Abstract
Objective
To determine whether a collaborative learning strategy derived clinical practice guideline (CPG) can reduce the duration of endotracheal intubation following infant heart surgery.
Design
Prospective and retrospective data collected from the Pediatric Heart Network in the 12 months pre- and post-CPG implementation at the four sites participating in the collaborative (active sites) compared to data from five PHN centers not participating in collaborative learning (control sites).
Patients
Data were collected for infants following 2 index operations: 1) repair of isolated coarctation of the aorta (birth-365 days) and 2) repair of tetralogy of Fallot (29–365 days). There were 240 subjects eligible for the CPG at active sites and 259 subjects at control sites.
Measurements and Main Results
After CPG implementation, the rate of early extubation at active sites increased significantly from 11.7% to 66.9% (p<0.001) with no increase in reintubation rate. The median duration of post-operative intubation among active sites decreased from 21.2 hours to 4.5 hours (p<.001). No statistically significant change in early extubation rates was found in the control sites 11.7% to 13.7% (p=0.63). At active sites CPG implementation had no statistically significant impact on median intensive care unit (ICU) length of stay (LOS) (71.9 hours pre- vs. 69.2 post-implementation, p=0.29) for the entire cohort. There was a trend toward shorter ICU LOS in the tetralogy of Fallot subgroup (71.6 hours pre- vs. 54.2 post-implementation, p=0.068).
Conclusions
A collaborative learning strategy designed CPG significantly increased the rate of early extubation with no change in the rate of reintubation. The early extubation CPG did not significantly change post-operative ICU LOS.
Keywords: mechanical ventilation, tetralogy of Fallot, outcomes
Collaborative learning is a technique based upon principles from the manufacturing industry designed to bring low-performing processes into line with the highest-performing processes. Collaborative learning involves several key steps including: 1) topic selection; 2) learning sessions; and 3) action periods in which teams test and implement changes in their local centers. Through benchmarking, locations with superior outcomes are identified. In the medical community, this is achieved by site visits, sharing protocols, and ongoing communication.
Investigators have applied collaborative learning to improve results for coronary artery bypass graft surgery by creating a regional group willing to compare outcomes.1 This voluntary program, which involved reciprocal site visits, was undertaken with the goal of exchanging information concerning the treatment of cardiovascular disease so that each participating institution would benefit. A similar strategy has been used by other adult cardiovascular consortia to improve outcomes following valve surgery.2,3 Collaborative learning using site visits has not yet been applied to pediatric cardiac care and thus it is unknown if it can improve outcomes in pediatric cardiac surgery. A national structure for collaborative site visits has never been tried, to our knowledge, in any field.
We sought to apply the strategy of collaborative learning to improve the post-operative care of children with congenital heart disease. More specifically, we used the collaborative learning approach to develop a clinical practice guideline (CPG) to increase the rate of early extubation following two index heart operations in infants. Early extubation is an attractive target to improve post-operative care. There is wide variability in the approach to post-operative extubation and several quality initiatives have focused on decreasing the length of mechanical ventilation.4 We hypothesized that the collaborative learning model would produce a high rate of compliance with a CPG, which in turn would translate in demonstrable improvements in the rate of early extubation and other key clinical outcome variables.
Methods
The Collaborative Learning Study was initiated with the goal of employing collaborative learning techniques with specific emphasis on site visits to identify the major components of post-operative care that might be improved by sharing expertise. An extensive description of this process has previously been reported by our investigative team.5 Briefly, the Pediatric Heart Network (PHN) has 9 core centers representing a total of 10 children’s hospitals. All of the PHN centers have a dedicated cardiac intensive care unit (ICU). Five of the hospitals were selected to participate in this collaborative learning project and are 'Active Sites'. The centers were chosen to provide geographic and institutional surgical volume variation. Five remaining hospitals were not directly involved in the collaborative learning element of the study and represent “Control Sites” (Figure 1). Investigators from Emory University and Georgia Institute of Technology conducted a series of preliminary site visits to all 5 active sites to assess institutional resources/staffing, clinical protocols, innovative approaches and clinical outcomes data. Using the data from these initial site visits, the collaborative elected to address the approach to the timing of post-operative extubation for infant heart surgery because this practice varied widely among the 5 centers. One of the five active sites had already devoted time and effort to advance the concept of early extubation. Observation of care at this site suggested that early extubation could lead to an acceleration of the post-operative care process including earlier introduction of enteral feeds and shorter duration of sedation. As such, this practice became an attractive target for the collaborative learning model. This insitution is referred to as the “Model Site” owing to its expertise in early extubation, and this expertise was used to guide CPG protocol development for the four other active sites (none of which routinely used the strategy of early extubation in the target patient populations).
