Abstract
Chronic pain occurs in as many as 85% of individuals with HIV and is associated with substantial functional impairment. Little guidance is available for HIV providers seeking to address their patients’ chronic pain. We conducted a systematic review to identify clinical trials and observational studies that examined the impact of pharmacologic or non-pharmacologic interventions on pain and/or functional outcomes among HIV-infected individuals with chronic pain in high-development countries. Eleven studies met inclusion criteria and were mostly low or very low quality. Seven examined pharmacologic interventions (gabapentin, pregabalin, capsaicin, analgesics including opioids) and four examined non-pharmacologic interventions (cognitive behavioral therapy, self-hypnosis, smoked cannabis). The only controlled studies with positive results were of capsaicin and cannabis, and had short-term follow-up (≤12 weeks). Among the seven studies of pharmacologic interventions, five had substantial pharmaceutical industry sponsorship. These findings highlight several important gaps in the HIV/chronic pain literature that require further research.
Keywords: HIV, chronic pain, cognitive therapy, medical marijuana, hypnosis, systematic review
Introduction
Chronic pain lasting at least three months (Turk & Rudy, 1987) affects up to 30% of the US population (Institute of Medicine, 2011). Prevalence estimates of pain in individuals with HIV are high, ranging from 39% to 85% (Merlin et al., 2012; Miaskowski et al., 2011). In the modern treatment era, chronic pain in HIV includes the classically described syndromes of HIV neuropathy (Robinson-Papp & Simpson, 2009) and avascular necrosis, (Mazzotta et al., 2011) but also a high burden of regional and widespread musculoskeletal pain (Miaskowski et al., 2011). Emerging evidence suggests that chronic pain among some patients is associated with suboptimal retention in HIV primary care (Merlin et al., 2012) and has serious health consequences, including up to 10 times greater odds of functional impairment (Merlin et al., October 2012). Individuals with HIV who have chronic pain experience a distinct biological, psychological, and social context, as described in an adapted Biopsychosocial Framework for Chronic Pain in HIV (Merlin et al., 2013).
Despite the uniqueness of chronic pain in individuals with HIV, little guidance is available for HIV providers seeking to address patients’ pain (Dworkin et al., 2010; Finnerup et al., 2015; Krashin, Merrill, & Trescot, 2012; Parker, Stein, & Jelsma, 2014). To address this gap, we conducted a systematic review of studies examining the impact of pharmacologic or non-pharmacologic interventions on pain or function among HIV-infected individuals with chronic pain.
Methods
Search strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses recommendations (PRISMA) (Liberati et al., 2009). A medical librarian (LAV) searched five electronic databases. The complete PubMed search strategy, which was peer reviewed by a second librarian, is provided in the appendix, and search strategies for other databases were similar. We also reviewed articles referenced in the bibliographies of articles selected for inclusion and their forward citations, and the references of relevant systematic reviews.
Study selection
Articles were eligible for inclusion if: (1) the article reported original research (i.e., not a review or commentary); (2) subjects were HIV-infected adult human subjects; (3) subjects had chronic pain (≥3 months’ duration by study inclusion criteria, or reported a mean duration of pain of ≥3 months) (Institute of Medicine, 2011); (4) study examined the impact of any exposure or intervention on a pain or functional outcome (such as pain intensity or functional status/disability), (Turk et al., 2003); (5) sample included ≥25 individuals with HIV, and (6) study was conducted in a “very high human development” country based on the United Nations Human Development report (Malik, 2013). When considering whether a study was about chronic pain, we excluded studies that did not specify whether the duration of participants’ pain was ≥3 months. Concurrently, we excluded studies that were not focused on pain (i.e., did not include pain as a major objective or hypothesis), and studies that had met inclusion criteria to this point but did not have at least 25 participants.
Two reviewers (JSM and HWB) screened titles and abstracts of 100 randomly selected articles for potential eligibility. Articles were excluded at this level of review if any of the six inclusion criteria were clearly unmet. Discrepancies were reviewed and resolved through refinement in inclusion criteria language. One reviewer (HWB) applied these criteria to the full set of titles/abstracts. Next, the remaining articles were subject to full review by two independent reviewers (JSM and HWB). A third reviewer (either JLS or EJE) resolved disagreements.
