Abstract
Aims
To determine whether the CHA2DS2-VASc score can predict adverse outcomes such as death, ischemic stroke, and major hemorrhage, in patients with systolic heart failure in sinus rhythm.
Methods and Results
CHA2DS2-VASc scores were calculated for 1,101 patients randomized to warfarin and 1,123 patients randomized to aspirin. Adverse outcomes were defined as death or ischemic stroke, death alone, ischemic stroke alone, and major hemorrhage. Using proportional hazards models, we found that each 1-point increase in the CHA2DS2-VASc score was associated with increased hazard of death or ischemic stroke events (hazard ratio [HR] for the warfarin arm = 1.21 [1.13–1.30], p<0.001; for aspirin, HR = 1.20 [1.11–1.29], p<0.001). Similar increased hazards for higher CHA2DS2-VASc scores were observed for death alone, ischemic stroke alone, and major hemorrhage. Overall performance of the CHA2DS2-VASc score was assessed using c-statistics for full models containing the risk score, treatment assignment, and score-treatment interaction, with the c-statistics for the full models ranging from 0.57 for death to 0.68 for major hemorrhage.
Conclusions
The CHA2DS2-VASc score predicted adverse outcomes in patients with systolic HF in sinus rhythm, with modest prediction accuracy.
Keywords: heart failure, warfarin, sinus rhythm, stroke, bleeding
Background
Patients with heart failure with reduced ejection fraction (HFrEF) are at increased risk for adverse outcomes, including death and stroke.(1, 2) For patients with HFrEF in sinus rhythm, there has been recent interest in assessing whether the risk of these adverse outcomes can be accurately predicted by existing risk scores that incorporate established risk factors.(3–5) In particular, although the CHA2DS2-VASc risk score (incorporating risk factors including congestive heart failure, hypertension, age ≥75 years [2 points], age 65–75 years, diabetes, stroke/transient ischemic attack/thromboembolism [2 points], vascular disease [prior myocardial infarction, peripheral artery disease, or aortic plaque], and female sex) was developed for patients with atrial fibrillation,(6) Melgaard and colleagues recently described its use for predicting the risk of death and stroke in HFrEF patients in sinus rhythm.(3) We therefore undertook this analysis of patients enrolled in the Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction Trial (WARCEF),(7) to evaluate whether the CHA2DS2-VASc risk score can predict death, ischemic stroke, and major hemorrhage in patients with HFrEF in sinus rhythm who received warfarin or aspirin.
Methods
The protocol for the randomized, double blinded WARCEF trial (http://www.ClinicalTrials.gov No. NCT00041938) has been described previously.(7, 8) Briefly, patients with left ventricular ejection fraction (LVEF) ≤35% and who were in SR were randomized to receive warfarin or aspirin. Additional eligibility criteria included age ≥18 years old, having no contraindications to warfarin, having a modified Rankin score of 4 or less, and on evidence-based heart failure medications (beta-blocker, angiotensin-converting-enzyme inhibitor or angiotensin-receptor blocker, or hydralazine and nitrates). Patients were excluded if they had a clear indication for warfarin or aspirin, or if they had a condition that conferred a high risk of cardiac embolism. A total of 2,305 participants were recruited from 168 centers in 11 countries from October 2002 to January 2010. The investigation conforms with the principles outlined in the Declaration of Helsinki.(9) All subjects provided informed consent.
For the WARCEF trial, ischemic stroke was defined as a clinically relevant new lesion detected on computed tomography or magnetic resonance imaging or, in the absence of a new lesion, clinical findings that were consistent with the occurrence of clinical stroke and that lasted for longer than 24 hours. Major bleeding was defined as intracerebral, epidural, subdural, subarachnoid, spinal intramedullary, or retinal hemorrhage; any other bleeding causing a decline in the hemoglobin level of more than 2 g per deciliter in 48 hours; or bleeding requiring transfusion of 2 or more units of whole blood, hospitalization, or surgical intervention. A blinded independent end-point adjudication committee adjudicated all stroke and major bleeding events.
