Abstract
Following pediatric traumatic brain injury (TBI), longitudinal diffusion tensor imaging may characterize alterations in initial recovery and subsequent trajectory of white matter development. Our primary aim examined effects of age at injury and time since injury on pathway microstructure in children ages 6–15 scanned 3 and 24 months after TBI. Microstructural values generated using tract‐based spatial statistics extracted from core association, limbic, and projection pathways were analyzed using general linear mixed models. Relative to children with orthopedic injury, the TBI group had lower fractional anisotropy (FA) bilaterally in all seven pathways. In left‐hemisphere association pathways, school‐aged children with TBI had the lowest initial pathway integrity and showed the greatest increase in FA over time suggesting continued development despite incomplete recovery. Adolescents showed limited change in FA and radial diffusivity and had the greatest residual deficit suggesting relatively arrested development. Radial diffusivity was persistently elevated in the TBI group, implicating dysmyelination as a core contributor to chronic post‐traumatic neurodegenerative changes. The secondary aim compared FA values over time in the total sample, including participants contributing either one or two scans to the analysis, to the longitudinal cases contributing two scans. For each pathway, FA values and effect sizes were very similar and indicated extremely small differences in measurement of change over time in the total and longitudinal samples. Statistical approaches incorporating missing data may reliably estimate the effects of TBI and provide increased power to identify whether pathways show neurodegeneration, arrested development, or continued growth following pediatric TBI. Hum Brain Mapp 37:3929–3945, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: tract‐based spatial statistics, fractional anisotropy, radial diffusivity, axial diffusivity, children, microstructure, neurodegeneration, dysmyelination, chronic
INTRODUCTION
Despite significant advances in prevention, pediatric traumatic brain injury (TBI) remains a serious public health concern. Injury is the leading cause of mortality and morbidity in children and adolescents [Faul et al., 2010; Langlois et al., 2005]. Survivors of serious TBI are often left with residual, lifelong difficulties in a broad range of areas, including intellectual function, motor coordination, attention and memory, adaptive behavior, executive functions, academic achievement, social integration, and psychological adjustment that reduce quality of life [Di Battista et al., 2012; Koskinicmi et al., 1995; Rosema et al., 2015]. TBI sustained during childhood may alter the trajectory of brain development and connectivity and adversely affect neuropsychological functioning. TBI sustained at different ages during childhood may differentially affect the ongoing dynamic processes underlying brain maturation. Longitudinal studies of neuropsychological outcomes after pediatric TBI indicate that younger age at injury [Anderson et al., 2005; Ewing‐Cobbs et al., 1997] or injury during specific age ranges [Crowe et al., 2012] may be associated with greater vulnerability to adverse effects in a variety of cognitive and social domains. At present, it is unclear whether TBI sustained at different developmental stages, such as preschool, school‐age, or adolescent stages, produces similar or unique effects on brain microstructure. Moreover, there is little consensus regarding whether long‐term post‐traumatic changes in the development of specific pathways or structures are characterized by continued neurodegeneration, arrested or slowed development, or continued normal development. Longitudinal studies are essential to characterize the impact of TBI on the recovery of neural structures and the neuropsychological and functional outcomes they support. Therefore, the purpose of this article is to characterize the effects of age at injury and time since injury on subacute and chronic microstructural changes of several core association, limbic, and projection pathways during the first two years after TBI in school‐aged children and adolescents.
DIFFUSION TENSOR IMAGING OF TRAUMATIC BRAIN INJURY
The primary pathophysiological consequence of TBI is traumatic axonal injury (TAI). TAI is related to the combined effects of direct injury at the time of impact to myelinated and unmyelinated axons as well as to cell bodies [Reeves et al., 2005] and to a variety of secondary injury mechanisms. These mechanisms, including ionic flux, glutamate release, oxidative stress, apoptosis, and neuroinflammation, contribute substantially to cellular injury [Bramlett and Dietrich, 2015; Gennarelli et al., 1998; Giza and Hovda, 2014; Reeves et al., 2005]. Axonal degeneration is viewed as progressing from disruption in axonal transport, which produces swelling and progressing to disconnection and Wallerian degeneration [Gennarelli et al., 1998; Johnson et al., 2013b; Reeves et al., 2005]. Neurodegeneration of white matter has been documented years after a single TBI, particularly in cases with long‐term survival and evidence of inflammatory pathology characterized by reactive microglia [Johnson et al., 2013a]. In relation to pediatric TBI, the vulnerability of unmyelinated axons to injury [Reeves et al., 2005], and the ongoing rapid myelination of most pathways [Krogsrud, 2016; Lebel et al., 2008; Simmonds et al., 2014] may confer particular vulnerability when injury is sustained during school‐age and adolescent stages of development.
Recent advances in structural neuroimaging, particularly diffusion tensor imaging (DTI), have yielded better visualization of the nature and extent of injury to both white and gray matter. DTI metrics include fractional anisotropy (FA), which is affected by several factors including myelin thickness, the direction of water molecules moving in multiple directions, and the density of white matter fiber tracts [Pierpaoli et al., 2001]. Mean diffusivity (MD) may index several variables, including fiber density, axonal diameter, myelination, and expansion of extracellular space, which may indicate loss of neurons or glia. MD may be subdivided into clinically useful diffusivity metrics, including axial diffusivity (AD) and radial diffusivity (RD), which are believed to assess the status of axonal morphology and the myelin sheath, respectively [Sun et al., 2006].
In pediatric studies, DTI metrics have characterized changes in white matter microstructure across a wide range of injury severity at different time points after injury. Within the first month after TBI, FA is increased and mean diffusivity is decreased. In contrast, during subacute (approximately 1–5 months after injury) and chronic stages of recovery, FA is decreased and mean diffusivity is increased across a broad range of pathways and regions of interest [Roberts et al., 2014; Sidaros et al., 2008]. Relative to a comparison group of healthy children or children with orthopedic injury (OI), FA was significantly lower and MD was significantly elevated in the children and adolescents with TBI in a variety of pathways, including commissural [Ewing‐Cobbs et al., 2008; Wilde et al., 2006; Wu et al., 2010], projection [Caeyenberghs et al., 2010; Kurowski et al., 2009; Scheibel et al., 2011; Wilde et al., 2008], and association pathways [Johnson et al., 2011, 2015; Wilde et al., 2008, 2011, 2010]. Similar patterns of decreased FA and increased MD were reported in regions of interest, including frontal lobe white matter in addition to the centrum semiovale, corona radiata, and temporal lobe white matter [Caeyenberghs et al., 2010; Kurowski et al., 2009; Levin et al., 2011; Scheibel et al., 2011]. Studies that have examined RD and AD following pediatric TBI indicated that during the chronic stage, RD is significantly increased in the majority of callosal, projection, and association pathways; AD appears to be less consistently disrupted [Dennis et al., 2015; Ewing‐Cobbs et al., 2008].
Very few studies have examined longitudinal changes in DTI metrics. In children, longitudinal studies are needed to examine how age at the time of injury and severity of injury may affect microstructural recovery. Two longitudinal studies evaluated changes in DTI metrics in children and adolescents with complicated‐mild to severe TBI or OI evaluated at 3 and 18 months postinjury. Wu et al. [2010] examined changes in the corpus callosum and its subregions using fiber tractography. At both time intervals, FA was significantly lower and MD was higher in the total corpus callosum and in the genu, body, and splenium in the TBI group compared to children with OI. Despite reduction in callosal volume over the follow‐up period, FA remained significantly lower in the TBI group. Increases in FA over time were noted in both groups in the splenium and posterior regions suggesting continued developmental changes. In the same sample, Wilde et al. [2012] used tract‐based spatial statistics (TBSS) to examine changes in white matter from 3 to 18 months after TBI. TBSS is a data‐driven approach allowing estimation of microstructural metrics in the core of white matter pathways and regions of interest [Smith et al., 2006]. Relative to an OI group, the TBI group had decreased FA and increased MD on the initial scan in regions of frontal, superior and/or posterior temporal, parietal, and occipital white matter, as well as the corpus callosum, cingulum bundle, and cerebellum. By the latter scan, the OI group showed typical developmental changes including diffuse increases in FA in the bilateral lobular white matter, cerebellum, and corpus callosum. Importantly, children with TBI failed to show expected increases in FA over time in widespread regions; rather, decreases in FA were observed in white matter from anterior and central temporal lobes, all other lobes, the genu and splenium of the corpus callosum, cingulum bundle, cerebellum, and brainstem.
Dennis et al. [2015] used a novel method of tract extraction to examine DTI metrics during postacute and chronic stages of recovery in a cross‐sectional sample of children with moderate and severe TBI. At the postacute assessment, FA in the children with TBI was significantly reduced in the left inferior fronto‐occipital and inferior longitudinal fasciculi; FA was similar in TBI and community comparison children in all other pathways after correction for multiple comparisons. By the chronic stage at least 1 year after injury, the TBI group showed significant elevation in MD and RD across 14–16 of the 18 tracts evaluated. FA and AD were less affected by TBI.
