Abstract
Background
Reduced arm swing is a well-known clinical feature of Parkinson’s disease (PD), often observed early in the course of the disease. We hypothesized that subtle changes in arm swing and axial rotation may also be detectable in the prodromal phase.
Objective
The purpose of this study was to evaluate the relationship between the LRRK2-G2019S mutation, arm swing, and axial rotation in healthy nonmanifesting carriers and noncarriers of the G2019S mutation and in patients with PD.
Methods
A total of 380 participants (186 healthy nonmanifesting controls and 194 PD patients) from 6 clinical sites underwent gait analysis while wearing synchronized 3-axis body-fixed sensors on the lower back and bilateral wrists. Participants walked for 1 minute under the following 2 conditions: (1) usual walking and (2) dual-task walking. Arm swing amplitudes, asymmetry, variability, and smoothness were calculated for both arms along with measures of axial rotation.
Results
A total of 122 nonmanifesting participants and 67 PD patients were carriers of the G2019S mutation. Nonmanifesting mutation carriers walked with greater arm swing asymmetry and variability and lower axial rotation smoothness under the dual task condition when compared with noncarriers (P < .04). In the nonmanifesting mutation carriers, arm swing asymmetry was associated with gait variability under dual task (P = .003). PD carriers showed greater asymmetry and variability of movement than PD noncarriers, even after controlling for disease severity (P < .009).
Conclusions
The G2019S mutation is associated with increased asymmetry and variability among nonmanifesting participants and patients with PD. Prospective studies should determine if arm swing asymmetry and axial rotation smoothness may be used as motor markers of prodromal PD.
Keywords: arm swing, gait, LRRK2, G2019S, Parkinson’s disease
Prodromal motor features in Parkinson’s disease (PD) likely develop gradually, years before diagnosis.1 To be able to treat the disease earlier than is currently possible today, recent research has focused on the early identification of people who are likely to develop PD in the future. Reduced unilateral arm swing, a well-described feature of PD, was observed in healthy adults who have an increased risk of developing PD, as determined by substansia nigra hyperechogenicity and among carriers of PINK1 mutations.2,3 We reported subtle alterations in gait among another group of healthy adults who have an increased risk of developing PD, that is, carriers of the LRRK2-G2019S mutation, during challenging conditions.4 These findings support the possibility of identifying motor changes in the prodromal state. However, the relationships between arm swing, axial movements, gait changes, and the LRRK2-G2019S mutation have not been studied to date.
Body-fixed wearable sensors have great potential for augmenting the diagnosis, prognosis, and early identification of PD.5–7 We hypothesized that by using body-fixed sensors, we could identify increased arm swing asymmetry in healthy carriers of the LRRK2-G2019S mutation. As such, we addressed the following three aims: (1) to evaluate whether alterations in arm swing can be detected in healthy subjects who have an increased risk of developing PD, that is, nonmanifesting carriers of the LRRK2-G2019S mutation; (2) to investigate the relationship between arm swing and two potential mediators of arm swing, that is, axial rotation and gait speed, among nonmanifesting LRRK2-G2019S carriers; and (3) to assess the relationship between the presence of the G2019S mutation and arm swing and axial rotation in patients with PD.
Methods
Study Design and Ethical Approval
This observational study was conducted as part of the MJFF LRRK2 Gait Consortium. The multicenter consortium included participants from Israel (Tel Aviv Medical Center, n = 198), the United States (Mount Sinai-Beth Israel Medical Center, n = 107 and Columbia University, n = 3), Germany (University of Tubingen, n = 31), Norway (Norwegian University of Science and Technology, n = 15), and Spain (Hospital Clinic of Barcelona, n = 26) (Michael J Fox Foundation for Parkinson Research). Ethical approval was obtained from the local ethical boards at all sites. All participants provided informed written consent prior to participating in this study. To maximize uniformity across clinical sites, testing was performed using a predefined, structured protocol.
Participants
A convenience sample of 186 nonmanifesting relatives of patients with PD and 194 patients with PD were evaluated. Nonmanifesting participants were included if they were older than 30 years and had a relative diagnosed with LRRK2-G2019S PD. Nonmanifesting participants were excluded if they had a diagnosis of PD according to UK PD Society Brain Bank criteria8 or showed PD-related signs (Unified Parkinson’s Disease Rating Scale part III [UPDRS-III] > 4). Patients with PD were included if they were able to walk for 5 minutes unassisted. Patients and nonmanifesting participants were also excluded if they had a history of clinical stroke or neurological disorders other than PD, unstable cardiovascular disease, major depression, diagnosed dementia, major orthopedic disease (eg, acute pain, history of shoulder or upper extremity injury), clinically diagnosed psychiatric disorder according to the DSM-IV, or current or past exposure to dopamine-blocking agents.
Procedures
Demographic data and medical history were collected from all participants. Participants underwent clinical and neurological exams using the UPDRS-III.9 Patients were assessed in the “ON” medication state, approximately 1 hour after medication intake. PD motor subtypes were determined as previously defined.10 Cognitive function was evaluated using the Montreal Cognitive Assessment (MoCA).11 Assessors and participants were blinded to mutation status at the time of assessment.