For each participant, the CHA2DS2-VASc risk score was calculated based on the approach described by Lip and colleagues.(6) For this analysis, 81 participants were excluded because incomplete data precluded the calculation of the CHA2DS2-VASc score. Because stroke and bleeding risk are expected to differ for patients receiving warfarin and aspirin, we performed all analyses separately for the warfarin and aspirin arms of the WARCEF trial on an intent-to-treat (ITT) basis. Baseline characteristics of the study participants were compared by treatment group using two-sample t-test for continuous variables and Chi-squared test categorical variables. For each CHA2DS2-VASc score, we calculated incidence rate per 100 patient-years for the composite outcome of death and ischemic stroke, as well as for the individual outcomes of death, ischemic stroke, and major bleeding. Exact Poisson 95% confidence intervals of the incidence rates were provided. To determine the association between CHA2DS2-VASc score and each outcome above, as well as to investigate whether the CHA2DS2-VASc score influenced the treatment effect of warfarin versus aspirin, we constructed Cox proportional hazard models that incorporated treatment assignment, the CHA2DS2-VASc score, and their interaction term. Model discrimination was assessed using Harell’s c-index. Model calibration was assessed using the survival-adapted Hosmer-Lemeshow χ2 goodness of fit test.(10) The test statistic measures the difference between the predicted and the observed probabilities of risk. A small p-value suggests that the model is not well calibrated. P-values of <0.05 were considered statistically significant for all testing. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
For this analysis, a total of 2,204 participants were included, of whom 1,101 were enrolled in the warfarin arm and 1,123 were enrolled in the aspirin arm. The mean (SD) age of participants were 60.8 (11.3) years old, and 435 (19.6%) were female. Baseline characteristics of WARCEF participants by treatment arm are described in Table 1.
Table 1.
Baseline characteristics of the study participants, according to treatment group (warfarin versus aspirin). For continuous variables, mean ± SD were reported, and p-values were calculated using two-sample t-test. For categorical variables, number /total number (%) were reported, and p-values were calculated using Chi-squared test.