CURRENT STUDY
To date, pediatric studies have examined structural imaging data obtained at postacute and chronic time points separately without assessing the influence of age at injury or directly comparing change over time in TBI and comparison groups. To address these gaps in the literature, the primary aim of this study was to use TBSS to examine the impact of age at injury and time since injury on FA values obtained during subacute and chronic stages of recovery in children and adolescents with TBI relative to an orthopedic injury (OI) comparison group. In selected association, limbic, and projection pathways that support core academic skills and executive functions, we expected FA to be lower and RD to be elevated in TBI compared to OI groups at 3 and 24 months after injury. Although children with TBI were expected to show increases in FA over time, a persistent deficit was anticipated at the 24 month follow‐up. After controlling for age, we expected that younger children would be more vulnerable to the effects of TBI than older children and adolescents as indicated by lower FA values and less change over time. Given the sensitivity of RD to alteration in the status of myelin and neurodegeneration, we also expected that RD would be elevated in the TBI group.
A major issue in longitudinal studies involves subject loss at different time points. Given the high expenses associated with longitudinal studies, particularly those involving imaging in clinical samples, it is important to investigate whether analyses including cases with available data sets yield similar imaging findings to analyses restricted to longitudinal data sets. Therefore, the secondary aim was to examine the pattern of findings when using the longitudinal versus the total sample to characterize the impact of TBI on FA values.
METHODS
Participants
All participants were recruited from the Level 1 Pediatric Trauma Center at Children's Memorial Hermann Hospital/University of Texas Health Science Center for either a TBI or OI. The pool of participants was drawn from a larger pool of 119 children recruited for a prospective, longitudinal study of academic outcomes following pediatric injury from 2004 to 2009. Of the original 119 participants, two were removed for not meeting exclusion criteria after consent and one withdrew from the study. Thirteen participants did not receive an MRI. Following rigorous examination of each scan, an additional 16 cases were excluded from the final sample due to imaging concerns (n = 11 due to motion artifact, n = 2 to other artifact, n = 3 due to failure to register to the TBSS template; see p. 10). The remaining 87 participants in the total sample included 44 children with TBI and 43 with OI. The longitudinal sample consisted of a subset of participants with TBI (n = 16) and OI (n = 18) who had usable neuroimaging at both 3 and 24 month intervals.
Children with OI were used as a comparison group to account for possible preinjury characteristics, such as risk‐taking behavior, that may influence outcomes, and to control for exposure to the stresses of injury and hospitalization. All participants met the following inclusion criteria: (1) age at injury between 6 and 15 years, (2) hospitalization for TBI or OI, (3) proficiency in English, (4) residing within a 125 mile catchment radius, (5) no preinjury history of major neuropsychiatric disorder such as intellectual deficiency or low functioning autism spectrum disorder that would complicate assessment of the impact of injury on imaging or behavioral outcomes, and (6) no prior medically attended TBI. The latter two criteria were assessed during hospitalization using a brief parent questionnaire. Demographic and injury characteristics of the TBI and OI participants in the total sample are provided in Table 1 (see Supporting Information, Table S1 for longitudinal sample data). Several measures were used to estimate severity of TBI and bodily injury. The TBI group experienced acceleration–deceleration or blunt impact injuries. The severity of head injury was rated using the lowest postresuscitation Glasgow Coma Scale (GCS) score [Teasdale and Jennett, 1974], where severe TBI was a score of 3–8 and moderate TBI was a score of 9–12. Complicated mild TBI was classified as a GCS score of 13–15 combined with acute hemorrhage or parenchymal injury seen in acute neuroimaging [Levin et al., 2008]. Participants in the OI group also had no evidence of trauma to the head or symptoms of concussion. Severity of injury was based on the Injury Severity Scale (ISS) [Baker et al., 1974], which sums the squared Abbreviated Injury Scale score of the 3 most severely injured head and body regions. To compare the severity of bodily injury across the TBI and OI groups, we also computed the modified Injury Severity Scale (MISS) score [Mayer et al., 1980] that excludes injury to the head.
Table 1.
Demographic information and injury characteristics for traumatic brain and orthopedic injury groups
| Group | |||
|---|---|---|---|
| Traumatic Brain Injury | Orthopedic Injury | ||
| Demographic Variables | (n = 44) | (n = 43) | Statistic (p) |
| Months of age at injury M (SD) | 131.3 (34.4) | 124.2 (35.3) | t(85) = −0.95 (0.344) |
| Sex % male | 72.7 | 60.5 | Χ2(1, N = 87) = 1.47 (0.225) |
| Ethnicity/Race (n) | Χ2(3, N = 87) = 1.70 (0.736) | ||
| African‐American | 7 | 10 | |
| Hispanic | 13 | 14 | |
| Other/multiethnic | 4 | 4 | |
| Caucasian | 20 | 15 | |
| Maternal education (n) | X2(3, N = 87) = 3.03 (0.387) | ||
| <High school | 11 | 5 | |
| HS graduate | 11 | 13 | |
| Partial college | 12 | 16 | |
| College or graduate degree | 10 | 9 | |
| Injury characteristics | |||
| External cause of injury (n) | Χ2(4, N = 87) = 24.80 (0.000) | ||
| Fall | 8 | 17 | |
| Motor vehicle collision | 24 | 5 | |
| Vehicle‐pedestrian | 9 | 8 | |
| Sports/recreational | 2 | 13 | |
| Other | 1 | 0 | |
| Injury‐scan interval (m) M (SD) | |||
| Initial | 3.14 (1.42) | 3.24 (2.53) | t(83) = 0.067 (0.947) |
| Follow‐up | 24.83 (2.86) | 23.0 (4.97) | t(75) = −2.06 (0.042) |
| Injury Severity Score M (SD) | 22.52 (10.48) | 8.40 (1.76) | t(85) = −10.19 (0.000) |
| Modified Injury Severity Score M (SD) | 7.14 (8.89) | 8.40 (1.76) | t(85) = 0.91 (0.365) |
| Glasgow Coma Scale score (n) | |||
| 3–8 | 31 | – | |
| 9–12 | 10 | – | |
| 13–15 | 3 | – | |
Procedure
Participants were recruited during or shortly following hospitalization. The research was conducted in accordance with the Code of Ethics of the World Medical Association, the granting agency, and the University Institutional Review Board. Informed written consent was obtained from the child's guardian according to Institutional Review Board guidelines. Oral assent was obtained from children aged 6–7 years, and written assent was obtained from children 8 years and older.
As part of the longitudinal follow‐up protocol, cognitive and academic measures were administered at 3, 6, 12, and 24 months postinjury by a trained research assistant. Participants received an MRI scan at approximately 3 and 24 months postinjury.
NEUROIMAGING
Image Acquisition
All MRIs were performed on a Philips 3 T scanner with SENSE (Sensitivity Encoding) technology using an eight‐channel phase array head coil. After conventional scout, T1‐, and T2‐weighted images were collected, followed by a DTI sequence. The DTI sequence consisted of a single‐shot spin‐echo diffusion sensitized echoplanar imaging sequence with the following parameters: 21 noncollinear equally distributed diffusion encoding directions (e.g., Icosa21; Hasan & Narayana, 2003]; repetition time/echo time = 6100/84 ms; b = 0, and 1000 s/mm2; field‐of‐view = 240 × 240 mm2; acquisition matrix = (112, 112); reconstructed matrix = 256 × 256; slice thickness = 3.0 mm; reconstructed in‐plane pixel dimensions (x,y) = 0.94,0.94; SENSE acceleration factor = 2; a total scan time of approximately 7 min.
Image Processing
DTI data were processed using FMRIB's Software Library version 5.0.7 [FSL; Smith, Jenkinson, Woorich, Beckmann, Behrens, Johansen‐Berg et al., 2004] on a 64bit Linux workstation. FSL's workflow (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/TBSS/UserGuide) for TBSS [Smith et al., 2006] was utilized to extract FA, RD, and AD values from atlas‐based white matter pathways for statistical analyses in a third party package (SAS). All reconstructed directional and B0 volumes in the 4D image files were reviewed for motion artifacts. Participant scan sessions were eliminated if there was visually identifiable evidence of motion distortion present in any volume of the reconstructed 4D image file. As noted in the participants section, through this quality check 22 sessions were removed, which resulted in the loss of 11 complete participants from the original sample. Briefly, standard TBSS preprocessing included correction for eddy currents and head motion (ECC), extraction of brain tissue from the skull using Brain Extraction Tool (BET), followed by fitting a tensor model to the data using DTIFIT to generate spatial maps of FA values for each individual subject. Subsequently, FA data from each participant was then aligned onto the MNI152 standard‐space template using the nonlinear registration tool (FMRIB Nonlinear Image Registration Tool) [Andersson, 2007]. Next, a mean FA skeleton was created, which represents the centers of all white matter tracts common to all subjects included in the analyses. Each subject's aligned FA data (thresholded at FA values >0.15) was then projected onto this skeleton. Accuracy of registration was then visually checked for each participant. Registration was not successful in 9 scans from each group, which resulted in exclusion of 3 participants from further analysis. Included with FSL's atlas tools is the atlas of probabilistic tracts derived from the Johns Hopkins University white‐matter tractography data [Hua et al., 2008]. Binary masks were derived from each white matter tract of interest included with the JHU atlas. The intersection of each subject's skeletonized FA values and binary mask for each white matter tract of interest were used to record the mean value of each tract in each subject using FSL‐based command‐line utilities. Subsequently, FSL's non‐FA workflow for TBSS was used to generate AD and RD values for each tract.
