2013;54:1866–1876. doi: 10.1111/epi.12377. [DOI] [PubMed] [Google Scholar]
- 9.Galtrey CM, Mula M, Cock HR. Stress and epilepsy: fact or fiction, and what can we do about it? Pract Neurol. 2016 doi: 10.1136/practneurol-2015-001337. [DOI] [PubMed] [Google Scholar]
- 10.Attarian H, Vahle V, Carter J, Hykes E, Gilliam F. Relationship between depression and intractability of seizures. Epilepsy Behav. 2003;4:298–301. doi: 10.1016/s1525-5050(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 11.Harden CL, Goldstein MA. Mood disorders in patients with epilepsy: epidemiology and management. CNS Drugs. 2002;16:291–302. doi: 10.2165/00023210-200216050-00002. [DOI] [PubMed] [Google Scholar]
- 12.Harden CL. The co-morbidity of depression and epilepsy: epidemiology, etiology, and treatment. Neurology. 2002;59:S48–55. doi: 10.1212/wnl.59.6_suppl_4.s48. [DOI] [PubMed] [Google Scholar]
- 13.Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry. 2003;54:388–398. doi: 10.1016/S0006-3223(03)00469-4. [DOI] [PubMed] [Google Scholar]
- 14.Tellez-Zenteno JF, Patten SB, Jetté N, Williams J, Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007;48:2336–44. doi: 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- 15.Kanner AM. Can neurobiological pathogenic mechanisms of depression facilitate the development of seizure disorders? Lancet Neurol. 2012;11:1093–102. doi: 10.1016/S1474-4422(12)70201-6. [DOI] [PubMed] [Google Scholar]
- 16.Kanner AM, Schachter SC, Barry JJ, Hesdorffer DC, Hersdorffer DC, Mula M, et al. Depression and epilepsy: epidemiologic and neurobiologic perspectives that may explain their high comorbid occurrence. Epilepsy Behav. 2012;24:156–68. doi: 10.1016/j.yebeh.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Kandratavicius L, Ruggiero RN, Hallak JE, Garcia-Cairasco N, Leite JP. Pathophysiology of Mood Disorders in Temporal Lobe Epilepsy. Rev Bras Psiquiatr. 2012;34:233–259. doi: 10.1016/j.rbp.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Kanner AM. Depression and epilepsy: A bidirectional relation? Epilepsia. 2011;52(Suppl 1):21–7. doi: 10.1111/j.1528-1167.2010.02907.x. [DOI] [PubMed] [Google Scholar]
- 19.Hesdorffer DC, Ishihara L, Mynepalli L, Webb DJ, Weil J, Hauser WA. Epilepsy, suicidality, and psychiatric disorders: a bidirectional association. Ann Neurol. 2012;72:184–91. doi: 10.1002/ana.23601. [DOI] [PubMed] [Google Scholar]
- 20.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 21.Myers B, McKlveen JM, Herman JP. Neural Regulation of the Stress Response: The Many Faces of Feedback. Cell Mol Neurobiol. 2012 doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14:398–406. doi: 10.3109/10253890.2011.586446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Braz J Med Biol Res. 2012;45:292–8. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Gerrits M, Westenbroek C, Fokkema DS, Jongsma ME, Den Boer JA, Ter Horst GJ. Increased stress vulnerability after a prefrontal cortex lesion in female rats. Brain Res Bull. 2003;61:627–35. doi: 10.1016/j.brainresbull.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKlveen JM, Myers B, Flak JN, Bundzikova J, Solomon MB, Seroogy KB, et al. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74:672–9. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKlveen JM, Myers B, Herman JP. The Medial Prefrontal Cortex: Coordinator of Autonomic, Neuroendocrine, and Behavioral Responses to Stress. J Neuroendocrinol. 2015 doi: 10.1111/jne.12272. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones KR, Myers B, Herman JP. Stimulation of the prelimbic cortex differentially modulates neuroendocrine responses to psychogenic and systemic stressors. Physiol Behav. 2011;104:266–271. doi: 10.1016/j.physbeh.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–47. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Figueiredo HF, Bruestle A, Bodie B, Dolgas CM, Herman JP. The medial prefrontal cortex differentially regulates stress-induced c-fos expression in the forebrain depending on type of stressor. Eur J Neurosci. 2003;18:2357–64. doi: 10.1046/j.1460-9568.2003.02932.x. [DOI] [PubMed] [Google Scholar]
- 34.Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–24. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–34. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 36.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 37.Herman JP, Schäfer MK, Young EA, Thompson R, Douglass J, Akil H, et al. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. 1989;9:3072–82. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herman JP, Cullinan WE, Young EA, Akil H, Watson SJ. Selective forebrain fiber tract lesions implicate ventral hippocampal structures in tonic regulation of paraventricular nucleus corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) mRNA expression. Brain Res. 1992;592:228–238. doi: 10.1016/0006-8993(92)91680-d. [DOI] [PubMed] [Google Scholar]