Data extraction and quality assessment
All articles selected for inclusion were independently reviewed by two reviewers (JSM and either JLS, EJE, or HWB) using a data extraction instrument. Two methods were used to assess study quality. First, we used GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) (see Appendix Table A1) (Atkins et al., 2004). To further characterize studies based on quality indicators that were specific to the questions of this review, we developed a checklist of 20 quality indicators similar to prior systematic reviews (see Appendix Table A2) (Starrels et al., 2010). Discrepancies were resolved by a third reviewer.
Results
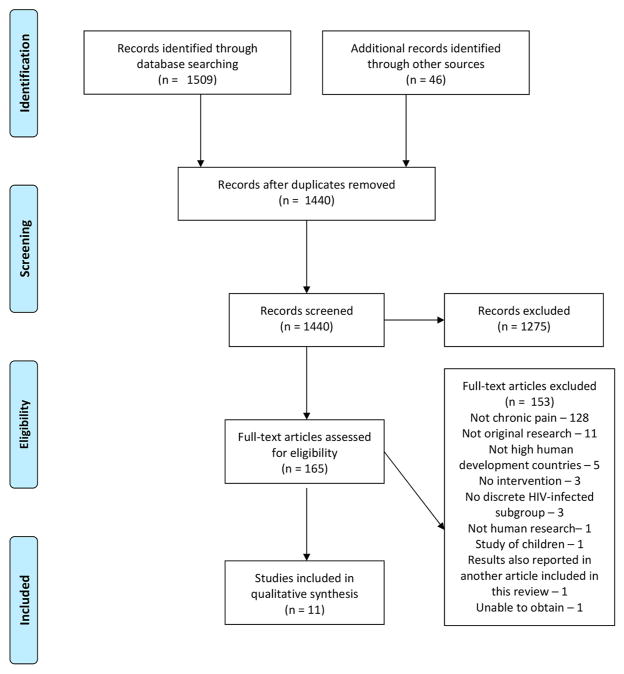
Of the 1440 abstracts screened, 165 articles were eligible for full review, and 11 met criteria for inclusion (see Figure 1). In the text and tables, we summarize the seven studies that examined pharmacologic interventions (Table 1) (Blinderman, Sekine, Zhang, Nillson, & Shaiova, 2009; Clifford et al., 2012; Hahn et al., 2004; Koeppe, Lyda, Johnson, & Armon, 2012; Newshan & Lefkowitz, 2001; Simpson, Brown, Tobias, & Group, 2008; Simpson et al., 2010) and the four studies that examined non-pharmacologic interventions (Table 2) (Abrams et al., 2007; Cucciare, Sorrell, & Trafton, 2009; Dorfman et al., 2013; Trafton et al., 2012).
Figure 1.
PRISMA 2009 Flow Diagram.
Table 1.
Studies of pharmacologic treatments for chronic pain in patients with HIV.
| Study, year | Exposure/intervention description | Design | Locationa | N | Chronic pain type (s) | Outcome measuresb | Follow-up time, in weeks | Sample characteristics | Pain results | Function results | GRADE score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hahn, Journal of Neurology, 2004 | Gabapentin (up to 2400 mg./day) vs. placebo | RCT | Five outpatient centers | 26 | Neuropathic | Pain: McGill-SF (VAS) |
4 | All HIV+; intervention group: median age 46, 67% male, median CD4 395 cells/mL | No significant difference | N/A | 2 |
| Simpson, Neurology, 2010 | Pregabalin (flexible dose) vs. placebo | RCT | Not specified | 302 | Neuropathic | Pain: Patient Global Impression of Change (PGIC) Neuropathic Pain Symptom Inventory (NPSI) Gracely Pain Scale (GPS) Function: Hospital Anxiety and Depression Scale (HADS) |
14 | All HIV+; intervention group: mean age 48, 56% white, 83% male, mean CD4 483 cells/mL, mean HIV viral load 8762 copies/mL | No significant differences | No significant difference | 4 |
| Simpson, Neurology, 2008 | Capsaicin patch high (8%) vs. low (<0.1%) concentration, single application | RCT | Not specified, multicenter | 307 | Neuropathic | Pain: Numeric Pain Rating Scores (NPRS) GPS BPI McGill-SF Function PGIC |
12 | All HIV+; intervention group: mean age 47.7, 60% white, 92% male, median CD4 388 cells/mL, median HIV viral load log10 copies/mL 3.01 | Greater improvement in NPRS in high vs. low concentration groups (22.8% vs. 10.7% reduction, p = .0026) at 12 weeks. Statistically significant differences between high vs. low concentration groups for GPS, McGill-SF, but not BPI | No significant difference | 3 |
| Clifford, Journal of Acquired Immune Deficiency Syndromes, 2012 | Capsaicin patch high (8%) vs. low concentration, single application for 30 or 60 minutes | RCT | Not specified, clinic | 494 | Neuropathic | Pain: NPRS McGill-SF Function: PGIC Clinical Global Impression of Change (CGIC) |
12 | All HIV+; intervention group: mean age 50, 68% white, 87% male, mean CD4 424 cells/mL, mean HIV viral load 17,746 copies/mL | Greater improvement in NPRS in high vs. low concentration groups (31.2% vs. 25.3%, p = .038) Statistically significant differences seen between high vs. low concentration groups not seen for the McGill-SF | Greater improvement in PGIC in 30 minute group vs. placebo (p = .0003). No significant difference in 60 minute group vs. placebo. Similar results for CGIC. | 2 |