DTI Metrics and Pathways of Interest
Following the TBSS analysis for group differences, mean values for FA, RD, and AD were extracted from the left and right hemisphere for the seven tracts of interest and then exported to SAS 9.3 for further statistical analyses. Based on Simmonds et al. [2014], pathways were divided into association, limbic, and projection categories. Long association pathways interconnecting cortical areas included superior and inferior longitudinal, inferior frontal‐occipital, and uncinate fasciculi. Limbic pathways included two sections of the cingulum bundle, the cingulate portion and the hippocampal extension. DTI metrics were also extracted from the corticospinal tract, a projection pathway. Figure 1 shows the mean skeletonized FA image with masks corresponding to the association and limbic pathways investigated.
Figure 1.
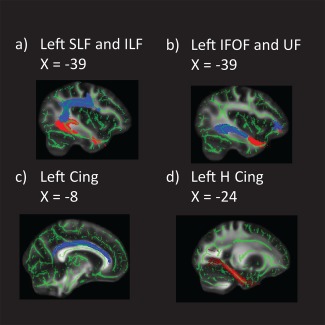
Mean skeletonized FA image with masks corresponding to (a) SLF (blue) and ILF (red); (b) IFOF (blue) and UF (red); (c) cingulum; (d) cingulum hippocampal.
FA was the primary metric of interest. To minimize the number of analyses using correlated metrics, we opted to follow up any significant group differences in FA by completing post‐hoc analysis of RD and AD. This option reduces the number of overlapping analyses and permits better characterization of the type of microstructural injury contributing to changes in FA.
STATISTICAL APPROACH
Demographic and injury variables were compared across the TBI and OI groups using chi‐square and general linear model analysis.
The distributions of the DTI metrics were examined. Group differences for FA values from each pathway were then investigated for the total sample and the longitudinal sample. For the total sample, a mixed model with a compound symmetry covariance structure was used to examine the main effects of group (TBI, OI), age, time of scanning (3 and 24 months), and their interactions on FA values from seven association and projection pathways. For all analyses, left and right hemisphere values were examined in separate models. For the longitudinal sample with DTI data at both 3 and 24 months, FA of each pathway from the left and right hemisphere was examined using repeated measures ANOVA with group (TBI, OI) and age at scan as the between‐subjects factors and time of scanning (3 and 24 months) and interactions of time with age and group as within‐subjects factors. To identify the most parsimonious model, if the three‐way interaction of group, age, and time was nonsignificant, it was trimmed from each model. Subsequently, the two‐way interactions were examined and trimmed if nonsignificant for all analyses. Secondary analyses of RD and AD were completed only for pathways where significant group differences in FA results were obtained. The Type I error rate for the secondary analyses was controlled using the Bonferroni correction for the seven dependent variables from each hemisphere separately.
Our secondary aim was to examine the comparability of results based on using mixed models versus repeated measures analyses. General linear mixed models allow inclusion of all available data which increases power, whereas repeated measures approaches entail listwise deletion of missing data. Measures of effect size, which are less influenced by sample size than are p values, were included to index the strength or magnitude of findings [Durlak, 2009; Lipsey, 1990]. First, Cohen's d [Cohen, 1998] was used to calculate effect sizes for change from 3 to 24 months for TBI and OI groups for each pathway for the total and longitudinal samples. The effect size was derived by dividing the estimate for the difference in time for each group by the standard deviation at the 3 month interval. Cohen's d is interpreted as follows 0.2, small effect; 0.5, medium effect, and 0.8 large effect. Second, to assess whether the change over time was similar across the samples, the estimate and effect size from the longitudinal sample were subtracted from the values for the total sample. This yielded measures of the mean difference and the effect size for those differences. The effect size for the difference in total and longitudinal samples from 3 to 24 months for each pathway is reported in standard deviation units such that an effect size of 0.05 indicates that the estimate of change across time in the two samples differed by 5/100 of a standard deviation, which would indicate a very small difference.
RESULTS
Participants
As indicated in Table 1, the distribution of demographic variables for the TBI and OI groups in the total sample did not differ for age at injury, sex, ethnicity, or maternal education. For the longitudinal sample, the TBI group was significantly older than the OI group at the time of injury (Supporting Information, Table SI). Otherwise, demographic variables were similar across the two injury groups. Injury‐related variables differed in TBI and OI participants for the total sample for external cause of injury. The TBI group was more likely to be injured in motor vehicle collisions, while falls and sports/recreational injuries predominated in the OI group. The first MRI scan was obtained approximately 3.1 months after the injury for both groups; in contrast, the interval between the injury and the follow‐up scan was significantly longer for the TBI than OI group. A GLM covarying age at the follow‐up scan indicated that neither the injury to test interval nor the group by injury test interval interaction was significantly related to the FA values, Ps > 0.7. Therefore, the difference in the injury test interval did not appear to significantly impact the results. As expected, the ISS was higher in the TBI than OI group because it includes the codes for injury to the brain. The MISS score, which excluded injury to the head, indicated that the severity of body injury was similar across the TBI and OI groups. The majority of TBI patients had a severe injury; 70% had GCS scores ≤ 8. Age at injury was not significantly related to the GCS score, suggesting that the severity of TBI was comparable across age, rs (42) = 0.07, p = 0.64. Injury‐related variables for the longitudinal sample showed a similar pattern of group differences. The TBI and OI groups differed on external cause and the ISS but not on the MISS score. Sixty‐nine percent of the TBI group had a GCS score ≤8.
To assess any selective differences in demographic and injury variables, we compared the patients with TBI who were in the longitudinal sample and contributed two scans to the analysis (n = 16) with those who contributed one scan to the analysis (n = 28). The TBI subgroups did not differ significantly on age at injury, t(42) = 1.07, P = 0.290, gender, Χ 2 (1, N = 44) = 0.065, P = 0.798, ethnicity Χ 2 (3, N = 44) = 2.77, P = 0.429, maternal education, Χ 2 (3, N = 44) = 0.163, P = 0.653, GCS score, Χ 2 (2, N = 44) =1.04, P = 0.594, or cause of injury, Χ 2 (4, N = 44) = 2.41, P = 0.661. Similarly, there were no significant differences in demographic or injury variables for OI participants contributing one (n = 25) or two scans (n = 18) to the analyses. Age at injury, t(41) = −1.37, P = 0.177, gender, Χ 2 (1, N = 43) = 0.312, P = 0.576, ethnicity Χ 2 (3, N = 44) = 1.57, P = 0.667, maternal education, Χ 2 (3, N = 44) = 1.54, P = 0.673, and cause of injury, Χ 2 (3, N = 43) = 1.45, P = 0.694, did not differ. This set of analyses suggests that there is no bias in evident in the demographic or injury variables affecting whether TBI or OI participants contributed one or two scans to the analyses. Table 2 shows the number of children ages < 11 ≥ years of age in the TBI and OI groups with usable scans at 3‐ and 24‐month time points.
Table 2.
Distribution of usable scans by age and group
| Time of scanning | ||||
|---|---|---|---|---|
| Group | 3 months | 24 months | ||
| Years of age at injury | ||||
| TBI | 6–10 | 11–15 | 6–10 | 11–15 |
| GCS 3–8 | 12 | 9 | 13 | 8 |
| GCS 9–15 | 4 | 3 | 5 | 6 |
| OI | 19 | 14 | 17 | 11 |
| Total | 35 | 26 | 35 | 25 |
Acute CT scans were examined to provide additional information regarding the type and location of brain injury. Intracranial abnormalities were visualized in 40 of the 44 patients with TBI. Lesions were classified as parenchymal and extra‐axial; location of lesion was noted as left, right, bilateral, or midline. Parenchymal lesions included hemorrhagic contusion, intracerebral hemorrhage, shear, and edema. Extra‐axial findings were classified as subarachnoid or intraventricular, subdural, or epidural hemorrhages. As indicated in Table 3, the distribution of both types of lesions to the left and right hemispheres was similar; the majority of lesions were bilateral.
Table 3.
Number of parenchymal and extra‐axial lesions visualized on acute CT scans for the total sample of TBI participants
| Lesion location | ||||
|---|---|---|---|---|
| Lesion type | Left | Right | Bilateral | Midline |
| Parenchymal | ||||
| Contusion | 3 | 3 | 13 | 0 |
| Intracerebral hemorrhage | 0 | 0 | 4 | 0 |
| Shear | 0 | 1 | 7 | 1 |
| Edema | 2 | 1 | 5 | 0 |
| Extra‐axial hemorrhage | ||||
| Subarachnoid/IVH | 3 | 3 | 8 | 0 |
| Subdural | 3 | 2 | 2 | 4 |
| Epidural | 2 | 3 | 3 | 0 |
DTI FINDINGS FOR TOTAL AND LONGITUDINAL SAMPLES
Fractional Anisotropy
Least square means adjusted for age at scan for FA from each pathway at the 3‐ and 24‐month follow‐up TBSS analyses are reported in Table 4 for the TBI and OI participants in the total and Table SII for the longitudinal samples. The mean values were similar in the total and longitudinal samples across the association, limbic, and projection pathways.
Table 4.