- 39.Herman JP, Spencer R. Regulation of Hippocampal Glucocorticoid Receptor Gene Transcription and Protein Expression In Vivo. J Neurosci. 1998;18:7462–7473. doi: 10.1523/JNEUROSCI.18-18-07462.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ. Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. J Neuroendocrinol. 1995;7:475–82. doi: 10.1111/j.1365-2826.1995.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 41.Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci. 2011;31:9683–95. doi: 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sah P, Faber ESL, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–34. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 43.Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo- pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 44.Beaulieu S, Di Paolo T, Barden N. Control of ACTH secretion by the central nucleus of the amygdala: implication of the serotoninergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology. 1986;44:247–54. doi: 10.1159/000124652. [DOI] [PubMed] [Google Scholar]
- 45.Feldman S, Conforti N, Itzik A, Weidenfeld J. Differential effect of amygdaloid lesions on CRF-41, ACTH and corticosterone responses following neural stimuli. Brain Res. 1994;658:21–6. doi: 10.1016/s0006-8993(09)90005-1. [DOI] [PubMed] [Google Scholar]
- 46.Van de Kar LD, Piechowski RA, Rittenhouse PA, Gray TS. Amygdaloid lesions: differential effect on conditioned stress and immobilization-induced increases in corticosterone and renin secretion. Neuroendocrinology. 1991;54:89–95. doi: 10.1159/000125856. [DOI] [PubMed] [Google Scholar]
- 47.Davis M. The role of the amygdala in fear and anxiety. Annu Rev Neurosci. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 48.Fujita S, Toyoda I, Thamattoor AK, Buckmaster PS. Preictal activity of subicular, CA1, and dentate gyrus principal neurons in the dorsal hippocampus before spontaneous seizures in a rat model of temporal lobe epilepsy. J Neurosci. 2014;34:16671–87. doi: 10.1523/JNEUROSCI.0584-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toyoda I, Bower MR, Leyva F, Buckmaster PS. Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J Neurosci. 2013;33:11100–15. doi: 10.1523/JNEUROSCI.0472-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curia G, Lucchi C, Vinet J, Gualtieri F, Marinelli C, Torsello A, et al. Pathophysiogenesis of mesial temporal lobe epilepsy: is prevention of damage antiepileptogenic? Curr Med Chem. 2014;21:663–88. doi: 10.2174/0929867320666131119152201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calabrese VP, Gruemer HD, Tripathi HL, Dewey W, Fortner Ca, DeLorenzo RJ. Serum cortisol and cerebrospinal fluid beta-endorphins in status epilepticus. Their possible relation to prognosis. Arch Neurol. 1993;50:689–693. doi: 10.1001/archneur.1993.00540070009006. [DOI] [PubMed] [Google Scholar]
- 52.Meldrum BS, Horton RW, Bloom SR, Butler J, Keenan J. Endocrine factors and glucose metabolism during prolonged seizures in baboons. Epilepsia. 1979;20:527–33. doi: 10.1111/j.1528-1157.1979.tb04835.x. [DOI] [PubMed] [Google Scholar]
- 53.Daniels WMU, Jaffer A, Engelbrecht AH, Russell VA, Taljaard JJF. The effect of intrahippocampal injection of kainic acid on corticosterone release in rats. Neurochem Res. 1990;15:495–499. doi: 10.1007/BF00966206. [DOI] [PubMed] [Google Scholar]
- 54.O’Toole KK, Hooper A, Wakefield S, Maguire J. Seizure-induced disinhibition of the HPA axis increases seizure susceptibility. Epilepsy Res. 2014;108:29–43. doi: 10.1016/j.eplepsyres.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piekut D, Phipps B, Pretel S, Applegate C. Effects of generalized convulsive seizures on corticotropin-releasing factor neuronal systems. Brain Res. 1996;743:63–69. doi: 10.1016/S0006-8993(96)00970-5. [DOI] [PubMed] [Google Scholar]
- 56.King LR, McLaurin RL, Lewis HP, Knowles HC. Plasma cortisol levels after head injury. Ann Surg. 1970;172:975–84. doi: 10.1097/00000658-197012000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pentelényi T, Kammerer L. Alterations of the serum cortisol and blood glucose in brain-injured patients. Injury. 1984;15:397–402. doi: 10.1016/0020-1383(84)90205-5. [DOI] [PubMed] [Google Scholar]
- 58.McCullers DL, Sullivan PG, Scheff SW, Herman JP. Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res. 2002;947:41–9. doi: 10.1016/s0006-8993(02)02904-9. [DOI] [PubMed] [Google Scholar]