| Newshan, Journal of Pain and Symptom Management, 2001 | Transdermal fentanyl and immediate release morphine | Pre-post (open-label) | Outpatient | 35 | Mixed: lower limb 43%; head or face, 14%; abdominal or pelvic 17%; upper limb 6%; low back 11%; thoracic region 3% | Pain and function: BPI |
2 | All HIV+ and taking oral opioids at baseline; mean age 39, 23% white, 74% male, median CD4 count 178 cells/mL | Improvement in BPI average pain (5.4–2.4, p < .001) | Improvement in BPI general activity (from 6.8 to 2.5, p < .001) | 1 |
| Blinderman, Journal of Opioid Management, 2009 | Methadone | Observational (no control group) | HIV/pain specialty clinic | 53 | Mixed: 44% neuropathic, 29% visceral, 27% musculoskeletal, 4% central | Pain NPRS |
52 | All HIV+ and in methadone treatment for opioid use disorder at baseline; 9% white, 68% male | Improvement in NPRS (9.4–4.2, p < .001) | N/A | 1 |
| Koeppe, Clinical Journal of Pain, 2012 | Opioid analgesics, neuromodulating analgesics, or other analgesics | Observational (no control group) | HIV primary care | 27 | Mixed: 40% peripheral neuropathy, 32% back pain, 16% arthritis, 5% unspecified chronic pain, 3% headache, 4% abdominal | Pain: NPRS |
270 (median) | All HIV+; median age 42, 72% white, 76% male, median CD4 413 cells/mL, median viral load log 10 copies/mL = 2.9 | Decreasing pain on NPRS negatively associated with opioid use (OR = 0.24, P = .003). No significant differences for neuromodulating or other analgesics | N/A | 2 |
If Veterans were included, this was noted.
Only pain and function outcomes were included in this table.
RCT = Randomized controlled trial.
Table 2.
Studies of non-pharmacologic treatments for chronic pain in patients with HIV.
| Study, year | Exposure/intervention description | Design | N | Locationa | Chronic pain type (s) | Outcome measuresb | Follow-up time, in weeks | Sample characteristics | Pain results | Function results | GRADE score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abrams, Neurology, 2007 | Cannabis vs. placebo cigarettes | RCT | 55 | Clinical research unit | Neuropathic | Pain: VAS Function: Profile of Mood States (POMS) |
1 | All HIV+; in intervention group, mean age 50, 52% white, 81% male, median CD4 = 355 cells/mL, 70% undetectable viral load | Greater improvement in VAS in cannabis vs. placebo group (34% vs. 17%, p = .03). | No significant difference | 2 |
| Trafton, Journal of Behavioral Health Services Research, 2012 | CBT, 12 group sessions | Pre-post | 60 | HIV primary carec | Mixed: leg 81.7%, foot 73.3%, low back 68.3%, arm/hand 63.3%, neck 51.7%; | Pain and function: POQ |
24 | All HIV+; for intervention group: mean age 50, 33% white, 45% black, 10% other, 65% male | Improvement in POQ pain intensity (7.3–5.2, p < .001) | Improvement in POQ function (87.2–72.0, p < .001) | 1 |
| Cucciare, Journal of Behavioral Medicine, 2009 | CBT, 12 group sessions | Pre-post | 60 | HIV primary care, included VA patients | Mixed | Pain and function: POQ |
12 | All HIV+, mean age 50, 33% white, 65% male, | Improvement in total POQ score (85–75, p ≤ .05) | Improvement in POQ psychologic subscale (41 to 33, p < .01). No difference in physical subscale | 1 |
| Dorfman, Pain Medicine, 2013 | Self-hypnosis 3 sessions | Pre-post | 41 | Outpatients | Neuropathic | Pain: McGill-SF Function Medical Outcomes Study Quality of Life measure for HIV (MOS-QOL) Center for Epidemiologic Studies Depression scale (CES-D) State Trait Anxiety Inventory (STAI) |
10 | All HIV+, mean age 48, 17% white, 73% male, median CD4 409 cells/mL, median HIV viral load 116 copies/mL | Improvement in McGill-SF total pain score (17.8–13.2, p < .0001) | Improvement in MOS-QOL. Improvement in CES-D among individuals with high pretreatment depression scores (23.7–18.0. No difference in STAI | 1 |
If Veterans were included, this was noted.