| Characteristic | Warfarin (n=1101) | Aspirin (n=1123) | p-value |
|---|---|---|---|
| Age - years | 60.9± 11.5 | 60.7±11.1 | 0.647 |
| Male sex | 879/1101 (79.8) | 910/1123 (81.0) | 0.477 |
| Race or ethnic group | 0.862 | ||
| Non-Hispanic white | 830/1101 (75.4) | 856/1123 (76.2) | . |
| Non-Hispanic black | 158/1101 (14.4) | 153/1123 (13.6) | . |
| Hispanic | 83/1101 (7.5) | 79/1123 (7.0) | . |
| Other | 30/1101 (2.7) | 35/1123 (3.1) | . |
| Body-mass index – kg / m2 | 28.9± 5.9 | 29.2± 6.0 | 0.197 |
| Systolic blood pressure - mmHg | 124.0±19.4 | 124.2±18.4 | 0.832 |
| Diastolic blood pressure - mmHg | 74.1±11.6 | 74.5±11.3 | 0.402 |
| Pulse - beats/min | 71.8±11.3 | 72.1±12.5 | 0.479 |
| Hypertension | 669/1101 (60.8) | 692/1123 (61.6) | 0.678 |
| Diabetes Mellitus | 359/1101 (32.6) | 337/1123 (30.0) | 0.187 |
| Atrial Fibrillation | 42/1101 (3.8) | 38/1123 (3.4) | 0.585 |
| Peripheral Vascular Disease | 130/1101 (11.8) | 122/1123 (10.9) | 0.483 |
| Prior stroke or TIA | 148/1101 (13.4) | 137/1123 (12.2) | 0.381 |
| Ischemic Cardiomyopathy | 475/1101 (43.1) | 488/1123 (43.5) | 0.882 |
| Smoking status | 0.424 | ||
| Current smoker | 211/1099 (19.2) | 193/1122 (17.2) | . |
| Former smoker | 563/1099 (51.2) | 579/1122 (51.6) | . |
| Never smoked | 325/1099 (29.6) | 350/1122 (31.2) | . |
| NYHA classification | 0.502 | ||
| 1 | 145/1098 (13.2) | 160/1118 (14.3) | . |
| 2 | 595/1098 (54.2) | 626/1118 (56.0) | . |
| 3 | 343/1098 (31.2) | 319/1118 (28.5) | . |
| 4 | 15/1098 (1.4) | 13/1118 (1.2) | . |
| Ejection fraction - % | 24.6± 7.5 | 24.9± 7.5 | 0.319 |
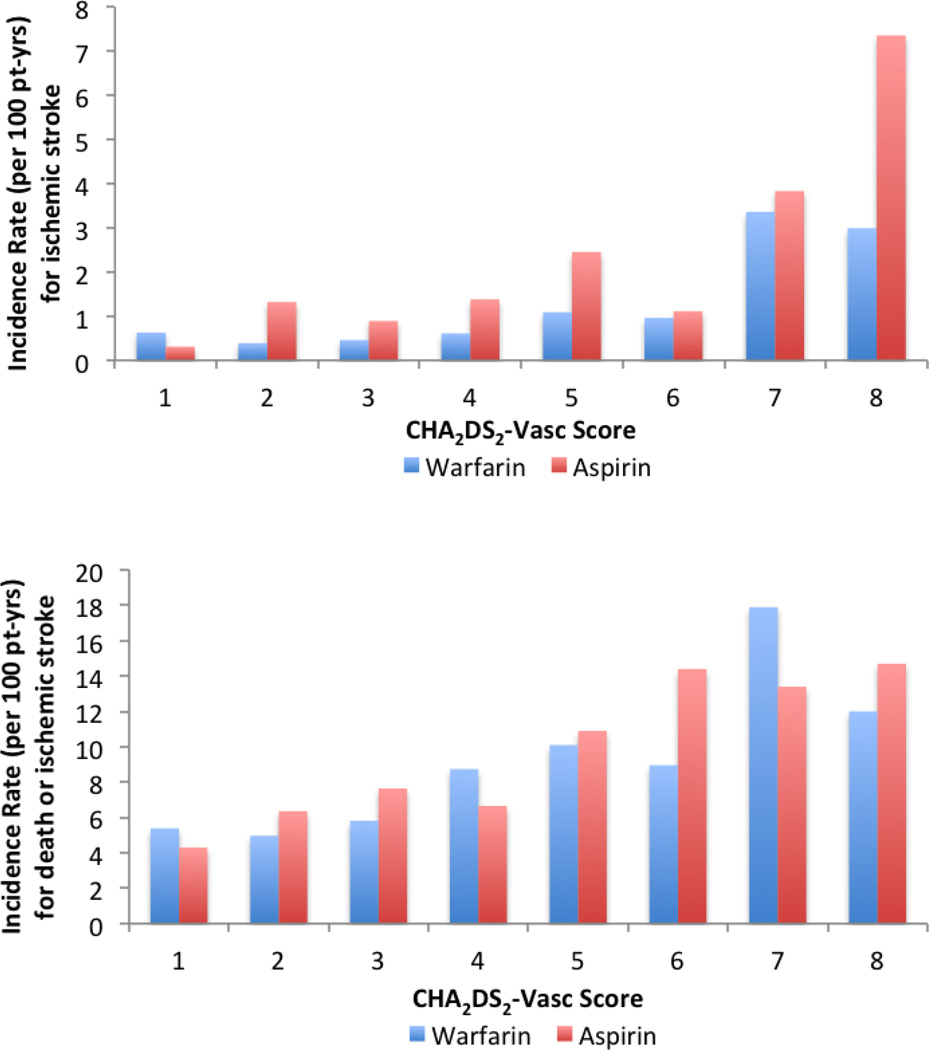
Because all WARCEF participants had a diagnosis of heart failure, the minimum CHA2DS2-VASc score was 1; the maximum was 9. The composite outcome of death and ischemic stroke occurred in 588 (27%) of the 2,204 participants; the risk significantly increased with each 1-point increase in CHA2DS2-VASc score (Figure) for participants enrolled in both warfarin (hazard ratio [HR] 1.21, 95% confidence interval [CI] 1.13–1.30, p<0.001) and aspirin arms (HR 1.20, 95% CI 1.11–1.29, p<0.001). Similar trends were observed for the individual outcomes of ischemic stroke (Table 2), death alone and major hemorrhage (Table 3). For ischemic stroke, the incidence rate per 100 patient-years was largely above 1% for CHA2DS2-VASc scores of 5 and above for patients receiving warfarin, and for CHA2DS2-VASc scores of 2 and above for patients receiving aspirin.