Assessment of Gait, Arm Swing, and Axial Rotation
A lightweight body-fixed sensor (Opal APDM, Portland, Oregon) was adhered to the lower back (lumbar vertebrae 4–5) and to each wrist using Velcro straps (Velcro Companies, Manchester, New Hampshire) to quantify gait, arm swing, and axial rotation.12 The sensors were synchronized and precalibrated referenced to the vertical axis in the anatomical position. The participants walked back and forth in a well-lit corridor of 15 m in each site for 1 minute under the following 2 walking conditions: (1) preferred, usual-walking speed and (2) dual-task (DT; serially subtracting 3 seconds from a predefined 3 digit number while walking, with no task prioritization). Testing order was fixed. Signals were filtered using a low-pass Butterworth filter with a frequency cutoff of 3.5 Hz with a bandpass of less than 0.5 db. Turns were identified from the gyroscope signal and were removed. Only straight-line walking, defined as sagittal progression walking, was analyzed.13
Mean gait speed was determined by the average time taken to walk 10 m. Stride time was determined by automatic identification of the time between 2 consecutive strikes of the same foot, detected from the trunk acceleration.12,14,15 Stride time variability was calculated as the magnitude of stride-to-stride fluctuations, normalized to each participant’s mean stride time, coefficient of variation = (standard deviation/mean) × 100.14,15
Arm swing amplitudes (degrees) for each arm were calculated as the range from peak flexion to peak extension in the sagittal plane accounting for the rotation of 1 segment relative to a reference segment using an Euler sequence.12 For patients with PD, the upper extremity exhibiting more (or less) signs based on the UPDRS (items 20–26) was defined as the more or less affected side. For the asymptomatic participants, arm swing amplitudes of the dominant and nondominant hands were evaluated separately. Asymmetry was evaluated as previously described,12,16 with a value of 0 indicating complete symmetry.16 Arm swing variability was determined as the coefficient of variation across the entire range of movement. Arm swing jerk was calculated to assess smoothness, that is, lower jerk represents smoother movement.17
The gyroscope of the back sensor quantified the transverse plane angular axial rotation around the vertical axis.12 The magnitude of the rotation was quantified as the rotation range (deg) during a stride. Axial rotation jerk (deg/s3) was assessed and axial rotation asymmetry (%) was evaluated as the symmetry in side-to-side rotation, with a value of 0 indicating perfect symmetry.16
Genetic Testing
All participants were screened for the LRRK2-G2019S mutation and for the common glucocerebrosidase (GBA) mutations among Ashkenazi Jews as previously reported.18,19 GBA mutation carriers, including those who also carried the LRRK2-G2019S mutation were excluded from the analyses.
Statistical Analysis
Demographics, disease characteristics, UPDRS, MoCA, and gait speed performance of both patients and nonmanifesting LRRK2-G2019S carriers and noncarriers were compared using chi-square tests and Student t tests and compared among the 5 recruitment sites. Linear mixed-model analyses were used for each group separately (ie, nonmanifesting participants and PD patients) for each of the outcome measures, with genotype status and condition (ie, usual walking vs DT walking) entered as fixed effects. The models were adjusted for age, disease duration, disease severity (based on the UPDRS), baseline gait speed, cognitive function (MoCA), and recruitment site. Bonferroni corrections were used to account for multiple comparisons within each of the 3 constructs (arm swing, axial, and gait measures) with alpha levels of .04, .02, and .02, respectively. Pearson correlations were used to assess the interplay between genetic status, disease characteristics, gait, arm swing, and axial rotation. Linear regression models explored the associations between gait, arm swing, and axial rotation; beta values and 95% confidence intervals were determined. Data processing and analysis was done at Tel Aviv Medical Center. Data reported here were not used in previous publications. Statistical analyses were performed using SPSS version 21 (IBM Corp., Armonk, New York).
Results
Demographic characteristics of the nonmanifesting participants and the patients are presented in Table 1. Nonmanifesting carriers (NM-C) and nonmanifesting noncarriers (NM-NC) had comparable demographic characteristics. Likewise, the 2 PD groups were similar with respect to age, gender, cognitive function, height, weight, and body mass index. PD carriers had slightly longer disease duration than PD noncarriers and slightly lower scores on the UPDRS-III (see Table 1). The postural instability and gait disturbance (PIGD) motor subtype was more common among PD carriers when compared with PD noncarriers (P = .01). Stratification by site revealed no differences in the characteristics of the nonmanifesting participants across sites with the exception of younger age in Spain (43.4 ± 4.7 years vs 54.75 ± 10.92 years in all other sites; P = .01). Patient characteristics were also similar across sites with the exception of gender (P = .003), with 92% male patients in Spain compared to 54% in all other centers.
TABLE 1.