- 59.Griesbach GS, Hovda Da, Tio DL, Taylor aN. Heightening of the stress response during the first weeks after a mild traumatic brain injury. Neuroscience. 2011;178:147–158. doi: 10.1016/j.neuroscience.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor AN, Rahman SU, Sanders NC, Tio DL, Prolo P, Sutton RL. Injury severity differentially affects short- and long-term neuroendocrine outcomes of traumatic brain injury. J Neurotrauma. 2008;25:311–23. doi: 10.1089/neu.2007.0486. [DOI] [PubMed] [Google Scholar]
- 61.Olsson T, Marklund N, Gustafson Y, Näsman B. Abnormalities at different levels of the hypothalamic-pituitary-adrenocortical axis early after stroke. Stroke. 1992;23:1573–6. doi: 10.1161/01.str.23.11.1573. [DOI] [PubMed] [Google Scholar]
- 62.El Husseini N, Laskowitz DT. The role of neuroendocrine pathways in prognosis after stroke. Expert Rev Neurother. 2014;14:217–32. doi: 10.1586/14737175.2014.877841. [DOI] [PubMed] [Google Scholar]
- 63.de la Tremblaye PB, Raymond J, Milot MR, Merali Z, Plamondon H. Evidence of lasting dysregulation of neuroendocrine and HPA axis function following global cerebral ischemia in male rats and the effect of Antalarmin on plasma corticosterone level. Horm Behav. 2014;65:273–84. doi: 10.1016/j.yhbeh.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 64.Krugers HJ, Knollema S, Kemper RHA, Ter Horst GJ, Korf J. Down-regulation of the hypothalamo-pituitary-adrenal axis reduces brain damage and number of seizures following hypoxia/ischaemia in rats. Brain Res. 1995;690:41–47. doi: 10.1016/0006-8993(95)00585-E. [DOI] [PubMed] [Google Scholar]
- 65.Rao ML, Stefan H, Bauer J. Epileptic but not psychogenic seizures are accompanied by simultaneous elevation of serum pituitary hormones and cortisol levels. Neuroendocrinology. 1989;49:33–9. doi: 10.1159/000125088. [DOI] [PubMed] [Google Scholar]
- 66.Pritchard PB. The effect of seizures on hormones. Epilepsia. 1991;32(Suppl 6):S46–S50. doi: 10.1111/j.1528-1157.1991.tb05892.x. [DOI] [PubMed] [Google Scholar]
- 67.Abbott RJ, Browning MC, Davidson DL. Serum prolactin and cortisol concentrations after grand mal seizures. J Neurol Neurosurg Psychiatry. 1980;43:163–7. doi: 10.1136/jnnp.43.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aminoff MJ, Simon RP, Wiedemann E. The hormonal responses to generalized tonic-clonic seizures. Brain. 1984;107(Pt 2):569–78. doi: 10.1093/brain/107.2.569. [DOI] [PubMed] [Google Scholar]
- 69.Culebras A, Miller M, Bertram L, Koch J. Differential response of growth hormone, cortisol, and prolactin to seizures and to stress. Epilepsia. 1987;28:564–70. doi: 10.1111/j.1528-1157.1987.tb03689.x. [DOI] [PubMed] [Google Scholar]
- 70.Takeshita H, Kawahara R, Nagabuchi T, Mizukawa R, Hazama H. Serum prolactin, cortisol and growth hormone concentrations after various epileptic seizures. Jpn J Psychiatry Neurol. 1986;40:617–23. doi: 10.1111/j.1440-1819.1986.tb03176.x. [DOI] [PubMed] [Google Scholar]
- 71.Casady RL, Taylor AN. Effect of electrical stimulation of the hippocampus upon corticosteroid levels in the freely-behaving, non-stressed rat. Neuroendocrinology. 1976;20:68–78. doi: 10.1159/000122470. [DOI] [PubMed] [Google Scholar]
- 72.Szafarczyk a, Caracchini M, Rondouin G, Ixart G, Malaval F, Assenmacher I. Plasma ACTH and corticosterone responses to limbic kindling in the rat. Exp Neurol. 1986;92:583–590. doi: 10.1016/0014-4886(86)90300-6. [DOI] [PubMed] [Google Scholar]
- 73.Zobel A, Wellmer J, Schulze-Rauschenbach S, Pfeiffer U, Schnell S, Elger C, et al. Impairment of inhibitory control of the hypothalamic pituitary adrenocortical system in epilepsy. Eur Arch Psychiatry Clin Neurosci. 2004;254:303–11. doi: 10.1007/s00406-004-0499-9. [DOI] [PubMed] [Google Scholar]
- 74.Gallagher BB. Endocrine abnormalities in human temporal lobe epilepsy. Yale J Biol Med. 1987;60:93–7. [PMC free article] [PubMed] [Google Scholar]
- 75.Gallagher BB, Murvin a, Flanigin HF, King DW, Luney D. Pituitary and adrenal function in epileptic patients. Epilepsia. 1984;25:683–689. doi: 10.1111/j.1528-1157.1984.tb03477.x. [DOI] [PubMed] [Google Scholar]
- 76.Allendorfer JB, Heyse H, Mendoza L, Nelson EB, Eliassen JC, Storrs JM, et al. Physiologic and cortical response to acute psychosocial stress in left temporal lobe epilepsy - a pilot cross-sectional fMRI study. Epilepsy Behav. 2014;36:115–23. doi: 10.1016/j.yebeh.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 77.Cavallo A, Moore DC, Nahori A, Beaumanoir A, Sizonenko PC. Plasma prolactin and cortisol concentrations in epileptic patients during the night. Arch Neurol. 1984;41:1179–82. doi: 10.1001/archneur.1984.04050220077018. [DOI] [PubMed] [Google Scholar]