Figure 1.
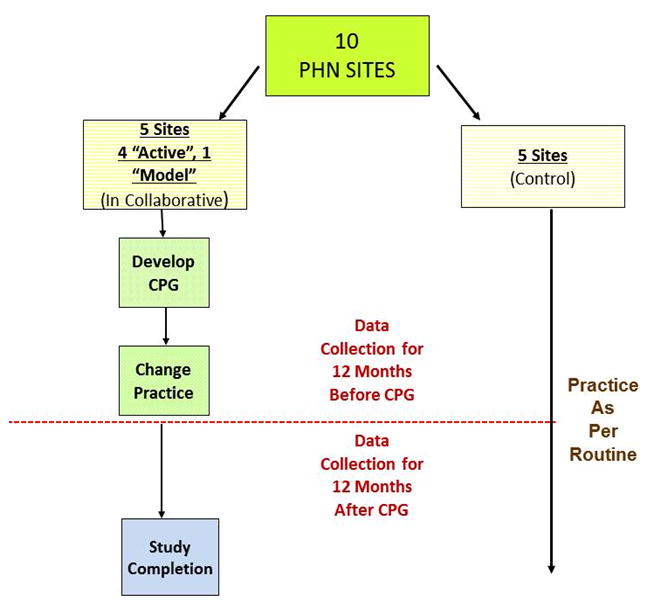
Scheme of the Study Design (PHN = Pediatric Heart Network; CPG = clinical practice guideline).
After rotational site visits in which a team of clinicians including a cardiac surgeon, intensivist, respiratory therapist and cardiac ICU nurse from each of the 5 Collaborative Learning active sites visited one other active center and hosted a second center, the collaborative set out to construct a CPG for early extubation. Two index operations were selected: complete repair of tetralogy of Fallot and repair of isolated coarctation of the aorta. These lesions were chosen since both would result in two-ventricle circulations and normal arterial saturation following repair. Moreover, analysis of data from the active sites suggested that early extubation was uncommon (except at the model site that was already practicing early extubation) following these index operations and the usual total duration of post-operative ventilation exceeded 20 hours.
The primary outcome measure was the proportion of subjects extubated within 6 hours of return to the ICU from the operating room following one of the index operations. Duration (in hours) of intubation was defined as time from leaving operating room (OR) to removal of the endotracheal tube. Extubation in the operating room was defined as 0 hours of post-operative ventilation. Prospectively defined secondary outcome measures included: the need for reintubation within 48 hours of initial extubation; total duration of post-operative intravenous sedation; and the post-operative pain scores (median and range) using the FLACC6 pain scale during the first 48 hours following ICU admission. In addition, data were gathered on sedation and analgesia use. Other variables of interest included timing of first enteral feeds, postoperative medical ICU length of stay - defined as the time of ICU admission until the time that the patient was ready for transfer to a lower acuity unit, as well as the postoperative physical ICU LOS, defined as time from ICU admission until the time the patient physically left the ICU.
To identify all subjects that would meet eligibility criteria for the CPG, we screened all children undergoing reparative surgery for tetralogy of Fallot or coarctation of the aorta. Subjects meeting CPG eligibility criteria were considered enrolled in the study. “Enrolled” represents those subjects that met all inclusion and no exclusion criteria for the CPG. Although the CPG was not applied at control sites, the specificity of the CPG allowed us to determine which subjects at these sites would have met enrollment criteria.
All outcome measures were collected for the 12 months immediately preceding (pre-implementation) and 12 months immediately following (post-implementation) the introduction of the CPG at active sites. Data on the same outcome measures at control sites were collected during contemporaneous time intervals, though the CPG was not implemented at these sites. There was an 8 week implementation phase at each site during which centers instituted the CPG. This allowed for troubleshooting and refinement of CPG process. For the purposes of data analysis this 8 week period was not included in either the pre-implementation or post-implementation data reporting.