Participant characteristics
| Parkinson’s disease patients | Noncarriers (n = 127) | Carriers (n = 67) | P value |
|---|---|---|---|
| Age, years | 65.7 ± 9.7 (27–85) | 64.9 ± 10.6 (26–89) | .42 |
| Gender, % women | 42 | 48 | .42 |
| Body mass index, kg/m2 | 26.4 ± 5.4 | 25.9 ± 4.3 | .55 |
| Disease duration, years | 5.7 ± 4.2 (1–21) | 7.9 ± 6.2 (1–31) | .01* |
| UPDRS motor part III | 19.9 ± 10.7 (8–59) | 15.9 ± 6.9 (9–31) | .007* |
| Hoehn and Yahr Stage, % | .89 | ||
| 1 | 24.5 | 23.8 | |
| 2 | 51.1 | 49.0 | |
| 2.5 | 7.4 | 6.9 | |
| 3 | 17.0 | 20.3 | |
| Motor subtypes | |||
| PIGD, % | 54.3 | 68.2 | .01* |
| Tremor predominant, % | 19.2 | 15.1 | |
| Intermediate, % | 26.5 | 16.7 | |
| MOCA | 25.6 ± 3.1 (20–30) | 26.1 ± 2.6 (18–30) | .24 |
| Usual walking speed, m/s | 1.1 ± 0.2 (0.6–1.8) | 1.1 ± 0.2 (0.6–1.8) | .19 |
| Nonmanifesting | Noncarriers (n = 64) | Carriers (n = 122) | |
| Age, years | 52.8 ± 14.2 (31–84) | 51.89 ± 12.86 (30–85) | .66 |
| Gender, % women | 57 | 55 | .91 |
| Body mass index, kg/m2 | 25.8 ± 5.5 | 26.0 ± 6.1 | .35 |
| UPDRS III | 1.73 ± 1.3 (0–4) | 1.8 ± 2.1 (0–4) | .89 |
| MOCA | 27.9 ± 1.7 (21–30) | 27.3 ± 2.3 (21–30) | .48 |
| Usual walk speed, m/s | 1.23 ± 0.2 (0.8–1.7) | 1.24 ± 0.2 (0.7–1.9) | .84 |
Means ± standard deviations and range are presented. MOCA, Montreal Cognitive Assessment (maximum score = 30); UPDRS, Unified Parkinson’s Disease Rating Scale; PIGD, Postural Instability Gait Difficulty (data available for 104 PD noncarriers and 56 PD carriers).
Statistically significant difference between groups.
Aim 1: Can Arm Swing Alterations Be Detected in Individuals at Risk of Developing PD?
No differences between groups were observed in gait speed during usual walking. NM-C had significantly higher (worse) stride time variability during DT walking when compared with NM-NC (F = 3.79, P = .024).
Arm swing measures were similar between NM-C and NM-NC under usual gait condition (Table 2). In contrast, in the DT condition, NM-C walked with higher arm swing asymmetry (F = 4.11, P = .020), higher arm swing variability (F = 3.33, P = .038) and reduced smoothness of movement (F = 5.39, P = .006) (Table 2 and Figs. 1 and 2). Axial rotation amplitude was similar between the groups under both conditions; however, axial jerk was higher in the NM-C in the DT condition, reflecting reduced smoothness (F = 4.13, P = .04; Table 2).
TABLE 2.
Gait, arm swing, and axial rotation in the nonmanifesting subjects (mean ± standard deviation)
| NM-NC | NM-C | Condition effect | Interaction effect | Group effect | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Arm swing | ||||||
| Arm swing amplitude dominant hand, deg | Usual walk | 40.27 ± 17.39 | 39.98 ± 16.89 | 0.003 | 0.191 | 0.059 |
| DT walk | 37.42 ± 19.43 | 34.03 ± 17.57a | ||||
| Arm swing amplitude nondominant hand, deg | Usual walk | 43.70 ± 18.68 | 42.34 ± 16.78 | 0.001 | 0.089 | 0.053 |
| DT walk | 37.99 ± 19.45a | 36.15 ± 17.74a | ||||
| Average arm swing variability, % | Usual walk | 17.54 ± 7.60 | 18.61 ± 8.24 | 0.000 | 0.165 | 0.007b |
| DT walk | 20.47 ± 10.42a | 24.53 ± 10.45a | ||||
| Arm swing asymmetry, % | Usual walk | 7.83 ± 5.90 | 9.40 ± 5.87 | 0.006 | 0.148 | 0.004b |
| DT walk | 7.27 ± 5.83 | 12.48 ± 6.67a | ||||
| Arm swing jerk, m/s) | Usual walk | 0.07 ± 0.09 | 0.09 ± 0.11 | 0.019 | 0.195 | 0.001b |
| DT walk | 0.05 ± 0.05 | 0.11 ± 0.39a | ||||
| Axial measures | ||||||
| Axial rotation amplitude, deg | Usual walk | 7.28 ± 3.21 | 5.79 ± 2.92 | 0.021 | 0.156 | 0.057 |
| DT walk | 6.51 ± 3.23a | 5.88 ± 3.22 | ||||
| Axial rotation jerk, deg/s3 | Usual walk | 0.51 ± 0.34 | 0.56 ± 0.38 | 0.000 | 0.053 | 0.013b |
| DT walk | 0.26 ± 0.18a | 0.29 ± 0.23a | ||||
| Trunk rotation asymmetry, % | Usual walk | 15.25 ± 7.35 | 15.58 ± 7.97 | 0.078 | 0.292 | 0.095 |
| DT walk | 14.40 ± 7.41 | 17.01 ± 6.10a | ||||
| Gait measures | ||||||
| Gait speed, m/sec | Usual walk | 1.23 ± 0.21 | 1.24 ± 0.23 | 0.001 | 0.332 | 0.323 |
| DT walk | 1.17 ± 0.25a | 1.17 ± 0.31a | ||||
| Stride time variability, % | Usual walk | 1.73 ± 0.98 | 1.81 ± 1.06 | 0.000 | 0.367 | 0.012b |
| DT walk | 2.05 ± 1.03a | 2.38 ± 1.58a | ||||
Condition effect is the comparison between usual and dual-task (DT) walking conditions; group effect is the comparison between carriers and noncarriers.