Only pain and function outcomes were included in this table.
Study received Institutional Review Board approval from VA but unclear whether Veterans were enrolled.
RCT = Randomized controlled trial.
Study outcomes and quality
Instruments used to assess pain and function outcomes varied considerably among studies, and included the McGill Questionnaire (Clifford et al., 2012; Dorfman et al., 2013; Hahn et al., 2004; Simpson et al., 2008), the Brief Pain Inventory (BPI) (Newshan & Lefkowitz, 2001), the Pain Outcomes Questionnaire (POQ) (Cucciare et al., 2009; Trafton et al., 2012), and others. Of the 11 studies, 5 were randomized controlled trials (RCTs), 4 were pre-post studies, and 2 were observational studies without a control condition. Nine of the 11 studies had a GRADE score of 1 (very low) or 2 (low) (see Tables A1 and A2 in the Appendix). The median number of quality indicators met was 9 out of 20 (range 4–18). Common quality concerns were lack of long-term follow-up, intention-to-treat analyses, or randomization.
Studies were generally small; only three had more than 100 participants (Clifford et al., 2012; Simpson et al., 2008; Simpson et al., 2010). No studies were conducted completely in the current HIV treatment era, defined as 2006-present. Only five studies followed participants for six months or longer (Blinderman et al., 2009; Koeppe et al., 2012; Simpson et al., 2008; Simpson et al., 2010; Trafton et al., 2012), and two followed participants for a year or longer (Blinderman et al., 2009; Koeppe et al., 2012). Of the seven studies of pharmacologic interventions, five had substantial pharmaceutical company sponsorship (Clifford et al., 2012; Hahn et al., 2004; Newshan & Lefkowitz, 2001; Simpson et al., 2008; Simpson et al., 2010).
Summary of study findings
Pharmacologic interventions
Of the seven studies of pharmacologic interventions, four were RCTs, all of which evaluated non-opioid medications. In two randomized trials of anticonvulsants, neither gabapentin nor pregabalin was more effective than placebo in treating HIV-associated neuropathic pain (Hahn et al., 2004; Simpson et al., 2010). Two randomized trials of a high dose capsaicin patch vs. placebo for HIV-infected individuals with neuropathic pain found a greater reduction in pain on the numeric pain scale in the intervention group than in the placebo group (mean reduction 22.8% vs. 10.7% in one study, and 31.2% vs. 25.3%); however, follow-up was short (12 weeks) and both studies allowed participants to pre-medicate with lidocaine and address capsaicin-related pain with opioids (Clifford et al., 2012; Simpson et al., 2008). Of the remaining three pharmacologic studies, two found reductions in pain after adding opioids for individuals already taking opioids (Blinderman et al., 2009; Newshan & Lefkowitz, 2001), and one found higher pain scores after a mean of five years on opioids (Koeppe et al., 2012).
Non-pharmacologic interventions
Of the four studies of non-pharmacologic interventions, the only RCT examined smoked cannabis vs. placebo in patients with neuropathic pain over a one week follow-up period. This study found that median pain on a visual analog scale (VAS) decreased twice as much (34% vs. 17%) in the cannabis group compared to the placebo group. The other three non-pharmacologic studies were pre-post studies of behavioral interventions – two studies of cognitive behavioral therapy (CBT) found small improvements in pain at 12 and 24 weeks but were limited by poor adherence, and a study of self-hypnosis for neuropathic pain found a small reduction in pain at 10 weeks.