Clinical Practice Guideline
The specific inclusion criteria for the tetralogy of Fallot cohort were: diagnosis of tetralogy of Fallot, patient age >28 and ≤365 days on the date of index surgery and planned complete surgical repair (including those patients who had undergone prior palliative Blalock-Taussig shunt). For the coarctation of the aorta cohort the inclusion criteria were: planned complete surgical repair without the need for cardiopulmonary bypass and age < 365 days on the date of index surgery. Patients with coarctation of the aorta with ventricular septal defect undergoing a concomitant pulmonary artery band were eligible for the CPG.
CPG exclusion criteria included corrected gestational age < 36 weeks, known primary lung disease, known airway anomalies, pulmonary hypertension requiring medication therapy, mechanical ventilation immediately prior to surgery, known congenital or acquired neurological injury likely to impact respiratory function, known chromosomal abnormality or syndrome likely to impact airway or lung function. Patients meeting all inclusion criteria and no exclusion criteria were enrolled into the CPG preoperatively (Supplemental Appendix). For those patients considered eligible for early extubation, an anesthetic plan of care that would allow for early extubation was utilized. This anesthetic approach was guided by several principles: 1) choice and dose of premedication and anesthetic agents that would allow for early extubation; 2) anesthesia with volatile anesthetic with low dose opioids and 3) balanced sedation/analgesia in the early post-operative period including low dose opioids and benzodiazepines along with adjunctive therapy such as dexmedetomidine or intravenous acetaminophen. The recommended anesthetic approach was as follows: the cumulative dose of intra-operative fentanyl (or alternative opioid equivalent analgesic dose) should not exceed 10 mcg/kg per hour. The choice of volatile anesthetic agent and minimum alveolar concentration (MAC) was at the discretion of the attending anesthesiologist. For those patients having a thoracotomy, a regional intercostal nerve block using local anesthetic was employed at the discretion of the operative team. The post-operative analgesia and sedation regimen was targeted at producing adequate pain relief and anxiolysis without excessive sedation to the point of precluding tracheal extubation or necessitating re-intubation. Typically this included one or more agents such as dexmedetomidine, acetaminophen (intravenous or rectal), as well as intravenous opioids, or benzodiazepines. Clearly if the trajectory of the patient changed at any point in the operating room or the ICU such that they were no longer deemed candidates for early extubation, then the choice and dose of agents was modified accordingly.
For those subjects enrolled in the CPG, we defined parameters to guide early extubation following surgery. The CPG did not recommend early extubation if the patient manifested postoperative hemodynamic instability (requiring significant or escalating vasoactive support or fluid resuscitation), refractory arrhythmias, significant lactic acidosis and/or ongoing bleeding. All subjects enrolled in the CPG were included in analysis of clinical outcomes even if early extubation was not recommended by the CPG due to the clinical features noted above.
Implementation of the CPG took place at each individual center after review by the PHN’s Data and Safety Monitoring Board, approval from the individual center’s quality committees, and institutional review board (IRB) approval for data collection from subjects. The need for informed consent was waived by the IRBs because of the quality improvement nature of the study.
Statistical Methods
To examine the effect of the implementation of the CPG developed through collaborative learning on the primary aim, the proportion of subjects extubated within 6 hours of ICU arrival before and after the implementation of the CPG using Chi-squared test. As the 'Model Site' routinely undertook early extubation in nearly all infant surgery cases, the primary analysis was limited to the 4 active sites that did not routinely use this strategy prior to the application of the CPG. A preliminary analysis compared the 12 month cumulative early extubation ratios at control sites and active sites separately. After this preliminary analysis indicated there was a significant difference between pre- and post-implementation in active sites but not in control sites, an interrupted time series approach (ITS) was utilized. ITS analyses are commonly used in quasi-experimental research designs where there is no randomization either because it is unethical or not feasible. This approach tests for a change in the outcome rate before and after implementation of the program controlling for secular trends. ITS uses a segmented regression, where the first regression is the period before the implementation of the program and the second regression is the period after the implementation of the program, where the early extubation rate for each month provides a unique data point. An ANCOVA was used to test for difference between the adjusted means (y-intercept).