Least square means adjusted for age and standard errors for pathway fractional anisotropy for traumatic brain injury and orthopedic injury groups: left and right hemisphere values at 3 and 24 months after injury for total sample
| Traumatic brain injury | Orthopedic injury | |||||||
|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | |||||
| Months after injury | Months after injury | |||||||
| Pathway | 3 | 24 | 3 | 24 | 3 | 24 | 3 | 24 |
| Superior longitudinal | 0.437 (0.0045) | 0.456 (0.0037) | 0.447 (0.0041) | 0.465 (0.0040) | 0.461 (0.0044) | 0.477 (0.0038) | 0.466 (0.0040) | 0.482 (0.0041) |
| Inferior longitudinal | 0.473 (0.0050) | 0.489 (0.0041) | 0.480 (0.0043) | 0.497 (0.0039) | 0.509 (0.0049) | 0.519 (0.0042) | 0.512 (0.0041) | 0.524 (0.0040) |
| Inferior frontal occipital | 0.481 (0.0053) | 0.495 (0.0044) | 0.485 (0.0048) | 0.501 (0.0044) | 0.518 (0.0052) | 0.530 (0.0045) | 0.519 (0.0047) | 0.530 (0.0045) |
| Uncinate | 0.446 (0.0053) | 0.459 (0.0047) | 0.438 (0.0054) | 0.454 (0.0054) | 0.483 (0.0052) | 0.498 (0.0049) | 0.470 (0.0052) | 0.489 (0.0056) |
| Cingulum | 0.492 (0.0069) | 0.513 (0.0074) | 0.451 (0.0072) | 0.477 (0.0079) | 0.530 (0.0067) | 0.552 (0.0076) | 0.489 (0.0071) | 0.516 (0.0081) |
| Cingulum hippocampal | 0.397 (0.0050) | 0.410 (0.0044) | 0.442 (0.0047) | 0.456 (0.0043) | 0.414 (0.0048) | 0.426 (0.0045) | 0.468 (0.0045) | 0.477 (0.0044) |
| Corticospinal | 0.582 (0.0048) | 0.597 (0.0041) | 0.582 (0.0041) | 0.595 (0.0035) | 0.606 (0.0046) | 0.615 (0.0042) | 0.604 (0.0039) | 0.615 (0.0036) |
Total sample
Results of general linear mixed model analysis examining FA values for each hemisphere for the total sample are provided in Table 5. Age at injury was treated as a continuous variable; midpoints for the younger, mid, and older ages were approximately 8, 10, and 13.5 years of age. For left‐hemisphere FA, group × age × time interactions were significant at the 0.05 level for the superior and inferior longitudinal, inferior frontal‐occipital, cingulum‐hippocampal extension, and uncinate fasciculi. The pattern of findings was similar across these pathways. To illustrate, Figure 2 shows that after covarying for age at scan, FA was the lowest at the initial scan in the left superior longitudinal fasciculus in the young and middle age groups of children with TBI. FA values increased over the follow‐up period more in the youngest children with TBI than the youngest children with OI. In contrast, the increase over time was less in the older TBI group than in the older OI group. Two years after injury, the relative deficit was larger in the older than younger children with TBI. For the two remaining pathways, the cingulum and corticospinal tract, the three‐way interaction trended toward significance, with P values of 0.051 and 0.094, respectively. For both pathways, the main effects of time, group, and age were significant when nonsignificant interaction effects were trimmed.
Table 5.
Final models from mixed model analysis of fractional anisotropy values for group, age, and time of scan for left and right hemisphere pathways
| Within‐subjects effects | Between‐subjects effects | |||||
|---|---|---|---|---|---|---|
| Group × time × age | Time × age | Time × group | Time | Group | Age | |
| Pathway and hemisphere | df (1,30) F (p) | df (1,30) F (p) | df (1,30) F (p) | df (1,30) F (p) | df (1,83) F (p) | df (1,83) F (p) |
| Superior longitudinal | ||||||
| Left | 5.16 (0.030) | 0.05 (0.817) | 5.97 (0.021) | 11.34 (0.002) | 1.72 (0.193) | 9.15 (0.003) |
| Right | 74.11 (<0.0001) | 10.76 (0.002) | 12.19 (0.001) | |||
| Inferior longitudinal | ||||||
| Left | 4.88 (0.035) | 0.20 (0.657) | 5.91 (0.021) | 3.69 (0.064) | 0.20 (0.007) | 3.69 (0.654) |
| Right | 42.61 (<0.0001) | 30.17 (<0.0001) | 9.34 (0.003) | |||
| Inferior frontal occipital | ||||||
| Left | 4.67 (0.039) | 1.49 (0.231) | 5.20 (0.030) | 6.99 (0.013) | 0.28 (0.597) | 6.64 (0.012) |
| Right | 42.6 (<0.0001) | 26.34 (<0.001) | 12.32 (0.001) | |||
| Uncinate | ||||||
| Left | 4.51 (0.042) | 6.32 (0.012) | 4.76 (0.037) | 16.55 (<0.001) | 0.96 (0.331) | 4.75 (0.032) |
| Right | 32.83 (<0.0001) | 23.03 (<0.0001) | 4.62 (0.035) | |||
| Cingulum | ||||||
| Left | 27.37 (<0.0001) | 17.60 (<0.0001) | 6.61 (0.012) | |||
| Right | 45.42 (<0.0001) | 14.95 (0.001) | 10.92 (0.001) | |||
| Cingulum hippocampal | ||||||
| Left | 4.13 (0.051) | 0.14 (0.707) | 4.02 (0.054) | 2.27 (0.143) | 0.060 (0.811) | 8.24 (0.005) |
| Right | 18.02 (0.001) | 16.75 (<0.0001) | 9.57 (0.003) | |||
| Corticospinal | ||||||
| Left | 16.83 (0.001) | 15.44 (0.001) | 6.28 (0.014) | |||
| Right | 31.85 (<0.0001) | 18.14 (<0.0001) | 3.06 (0.084) | |||
Figure 2.
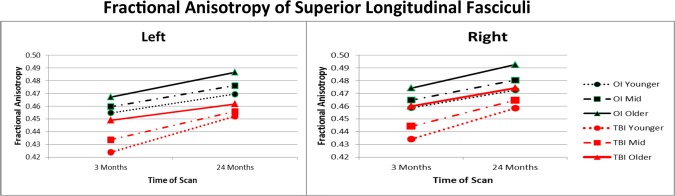
The three‐way interaction of group, age at injury, and time of scanning is plotted for left‐ and right‐hemisphere FA values from the superior longitudinal fasciculus for the total sample. After controlling for age at scan, younger children with TBI had the lowest initial pathway integrity and showed the greatest increase in left hemisphere FA over time. The adolescents with TBI showed the least increase in FA from 3 to 24 months after injury and the greatest deficit at 24 months relative to the OI group. Right hemisphere values showed main effects for group, age, and time but no interactions.
Analysis of the right‐hemisphere FA values for the total sample did not yield significant three‐way interactions, although trends were noted for the superior and inferior longitudinal fasciculi, with P values of 0.076 and 0.074, respectively. The two‐way interactions were nonsignificant and were trimmed. Only after trimming these terms, the main effects of group, time, and age were found to be significant. FA values were significantly and consistently lower in the TBI than OI groups across all pathways. Similarly, FA increased significantly over time in all pathways. Higher age at scan was significantly associated with higher FA values in all pathways except for the corticospinal tract.
Longitudinal sample
For the longitudinal sample, findings from final models from each repeated measures ANOVA are provided in Supporting Information, Table SII. The pattern of performance was similar to the total sample, with FA lowest in younger children with TBI and the older children with TBI showing less increase in FA over the follow‐up.
EFFECT SIZES FOR FRACTIONAL ANISOTROPY FOR TOTAL AND LONGITUDINAL SAMPLES
To address the secondary aim, we first examined effect sizes for change in FA values across time for the OI and TBI groups for the total and longitudinal samples separately. Table 6 contains the estimates and effect sizes for OI and TBI groups from each sample across time of scan. The effect sizes based on Cohen's d indicated that the magnitude of change over time within each group was in the small to medium range. Based on the total sample, the effect size was smallest in the left corticospinal tract (0.343) and inferior longitudinal fasciculus (0.335) of the OI group and largest in the right superior longitudinal fasciculus (0.716) in the TBI group.
Table 6.