NM-NC, nonmanifesting noncarriers; NM-C, nonmanifesting carriers.
Significant difference between conditions in post hoc analysis.
Significant difference after Bonferroni correction for multiple comparisons.
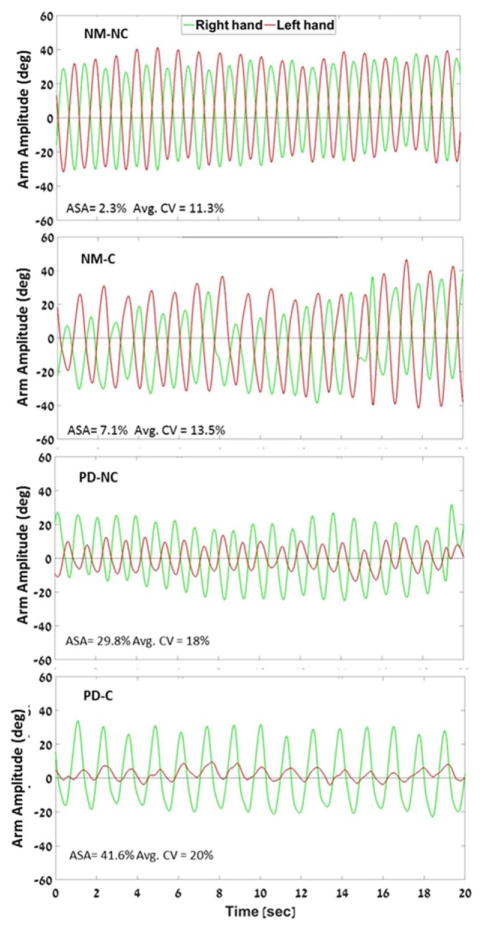
FIG. 1.
Example of arm swing acceleration traces during dual-task walking. Representative participants are matched within group (Parkinson’s disease [PD] or nonmanifesting [NM]) by age, gender, UPDRS-III, and disease duration. Carriers in both groups had decreased amplitude, higher asymmetry, and higher variability than noncarriers. ASA, arm swing asymmetry; CV, coefficient of variation; NM-C, nonmanifesting carriers; NM-NC, nonmanifesting noncarriers; PD-NC, PD noncarriers; PD-C, PD carriers. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIG. 2.
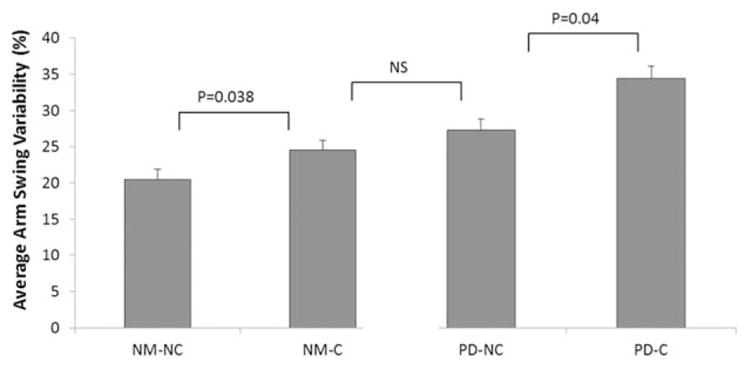
Differences in arm-swing variability (coefficient of variation [CV]) during dual-task walking. Carriers had higher (worse) variability than noncarriers. Nonmanifesting carriers (NM-C) showed similar variability as patients with PD (PD noncarriers), suggesting subtle motor impairments indicative of disease in at least some individuals in this group. NM-NC, nonmanifesting noncarriers; NS, not significant; PD-NC, PD noncarriers; PD-C, PD carriers.
Aim 2: The Relationship Between Arm Swing, Axial Rotation, and Gait Measures in LRRK2-G2019S Nonmanifesting Mutation Carriers
In the nonmanifesting participants, arm swing asymmetry was related to age (r = .36, P = .02) and gait speed (r = −.33, P = .001). Analysis adjusted for age and UPDRS-III revealed that in NM-C, arm swing amplitude, asymmetry, variability, and jerk were not related to DT gait speed (P > 0.09); however, arm swing asymmetry was associated with gait variability under the DT condition (B = 0.34, CI = 3.13, 0.45, P = .003).
Aim 3: Are Alterations in Arm Swing Related to the LRRK2-G2019S Mutation Among Patients With PD?
An example of the arm swing raw accelerometer signal is presented in Figure 1. Differences in arm swing measures were observed between representative PD carriers and PD noncarriers despite similarities in age, gender, disease duration, and disease severity. These differences were also seen on a group level. Adjusted models show that the PD carriers walked with larger arm swing amplitude in the least-affected side when compared with PD noncarriers under both usual and DT conditions. PD carriers also had higher arm swing variability (F = 4.31, P = .04; Fig. 2) and higher arm swing asymmetry when compared with PD noncarriers (F = 8.36, P = .001; Table 3).