Secondary outcomes were similarly compared using the pre- and post-implementation rates at active and control sites separately. Categorical outcomes were analyzed the same as the early extubation rates (via chi-squared tests). Continuous outcomes for pre-and post-implementation values were compared using a Wilcoxon two sample test. All analyses were performed in SAS 9.3 (SAS Institute, Cary NC) and R studio.
Results
The number of subjects enrolled at the 4 active sites was 240 (119 in pre- implementation and 121 in post-implementation) and at the 5 control sites was 259 (119 in pre-implementation and 121 in post-implementation). The proportion of subjects that achieved early extubation in the pre-implementation phase was nearly identical in active sites as compared to control sites, 11.8% vs. 11.7% (p=0.98) (Table 1). Following the implementation of the CPG, the active sites achieved a significantly higher rate of early extubation, increasing from 11.8% to 66.9% (p<0.001). Of the 81 subjects with early extubation, 28 (34.6%) were extubated in the operating room. ITS demonstrated an increasing trend in early extubation rates in the 12 months following implementation of the CPG at the active sites (Figure 2). The control sites were essentially unchanged (11.7% vs. 13.7%). Of note, the rate of early extubation for the model site was 34/44 (77.3%) in the pre-implementation phase and remained high at 36/38 (94.7%) in the post-implementation phase. The median duration of post-operative intubation among the 4 active sites decreased from 21.2 hours in the pre-implementation phase to 4.5 hours in the post-implementation phase (p<.001). There was no significant change in time to extubation among control sites (19.8 hours vs. 17.3 hours, p=0.12) (Table 2). The rate of reintubation was low at baseline (2.5%) among active sites and did not change following implementation of the CPG (3.3%, p=1.00). There was no difference in the use of high flow nasal cannula (HNC) support after extubation. HFNC was utilized in 26.1% of subjects in pre-implementation phase and 24.7% of subjects in post-implementation phase (p=0.82)
Table 1.
Early Extubation Proportions, Pre- v Post-implementation by Procedure Excluding the Single Active Site with Historical High Rate of Extubation
| Procedure | Pre-Implementation | Post-Implementation | P-Value |
|---|---|---|---|
| Active Sites† | 14/119 (11.8%) | 81/121 (66.9%) | <.0001 |
| TOF | 9/69 (13.0%) | 57/78 (73.1%) | |
| CoA | 5/50 (10.0%) | 24/43 (55.8%) | |
| Control Sites | 14/120 (11.7%) | 19/139 (13.7%) | 0.6299 |
| TOF | 7/59 (11.8%) | 11/90 (12.2%) | |
| CoA | 7/61 (11.5%) | 8/49 (16.3%) |
Abbreviations: TOF = tetralogy of Fallot; CoA = coarctation of the aorta.
Does not include one Model Site
Figure 2.
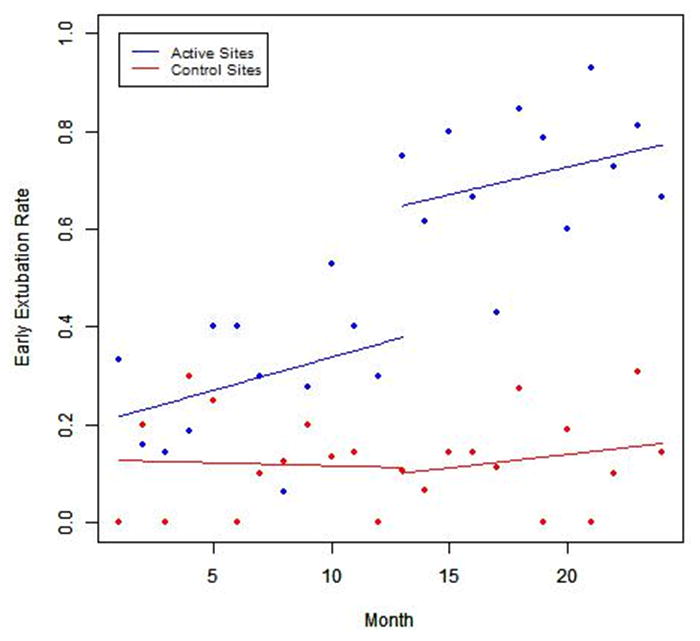
Interrupted Time Series Analyses of Early Extubation Rates at Active and Control Sites
Table 2.