Comparison of estimates and effect sizes (d) of pathway fractional anisotropy for total and longitudinal groups by time of scan
| Total samplea | Longitudinal samplea | Difference in samples from 3 to 24 monthsb | Standardized difference in effect size between injury groupsc | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pathway | Group | Estimate | d | Estimate | d | Estimate | d | Mean | |
| Superior longitudinal | |||||||||
| Left | OI | 0.016 | 0.586 | 0.017 | 0.529 | −0.001 | 0.057 | 0.07 | |
| TBI | 0.019 | 0.673 | 0.019 | 0.591 | 0 | 0.082 | |||
| Right | OI | 0.015 | 0.602 | 0.016 | 0.56 | −0.001 | 0.042 | 0.055 | |
| TBI | 0.018 | 0.716 | 0.018 | 0.648 | 0 | 0.068 | |||
| Inferior longitudinal | |||||||||
| Left | OI | 0.01 | 0.335 | 0.011 | 0.352 | −0.001 | 0.017 | 0.033 | |
| TBI | 0.016 | 0.523 | 0.015 | 0.474 | 0.001 | 0.049 | |||
| Right | OI | 0.012 | 0.451 | 0.012 | 0.473 | 0 | 0.022 | 0.021 | |
| TBI | 0.017 | 0.671 | 0.017 | 0.69 | 0 | 0.019 | |||
| Inferior frontal occipital | |||||||||
| Left | OI | 0.012 | 0.364 | 0.013 | 0.392 | −0.001 | 0.027 | 0.02 | |
| TBI | 0.014 | 0.432 | 0.014 | 0.419 | 0 | 0.013 | |||
| Right | OI | 0.011 | 0.379 | 0.011 | 0.424 | 0 | 0.044 | 0.059 | |
| TBI | 0.016 | 0.553 | 0.017 | 0.627 | 0 | 0.074 | |||
| Uncinate | |||||||||
| Left | OI | 0.015 | 0.463 | 0.015 | 0.498 | 0 | 0.035 | 0.033 | |
| TBI | 0.013 | 0.413 | 0.014 | 0.445 | 0 | 0.031 | |||
| Right | OI | 0.019 | 0.583 | 0.02 | 0.612 | −0.001 | 0.03 | 0.036 | |
| TBI | 0.016 | 0.511 | 0.018 | 0.553 | −0.001 | 0.042 | |||
| Cingulum | |||||||||
| Left | OI | 0.022 | 0.532 | 0.023 | 0.537 | −0.001 | 0.005 | 0.003 | |
| TBI | 0.021 | 0.502 | 0.022 | 0.504 | −0.001 | 0.002 | |||
| Right | OI | 0.026 | 0.595 | 0.026 | 0.551 | 0.001 | 0.045 | 0.026 | |
| TBI | 0.026 | 0.583 | 0.027 | 0.575 | −0.001 | 0.007 | |||
| Cingulum hippocampal | |||||||||
| Left | OI | 0.012 | 0.408 | 0.013 | 0.395 | −0.001 | 0.013 | 0.077 | |
| TBI | 0.013 | 0.428 | 0.01 | 0.286 | 0.003 | 0.142 | |||
| Right | OI | 0.01 | 0.354 | 0.01 | 0.316 | 0 | 0.038 | 0.057 | |
| TBI | 0.014 | 0.492 | 0.013 | 0.416 | 0.001 | 0.076 | |||
| TBI | 0.016 | 0.511 | 0.018 | 0.553 | −0.001 | 0.042 | |||
| Corticospinal | |||||||||
| Left | OI | 0.01 | 0.343 | 0.012 | 0.393 | −0.002 | 0.05 | 0.032 | |
| TBI | 0.015 | 0.539 | 0.016 | 0.553 | −0.001 | 0.014 | |||
| Right | OI | 0.011 | 0.453 | 0.012 | 0.425 | −0.001 | 0.027 | 0.035 | |
| TBI | 0.013 | 0.514 | 0.013 | 0.471 | −0.001 | 0.042 | |||
Second, we then compared d estimating the difference in magnitude of change over time across the total and longitudinal samples for the TBI and OI groups separately. The difference in effect size was extremely small for the left cingulum in the TBI (ES = 0.002) and OI groups (ES = 0.005). This suggests that the estimates of change in FA over time were very similar across the total and longitudinal samples for both groups. The greatest variation was evident for the left superior longitudinal fasciculus, with difference in effect sizes of 0.082 for the TBI and 0.057 for the OI groups. Finally, we compared the d values obtained in the second set of analyses above between groups. The resulting standardized difference in effect sizes across groups is expressed in standard deviation units. Across all pathways, the average difference in effect size was 0.04, suggesting that the change in FA values over time from the total and longitudinal samples was similar within each group.
Radial and Axial Diffusivities
To further characterize the impact of TBI on pathway microstructure, we completed post‐hoc analyses of RD and AD on pathways for which we identified significant findings based on general linear mixed model analyses of FA. The Type I error rate was controlled for analyses of pathways within each hemisphere at a critical value of 0.007 (0.05/7). Tables 7 and 8 contain the significant findings for the trimmed models for RD and AD, respectively. The pattern of findings was similar in left and right hemispheres. For RD, none of the three‐way interactions of group, time, and age survived correction. Several time × age interaction terms were significant; RD decreased more across the follow‐up in younger than in older participants for left superior longitudinal fasciculus, left uncinate, and bilateral cingulum bundles. No other two‐way interactions remained significant. In contrast, the main effects of group and time were significant for all pathways, suggesting that RD values were higher in the TBI than OI participants and changed to the same degree over time in both groups. The main effect of age was significant for most of the comparisons; RD was higher in younger than in older children. Figure 3 shows the pattern of change over time in the total sample for RD and AD of the left superior longitudinal fasciculus. Given the lack of change over time in RD in the oldest TBI group, it is likely that this accounts in part for the less pronounced change over time in FA.
Table 7.
Final models from mixed model analysis of radial diffusivity values for group, age, and time of scan for left and right hemisphere pathways
| Within‐subjects effects | Between‐subjects effects | |||||
|---|---|---|---|---|---|---|
| Group × time × age | Time × age | Time × group | Time | Group | Age | |
| Pathway | df (1,30) F (p) | df (1,83) F (p) | df (1,30) F (p) | df (1,30) F (p) | df (1,83) F (p) | df (1,83) F (p) |
| Superior longitudinal | ||||||
| Left | 13.33 (0.001) | 22.66 (<0.0001) | 17.18 (<0.0001) | 14.76 (0.001) | ||
| Right | 6.51 (0.016) | 18.59 (0.0001) | 15.66 (0.0002) | 18.73 (<0.0001) | ||
| Inferior longitudinal | ||||||
| Left | 9.48 (0.004) | 26.76 (<0.0001) | 8.21 (0.005) | |||
| Right | 4.35 (0.045) | |||||
| Inferior frontal occipital | ||||||
| Left | 8.43 (0.007) | 12.47 (0.002) | 30.56 (<0.0001) | 7.93 (0.006) | ||
| Right | 37.72 (<0.0001) | 29.46 (<0.0001) | 16.70 (<0.0001) | |||
| Uncinate | ||||||
| Left | 19.53 (0.0001) | 27.72 (<0.0001) | 29.89 (<0.0001) | 5.17 (0.026) | ||
| Right | 29.87 (<0.0001) | 30.99 (<0.0001) | 8.86 (0.004) | |||
| Cingulum | ||||||
| Left | 11.68 (0.002) | |||||
| Right | 13.65 (0.0008) | 30.18 (<0.0001) | 21.22 (<0.0001) | 15.81 (0.0001) | ||
| Cingulum hippocampal | ||||||
| Left | 11.26 (0.002) | 12.43 (0.001) | 17.14 (<0.0001) | |||
| Right | 29.58 (<0.0001) | 22.00 (<0.0001) | 19.72 (<0.0001) | |||
| Corticospinal | ||||||
| Left | ||||||
| Right | 33.48 (<0.0001) | 24.55 (<0.0001) | 7.37 (0.008) | |||
Note: Values in bold survived Bonferroni correction at a critical value of 0.007.
Table 8.
Final models from mixed model analysis of axial diffusivity values for group, age, and time of scan
| Within‐subjects effects | Between‐subjects effects | |||||
|---|---|---|---|---|---|---|
| Group × time × age | Time × age | Time × group | Time |
Group (1,31) |
Age | |
| Pathway | df (1,30) F (p) | df (1,31) F (p) | df (1,31) F (p) | df (1,31) F (p) | df (1,84) F (p) | df (1,84) F (p) |
| Superior longitudinal | ||||||
| Left | 5.86 (0.021) | 16.56 (0.001) | 1.98 (0.164) | 4.17 (0.044) | ||
| Right | ||||||
| Inferior longitudinal | ||||||
| Left | 5.36 (0.027) | 17.14 (0.001) | 0.13 (0.716) | 0.34 (0.560) | ||
| Right | ||||||
| Inferior frontal occipital | ||||||
| Left | 3.45 (0.073) | 6.47 (0.016) | 23.61 (<0.0001) | 0.09 (0.759) | 0.79 (0.378) | |
| Right | 4.98 (0.033) | |||||
| Uncinate | ||||||
| Left | 4.67 (0.038) | 20.07 (<0.0001) | 0.05 (0.825) | 0.95 (0.333) | ||
| Right | ||||||
| Cingulum | ||||||
| Left | 2.55 (0.120) | 0.84 (0.362) | 0.70 (0.404) | |||
| Right | ||||||
| Cingulum hippocampal | ||||||
| Left | 0.48 (0.493) | 0.31 (0.579) | 5.93 (0.017) | |||
| Right | ||||||
| Corticospinal | ||||||
| Left | 2.89 (0.099) | 2.29 (0.134) | 7.15 (0.009) | |||
| Right | ||||||
Note: Values in bold survived Bonferroni correction at a critical value of 0.007.
Figure 3.
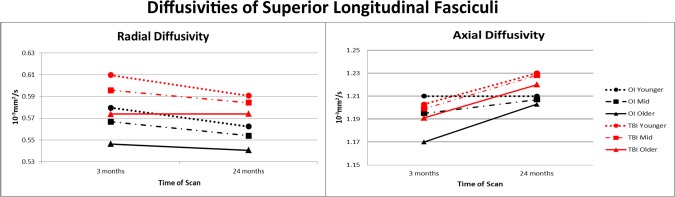
After controlling for age at scan, both radial and axial diffusivities of the left superior longitudinal fasciculus showed more change over time in children than adolescents in both the TBI and OI groups. RD values were significantly elevated in the TBI group suggesting dysmyelination; AD values did not vary by group.