Figure 1.
Adverse outcomes by CHA2DS2-VASc score.
Incidence rate of ischemic stroke (top panel), and death or ischemic stroke (bottom panel), by categories of CHA2DS2-VASc score. Because only 1 patient had a CHA2DS2-VASc score of 9, data are not shown for that category.
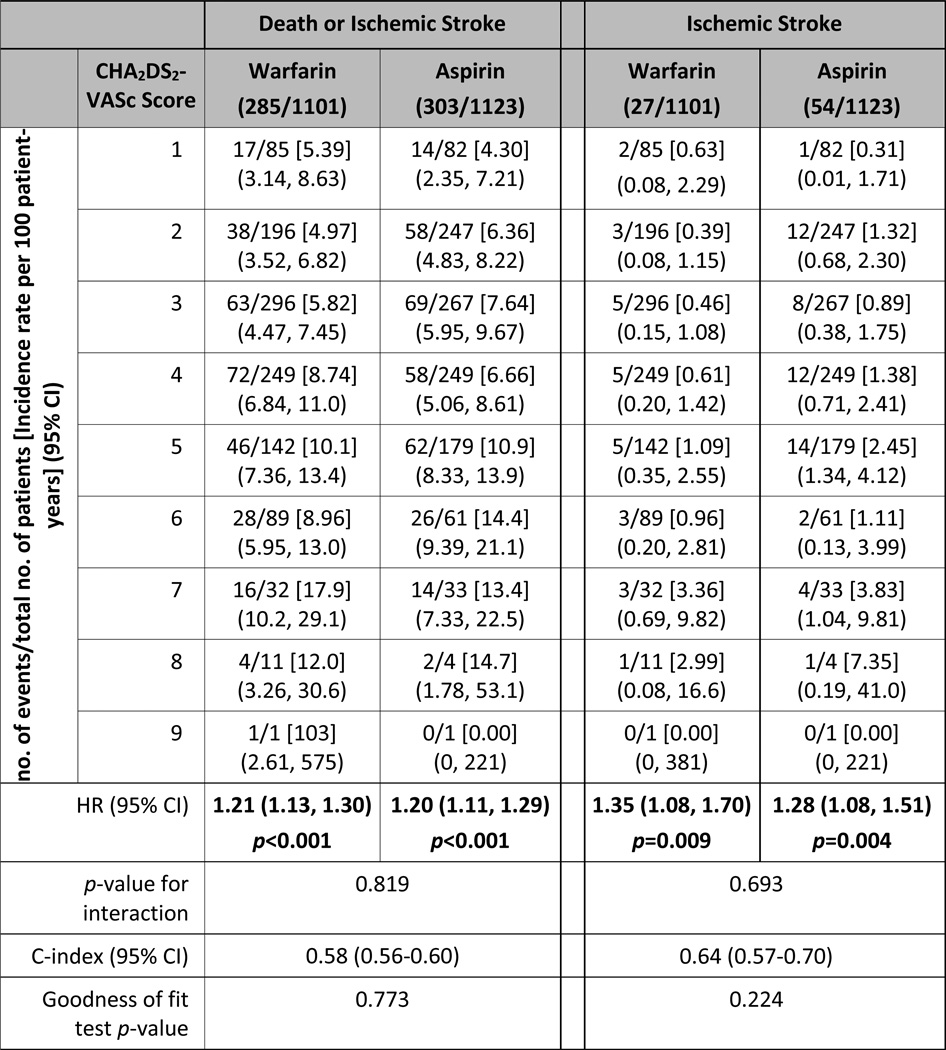
Table 2.
Incidence rate per 100 patient-years for death or ischemic stroke and ischemic stroke only, by categories of CHA2DS2-VASc score. Hazard ratios are for each 1-point increase in CHA2DS2-VASc score, using models that contained treatment assignment, CHA2DS2-VASc score and their interaction.