- 78.Mazarati AM, Shin D, Kwon YS, Bragin A, Pineda E, Tio D, et al. Elevated plasma corticosterone level and depressive behavior in experimental temporal lobe epilepsy. Neurobiol Dis. 2009;34:457–61. doi: 10.1016/j.nbd.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pineda E, Shin D, Sankar R, Mazarati AM. Comorbidity between epilepsy and depression: experimental evidence for the involvement of serotonergic, glucocorticoid, and neuroinflammatory mechanisms. Epilepsia. 2010;51(Suppl 3):110–4. doi: 10.1111/j.1528-1167.2010.02623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keller SS, Schoene-Bake JC, Gerdes JS, Weber B, Deppe M. Concomitant fractional anisotropy and volumetric abnormalities in temporal lobe epilepsy: cross-sectional evidence for progressive neurologic injury. PLoS One. 2012;7:e46791. doi: 10.1371/journal.pone.0046791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mathern GW, Adelson PD, Cahan LD, Leite JP. Hippocampal neuron damage in human epilepsy: Meyer’s hypothesis revisited. Prog Brain Res. 2002;135:237–51. doi: 10.1016/s0079-6123(02)35023-4. [DOI] [PubMed] [Google Scholar]
- 82.Ben-Ari Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14:375–403. doi: 10.1016/0306-4522(85)90299-4. [DOI] [PubMed] [Google Scholar]
- 83.Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, Arnold DL. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126:462–9. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- 84.Bonilha L, Edwards JC, Kinsman SL, Morgan PS, Fridriksson J, Rorden C, et al. Extrahippocampal gray matter loss and hippocampal deafferentation in patients with temporal lobe epilepsy. Epilepsia. 2010;51:519–28. doi: 10.1111/j.1528-1167.2009.02506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fujikawa DG. The temporal evolution of neuronal damage from pilocarpine-induced status epilepticus. Brain Res. 1996;725:11–22. doi: 10.1016/0006-8993(96)00203-x. [DOI] [PubMed] [Google Scholar]
- 86.Knopp A, Kivi A, Wozny C, Heinemann U, Behr J. Cellular and network properties of the subiculum in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2005;483:476–88. doi: 10.1002/cne.20460. [DOI] [PubMed] [Google Scholar]
- 87.Aliashkevich AF, Yilmazer-Hanke D, Van Roost D, Mundhenk B, Schramm J, Blümcke I. Cellular pathology of amygdala neurons in human temporal lobe epilepsy. Acta Neuropathol. 2003;106:99–106. doi: 10.1007/s00401-003-0707-0. [DOI] [PubMed] [Google Scholar]
- 88.Tuunanen J, Lukasiuk K, Halonen T, Pitkänen A. Status epilepticus-induced neuronal damage in the rat amygdaloid complex: distribution, time-course and mechanisms. Neuroscience. 1999;94:473–495. doi: 10.1016/S0306-4522(99)00251-1. [DOI] [PubMed] [Google Scholar]
- 89.Yilmazer-Hanke DM, Wolf HK, Schramm J, Elger CE, Wiestler OD, Blümcke I. Subregional pathology of the amygdala complex and entorhinal region in surgical specimens from patients with pharmacoresistant temporal lobe epilepsy. J Neuropathol Exp Neurol. 2000;59:907–20. doi: 10.1093/jnen/59.10.907. [DOI] [PubMed] [Google Scholar]
- 90.Faure JB, Marques-Carneiro JE, Akimana G, Cosquer B, Ferrandon A, Herbeaux K, et al. Attention and executive functions in a rat model of chronic epilepsy. Epilepsia. 2014;55:644–53. doi: 10.1111/epi.12549. [DOI] [PubMed] [Google Scholar]
- 91.Wiebe S. Epidemiology of temporal lobe epilepsy. Can J Neurol Sci. 2000;27(Suppl 1):S6–10. doi: 10.1017/s0317167100000561. discussion S20–1. [DOI] [PubMed] [Google Scholar]
- 92.Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–7. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- 93.Curia G, Longo D, Biagini G, Jones RSG, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–57. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Danzer SC, He X, Loepke AW, McNamara JO. Structural plasticity of dentate granule cell mossy fibers during the development of limbic epilepsy. Hippocampus. 2010;20:113–24. doi: 10.1002/hipo.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu J, Tang F, Liu Y. Neuron activation, degeneration and death in the hippocampus of mice after pilocarpine induced status epilepticus. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:1071–8. doi: 10.3969/j.issn.1672-7347.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 96.Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73:1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 97.Murphy BL, Hofacer RD, Faulkner CN, Loepke AW, Danzer SC. Abnormalities of granule cell dendritic structure are a prominent feature of the intrahippocampal kainic acid model of epilepsy despite reduced postinjury neurogenesis. Epilepsia. 2012;53:908–21. doi: 10.1111/j.1528-1167.2012.03463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scharfman HE. The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 2007;7:348–54. doi: 10.1007/s11910-007-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sloviter RS. Hippocampal epileptogenesis in animal models of mesial temporal lobe epilepsy with hippocampal sclerosis: the importance of the “latent period” and other concepts. Epilepsia. 2008;49(Suppl 9):85–92. doi: 10.1111/j.1528-1167.2008.01931.x. [DOI] [PubMed] [Google Scholar]