Mixed model analysis of AD values did not yield any significant interaction effects. The main effect of time remained significant for the left hemisphere superior and inferior longitudinal, inferior frontal occipital, and uncinate fasciculi; AD increased over time in these pathways irrespective of group or age. Neither group nor age main effects were significant, with the exception of significant effect of age for the left corticospinal tract.
DISCUSSION
The primary aim of this prospective, longitudinal study was to examine the influence of age at injury and time since injury on the trajectory of change in white matter microstructure from selected pathways during the first two years after complicated‐mild to severe TBI in school‐aged children and adolescents. The secondary aim addressed the comparability of initial FA values and change over time when using the longitudinal sample with complete data versus the total sample including data from participants contributing a single scan to the analysis. In the following section, findings from the secondary aim will be discussed first because these results influence interpretation of the clinical data. We will then address our clinical findings related to the primary aim with a discussion of changes to brain microstructure following injury in a developmental population.
TOTAL VERSUS LONGITUDINAL SAMPLE: IS THE CHANGE IN FA SIMILAR OVER TIME?
We examined effect sizes, as well as the difference in the effect sizes for change in FA values from 3 to 24 months after injury from the total and longitudinal TBI and OI samples. For each pathway, ES were very similar whether based solely on longitudinal cases versus on the total sample. When data are likely missing at random, estimates of microstructure based on mixed models approaches to analysis of variance may provide similar estimates of the impact of TBI on FA values derived from TBSS for the total sample relative to those based on longitudinal data. In addition, direct comparison of the difference in effect sizes estimating change over time across the two samples suggested very similar sensitivity. The change in effect sizes over time was measured in standard deviation units; the average standardized difference in ES over time between the TBI and OI groups ES was 0.040. The estimates were very similar, suggesting extremely small differences in measurement of change over time in the total and longitudinal samples. The largest difference was noted for the left superior longitudinal fasciculus of the TBI group (0.082); the smallest differences were obtained for the left cingulum‐cingulate portion for both TBI (0.002) and OI (0.005) groups. The similarity of estimates of change over time for the total and longitudinal samples is a positive finding that has a number of implications for longitudinal imaging research. Given the larger sample size, increased stability of the covariance matrix, and enhanced power, mixed model approaches have the potential to contribute to identification of injury effects and recovery, particularly for longitudinal clinical research applications in which missing data are often unavoidable.
Loss of subjects in longitudinal clinical imaging studies requiring outpatient visits may occur due a variety of reasons. Illness of the patient, transportation difficulties, implantation of imaging‐incompatible metal or devices, orthodontic braces, family upheaval, inability to contact family, and loss of interest in a study may contribute to attrition. Motion artifact, scan quality, and scanner issues may also reduce the number of scans available at each time point. Using only longitudinal cases often means losing up to 30–50% of available data. Assuming that data are missing at random, the total sample has the potential to provide a reasonable estimate of the magnitude of an effect and of change over time. This comparative analysis of effect sizes for a total sample compared to a longitudinal sample advocates that the use of mixed models should be investigated for application to other DTI metrics and samples. Based on the comparability of findings across the total and longitudinal samples, and the increased power to identify interactions among key developmental variables, the discussion focuses on findings from the general linear mixed model analysis of the total sample.
INTERACTION OF BRAIN INJURY, AGE, AND TIME OF SCANNING
Our findings are similar to those of longitudinal studies of children and adults with TBI that showed reduction in FA and elevation in RD in the majority of regions and pathways investigated [Bendlin et al., 2008; Dinkel et al., 2014; Sidaros et al., 2008]. We identified increase in AD in several left hemisphere association pathways in both the TBI and OI groups. In prior studies, the impact of TBI on longitudinal AD has been inconsistent; values have decreased [Farbota et al., 2012], increased [Sidaros et al., 2008] or remained unchanged [Dinkel et al., 2014].
In this study, FA was significantly lower in the TBI group compared to the OI group in all pathways. FA increased significantly from 3 to 24 months after injury in both groups. Importantly, age at injury exerted a significant influence on the patterns of findings from the association and limbic pathways. For all association pathways in the left hemisphere, age × group × time interactions were obtained for FA. FA was lower in the TBI than OI group in the superior and inferior longitudinal, inferior fronto‐occipital, and uncinate fasciculi. Investigation of effects related to age showed that the youngest children with TBI were disproportionately affected initially but showed the greatest increase in FA over the follow‐up while the adolescents with TBI showed less initial impact but limited change over time. The superior longitudinal fasciculus is a dorsal pathway that incorporates the arcuate fasciculus and contains both long and short fibers connecting perisylvian regions of frontal, temporal, and parietal lobes. The inferior longitudinal fasciculus courses along a ventral path with short and long fibers connecting the occipital and temporal lobes [Mori et al., 2005]. The inferior longitudinal fasciculus also connects with limbic regions via the projections to temporal lobe, as well as to the amygdala and hippocampus [Catani and de Schotten, 2012]. The inferior fronto‐occipital fasciculus is a ventral pathway connecting occipital regions to the orbitofrontal cortex. Based on recent studies examining growth and timing of white matter development using DTI, growth in these three cortico‐cortical association pathways peaks relatively early in adolescence [Lebel et al., 2008; Simmonds et al., 2014]. The uncinate fasciculus, a ventral pathway connecting anterior temporal with medial and lateral orbitofrontal cortex [Mori et al., 2005], continues to develop after adolescence into adulthood [Lebel et al., 2008; Simmonds et al., 2014]. These association pathways share the attribute of connecting prefrontal lobes with other lobes. It is possible that the frequent injury to frontal lobes in patients with TBI renders association pathways particularly vulnerable to disruption. In addition, the rapid development of these pathways in school‐aged children may be associated with initial disruption but some catch‐up growth over time while the more stable pathway development in adolescents may limit microstructural recovery or reorganization.
FA from the limbic and projection pathways was lower in TBI group, increased with increasing age, and increased over time. However, in contrast to the pattern in the association pathways, the developmental variables did not interact with group. Similar to other projection tracts integrating cortical and subcortical structures, the corticospinal tract matures relatively early in late childhood. The cingulum is a medial tract coursing within the cingulate gyrus that connects medial frontal, parietal, occipital, and temporal lobes and portions of the cingulate cortex as well as the posterior hippocampal extension into the medial temporal lobe [Catani and de Schotten, 2012; Mori et al., 2005]. The cingulum‐cingulate and cingulum‐hippocampal have different growth trajectories. The former shows biphasic changes, with peak growth periods at ages 8 and 20; the latter peaks at 13.5 [Simmonds et al., 2014]. Despite the broad range of ages of peak maturation, these pathways did not appear to be preferentially disrupted at any age or to show reduced growth over time in the participants with TBI.
Since FA was significantly lower in the TBI than OI groups for all pathways examined, we completed follow‐up examination of the diffusivities. RD, which is believed to relate to myelination status, was substantially elevated across pathways in the TBI group. RD decreased over time in virtually all pathways and showed greater reduction in younger than older children for the left superior longitudinal fasciculus, left uncinate, and bilateral cingulum bundle‐cingulate. However, RD values remained significantly elevated two years after TBI. This finding supports prior studies highlighting the sensitivity of RD to microstructural changes following TBI [Caeyenberghs et al., 2010; Dennis et al., 2015; Dinkel et al., 2014; Ewing‐Cobbs et al., 2008; Farbota et al., 2012]. Chronic elevation of RD implicates dysmyelination as a significant long‐term factor contributing to chronic differences in white matter after TBI. In contrast to FA and RD values, AD, which is believed to relate to axonal status, was not significantly related to TBI but showed age‐related increases over time in left hemisphere association pathways, including the inferior longitudinal, inferior frontal occipital, and uncinate fasciculi.
CHANGE OVER TIME
A major challenge in studying the changes in brain microstructure following injury is characterizing how damage to pathway microstructure evolves over time and interacts with ongoing developmental changes. The trajectory of change over time must be compared to what is expected at different developmental stages. In contrast to the relatively stable development of white matter in middle adulthood, TBI sustained during childhood and adolescence is superimposed on a “moving target” of brain development [Giza et al., 2009]. To assess the impact of TBI on subsequent growth and integrity of white matter, change over time must be compared to what is expected at different developmental stages, i.e., by the change in the comparison groups in longitudinal studies. In this study, FA increased in both the OI and TBI groups across in the younger (0.015, 0.028), mid (0.017, 0.022), and older (0.019, 0.013) age ranges. In studies of adults, healthy comparison groups had no significant change in DTI metrics over one to several years. Adults with TBI showed reduction in FA in most regions of interest [Dinkel et al., 2014; Farbota et al., 2012]; with restricted increase in FA in the superior longitudinal fasciculus and portions of the optic radiation [Farbota et al., 2012].