Post-operative Clinical Outcomes for All Subjects
| Outcome | Pre-Implementation
|
Post-Implementation
|
|||
|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | P-value | |
| Postoperative LOS, days | |||||
| Active | 119 | 6.0 (4.0, 8.0) | 121 | 5.0 (4.0, 8.0) | 0.3912 |
| Control | 120 | 6.0 (5.0, 8.0) | 139 | 5.0 (4.0, 8.0) | 0.0730 |
| Postoperative ICU LOS, days | |||||
| Active | 118 | 3.0 (2.0, 5.1) | 121 | 2.9 (1.8, 4.8) | 0.2865 |
| Control | 120 | 2.8 (1.8, 3.9) | 139 | 2.2 (1.8, 3.6) | 0.2837 |
| Postoperative ICU LOS, hours | |||||
| Active | 118 | 71.9 (47.0, 121.6) | 121 | 69.2 (44.1, 116.1) | 0.2865 |
| Control | 120 | 66.2 (44.3, 93.9) | 139 | 51.7 (42.7, 86.7) | 0.2837 |
| Time to extubation, hours | |||||
| Active | 118 | 21.2 (13.5, 36.0) | 121 | 4.5 (0.1, 9.3) | <.0001 |
| Control | 120 | 19.8 (11.8, 30.1) | 139 | 17.3 (9.0, 24.8) | 0.1181 |
| Postoperative critical care LOS, hours | |||||
| Active | 117 | 59.3 (39.7, 94.6) | 119 | 50.0 (26.6, 98.6) | 0.4439 |
| Control | 120 | 62.2 (43.3, 90.4) | 139 | 51.2 (41.2, 86.7) | 0.3499 |
| Time to continuous sedation/analgesia, hours | |||||
| Active | 85 | 43.6 (23.3, 68.8) | 103 | 19.3 (11.4, 39.5) | <.0001 |
| Time to first introduction of enteral feeds, hours | |||||
| Active | 89 | 30.7 (22.0, 46.6) | 88 | 19.2 (10.9, 27.8) | <.0001 |
| Average FLACC (pain) score, where at least three | |||||
| Active | 93 | 1.3 (0.4, 2.2) | 88 | 2.1 (1.0, 3.1) | 0.0002 |
| Cumulative opioid, mg/kg* | |||||
| Active | 118 | 0.8 (0.4, 3.0) | 118 | 0.5 (0.3, 1.0) | 0.0003 |
| Cumulative benzodiazepine, mg/kg** | |||||
| Active | 80 | 0.3 (0.1, 2.2) | 69 | 0.2 (0.1, 0.4) | 0.0108 |
| Cumulative dose of dex /kg | |||||
| Active | 50 | 5.4 (2.1, 14.5) | 91 | 2.9 (1.5, 6.9) | 0.0351 |
Abbreviations: dex= dexmedetomidine, ICU = intensive care unit, LOS = length of stay
Mg of morphine (converted to equianalgesic dose for other opioids),
Mg of midazolam
The post-operative physical ICU LOS did not change significantly following implementation of the CPG among active sites. ICU medical LOS, which assesses ICU discharge readiness, a measure which is not generally impacted by non-clinical factors such as bed availability also did not change significantly following CPG implementation. Total hospital LOS also did not significantly decrease in the post-implementation phase. In examining the subgroup of subjects undergoing TOF repair (Table 3), there was a non-significant trend toward shorter physical and medical ICU LOS (p=0.068 & p=0.076, respectively).
Table 3.