TABLE 3.
Gait, arm swing, and axial rotation in patients with PD (mean ± standard deviation)
| PD noncarriers | PD carriers | Condition effect | Interaction effect | Group effect | ||
|---|---|---|---|---|---|---|
| Arm swing | ||||||
| Arm swing amplitude most affected side, deg | Usual walk | 24.73 ± 16.36 | 19.35 ± 18.03 | 0.000 | 0.095 | 0.062 |
| DT walk | 18.89 ± 15.32a | 16.16 ± 19.23a | ||||
| Arm swing amplitude least affected side, deg | Usual walk | 28.59 ± 16.91 | 40.49 ± 21.70 | 0.000 | 0.123 | 0.0001b |
| DT walk | 21.46 ± 16.76a | 31.90 ± 22.38a | ||||
| Average arm swing variability, % | Usual walk | 21.88 ± 10.46 | 24.79 ± 11.50 | 0.000 | 0.181 | 0.001b |
| DT walk | 28.06 ± 13.98a | 35.88 ± 17.68a | ||||
| Arm swing asymmetry, % | Usual walk | 18.03 ± 11.25 | 19.01 ± 12.61 | 0.036 | 0.179 | 0.009b |
| DT walk | 19.02 ± 11.11 | 26.21 ± 8.14a | ||||
| Arm swing jerk, m/s3 | Usual walk | 0.09 ± 0.14 | 0.08 ± 0.09 | 0.068 | 0.048 | 0.041 |
| DT walk | 0.09 ± 0.15 | 0.07 ± 0.11 | ||||
| Axial measures | ||||||
| Axial rotation amplitude, deg | Usual walk | 6.14 ± 3.49 | 5.46 ± 2.72 | 0.197 | 0.278 | 0.139 |
| DT walk | 5.62 ± 3.27 | 5.44 ± 2.71 | ||||
| Axial rotation jerk, m/s3 | Usual walk | 0.38 ± 0.28 | 0.55 ± 0.47 | 0.000 | 0.208 | 0.016b |
| DT walk | 0.21 ± 0.14a | 0.23 ± 0.18a | ||||
| Trunk rotation asymmetry, % | Usual walk | 14.30 ± 6.64 | 16.12 ± 7.74 | 0.227 | 0.043 | 0.041 |
| DT walk | 12.64 ± 6.29 | 17.43 ± 6.87 | ||||
| Gait measures | ||||||
| Gait speed, m/sec | Usual walk | 1.08 ± 0.21 | 1.06 ± 0.23 | 0.163 | 0.002b | 0.051 |
| DT walk | 1.10 ± 0.33 | 1.03 ± 0.29 | ||||
| Stride time variability, % | Usual walk | 1.77 ± 0.72 | 2.18 ± 1.16 | 0.001 | 0.006b | 0.003b |
| DT walk | 2.66 ± 1.78a | 3.13 ± 2.48a | ||||
Condition effect is the comparison between usual and dual-task (DT) walking conditions; group effect is the comparison between carriers and noncarriers.
Significant difference between conditions in post hoc analysis.
Significant difference between groups after Bonferroni correction for multiple comparisons.
Axial rotation amplitude was similar in the 2 groups in both conditions, whereas the PD carriers had significantly higher axial jerk in usual walking, but not during dual tasking (P = .02 and P = .12, respectively; Table 3). Gait speed was similar between groups in both walking conditions. PD carriers had greater stride time variability when compared with PD noncarriers in the DT condition (F = 4.76, P = .031) consistent with previous findings.20
Arm swing amplitude was inversely correlated with disease severity in both PD carriers and PD noncarriers (r = −.31, P = .008 and r = −.28, P = .023, respectively) and positively correlated with gait speed (r = .11, P = .012) and LRRK2 status (r = .13, P = .001). Arm swing variability and asymmetry were not associated with gait measures in either group, whereas axial jerk was positively correlated with disease severity but only in the carriers group (r = .34, P = .001).
Under the DT condition, stride time variability was similar in NM-C and PD noncarriers (2.38 ± 1.58% vs 2.66 ± 1.78%, P = .213, respectively). This similarity was also observed in arm swing variability (24.53 ± 10.45% vs 28.06 ± 13.98%, P = .127; see Fig. 2).
Discussion
We observed arm swing alterations associated with the LRRK2-G2019S mutation among both healthy nonmanifesting carriers and patients with PD. These findings extend previous reports on specific gait changes in asymptomatic carriers of the LRRK2-G2019S mutation.4,20 The results from this relatively large, multicenter study also highlight the potential of using sensitive gait analysis to identify persons at risk for PD.
Arm Swing: A Potential Motor Marker of Disease in LRRK2
There has been a great deal of effort devoted to detecting PD symptoms in prodromal states using a variety of nonmotor biological markers (eg, olfaction, autonomic dysfunction, sleep disorders).21–23 Although such nonmotor features may one day be suitable for the early detection of PD, they are not yet sufficiently accurate, robust, or specific, and new features are needed.23,24 Because PD is still diagnosed by its motor features, it is reasonable to speculate that subtle changes in motor function will be present prior to the appearance of the cardinal motor signs required for diagnosis.25 Here, we evaluated arm swing measures that represent the following 4 properties of movement that are often affected in PD: amplitude, asymmetry, variability, and smoothness.