Discussion
Despite the clinical burden of chronic pain in this population, this systematic review identified only 11 studies that examined treatments for chronic pain in individuals with HIV. Most were low or very low quality. Although much of chronic pain in the current HIV treatment era is musculoskeletal, over half of the studies focused on neuropathic pain. Additionally, despite prevalent use of opioids among HIV-infected patients with pain, only three low or very low quality studies examined opioids. The only higher quality study (GRADE 3, moderate quality) that found significant improvements in pain was a short-term study of capsaicin. Based on these findings, though some interventions hold promise, there is insufficient evidence to guide providers in the care of individuals with HIV and chronic pain, and future research is needed.
Though opioids are commonly prescribed in individuals with HIV and chronic pain, the evidence to support this is limited and results are mixed. These findings are consistent with the state of evidence about opioids in general populations (Chou et al., 2015). Notably, among the seven studies of pharmacologic interventions, the five highest quality studies had substantial pharmaceutical industry sponsorship (Clifford et al., 2012; Hahn et al., 2004; Newshan & Lefkowitz, 2001; Simpson et al., 2008; Simpson et al., 2010). While such sponsorship is often necessary for such trials to be carried out, it is important to note the potential for conflict of interest.
Surprisingly, we only identified two low quality, short-term studies of behavioral interventions, which are among the most effective chronic pain treatments in the general population (Turk, Wilson, & Cahana, 2011). The success of a behavioral intervention is heavily influenced by how well it is tailored to the target population (Bartholomew, Parcel, Kok, Gottleib, & Fernandez, 2011). Therefore, future investigations should focus on developing behavioral interventions specifically for individuals with HIV. It is also noteworthy that while HIV is an indication for prescribing medical cannabis in some states, we identified only one study that met our inclusion criteria and investigated smoked cannabis for chronic pain in individuals with HIV.
The primary limitation of this study is that the inclusion criteria employed to ensure methodological rigor may have excluded relevant studies. For example, we excluded studies that did not report sufficient detail to identify participants as having chronic pain (Evans et al., 2007; Shlay et al., 1998), including studies that may have been included in prior systematic reviews on neuropathic pain. This highlights an additional limitation of the literature in this area, specifically, that there is no standardized research definition of chronic pain and high variability across studies. Similarly, there was a lack of consistency in measurement of pain and function outcomes across studies.
In sum, despite the high prevalence and unique features of chronic pain in HIV, the pace of research on chronic pain in HIV-infected individuals has not matched the clinical need. Future studies of pharmacologic and non-pharmacologic interventions in individuals with HIV are urgently needed.
Acknowledgments
Funding information
This work was supported by the Agency for Healthcare Research and Quality [grant number K12 HS019465 (JSM)], the National Institute of Mental Health [K23MH104073 (JSM)] and the National Institute on Drug Abuse [K23DA027719 (JLS) and K12DA033312 (EJE)].
Appendix
Databases
(1) PubMed (earliest index date to 1/2015), (2) the Cumulative Index to Nursing and Allied Health Literature Plus with Full Text (1937 to 1/2015), (3) Proquest PsycInfo (1806-1/2015), (4) the Cochrane Central Register of Controlled Trials (6/2014), and (5) Scopus (earliest index date-1/2015).
Chronic pain
-
(1)
(pain AND (chronic OR chronic disease OR refractory OR recalcitrant OR widespread OR “wide spread” OR intractable OR neuropath* OR polyneuropathies OR polyneuropath* OR back OR facial OR dental OR psychological OR musculoskeletal OR referred OR nociceptive OR visceral)) OR (chronic pain OR Pain, intractable OR facial pain OR back pain OR Nociceptive Pain OR pain, referred OR musculoskeletal pain OR neuralgia OR mastodynia OR osteoarthritis OR fibromyalgia OR (fibromyalgia OR peripheral neuropathy OR avascular necrosis OR migraine OR chronic headache OR arthritis OR neuralgia OR mastodynia))
HIV-infected individuals
-
(2)
(“HIV infections”[MeSH Terms] OR (HIV-infections OR HIV-patients OR HIV-infected OR HIV-infection OR HIV-positive OR HIV-seropositive OR HIV/AIDS OR HIV-related OR AIDS-related OR HIV-associated OR AIDS-associated OR acquired immune deficiency syndrome))
Pharmacologic or non-pharmacologic pain treatments
-
(3)
(analgesics OR antidepressive agents OR antiinflammatory agents OR sensory system agents OR anticonvulsants[Pharmacological Action]) OR (analgesia OR therapy OR therapeutics OR radiotherapy OR homeopath* OR mind-body therapy) OR (exercise OR exercise therapy OR behavioral therapy OR treatment OR drug therapy OR diet therapy) OR (spinal fusion OR spinal injections OR splints OR splinting OR nerve growth factor OR plant extracts)) OR (cannabis OR cannabinoids OR marijuana OR implantable device OR implantable devices OR nerve block OR nerve blocks OR pain management OR opioid OR opioids OR opiate OR opiates) OR (narcotic OR narcotics OR ointments OR anti-HIV agents OR chiropractic OR chiropractor OR massage OR manipulation OR relaxation therapy)
-
(4)
“animals”[MeSH] NOT “humans”[MeSH]
-
(5)
“clinical trial”[Publication Type] OR “comparative study”[Publication Type] OR “controlled clinical trial”[-Publication Type] OR “evaluation studies”[Publication Type] OR “observational study”[Publication Type] OR Epidemiologic Studies
-
(6)
#1 AND #2 AND #3
-
(7)
#5 NOT #4
-
(8)
#6 AND #7
Note: We also reviewed articles referenced in the bibliographies of articles selected for inclusion and their forward citations, and the references of relevant systematic reviews.