Our findings suggest that by the subacute to chronic stage of recovery, the core of association and projection pathways identified using TBSS shows continued developmental changes over the first 2 years after TBI in school‐aged children and adolescents. Over longer follow‐up intervals, it remains to be seen what developmental function best characterizes longer term changes in tissue microstructure in different pathways and structures. Following the usual pattern of initial deficit and partial recovery after TBI, it is possible that metrics in some pathways will plateau, reflecting a stable deficit. It is also possible that metrics in other pathways may show increasing normalization over time and continue to close the gap as a result of compensatory reorganization or other mechanism. Our current knowledge about pathophysiological processes such as apoptosis and neuroinflammation occurring in TBI patients postinjury is far more advanced for the acute/subacute stages relative to the chronic period. Particularly in pediatric patients at different stages of white matter maturation at the time of injury, it is difficult to differentiate between the complex interplay of separate neurobiological processes such as ongoing neurodegeneration (decreased FA, increased RD), arrested development (lack of expected signs of white matter maturation), and possible compensatory mechanisms to overcome brain injury (signs of cortical plasticity). The need to pursue this area is highlighted by our current findings of the youngest TBI group (mean = 8 yo) exhibiting the lowest FA values at 3 months, yet demonstrated the greatest increase in FA values over the first 2 years after injury. This suggests that less mature white matter tracts at this age may be more vulnerable, but also possibly more resilient to pathophysiological processes via increased neuroprotective mechanisms and/or increased neural plasticity to overcome structural deficits following head injury. This finding underscores the need for longitudinal follow‐up studies extending several years after injury to characterize the trajectory of change and identify factors predicting which clinical characteristics predict which trajectory.
HEMISPHERIC EFFECTS
Despite similar rates of left, right, and bilateral hemispheric injury in participants with TBI, interactions of injury group with age at injury and time since injury occurred more frequently in left than right hemisphere pathways for the total sample. For left hemisphere FA, significant three‐way interactions were found in 5 of 7 pathways and trends (P < 0.10) were found in the remaining 2. For right‐hemisphere pathways, none of the three‐way interactions was significant and trends were noted for 2 pathways. It is unclear why the effects of TBI and age were more pronounced in the left than right hemisphere. In healthy children, FA tends to be higher in a number of left than right hemisphere structures, which may reflect different rates of maturation and connectivity. Studies using a variety of methods, age ranges, and imaging parameters have reported inconsistent findings regarding asymmetry of FA in a number of pathways. Leftward asymmetry, indicating higher left than right hemisphere values, was noted in several regions, including the cingulum [Wilde et al., 2010; Yu et al., 2014], posterolateral corpus callosum, basal ganglia [Simmonds et al., 2014], uncinate fasciculus [Hasan et al., 2009], as well as in the anterior and posterior limbs of the internal capsule, cingulum, corticospinal tract, inferior longitudinal fasciculus, and/or centrum semiovale [Bonekamp et al., 2007; Dennis et al., 2015; Snook et al., 2005; Wilde et al., 2009]. In contrast, other studies noted symmetry in FA from the superior longitudinal fasciculus [Urger et al., 2015] as well as very small absolute differences in FA across left and right hemispheres for major white matter tracts, deep gray matter structures, and subcortical white matter [Lebel et al., 2008]. Higher FA and/or lower diffusivity have been interpreted as indicating effects of maturation on fiber organization and myelination status. The functional significance of different rates of maturation in pathways in left and right hemispheres remains an area of investigation. Recent DTI studies show that structural connectivity increases more rapidly with age in the left hemisphere in children [Dennis and Thompson, 2013; Krogsrud et al., 2016] and decreases in the right hemisphere during adolescence [Dennis et al., 2013]. It is possible that left hemispheric structures that are developing rapidly during school‐age and adolescent years may be particularly vulnerable to the effects of acquired injury. Clearly, additional research addressing hemispheric differences in microstructure, as well as functional implications of any difference in vulnerability of left hemisphere structures, is needed.
LIMITATIONS AND FUTURE DIRECTIONS
Several methodological issues limit the strength of our findings. First, as with many clinical studies, the sample size is relatively small, which reduces power to detect significant effects related to brain injury and to normal developmental changes. Given heterogeneity in the distribution of shear injury and other pathological focal and diffuse clinical findings, larger samples provide more robust estimates of the impact of TBI on various pathways. Second, although acceptable at the time this longitudinal imaging study started acquisitions, the DTI protocol used 21 gradient sampling orientations, which is insufficient for robust estimates of diffusivity measures by current standards. Future studies would benefit from increasing the number of gradient directions to improve angular resolution which would enhance the estimation of fiber orientation and diffusivity metrics, particularly in regions with complex architectures of crossing fibers [Jones, 2004]. Third, TBSS, along with other quantitative structural imaging techniques, are limited in investigation of patients with gross lesion loads. Scans of patients with large focal lesions may not register adequately to study templates, which often results in trimming severely injured patients from the sample and reducing the effect size. Fourth, TBSS includes only the core portion of each pathway. Therefore, it is not possible to ascertain whether microstructural changes in the peripheral regions of pathways are similar to or diverge from those identified by TBSS. For example, although DTI metrics reflecting the core of a pathway may show typical developmental changes, there may be different pathological processes, such as ongoing neurodegeneration, in distal regions, that are not detectable. Additionally, the values extracted from the TBSS analysis were averaged across the whole pathway based on the JHU atlas. Examination of segments of pathways [Dennis and Thompson, 2013; Park and Friston, 2013] may lead to the identification of specific portions of pathways which are differentially related to the effects of injury, age, change over time, and their interactions.
DTI and TBSS have great potential for characterizing factors affecting developmental trajectories of recovery in both natural history and intervention studies. Future studies should include additional extended follow‐up scans to characterize whether changes in microstructural metrics in children with TBI are linear or nonlinear and to determine whether there are later‐developing changes in the magnitude of residual deficit in relation to age at injury and severity of TBI. In addition, the use of multimodal imaging approaches will better characterize the complexity of microstructural and macrostructural pathological changes. Examination of indices of pathway integrity in relation to neuropsychological outcomes and growth in cognitive abilities over time is essential to understanding the cumulative impact of injury on clinical outcomes.
CONCLUSIONS
Developmental variables, including age at injury and time since injury, are critically important to inform our understanding of the impact of TBI on both initial recovery and on the subsequent development of white matter connectivity. FA values from several association pathways showed a 3‐way interaction of group, age at injury, and time of scanning. In the left hemisphere, superior and inferior longitudinal, inferior frontal‐occipital, cingulum‐hippocampal extension, and uncinate fasciculi, school‐aged children with TBI had the lowest initial pathway integrity and showed the greatest increase in FA over time suggesting continued development. However, it is unknown whether this steeper recovery slope persists and whether it is associated with enhanced microstructural recovery in school‐aged children relative to adolescents. Contrary to expectation, the adolescents with TBI in this sample showed limited change in both FA and RD compared to older participants with OI across the 2 years follow‐up. It remains to be seen whether their reduced growth trajectory in some pathways is associated with specific neuropsychological impairment or to the emergence of deficits over time. Despite continued increases in FA across the follow‐up, the participants with TBI showed a persistent deficit in pathway microstructure and did not close the gap between the integrity of the association, limbic, and projection pathways and that of the children with OI. The pattern of DTI findings is strikingly similar to the pattern often noted in behavioral outcomes. Following an initial deficit and initial recovery during the first several months after TBI, neuropsychological abilities continue to develop, albeit at a reduced rate relative to preinjury levels. Clearly, longer follow‐up intervals are essential to characterize the long‐term impact of injury on the trajectory of both brain and behavioral outcomes as children transition into adolescence and adulthood.
Supporting information
Supporting Information
ACKNOWLEDGMENTS AND DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the granting institute. The authors report no financial or other conflict of interest.
REFERENCES
- Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J (2005): Functional plasticity or vulnerability after early brain injury? Pediatrics 116:1374–1382. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S (2007): Non‐linear registration aka spatial normalization. Oxford UK: FMRIB Centre. Report nr TR07JA2.