- 100.Tang FR, Loke WK, Ling EA. Comparison of status epilepticus models induced by pilocarpine and nerve agents - a systematic review of the underlying aetiology and adopted therapeutic approaches. Curr Med Chem. 2011;18:886–99. doi: 10.2174/092986711794927720. [DOI] [PubMed] [Google Scholar]
- 101.Scharfman HE, Myers CE. Hilar mossy cells of the dentate gyrus: a historical perspective. Front Neural Circuits. 2012;6:106. doi: 10.3389/fncir.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Myers CE, Bermudez-Hernandez K, Scharfman HE. The influence of ectopic migration of granule cells into the hilus on dentate gyrus-CA3 function. PLoS One. 2013;8:e68208. doi: 10.1371/journal.pone.0068208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fabene PF, Merigo F, Galiè M, Benati D, Bernardi P, Farace P, et al. Pilocarpine-induced status epilepticus in rats involves ischemic and excitotoxic mechanisms. PLoS One. 2007;2:e1105. doi: 10.1371/journal.pone.0001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scorza FA, Arida RM, da Naffah-Mazzacoratti MG, Scerni DA, Calderazzo L, Cavalheiro EA. The pilocarpine model of epilepsy: what have we learned. An Da Acad Bras Ci ncias. 2009;81:345–65. doi: 10.1590/s0001-37652009000300003. [DOI] [PubMed] [Google Scholar]
- 105.Hester MS, Danzer SC. Hippocampal granule cell pathology in epilepsy - A possible structural basis for comorbidities of epilepsy? Epilepsy Behav. 2014 doi: 10.1016/j.yebeh.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spencer SS. Neural Networks in Human Epilepsy: Evidence of and Implications for Treatment. Epilepsia. 2002;43:219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- 107.Allendorfer JB, Szaflarski JP. Contributions of fMRI towards our understanding of the response to psychosocial stress in epilepsy and psychogenic nonepileptic seizures. Epilepsy Behav. 2014;35:19–25. doi: 10.1016/j.yebeh.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 108.Haneef Z, Lenartowicz A, Yeh HJ, Levin HS, Engel J, Stern JM. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia. 2014;55:137–45. doi: 10.1111/epi.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haneef Z, Lenartowicz A, Yeh HJ, Engel J, Stern JM. Effect of lateralized temporal lobe epilepsy on the default mode network. Epilepsy Behav. 2012;25:350–7. doi: 10.1016/j.yebeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pittau F, Grova C, Moeller F, Dubeau F, Gotman J. Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia. 2012;53:1013–23. doi: 10.1111/j.1528-1167.2012.03464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maccotta L, He BJ, Snyder AZ, Eisenman LN, Benzinger TL, Ances BM, et al. Impaired and facilitated functional networks in temporal lobe epilepsy. NeuroImage Clin. 2013;2:862–72. doi: 10.1016/j.nicl.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peng Z, Houser CR. Temporal patterns of fos expression in the dentate gyrus after spontaneous seizures in a mouse model of temporal lobe epilepsy. J Neurosci. 2005;25:7210–7220. doi: 10.1523/JNEUROSCI.0838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gould E, Woolley C, Frankfurt M, McEwen B. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–53. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 115.Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–31. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 116.McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala and Prefrontal Cortex. Neuropsychopharmacology. 2015:1–21. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev Neurosci. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Herman JP, Patel PD, Akil H, Watson SJ. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol. 1989;3:1886–94. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- 119.Sapolsky RM. Glucocorticoid toxicity in the hippocampus: temporal aspects of neuronal vulnerability. Brain Res. 1985;359:300–5. doi: 10.1016/0006-8993(85)91440-4. [DOI] [PubMed] [Google Scholar]
- 120.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–77. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 121.Datson NA, van der Perk J, de Kloet ER, Vreugdenhil E. Identification of corticosteroid-responsive genes in rat hippocampus using serial analysis of gene expression. Eur J Neurosci. 2001;14:675–89. doi: 10.1046/j.0953-816x.2001.01685.x. [DOI] [PubMed] [Google Scholar]
- 122.Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: Involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-L. [DOI] [PubMed] [Google Scholar]
- 123.Magariños AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997;94:14002–8. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McEwen BS, Magariños AM. Stress effects on morphology and function of the hippocampus. Ann N Y Acad Sci. 1997;821:271–284. doi: 10.1111/j.1749-6632.1997.tb48286.x. [DOI] [PubMed] [Google Scholar]