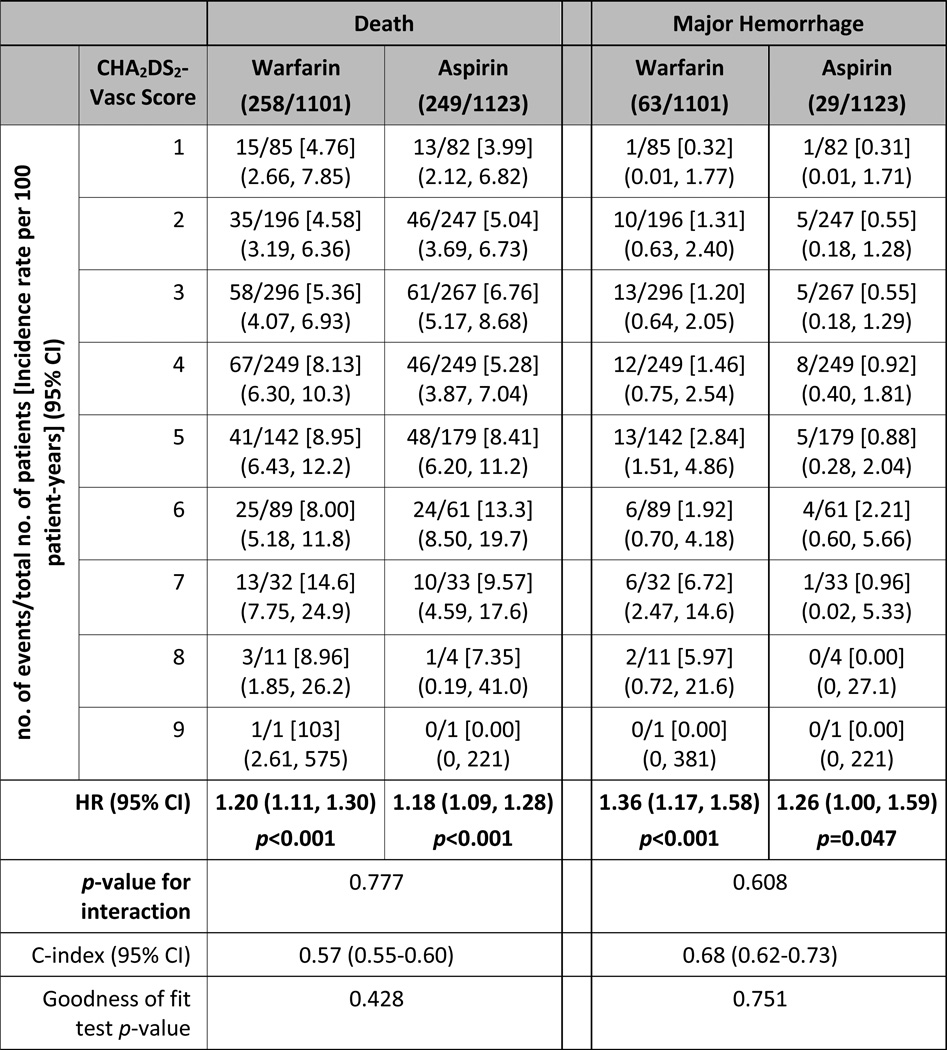
Table 3.
Incidence rate per 100 patient-years for death and major hemorrhage, by categories of CHA2DS2-VASc score. Hazard ratios are for each 1-point increase in CHA2DS2-VASc score, using models that contained treatment assignment, CHA2DS2-VASc score and their interaction.
The treatment effect of warfarin versus aspirin did not significantly differ according to the CHA2DS2-VASc score for all outcomes examined. Model discrimination was modest for the composite outcome of death and ischemic stroke (c-index 0.58, 95% CI 0.56–0.60) and the individual outcome of death (c-index 0.57, 95% CI 0.55–0.60), but was higher for ischemic stroke (c-index 0.64, 95% CI 0.57–0.70) and for major hemorrhage (c-index 0.68, 95% CI 0.62–0.73) (Tables 2 and 3). The survival-adapted Hosmer-Lemeshow goodness-of-fit test p-values were larger than 0.05 for all outcomes, suggesting that none of the models had a significant lack of fit.
Discussion
In this retrospective analysis of the WARCEF trial, we found that in patients with heart failure in sinus rhythm, the CHA2DS2-VASc score was moderately predictive of adverse outcomes, including death or ischemic stroke, and the individual outcomes of death, ischemic stroke, and major hemorrhage. Increased CHA2DS2-VASc risk score was consistently associated with increased stroke risk for both the aspirin and warfarin arms of the WARCEF cohort. There was no significant interaction between treatment assignment and the CHA2DS2-VASc score for these outcomes.