Table A1.
GRADE scoring.
| Author, year | Initial score* | Adjustment† | Reason for adjustment | Final GRADE score |
|---|---|---|---|---|
| Hahn, 2004 | 4 | −2 | Very serious limitations: short follow-up, inadequate adjustment for confounding, statistically significant differences were not between intervention and placebo but rather intervention pre-post | 2 |
| Simpson, 2010 | 4 | N/A | N/A | 4 |
| Simpson, 2008 | 4 | −1 | Serious limitations: inadequate blinding due to capsaicin effects, differences in opioid/other medication use | 3 |
| Clifford, 2012 | 4 | −2 | Serious limitations, inconsistency: Different pain meds not accounted for, inadequate blinding due to capsaicin effects, unexplained inconsistency of results (e.g., control condition responses of up to 30% improvement) | 2 |
| Newshan, 2001 | 2 | −1 | Serious limitations: no power calculation, no multivariate analyses | 1 |
| Blinderman, 2009 | 2 | −1 | Serious limitations: pre-post, no multivariable analysis, methadone titration protocol not given, no control group, no adjustment for confounding | 1 |
| Koeppe, 2012 | 2 | N/A | N/A | 2 |
| Abrams, 2007 | 4 | −2 | Very serious limitations: brief duration of follow-up; smoked cannabis provied by National Institute of Drug Abuse not generalizable to what patients can obtain | 2 |
| Trafton, 2012 | 2 | −1 | Serious limitations: Inadequate control condition/pre-post design, lack of long-term follow-up, concern for regression to the mean | 1 |
| Cucciare, 2009 | 2 | −1 | pre-post, small study, “p < .05”, regression to the mean issue, confounding because those with improved function more likely to attend, and not clear how drop-outs were different; that is, excluding them biases towards seeing an improvement when none may have existed, no power calculation, short follow-up | 1 |
| Dorfman, 2013 | 2 | −1 | pre-post, no multivariable analyses | 1 |
4 = randomized trial, 3 = quasi-randomized, 2 = observational (including pre-post), 1 = any other evidence.
Adjustments based on:
Study quality:
−1 if serious limitations
−2 if very serious limitations
−1 if important inconsistency
Directness:
−1 if some uncertainty
−2 if major uncertainty
−1 if sparse data
−1 if high probability of reporting bias
Strong association:
+1 if strong, no plausible confounders, consistent and direct evidence
+2 if very strong, no major threats to validity and direct evidence
+1 if evidence of a dose response gradient
+1 if all plausible confounders would have reduced the effect
Table A2.