- 125.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 126.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–48. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- 127.Radley JJ, Anderson RM, Hamilton BA, Alcock JA, Romig-Martin SA. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J Neurosci. 2013;33:14379–91. doi: 10.1523/JNEUROSCI.0287-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–20. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 129.Radley JJ, Rocher AB, Rodriguez A, Douglas B, Dammann M, Mcewen BS, et al. Repeated Stress Alters Dendritic Spine Morphology. J Comp Neurol. 2009;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. 20026655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schoenfeld TJ, Gould E. Differential effects of stress and glucocorticoids on adult neurogenesis. Curr Top Behav Neurosci. 2013;15:139–64. doi: 10.1007/7854_2012_233. [DOI] [PubMed] [Google Scholar]
- 132.Wong EYH, Herbert J. Raised circulating corticosterone inhibits neuronal differentiation of progenitor cells in the adult hippocampus. Neuroscience. 2006;137:83–92. doi: 10.1016/j.neuroscience.2005.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–9. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 134.Brummelte S, Galea LAM. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience. 2010;168:680–90. doi: 10.1016/j.neuroscience.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 135.Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- 136.Hu P, Oomen C, van Dam A-M, Wester J, Zhou J-N, Joëls M, et al. A single-day treatment with mifepristone is sufficient to normalize chronic glucocorticoid induced suppression of hippocampal cell proliferation. PLoS One. 2012;7:e46224. doi: 10.1371/journal.pone.0046224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meyer M, Gonzalez Deniselle MC, Hunt H, de Kloet ER, De Nicola AF. The selective glucocorticoid receptor modulator CORT108297 restores faulty hippocampal parameters in Wobbler and corticosterone-treated mice. J Steroid Biochem Mol Biol. 2014;143:40–8. doi: 10.1016/j.jsbmb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 138.Oomen CA, Mayer JL, de Kloet ER, Joëls M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J Neurosci. 2007;26:3395–401. doi: 10.1111/j.1460-9568.2007.05972.x. [DOI] [PubMed] [Google Scholar]
- 139.Smith-Swintosky VL, Pettigrew LC, Sapolsky RM, Phares C, Craddock SD, Brooke SM, et al. Metyrapone, an inhibitor of glucocorticoid production, reduces brain injury induced by focal and global ischemia and seizures. J Cereb Blood Flow Metab. 1996;16:585–98. doi: 10.1097/00004647-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 140.Stein BA, Sapolsky RM. Chemical adrenalectomy reduces hippocampal damage induced by kainic acid. Brain Res. 1988;473:175–80. doi: 10.1016/0006-8993(88)90332-0. [DOI] [PubMed] [Google Scholar]
- 141.Stein-Behrens B, Mattson MP, Chang I, Yeh M, Sapolsky R. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J Neurosci. 1994;14:5373–80. doi: 10.1523/JNEUROSCI.14-09-05373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Conrad C, Jackson J, Wise L. Chronic stress enhances ibotenic acid-induced damage selectively within the hippocampal CA3 region of male, but not female rats. Neuroscience. 2004;125:759–767. doi: 10.1016/j.neuroscience.2004.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, et al. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J Neurosci. 2007;27:8278–85. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Joëls M, de Kloet ER. Effects of glucocorticoids and norepinephrine on the excitability in the hippocampus. Science. 1989;245:1502–5. doi: 10.1126/science.2781292. [DOI] [PubMed] [Google Scholar]
- 145.Joëls M, Krugers HJ, Lucassen PJ, Karst H. Corticosteroid effects on cellular physiology of limbic cells. Brain Res. 2009;1293:91–100. doi: 10.1016/j.brainres.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 146.Joëls M. Stress, the hippocampus, and epilepsy. Epilepsia. 2009;50:586–97. doi: 10.1111/j.1528-1167.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- 147.Castro OW, Santos VR, Pun RYK, McKlveen JM, Batie M, Holland KD, et al. Impact of corticosterone treatment on spontaneous seizure frequency and epileptiform activity in mice with chronic epilepsy. PLoS One. 2012;7:e46044. doi: 10.1371/journal.pone.0046044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kumar G, Couper A, O’Brien TJ, Salzberg MR, Jones NC, Rees SM, et al. The acceleration of amygdala kindling epileptogenesis by chronic low-dose corticosterone involves both mineralocorticoid and glucocorticoid receptors. Psychoneuroendocrinology. 2007;32:834–42. doi: 10.1016/j.psyneuen.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 149.Lee PHK, Grimes L, Hong JS. Glucocorticoids potentiate kainic acid-induced seizures and wet dog shakes. Brain Res. 1989;480:322–325. doi: 10.1016/0006-8993(89)90200-X. [DOI] [PubMed] [Google Scholar]