Studies have shown that reduced arm swing and tremor of individual fingers best distinguished PD from age-matched controls26 and that in early stages of the disease, increased asymmetry and decreased coordination between the arms are more sensitive than reduced amplitude of movement.16,27 Similar to reports in early-stage PD,16,27 differences between nonmanifesting carriers and noncarriers were not observed in amplitude of movement. Rather, they were seen only in the control of movement (ie, asymmetry, variability, and smoothness). Variability has been shown to be a highly sensitive measure of gait impairment, more closely related to disease than measures based on the mean values of other walking parameters.28 Our findings suggest that arm swing variability and asymmetry have similar properties, differentiating between nonmanifesting mutation carriers and noncarriers, alluding to either an endophynotypic trait or perhaps to the existence of motor abnormalities in the prodromal phase.16,27
We hypothesized that to assess subtle changes in performance, it is necessary to test the neural system in challenging conditions when multiple resources are required to unmask compensatory strategies. Interestingly, arm swing variability in the NM-C was similar to that of the patients with PD in the DT condition (Fig. 2). It is possible that among the NM-C, there are individuals in the prodromal phase of disease and that the challenging condition exposed alterations in performance. Subtle gait abnormalities in NM-C of the LRRK2-G2019S mutation have been reported.4 The present findings suggest that subtle gait abnormalities may be accompanied by increased arm swing variability and asymmetry. The poorer performance of the mutation carriers may, therefore, be consistent with initial abnormalities in the central gait network.
Axial involvement in PD is customarily thought to be a feature of advanced PD (Hoehn and Yahr stage 3)1; however, this assumption may be a matter of sensitivity of commonly used assessment tools. Axial rigidity is usually evaluated through passive movement of the neck and observation of functional mobility. These may not be sufficiently sensitive to observe early axial changes involving translational movement around the body’s vertical axis.29 The quantification of axial movement revealed higher axial jerk in the nonmanifesting carriers during the DT condition. Interestingly, axial jerk was lower in patients with PD than controls, perhaps reflecting more rigid and constrained movement of the center of gravity. This observation parallels other sway findings in putative prodromal and manifesting state, where specific balance measures were nonlinearly related to disease state, showing increased sway in at-risk individuals and decreased sway as disease progresses.30 Still, further study is needed to better understand this somewhat counterintuitive finding.
Arm Swing Alterations and the Relationship to the LRRK2-G2019S Mutation in PD
Approximately 47% of our entire PD cohort had unilateral signs, reflected by their Hoehn and Yahr staging. When PD carriers were challenged, asymmetry and variability were more pronounced than in the PD noncarriers. PD carriers also showed increased axial variability and increased gait variability, suggesting more postural involvement and impaired axial control. These findings are consistent with a higher frequency of a PIGD subtype in this group (Table 1) and with recent studies reporting a possible motor phenotype in LRRK2-G2019S PD.20,31 The G2019S mutation may influence neural degeneration and increase the likelihood of developing the PIGD subtype of PD, in which gait impairment, rigidity, and postural instability are key signs.4,20,31 The current study demonstrates that alterations in arm swing may be an additional feature of this phenotype. Furthermore, an association was found between LRRK2-G2019S status, UPDRS motor score, arm swing amplitude, and axial jerk. Thus, the clinically perceived presence of motor asymmetry32 may be enhanced by objective, quantitative measures that can help delineate PD phenotypes.
The Interconnection Between Arm Swing and Gait
PD pathology may result in varied motor involvement.2,33 This suggests the presence of possibly partially independent, supraspinal control channels, whereby the dopaminergic systems has a relatively weaker influence on arm swing and preferential action on lower limb movements.34 Alternatively, differences in upper and lower extremity involvement may be related to the biomechanical properties of upper limb movements functioning in an open kinematic chain when compared with the coupled, closed kinematic chain motion used during gait.35 The higher magnitude of variability in arm swing when compared with stride time supports the influence of these biomechanical properties. We did not find an association between gait features and arm swing asymmetry or variability, suggesting that these motor features may be independent. Future longitudinal studies should explore the temporal relationship between gait variability and arm swing changes in PD development.
Limitations and Clinical Implications
Our study has several limitations. Selection and volunteer bias cannot be ruled out. Participating sites reached out to participants through established cohorts of an enriched LRRK2 population. To decrease the possibility of bias, the assessment of study participants was done without the examiners’ knowledge of mutation status. We only screened for G2019S-LRRK2 and GBA mutations, the most prevalent PD-associated mutations. Although other mutations may be relevant, their frequency is low (approximately 5% of all genetic-identified PD).36 Thus, we posit that if there were other mutation carriers, their incidence and influence would be negligible. Concomitant medication and levodopa equivalent dose were not collected for most participants, and, therefore, we could not assess the influence of medication on performance and its confounding effects. Still, all analyses were conducted while adjusting for age, gender, and disease severity to limit possible confounding effects. Importantly, the present study is not able to definitively determine whether the observed findings reflect a constitutional state of LRRK2-G2019S or whether they represent prodromal signs. Indeed, LRRK2-G2019S has only incomplete penetrance, ranging between 26% to 100% at age 80.12,37–40 Therefore, only about a quarter of our studied asymptomatic participants will likely develop PD. We speculate that these individuals, presenting with early changes, contributed to the findings of this study. Notwithstanding, longitudinal studies are needed to determine if the observed changes reflect changes in the prodromal state.