Study quality according to 20-item checklist.
| Author, year | Hahn, 2004 | Simpson, 2010 | Simpson, 2008 | Clifford, 2012 | Newshan, 2001 | Blinderman, 2009 | Koeppe, 2012 | Abrams, 2007 | Trafton, 2012 | Cucciare, 2009 | Dorfman, 2013 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Contemporary* | 0 | 0 | 0 | 0 | 0 | 0 | 0§ | 0 | 0 | 0 | 0 |
| Controlled | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Prospective | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Inclusion criteria specified a priori | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| Subjects from different groups recruited from same population | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Controls recruited concurrently | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| At least 80% power | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Follow-up ≥6 mo | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 |
| Multivarible analyses† | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| Adequate adjustment for confounding | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Concurrent pain treatments listed | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 |
| Primary outcome clearly identified | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | |
| Primary outcome measured using a valid instrument | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Intervention described clearly | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 |
| Intervention consistent within groups | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Randomized | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Blinded | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Intention to treat | 0 | 1§ | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 85% of participants completed study | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Free of substantial pharmaceutical company sponsorship|| | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total (/20) | 12 | 16 | 18 | 15 | 4 | 5 | 7 | 17 | 9 | 6 | 9 |
Contemporary considered 2006-present. If study concluded in 2006 we consider that to be pre-2006. Studies that straddle 2006 but at least half of the study was conducted pre-2006 were considered pre-2006. If the study was published pre-2006 but study dates were not specified we considered it pre-2006.
Marked as a positive response if multivariable analysis was conducted for at least 1 pain/function outcome.
Modified intention to treat.
We considered pharmaceutical support to be substantial if it included sponsorship (i.e., financial support to conduct the study), provision of study medication, or employment of a study author by the pharmaceutical company.
References
- Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, … Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology. 2007;68(7):515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, … Group GW. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew LK, Parcel GS, Kok G, Gottleib NH, Fernandez ME. Planning health promotion programs: An intervention mapping approach. San Francisco, CA: Jossey-Bass; 2011. [Google Scholar]
- Blinderman CD, Sekine R, Zhang B, Nillson M, Shaiova L. Methadone as an analgesic for patients with chronic pain in methadone maintenance treatment programs (MMTPs) Journal of Opioid Management. 2009;5(2):107–114. doi: 10.5055/jom.2009.0012. [DOI] [PubMed] [Google Scholar]
- Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, … Deyo RA. The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review for a National Institutes of Health Pathways to Prevention Workshop. Annals of Internal Medicine. 2015;162(4):276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Simpson DM, Brown S, Moyle G, Brew BJ, Conway B, Group N-CS. A randomized, double-blind, controlled study of NGX-4010, a capsaicin 8% dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2012;59(2):126–133. doi: 10.1097/QAI.0b013e31823e31f7. [DOI] [PubMed] [Google Scholar]
- Cucciare MA, Sorrell JT, Trafton JA. Predicting response to cognitive-behavioral therapy in a sample of HIV-positive patients with chronic pain. Journal of Behavioral Medicine. 2009;32(4):340–348. doi: 10.1007/s10865-009-9208-5. [DOI] [PubMed] [Google Scholar]
- Dorfman D, George MC, Schnur J, Simpson DM, Davidson G, Montgomery G. Hypnosis for treatment of HIV neuropathic pain: A preliminary report. Pain Medicine. 2013;14(7):1048–1056. doi: 10.1111/pme.12074. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, Wells CD. Recommendations for the pharmacological management of neuropathic pain: An overview and literature update. Mayo Clinic Proceedings. 2010;85(3 Suppl):S3–14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SR, Simpson DM, Kitch DW, King A, Clifford DB, Cohen BA, … Group ACT. A randomized trial evaluating Prosaptide for HIV-associated sensory neuropathies: Use of an electronic diary to record neuropathic pain. PLoS One. 2007;2(6):e551. doi: 10.1371/journal.pone.0000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, … Wallace M. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. The Lancet Neurology. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn K, Arendt G, Braun JS, von Giesen HJ, Husstedt IW, Maschke M, … Group GN-AW. A placebo-controlled trial of gabapentin for painful HIV-associated sensory neuropathies. Journal of Neurology. 2004;251(10):1260–1266. doi: 10.1007/s00415-004-0529-6. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Koeppe J, Lyda K, Johnson S, Armon C. Variables associated with decreasing pain among persons living with Human Immunodeficiency Virus: A longitudinal follow-up study. The Clinical Journal of Pain. 2012;28(1):32–38. doi: 10.1097/AJP.0b013e318220199d. [DOI] [PubMed] [Google Scholar]