- 150.Koe AS, Salzberg MR, Morris MJ, O’Brien TJ, Jones NC. Early life maternal separation stress augmentation of limbic epileptogenesis: the role of corticosterone and HPA axis programming. Psychoneuroendocrinology. 2014;42:124–33. doi: 10.1016/j.psyneuen.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 151.Joëls M. Steroid hormones and excitability in the mammalian brain. Front Neuroendocrinol. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- 152.Smith Ma, Weiss SR, Abedin T, Kim H, Post RM, Gold PW. Effects of amygdala kindling and electroconvulsive seizures on the expression of corticotropin-releasing hormone in the rat brain. Mol Cell Neurosci. 1991;2:103–16. doi: 10.1016/1044-7431(91)90002-6. http://www.ncbi.nlm.nih.gov/pubmed/19912788. [DOI] [PubMed] [Google Scholar]
- 153.Smith Ma, Weiss SRB, Berry RL, Zhang LX, Clark M, Massenburg G, et al. Amygdala-kindled seizures increase the expression of corticotropin-releasing factor (CRF) and CRF-binding protein in GABAergic interneurons of the dentate hilus. Brain Res. 1997;745:248–256. doi: 10.1016/S0006-8993(96)01157-2. [DOI] [PubMed] [Google Scholar]
- 154.Foradori CD, Lund TD, Nagahara AH, Koenig JI, Handa RJ. Corticotropin-releasing hormone heterogeneous nuclear RNA (hnRNA) and immunoreactivity are induced in extrahypothalamic brain sites by kainic-acid-induced seizures and are modulated by estrogen. Brain Res. 2007;1164:44–54. doi: 10.1016/j.brainres.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 155.Piekut DT, Phipps B. Increased corticotropin-releasing factor immunoreactivity in select brain sites following kainate-elicited seizures. Brain Res. 1998;781:100–113. doi: 10.1016/S0006-8993(97)01219-5. [DOI] [PubMed] [Google Scholar]
- 156.Baram TZ, Schultz L. Corticotropin-releasing hormone is a rapid and potent convulsant in the infant rat. Dev Brain Res. 1991;61:97–101. doi: 10.1016/0165-3806(91)90118-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ehlers CL, Henriksen SJ, Wang M, Rivier J, Vale W, Bloom FE. Corticotropin releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res. 1983;278:332–6. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- 158.Hollrigel GS, Chen K, Baram TZ, Soltesz I. The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience. 1998;84:71–9. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Maecker H, Desai A, Dash R, Rivier J, Vale W, Sapolsky R. Astressin, a novel and potent CRF antagonist, is neuroprotective in the hippocampus when administered after a seizure. Brain Res. 1997;744:166–70. doi: 10.1016/s0006-8993(96)01207-3. [DOI] [PubMed] [Google Scholar]
- 160.Piekut DT, Phipps B. Corticotropin-releasing factor - Immunolabeled fibers in brain regions with localized kainate neurotoxicity. Acta Neuropathol. 1999;98:622–628. doi: 10.1007/s004010051127. [DOI] [PubMed] [Google Scholar]
- 161.Wu J, Ma DL, Ling EA, Tang FR. Corticotropin releasing factor (CRF) in the hippocampus of the mouse pilocarpine model of status epilepticus. Neurosci Lett. 2012;512:83–8. doi: 10.1016/j.neulet.2012.01.057. [DOI] [PubMed] [Google Scholar]
- 162.Yang M, Ozturk E, Salzberg MR, Rees S, Morris M, O’Brien TJ, et al. Environmental enrichment delays limbic epileptogenesis and restricts pathologic synaptic plasticity. Epilepsia. 2016;57:484–94. doi: 10.1111/epi.13299. [DOI] [PubMed] [Google Scholar]
- 163.Feldman RG, Paul NL. Identity of emotional triggers in epilepsy. J Nerv Ment Dis. 1976;162:345–53. doi: 10.1097/00005053-197605000-00005. [DOI] [PubMed] [Google Scholar]
- 164.Gold PW, Loriaux DL, Roy A, Kling MA, Calabrese JR, Kellner CH, et al. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing’s disease. Pathophysiologic and diagnostic implications. N Engl J Med. 1986;314:1329–35. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- 165.Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress (1) N Engl J Med. 1988;319:348–53. doi: 10.1056/NEJM198808113190606. [DOI] [PubMed] [Google Scholar]
- 166.Carroll BJ, Curtis GC, Davies BM, Mendels J, Sugerman AA. Urinary free cortisol excretion in depression. Psychol Med. 1976;6:43–50. doi: 10.1017/s0033291700007480. [DOI] [PubMed] [Google Scholar]
- 167.Halbreich U, Asnis GM, Shindledecker R, Zumoff B, Nathan RS. Cortisol secretion in endogenous depression. I. Basal plasma levels. Arch Gen Psychiatry. 1985;42:904–8. doi: 10.1001/archpsyc.1985.01790320076010. [DOI] [PubMed] [Google Scholar]