Our results are largely consistent with those reported by Melgaard and colleagues, who found that the CHA2DS2-VASc score predicted death, ischemic stroke, and thromboembolism, in a nationwide cohort of 33,592 heart failure patients with reduced or preserved ejection fraction without concurrent atrial fibrillation.(3) In that study, the c-statistics for the CHA2DS2-VASc ranged from 0.63 to 0.69 for the individual outcomes. These values are slightly higher than those in our analysis. However, at each level of the CHA2DS2-VASc score, the absolute risk for ischemic strokes was higher in the Danish cohort, ranging from 1.5% per year for a score of 1 to 7% for a score of 6, where as across the same range of CHA2DS2-VASc scores in the WARCEF cohort the stroke risk was 0.3% to 1.1% in the aspirin arm and 0.4% to 1.1% in the warfarin arm. Potential reasons for this include the protective effects of aspirin and warfarin, inclusion of younger patients in WARCEF, and statistical imprecision due to the sample size. Regardless of the reason, these differences suggest additional analyses of independent cohorts are needed to better calibrate how the CHA2DS2-VASc score can predict stroke risk in heart failure patients in sinus rhythm, especially as the threshold for benefit from initiating anticoagulation is commonly thought to be a stroke risk of between 1–2%.(11, 12)
In addition to the CHA2DS2-VASc score, other approaches to risk stratification for heart failure patients in sinus rhythm have been examined recently. Abdul-Rahim and colleagues have found that in in patients with heart failure with reduced ejection fraction who are in sinus rhythm, risk scores that included clinical risk factors (age, New York Heart Association class, diabetes treated with insulin, body mass index, previous stroke) and N-terminal pro B-type natriuretic peptide accurately predicted strokes.(4) Similarly, we have previously demonstrated that major bleeding in WARCEF participants could be predicted by existing bleeding risk scores, such as the Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly (HAS-BLED) score and the Outpatient Bleeding Risk Index (OBRI).(5) Taken together, these findings all suggest that risk prediction in heart failure patients in sinus rhythm can be further refined. Although current guidelines do not recommend routine anticoagulation in this patient population,(13, 14) ongoing studies, such as the COMMANDER-HF randomized, controlled trial, are assessing the potential benefit of newer oral anticoagulants such as rivaroxaban.(15) Given the superior safety profile of these agents compared with warfarin, improved risk stratification of heart failure patients in sinus rhythm, especially with regards to stroke and bleeding risk,(16, 17) will greatly aid the identification of patients who may benefit from such therapy.(11)
The limitations of our analysis include its retrospective design and the modest number of stroke events. As mentioned previously, since WARCEF was a randomized, controlled trial, it is possible that healthier patients were selected, which could lead to underestimation of stroke risk and affect the generalizability of our findings. Similarly, since all patients in WARCEF received either warfarin or aspirin, the true stroke risk of heart failure patients in sinus rhythm who are not on anticoagulant therapy may be higher than described here. We did not construct a model including hemodynamic or laboratory parameters using WARCEF participants, and it is possible such a model may be superior to the CHA2DS2-VASc risk score. However, any such model would also need to be validated externally, while the CHA2DS2-VASc risk score is easy to calculate and already widely accepted, making it more likely to be clinically useful should future studies continue to confirm our findings. Finally, we did not formally monitor for the occurrence of asymptomatic atrial fibrillation during the WARCEF study, although a small number of participants (~10%) did develop clinical atrial fibrillation during the follow-up period.(18) Although this may have contributed to the relationship between the CHA2DS2-VASc score and clinical outcomes observed in our analysis, this does not change our finding that the CHA2DS2-VASc score represents a potentially useful tool for risk stratification in this patient population.
Despite these limitations, our findings are timely additions to the literature on understanding stroke risk in heart failure patients in sinus rhythm. Our analysis confirms that the CHA2DS2-VASc score can predict stroke risk in this population, and can potentially inform future studies on new therapeutic approaches for stroke prevention for heart failure patients in sinus rhythm.