- Bendlin BB, Ries ML, Lazar M, Alexander AL, Dempsey RJ, Rowley HA, Sherman JE, Johnson SC (2008): Longitudinal changes in patients with traumatic brain injury assessed with diffusion‐tensor and volumetric imaging. Neuroimage 42:503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, Mori S, Horska A (2007): Diffusion tensor imaging in children and adolescents: Reproducibility, hemispheric, and age‐related differences. Neuroimage 34:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD (2015): Long‐term consequences of traumatic brain injury: Current status of potential mechanisms of injury and neurological outcomes. J Neurotrauma 32:1834–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, Geurts M, Taymans T, Vander Linden C, Smits‐Engelsman BC, Sunaert S, Swinnen SP (2010): Brain‐behavior relationships in young traumatic brain injury patients: Fractional anisotropy measures are highly correlated with dynamic visuomotor tracking performance. Neuropsychologia 48:1472–1482. [DOI] [PubMed] [Google Scholar]
- Catani M, de Schotten MT (2012) Atlas of Human Brain Connections. New York: Oxford University Press. [Google Scholar]
- Cohen, J. (1998) Statistical Power Analysis for the Behavioral Sciences: Second Edition. Hillside: Erlbaum. [Google Scholar]
- Crowe LM, Catroppa C, Babl FE, Rosenfeld JV, Anderson V (2012): Timing of traumatic brain injury in childhood and intellectual outcome. J Pediatr Psychol 37:745–754. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Jahanshad N, McMahon KL, de Zubicaray GI, Martin NG, Hickie IB, Toga AW, Wright MJ, Thompson PM (2013): Development of brain structural connectivity between ages 12 and 30: A 4‐Tesla diffusion imaging study in 439 adolescents and adults. Neuroimage 64:671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Jin Y, Villalon‐Reina JE, Zhan L, Kernan CL, Babikian T, Mink RB, Babbitt CJ, Johnson JL, Giza CC, Thompson PM, Asarnow RF (2015): White matter disruption in moderate/severe pediatric traumatic brain injury: Advanced tract‐based analyses. Neuroimage Clin 7:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM (2013): Mapping connectivity in the developing brain. Int J Dev Neurosci 31:525–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Battista A, Anderson V, Catroppa C, Soo C (2012): Quality of life in children and adolescents post‐TBI: A systematic review and meta‐analysis. J Head Trauma Rehab 29:1717–1727. [DOI] [PubMed] [Google Scholar]
- Dinkel J, Drier A, Khalilzadeh O, Perlbarg V, Czernecki V, Gupta R, Gomas F, Sanchez P, Dormont D, Galanaud D, Stevens RD, Puybasset L, for NC (2014): Long‐term white matter changes after severe traumatic brain injury: A 5‐year prospective cohort. AJNR Am J Neuroradiol 35:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durlak JA (2009): How to select, calculate, and interpret effect sizes. J Pediatr Psychol 34:917–928. [DOI] [PubMed] [Google Scholar]
- Ewing‐Cobbs L, Fletcher JM, Levin HS, Francis DJ, Davidson K, Miner ME (1997): Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. J Int Neuropsych Soc 3:581–591. [PubMed] [Google Scholar]
- Ewing‐Cobbs L, Prasad MR, Swank P, Kramer L, Cox CS, Jr. , Fletcher JM, Barnes M, Zhang X, Hasan KM (2008): Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: Relation to neurobehavioral outcomes. Neuroimage 42:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbota KD, Bendlin BB, Alexander AL, Rowley HA, Dempsey RJ, Johnson SC (2012): Longitudinal diffusion tensor imaging and neuropsychological correlates in traumatic brain injury patients. Front Hum Neurosci 6:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG (2010): Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths 2002–2006: Centers for Disease Control and Prevention. Natl Center Injury Prevent Control. [Google Scholar]
- Gennarelli TA, Thibault LE, Graham DI (1998): Diffuse axonal injury: An important form of traumatic brain damage. Neuroscientist 4:202–215. [Google Scholar]
- Giza CC, Hovda DA (2014): The new neurometabolic cascade of concussion. Neurosurgery 75:S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza CC, Kolb B, Harris NG, Asarnow RF, Prins ML (2009): Hitting a moving target: Basic mechanisms of recovery from acquired developmental brain injury. Dev Neurorehabil 12:255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan K, Narayana PA (2003): Computation of the mean diffusivity and fractional anisotropy maps without tensor decoding and diagonalization: Theoretical analysis and experimental validation. Magn Reson Imaging 50: 589–598. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Iftikhar A, Kramer LA, Cirino PT, Papanicolaou AC, Fletcher JM, Ewing‐Cobbs L (2009): Quantification of the healthy human uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Research 1276:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S (2008): Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract‐specific quantification. Neuroimage 39:336–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP, Juranek J, Kramer LA, Prasad MR, Swank PR, Ewing‐Cobbs L (2011): Predicting behavioral deficits in pediatric traumatic brain injury through uncinate fasciculus integrity. J Int Neuropsychol Soc 17:663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP, Juranek J, Swank PR, Kramer L, Cox CS Jr., Ewing‐Cobbs L (2015): White matter and reading deficits after pediatric traumatic brain injury: A diffusion tensor imaging study. Neuroimage Clin 9:668–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W (2013a): Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136:28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH (2013b): Axonal pathology in traumatic brain injury. Exp Neurol 246:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK (2004): The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: A Monte Carlo study. Magn Reson Med 51:807–815. [DOI] [PubMed] [Google Scholar]
- Koskinicmi M, Kyyka T, Nybo T, Jarho L (1995): Long‐term outcome after severe brain injury in preschoolers is worse than expected. Arch Pediatr Adolesc Med 149:249–254. [DOI] [PubMed] [Google Scholar]
- Krogsrud SKF, A. M., Tamnes CK, Grydeland H, Mork L, Due‐Tonnessen P, Bjornerud A, Sampaio‐Baptista C, Andersson J, Johansen‐Berg H, Walhovd KB (2016): Changes in white matter microstructure in the developing brain–A longitudinal diffusion tensor imaging study of children from 4 to 11 years of age. Neuroimage 124:473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowski B, Wade SL, Cecil KM, Walz NC, Yuan W, Rajagopal A, Holland SK (2009): Correlation of diffusion tensor imaging with executive function measures after early childhood traumatic brain injury. J Pediatr Rehabil Med 2:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland‐Brown W, Thomas KE (2005): The incidence of traumatic brain injury among children in the United States. J Head Trauma Rehab 20:229–238. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008): Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40:1044–1055. [DOI] [PubMed] [Google Scholar]
- Levin HS, Hanten G, Roberson G, Li X, Ewing‐Cobbs L, Dennis M, Chapman S, Max JE, Hunter J, Schachar R, Luerssen TG, Swank P (2008): Prediction of cognitive sequelae based on abnormal computed tomography findings in children following mild traumatic brain injury. J Neurosurg Pediatr 1: 461–70. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde EA, Hanten G, Li X, Chu ZD, Vasquez AC, Cook L, Yallampalli R, Hunter JV (2011): Mental state attributions and diffusion tensor imaging after traumatic brain injury in children. Dev Neuropsychol 36:273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey MW (1990): Design Sensitivity: Statistical Power for Experimental Research. Newbury Park, CA: Sage Publications, Inc. [Google Scholar]
- Mayer T, Matlak ME, Johnson DG, Walker ML (1980): The modified injury severity scale in pediatric multiple trauma patients. J Pediatr Surg 15:719–726. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Nagae‐Poetscher LM, van Zijl PC (2005): MRI Atlas of Human White Matter. Oxford: Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- Park HJ, Friston K (2013): Structural and functional brain networks: From connections to cognition. Science 342:1238411. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajecic S, Chen R, Penix LR, Virta A, Basser PJ (2001): Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. NeuroImage 13:1174–1185. [DOI] [PubMed] [Google Scholar]
- Reeves TM, Phillips LL, Povlishock JT (2005): Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol 196:126–137. [DOI] [PubMed] [Google Scholar]
- Roberts RM, Mathias JL, Rose SE (2014): Diffusion tensor imaging (DTI) findings following pediatric non‐penetrating TBI: A meta‐analysis. Dev Neuropsychol 39:600–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosema S, Muscara F, Anderson V, Godfrey C, Hearps S, Catroppa C (2015): The trajectory of long‐term psychosocial development 16 years following childhood traumatic brain injury. J Neurotrauma 32:976–983. [DOI] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Wilde EA, McClelland MM, Hanten G, Krawczyk DC, Cook LG, Chu ZD, Vasquez AC, Yallampalli R, Lin X, Hunter JV, Levin HS (2011): Brain activation during a social attribution task in adolescents with moderate to severe traumatic brain injury. Soc Neurosci 6:582–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, Paulson OB, Jernigan TL, Rostrup E (2008): Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: A longitudinal study. Brain 131:559–572. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, Luna B (2014): Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. Neuroimage 92:356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1: S208–19. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. NeuroImage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C (2005): Diffusion tensor imaging of neurodevelopment in children and young adults. NeuroImage 26:1164–1173. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK (2006): Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med 55:302–308. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B (1974): Assessment of coma and impaired consciousness: A practical scale. Lancet 2:81–84. [DOI] [PubMed] [Google Scholar]
- Urger SE, De Bellis MD, Hooper SR, Woolley DP, Chen SD, Provenzale J (2015): The superior longitudinal fasciculus in typically developing children and adolescents: Diffusion tensor imaging and neuropsychological correlates. J Child Neurol 30:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Ayoub KW, Bigler ED, Chu ZD, Hunter JV, Wu TC, McCauley SR, Levin HS (2012): Diffusion tensor imaging in moderate‐to‐severe pediatric traumatic brain injury: Changes within an 18 month post‐injury interval. Brain Imag Behav 6:404–416. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, Newsome MR, Scheibel RS, Li X, Levin HS (2006): Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J Neurotraum 23:1412–1423. [DOI] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Chu Z, Hunter JV, Bigler ED, Yallampalli R, Wang ZJ, Hanten G, Li X, Ramos MA, Sabir SH, Vasquez AC, Menefee D, Levin HS (2009): Diffusion tensor imaging of hemispheric asymmetries in the developing brain. J Clin Exp Neuropsychol 31:205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Hunter JV, Bigler ED, Chu Z, Wang ZJ, Hanten GR, Troyanskaya M, Yallampalli R, Li X, Chia J, Levin HS (2008): Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 70:948–955. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Newsome MR, Bigler ED, Pertab J, Merkley TL, Hanten G, Scheibel RS, Li X, Chu Z, Yallampalli R, Hunter JV, Levin HS (2011): Brain imaging correlates of verbal working memory in children following traumatic brain injury. Int J Psychophysiol 82:86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Ramos MA, Yallampalli R, Bigler ED, McCauley SR, Chu Z, Wu TC, Hanten G, Scheibel RS, Li X, Vasquez AC, Hunter JV, Levin HS (2010): Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Dev Neuropsychol 35:333–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TC, Wilde EA, Bigler ED, Li X, Merkley TL, Yallampalli R, McCauley SR, Schnelle KP, Vasquez AC, Chu Z, Hanten G, Hunter JV, Levin HS (2010): Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev Neurosci 32:361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Peng Y, Mishra V, Ouyang A, Li H, Zhang H, Chen M, Liu S, Huang H (2014): Microstructure, length, and connection of limbic tracts in normal human brain development. Front Aging Neurosci 6:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