Conclusions
The present findings suggest that the quantitative assessment of arm swing may have the potential to contribute to an earlier clinical diagnosis of PD and perhaps to augment the monitoring of disease progression.
Acknowledgments
Funding agencies: This research was supported by grants from the Tel Aviv Medical Center Grant of Excellence, the Michael J. Fox Foundation for Parkinson’s Research, and NIH K02 NS073836. The funding sources had no involvement in the design, interpretation or writing of this manuscript. Drs. A.M. J.M.H., and N.G. report having submitted a patent application on the use of body-fixed sensors in Parkinson disease. The intellectual property rights for this patent application are held by the Tel Aviv Sourasky Medical Center.
We thank the participants of this project for their invaluable participation and the Michael J. Fox Foundation for Parkinson’s Research for their support.
Footnotes
Relevant conflicts of interests/financial disclosures: Nothing to report.
References
- 1.Evans JR, Mason SL, William-Gray CH, et al. The natural history of treated Parkinson’s disease in an incident, community based cohort. J Neurol Neurosurg Psychiatry. 2011;82:1112–1118. doi: 10.1136/jnnp.2011.240366. [DOI] [PubMed] [Google Scholar]
- 2.Liepelt I, Behnke S, Schweitzer K, et al. Pre-motor signs of PD are related to SN hyperechogenicity assessed by TCS in an elderly population. Neurobiol Aging. 2011;32(9):1599–1606. doi: 10.1016/j.neurobiolaging.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Nurnberger L, Klein C, Baudrexel S, et al. Ultrasound-based motion analysis demonstrates bilateral arm hypokinesia during gait in heterozygous PINK1 mutation carriers. Mov Disord. 2015;30(3):386–392. doi: 10.1002/mds.26127. [DOI] [PubMed] [Google Scholar]
- 4.Mirelman A, Gurevich T, Giladi N, Bar-Shira A, Orr-Urtreger A, Hausdorff JM. Gait alterations in healthy carriers of the LRRK2 G2019S mutation. Ann Neurol. 2011;69(1):193–197. doi: 10.1002/ana.22165. [DOI] [PubMed] [Google Scholar]
- 5.Cole BT, Roy SH, De Luca CJ, Nawab SH. Dynamical learning and tracking of tremor and dyskinesia from wearable sensors. IEEE Trans Neural Syst Rehabil Eng. 2014;22(5):982–991. doi: 10.1109/TNSRE.2014.2310904. [DOI] [PubMed] [Google Scholar]
- 6.Mancini M, Salarian A, Carlson-Kuhta P, et al. ISway: a sensitive, valid and reliable measure of postural control. J Neuroeng Rehabil. 2012;9:59. doi: 10.1186/1743-0003-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirelman A, Giladi N, Hausdorff JM. Body-fixed sensors for Parkinson disease. JAMA. 2015;314(9):873–874. doi: 10.1001/jama.2015.8530. [DOI] [PubMed] [Google Scholar]
- 8.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahn S, Elton R Members of the UPDRS Development Committee. Recent developments in Parkinson’s disease. Florham Park, NJ: Macmillan Health Care Information; 1987. Unified Parkinson’s disease rating scale; pp. 153–163. [Google Scholar]
- 10.Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson’s disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology. 1990;40(10):1529–1534. doi: 10.1212/wnl.40.10.1529. [DOI] [PubMed] [Google Scholar]
- 11.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 12.Marder K, Wang Y, Alcalay RN, et al. Age-specific penetrance of LRRK2 G2019S in the Michael J. Fox Ashkenazi Jewish LRRK2 Consortium. Neurology. 2015;85(1):89–95. doi: 10.1212/WNL.0000000000001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirelman A, Bernad-Elazari H, Nobel T, et al. Effects of aging on arm swing during gait: the role of gait speed and dual tasking. PLoS ONE. 2015;10(8):e0136043. doi: 10.1371/journal.pone.0136043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hausdorff JM. Stride variability: beyond length and frequency. Gait Posture. 2004;20(3):304. doi: 10.1016/j.gaitpost.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Hausdorff JM. Gait variability: methods, modeling and meaning. J Neuro Eng Rehabil. 2005;2:19. doi: 10.1186/1743-0003-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewek MD, Poole R, Johnson J, Halawa O, Huang X. Arm swing magnitude and asymmetry during gait in the early stages of Parkinson’s disease. Gait Posture. 2010;31(2):256–260. doi: 10.1016/j.gaitpost.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan N, Sternad D. Sensitivity of smoothness measures to movement duration, amplitude, and arrests. J Mot Behav. 2009;41(6):529–534. doi: 10.3200/35-09-004-RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gan-Or Z, Giladi N, Rozovski U, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70(24):2277–2283. doi: 10.1212/01.wnl.0000304039.11891.29. [DOI] [PubMed] [Google Scholar]