Acknowledgments
Funding:
The WARCEF trial was supported by grant #s U01-NS-043975 to S Homma, and U01-NS-039143 to JLP Thompson from the National Institute of Neurological Diseases and Stroke. Dr. Ye is supported by a NIH K23 career development award (K23 HL121144). Warfarin and warfarin placebo were provided by Taro Pharmaceuticals USA, and aspirin and aspirin placebo by Bayer HealthCare.
Dr. Homma reports receiving payment from AGA Medical (now St. Jude Medical) for his work as a member of a data and safety monitoring board and consulting fees from Boehringer Ingelheim; Dr. Levin, receiving consulting fees from United Healthcare; Dr. Teerlink, receiving research grants and/ or consulting fees from Amgen, Bayer, Cardio3 Bioscience, Cytokinetics, Mast Therapeutics, Medtronic, Novartis, St. Jude and Trevena; Dr. Graham, owning stock in March Pharmaceuticals, Medtronic, and Pfizer; Dr. Labovitz, receiving grant support from Boehringer Ingelheim on behalf of his institution, lecture fees from Boehringer Ingelheim, and fees for the development of educational presentations from the American College of Cardiology; Dr. Anker, receiving consulting fees from Amgen, Bosch Healthcare, GlaxoSmithKline, Helsinn, LoneStar Heart, Novartis, Professional Dietetics, PsiOxus, Relypsa, SHL Telemedicine, and Thermo Fisher, grant support from Vifor Pharma, and lecture fees from Novartis, holding patents with Brahms AG and Charité Berlin, and receiving royalties from Imperial College; Dr. Ponikowski, receiving consulting fees from Bayer, Boehringer Ingelheim, Coridea, Corthera, Johnson & Johnson, Pfizer, Respicardia, and Vifor Pharma, grant support from Vifor Pharma on behalf of himself and his institution, and lecture fees from Abbott, Boehringer Ingelheim, Merck Serono, Pfizer, Respicardia, Sanofi-Aventis, Servier, and Vifor Pharma; and Dr. Lip, receiving consulting fees from Astellas, AstraZeneca, Bayer, Biotronik, Boehringer Inhelheim, Bristol-Myers Squibb, Pfizer, Merck, Portola, and Sanofi-Aventis, speakers bureau fees from Bayer, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, and Sanofi-Aventis, and payment for the development of educational presentations from Bayer, Boehringer Ingelheim, and Merck.
Footnotes
Disclosures:
No other potential conflict of interest relevant to this article was reported.
References
- 1.Lip GY, Gibbs CR. Does heart failure confer a hypercoagulable state? Virchow's triad revisited. J Am Coll Cardiol. 1999 Apr;33(5):1424–1426. doi: 10.1016/s0735-1097(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 2.Kalaria VG, Passannante MR, Shah T, Modi K, Weisse AB. Effect of mitral regurgitation on left ventricular thrombus formation in dilated cardiomyopathy. American heart journal. 1998 Feb;135(2 Pt 1):215–220. doi: 10.1016/s0002-8703(98)70084-5. [DOI] [PubMed] [Google Scholar]
- 3.Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY. Assessment of the CHA2DS2-VASc Score in Predicting Ischemic Stroke, Thromboembolism, and Death in Patients With Heart Failure With and Without Atrial Fibrillation. JAMA. 2015 Sep 8;314(10):1030–1038. doi: 10.1001/jama.2015.10725. [DOI] [PubMed] [Google Scholar]
- 4.Abdul-Rahim AH, Perez AC, Fulton RL, Jhund PS, Latini R, Tognoni G, Wikstrand J, Kjekshus J, Lip GY, Maggioni AP, Tavazzi L, Lees KR, McMurray JJ. Risk of Stroke in Chronic Heart Failure Patients Without Atrial Fibrillation: Analysis of the Controlled Rosuvastatin in Multinational Trial Heart Failure (CORONA) and the Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca-Heart Failure (GISSI-HF) Trials. Circulation. 2015 Apr 28;131(17):1486–1494. doi: 10.1161/CIRCULATIONAHA.114.013760. discussion 1494. [DOI] [PubMed] [Google Scholar]