- 168.Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–8. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 169.De Mello ADAF, De Mello MF, Carpenter LL, Price LH. Update on stress and depression : the role of the hypothalamic-pituitary-adrenal ( HPA ) axis Uma atualização sobre estresse e depressão : o papel do eixo. Rev Bras Psiquiatr. 2003;25:231–238. doi: 10.1590/s1516-44462003000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sonino N, Fava GA. Psychiatric disorders associated with Cushing’s syndrome. Epidemiology, pathophysiology and treatment. CNS Drugs. 2001;15:361–73. doi: 10.2165/00023210-200115050-00003. [DOI] [PubMed] [Google Scholar]
- 171.DeBattista C, Belanoff J. The use of mifepristone in the treatment of neuropsychiatric disorders. Trends Endocrinol Metab. 2006;17:117–121. doi: 10.1016/j.tem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 172.Belanoff JK, Flores BH, Kalezhan M, Sund B, Schatzberg AF. Rapid reversal of psychotic depression using mifepristone. J Clin Psychopharmacol. 2001;21:516–21. doi: 10.1097/00004714-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 173.Flores BH, Kenna H, Keller J, Solvason HB, Schatzberg AF. Clinical and biological effects of mifepristone treatment for psychotic depression. Neuropsychopharmacology. 2006;31:628–36. doi: 10.1038/sj.npp.1300884. [DOI] [PubMed] [Google Scholar]
- 174.Young AH. Antiglucocoticoid treatments for depression. Aust N Z J Psychiatry. 2006;40:402–405. doi: 10.1080/j.1440-1614.2006.01813.x. [DOI] [PubMed] [Google Scholar]
- 175.Zhao Y, Ma R, Shen J, Su H, Xing D, Du L. A mouse model of depression induced by repeated corticosterone injections. Eur J Pharmacol. 2008;581:113–20. doi: 10.1016/j.ejphar.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 176.Iijima M, Ito A, Kurosu S, Chaki S. Pharmacological characterization of repeated corticosterone injection-induced depression model in rats. Brain Res. 2010;1359:75–80. doi: 10.1016/j.brainres.2010.08.078. [DOI] [PubMed] [Google Scholar]
- 177.Sterner EY, Kalynchuk LE. Behavioral and neurobiological consequences of prolonged glucocorticoid exposure in rats: relevance to depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:777–90. doi: 10.1016/j.pnpbp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 178.Park DI, Kim HG, Jung WR, Shin MK, Kim KL. Mecamylamine attenuates dexamethasone-induced anxiety-like behavior in association with brain derived neurotrophic factor upregulation in rat brains. Neuropharmacology. 2011;61:276–82. doi: 10.1016/j.neuropharm.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 179.Lussier AL, Lebedeva K, Fenton EY, Guskjolen A, Caruncho HJ, Kalynchuk LE. The progressive development of depression-like behavior in corticosterone-treated rats is paralleled by slowed granule cell maturation and decreased reelin expression in the adult dentate gyrus. Neuropharmacology. 2013;71:174–83. doi: 10.1016/j.neuropharm.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 180.Skupio U, Tertil M, Sikora M, Golda S, Wawrzczak-Bargiela A, Przewlocki R. Behavioral and molecular alterations in mice resulting from chronic treatment with dexamethasone: relevance to depression. Neuroscience. 2015;286:141–50. doi: 10.1016/j.neuroscience.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 181.Wulsin AC, Herman JP, Solomon MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 2010;35:1100–12. doi: 10.1016/j.psyneuen.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Solomon MB, Wulsin AC, Rice T, Wick D, Myers B, McKlveen J, et al. The selective glucocorticoid receptor antagonist CORT 108297 decreases neuroendocrine stress responses and immobility in the forced swim test. Horm Behav. 2014;65:363–71. doi: 10.1016/j.yhbeh.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Aisa B, Tordera R, Lasheras B, Del Río J, Ramírez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–66. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 184.Ledergerber D, Fritschy J-M, Kralic JE. Impairment of dentate gyrus neuronal progenitor cell differentiation in a mouse model of temporal lobe epilepsy. Exp Neurol. 2006;199:130–42. doi: 10.1016/j.expneurol.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 185.Hattiangady B, Shetty AK. Decreased neuronal differentiation of newly generated cells underlies reduced hippocampal neurogenesis in chronic temporal lobe epilepsy. Hippocampus. 2010;20:97–112. doi: 10.1002/hipo.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hester MS, Danzer SC. Hippocampal granule cell pathology in epilepsy - A possible structural basis for comorbidities of epilepsy? Epilepsy Behav. 2014:2–13. doi: 10.1016/j.yebeh.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bakshi VP, Kalin NH. Corticotropin-releasing hormone and animal models of anxiety: gene – environment interactions. Biol Psychiatry. 2000;48:1175–1198. doi: 10.1016/S0006-3223(00)01082-9. [DOI] [PubMed] [Google Scholar]