- 19.Orr-Urtreger A, Shifrin C, Rozovski U, et al. The LRRK2 G2019S mutation in Ashkenazi Jews with Parkinson disease: is there a gender effect? Neurology. 2007;69(16):1595–1602. doi: 10.1212/01.wnl.0000277637.33328.d8. [DOI] [PubMed] [Google Scholar]
- 20.Mirelman A, Heman T, Yasinovsky K, et al. Fall risk and gait in Parkinson’s disease: the role of the LRRK2 G2019S mutation. Mov Disord. 2013;28(12):1683–1690. doi: 10.1002/mds.25587. [DOI] [PubMed] [Google Scholar]
- 21.Becker G, Muller A, Braune S, et al. Early diagnosis of Parkinson’s disease. J Neurol. 2002;249(suppl 3):III/40–III/48. doi: 10.1007/s00415-002-1309-9. [DOI] [PubMed] [Google Scholar]
- 22.Maetzler W, Hausdorff JM. Motor signs in the prodromal phase of Parkinson’s disease. Mov Disord. 2012;27(5):627–633. doi: 10.1002/mds.24973. [DOI] [PubMed] [Google Scholar]
- 23.Siderowf A, Stern MB. Premotor Parkinson’s disease: clinical features, detection, and prospects for treatment. Ann Neurol. 2008;64(suppl 2):S139–S147. doi: 10.1002/ana.21462. [DOI] [PubMed] [Google Scholar]
- 24.Mirelman A, Alcalay RN, Saunders-Pullman R, et al. Nonmotor symptoms in healthy Ashkenazi Jewish carriers of the G2019S mutation in the LRRK2 gene. Mov Disord. 2015;30(7):981–986. doi: 10.1002/mds.26213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross GW, Abbott RD, Petrovitch H, Tanner CM, White LR. Premotor features of Parkinson’s disease: the Honolulu-Asia Aging Study experience. Parkinsonism Relat Disord. 2012;18(suppl 1):S199–S202. doi: 10.1016/S1353-8020(11)70062-1. [DOI] [PubMed] [Google Scholar]
- 26.Schneider SA, Drude L, Kasten M, Klein C, Hagenah J. A study of subtle motor signs in early Parkinson’s disease. Mov Disord. 2012;27(12):1563–1566. doi: 10.1002/mds.25161. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, Mahoney JM, Lewis MM, Guangwei D, Piazza SJ, Cusumano JP. Both coordination and symmetry of arm swing are reduced in Parkinson’s disease. Gait Posture. 2012;35(3):373–377. doi: 10.1016/j.gaitpost.2011.10.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov Disord. 1998;13(3):428–437. doi: 10.1002/mds.870130310. [DOI] [PubMed] [Google Scholar]
- 29.Collins SH, Adamczyk PG, Kuo AD. Dynamic arm swinging in human walking. Proc Biol Sci. 2009;276(1673):3679–3688. doi: 10.1098/rspb.2009.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maetzler W, Mancini M, Liepelt-Scarfone I, et al. Impaired trunk stability in individuals at high risk for Parkinson’s disease. PLoS ONE. 2012;7(3):e32240. doi: 10.1371/journal.pone.0032240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alcalay RN, Mirelman A, Saunders-Pullman R, et al. Parkinson disease phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Mov Disord. 2013;28(14):1966–1971. doi: 10.1002/mds.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 1992;42(6):1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 33.Carpinella I, Crenna P, Marzegan A, et al. Effect of L-dopa and subthalamic nucleus stimulation on arm and leg swing during gait in Parkinson’s Disease. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:6665–6668. doi: 10.1109/IEMBS.2007.4353888. [DOI] [PubMed] [Google Scholar]
- 34.Crenna P, Carpinella I, Lopiano L, et al. Influence of basal ganglia on upper limb locomotor synergies. Evidence from deep brain stimulation and L-DOPA treatment in Parkinson’s disease. Brain. 2008;131(Pt 12):3410–3420. doi: 10.1093/brain/awn272. [DOI] [PubMed] [Google Scholar]
- 35.Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: SLACK Inc; 1992. [Google Scholar]
- 36.Kalinderi K, Bostantjopoulou S, Fidani L. The genetic background of Parkinson’s disease: current progress and future prospects [published online ahead of print 2016] Acta Neurol Scand. doi: 10.1111/ane.12563. [DOI] [PubMed] [Google Scholar]
- 37.Goldwurm S, Tunesi S, Tesei S, et al. Kin-cohort analysis of LRRK2-G2019S penetrance in Parkinson’s disease. Mov Disord. 2011;26(11):2144–2145. doi: 10.1002/mds.23807. [DOI] [PubMed] [Google Scholar]
- 38.Healy DG, Falchi M, O’Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7(7):583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kachergus J, Mata IF, Hulihan M, et al. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations. Am J Hum Genet. 2005;76(4):672–680. doi: 10.1086/429256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trinh J, Amouri R, Duda JE, et al. A comparative study of Parkinson’s disease and leucine-rich repeat kinase 2 p.G2019S parkinsonism. Neurobiol Aging. 2014;35(5):1125–1131. doi: 10.1016/j.neurobiolaging.2013.11.015. [DOI] [PubMed] [Google Scholar]