- Krashin DL, Merrill JO, Trescot AM. Opioids in the management of HIV-related pain. Pain Physician. 2012;15(3 Suppl):ES157–168. [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, … Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik K. Human Development Report 2013-The rise of the south: Human progress in a diverse world. New York: United Nations Development Programme; 2013. p. 216. [Google Scholar]
- Mazzotta E, Agostinone A, Rosso R, Di Biagio A, De Socio GV, Cappelletti A, … Parruti G. Osteonecrosis in human immunodeficiency virus (HIV)-infected patients: A multicentric case-control study. Journal of Bone and Mineral Metabolism. 2011;29(3):383–388. doi: 10.1007/s00774-010-0245-5. [DOI] [PubMed] [Google Scholar]
- Merlin JS, Westfall AO, Chamot E, Overton T, Willig JH, Ritchie C, … Mugavero MJ. The relationship of pain to physical function in patients with HIV: An underappreciated phenomenon. Paper presented at the Infectious Diseases Society of America; San Diego, CA. 2012. Oct, [Google Scholar]
- Merlin JS, Westfall AO, Raper JL, Zinski A, Norton WE, Willig JH, … Mugavero MJ. Pain, mood, and substance abuse in HIV: Implications for clinic visit utilization, ART adherence, and virologic failure. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2012;61(2):164–170. doi: 10.1097/QAI.0b013e3182662215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin JS, Zinski A, Norton WE, Ritchie CS, Saag MS, Mugavero MJ, … Hooten WM. A conceptual framework for understanding chronic pain in patients with HIV. Pain Practice. 2013 doi: 10.1111/papr.12052. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, Kushel MB. Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. The Journal of Pain. 2011;12(9):1004–1016. doi: 10.1016/j.jpain.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newshan G, Lefkowitz M. Transdermal fentanyl for chronic pain in AIDS: A pilot study. Journal of Pain and Symptom Management. 2001;21(1):69–77. doi: 10.1016/s0885-3924(00)00238-4. [DOI] [PubMed] [Google Scholar]
- Parker R, Stein DJ, Jelsma J. Pain in people living with HIV/AIDS: A systematic review. Journal of the International AIDS Society. 2014;17:18719. doi: 10.7448/IAS.17.1.18719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Papp J, Simpson DM. Neuromuscular diseases associated with HIV-1 infection. Muscle & Nerve. 2009;40(6):1043–1053. doi: 10.1002/mus.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlay JC, Chaloner K, Max MB, Flaws B, Reichelderfer P, Wentworth D, … Cohn DL. Acupuncture and amitriptyline for pain due to HIV-related peripheral neuropathy: A randomized controlled trial. Terry Beirn Community Programs for Clinical Research on AIDS. JAMA. 1998;280(18):1590–1595. doi: 10.1001/jama.280.18.1590. [DOI] [PubMed] [Google Scholar]
- Simpson DM, Brown S, Tobias J, Group NCS. Controlled trial of high-concentration capsaicin patch for treatment of painful HIV neuropathy. Neurology. 2008;70(24):2305–2313. doi: 10.1212/01.wnl.0000314647.35825.9c. [DOI] [PubMed] [Google Scholar]
- Simpson DM, Schifitto G, Clifford DB, Murphy TK, Durso-De Cruz E, Glue P, … Group HNS. Pregabalin for painful HIV neuropathy: A randomized, double-blind, placebo-controlled trial. Neurology. 2010;74(5):413–420. doi: 10.1212/WNL.0b013e3181ccc6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrels JL, Becker WC, Alford DP, Kapoor A, Williams AR, Turner BJ. Systematic review: Treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Annals of Internal Medicine. 2010;152(11):712–720. doi: 10.1059/0003-4819-152-11-201006010-00004. [DOI] [PubMed] [Google Scholar]
- Trafton JA, Sorrell JT, Holodniy M, Pierson H, Link P, Combs A, Israelski D. Outcomes associated with a cognitive-behavioral chronic pain management program implemented in three public HIV primary care clinics. The Journal of Behavioral Health Services &; Research. 2012;39(2):158–173. doi: 10.1007/s11414-011-9254-y. [DOI] [PubMed] [Google Scholar]
- Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburg N, Carr DB, … Witter J. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–345. doi: 10.1016/j.pain.2003.08.001. S030439590300335X [pii] [DOI] [PubMed] [Google Scholar]
- Turk DC, Rudy TE. IASP taxonomy of chronic pain syndromes: Preliminary assessment of reliability. Pain. 1987;30(2):177–189. doi: 10.1016/0304-3959(87)91073-6. [DOI] [PubMed] [Google Scholar]
- Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. The Lancet. 2011;377(9784):2226–2235. doi: 10.1016/S0140-6736(11)60402-9. [DOI] [PubMed] [Google Scholar]