- 5.Ye S, Cheng B, Lip GY, Buchsbaum R, Sacco RL, Levin B, Di Tullio MR, Qian M, Mann DL, Pullicino PM, Freudenberger RS, Teerlink JR, Mohr JP, Graham S, Labovitz AJ, Estol CJ, Lok DJ, Ponikowski P, Anker SD, Thompson JL, Homma S. Bleeding Risk and Antithrombotic Strategy in Patients With Sinus Rhythm and Heart Failure With Reduced Ejection Fraction Treated With Warfarin or Aspirin. The American journal of cardiology. 2015 Sep 15;116(6):904–912. doi: 10.1016/j.amjcard.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 7.Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, Mohr JP, Massie BM, Labovitz AJ, Anker SD, Lok DJ, Ponikowski P, Estol CJ, Lip GY, Di Tullio MR, Sanford AR, Mejia V, Gabriel AP, del Valle ML, Buchsbaum R. Warfarin and aspirin in patients with heart failure and sinus rhythm. The New England journal of medicine. 2012 May 17;366(20):1859–1869. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pullicino P, Thompson JL, Barton B, Levin B, Graham S, Freudenberger RS. Warfarin versus aspirin in patients with reduced cardiac ejection fraction (WARCEF): rationale, objectives, and design. Journal of cardiac failure. 2006 Feb;12(1):39–46. doi: 10.1016/j.cardfail.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Rickham PP. HUMAN EXPERIMENTATION. CODE OF ETHICS OF THE WORLD MEDICAL ASSOCIATION. DECLARATION OF HELSINKI. British medical journal. 1964 Jul 18;2(5402):177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May S, Hosmer DW. A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime data analysis. 1998;4(2):109–120. doi: 10.1023/a:1009612305785. [DOI] [PubMed] [Google Scholar]
- 11.Homma S, Ye S. Stroke and anticoagulation in heart failure without atrial fibrillation: from risk to opportunity. Circulation. 2015 Apr 28;131(17):1465–1467. doi: 10.1161/CIRCULATIONAHA.115.016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckman MH, Singer DE, Rosand J, Greenberg SM. Moving the tipping point: the decision to anticoagulate patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2011 Jan 1;4(1):14–21. doi: 10.1161/CIRCOUTCOMES.110.958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European journal of heart failure. 2012 Aug;14(8):803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013 Oct 15;128(16):e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 15.Zannad F, Greenberg B, Cleland JG, Gheorghiade M, van Veldhuisen DJ, Mehra MR, Anker SD, Byra WM, Fu M, Mills RM. Rationale and design of a randomized, double-blind, event-driven, multicentre study comparing the efficacy and safety of oral rivaroxaban with placebo for reducing the risk of death, myocardial infarction or stroke in subjects with heart failure and significant coronary artery disease following an exacerbation of heart failure: the COMMANDER HF trial. European journal of heart failure. 2015 Jul;17(7):735–742. doi: 10.1002/ejhf.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eikelboom JW, Connolly SJ. Warfarin in Heart Failure. New England Journal of Medicine. 2012;366(20):1936–1938. doi: 10.1056/NEJMe1202504. [DOI] [PubMed] [Google Scholar]
- 17.Xiong Q, Lau YC, Senoo K, Lane DA, Hong K, Lip GY. Non-vitamin K antagonist oral anticoagulants (NOACs) in patients with concomitant atrial fibrillation and heart failure: a systemic review and meta-analysis of randomized trials. European journal of heart failure. 2015 Nov;17(11):1192–1200. doi: 10.1002/ejhf.343. [DOI] [PubMed] [Google Scholar]
- 18.Kato TS, Di Tullio MR, Qian M, Wu M, Thompson JL, Mann DL, Sacco RL, Pullicino PM, Freudenberger RS, Teerlink JR, Graham S, Lip GY, Levin B, Mohr JP, Labovitz AJ, Estol CJ, Lok DJ, Ponikowski P, Anker SD, Homma S. Clinical and Echocardiographic Factors Associated With New-Onset Atrial Fibrillation in Heart Failure- Subanalysis of the WARCEF Trial. Circulation journal : official journal of the Japanese Circulation Society. 2016 Feb 25;80(3):619–626. doi: 10.1253/circj.CJ-15-1054. [DOI] [PubMed] [Google Scholar]