Post-operative Clinical Outcomes for Tetralogy of Fallot Cohort
| Outcome | Pre-Implementation | Post-Implementation | |||
|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | P-value | |
| Postoperative LOS, days | |||||
| Active | 69 | 7.0 (4.0, 9.0) | 78 | 5.0 (4.0, 8.0) | 0.0931 |
| Control | 59 | 6.0 (6.0, 8.0) | 90 | 6.0 (5.0, 8.0) | 0.2170 |
| Postoperative ICU LOS, days | |||||
| Active | 68 | 3.0 (1.9, 6.0) | 78 | 2.3 (1.2, 4.2) | 0.0684 |
| Control | 59 | 2.9 (1.9, 4.0) | 90 | 2.8 (1.8, 3.7) | 0.3476 |
| Postoperative ICU LOS, hours | |||||
| Active | 68 | 71.6 (46.4, 143.6) | 78 | 54.2 (28.8, 100.8) | 0.0684 |
| Control | 59 | 69.4 (44.9, 95.9) | 90 | 67.5 (43.2, 90.0) | 0.3476 |
| Time to extubation, hours | |||||
| Active | 68 | 19.1 (13.9, 43.1) | 78 | 3.4 (0.0, 7.2) | <.0001 |
| Control | 59 | 19.8 (10.9, 28.8) | 90 | 18.0 (9.9, 26.3) | 0.5320 |
| Postoperative critical care LOS, hours | |||||
| Active | 68 | 56.1 (34.3, 117.8) | 77 | 45.0 (25.5, 85.0) | 0.0762 |
| Control | 59 | 68.6 (44.6, 93.8) | 90 | 65.8 (41.3, 90.0) | 0.3157 |
| Time to continuous sedation/analgesia, hours | |||||
| Active | 53 | 46.0 (22.4, 89.0) | 76 | 19.2 (11.6, 38.0) | <.0001 |
| Time to first introduction of enteral feeds, hours | |||||
| Active | 53 | 28.0 (21.2, 43.8) | 53 | 15.0 (9.9, 26.1) | <.0001 |
| Average FLACC (pain) score, where at least three | |||||
| Active | 52 | 1.3 (0.7, 2.2) | 61 | 2.3 (1.2, 3.2) | 0.0024 |
| Cumulative opioid, mg/kg* | |||||
| Active | 68 | 0.9 (0.5, 4.6) | 77 | 0.6 (0.3, 1.1) | 0.0003 |
| Cumulative benzodiazepine, mg/kg** | |||||
| Active | 53 | 0.3 (0.2, 2.2) | 47 | 0.2 (0.1, 0.3) | 0.0038 |
| Cumulative dose of dex /kg | |||||
| Active | 40 | 5.6 (2.3, 12.5) | 70 | 2.8 (1.7, 6.9) | 0.0128 |
Abbreviations: dex= dexmedetomidine, ICU = intensive care unit, LOS = length of stay
Mg of morphine (converted to equianalgesic dose for other opioids)
Mg of midazolam
Active sites do not include one Model Site
There were several clinical variables that did appear to be impacted by the CPG. The time to first introduction of enteral feeds following surgery was significantly less following implementation of the CPG, decreasing from a median (range) of 30.7 (21.9, 49.3) hours to 19.2 (10.9, 27.8) hours at the active sites (p<0.001). The time to discontinuation of all continuous IV analgesics significantly decreased following CPG implementation from median 43.6 hours to median 19.3 hours (p=0.001). The proportion of subjects receiving dexmedetomidine increased from 42.0% to 75.2%. The mean FLACC (pain) score was higher after implementation of the CPG 1.3 to 2.1, p=0.0002.
Examining the tetralogy of Fallot cohort alone, we found a reduction in the cumulative dose of both opioids and benzodiazepines following the CPG implementation among active sites (p<0.001 and p<0.001, respectively). Among the tetralogy of Fallot patients receiving dexmedetomidine, there was a significant decrease post-operative cumulative dosage (p=0.01) following CPG implementation.
In the coarctation of the aorta cohort the cumulative dose of opioids did not significantly decrease following the CPG implementation (Table 4). Among coarctation of the aorta patients, the proportion of subjects with significant hypertension did not increase significantly following implementation of the CPG (p=0.13).
Table 4.
Post-operative Clinical Outcomes for Coarctation of the Aorta Cohort
| Outcome | Pre-Implementation
|
Post-Implementation
|
|||
|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | P-value | |
| Postoperative LOS, days | |||||
| Active | 50 | 5.0 (4.0, 7.0) | 43 | 5.0 (4.0, 10.0) | 0.8065 |
| Control | 61 | 6.0 (4.0, 9.0) | 49 | 5.0 (4.0, 7.0) | 0.1124 |
| Postoperative ICU LOS, days | |||||
| Active | 50 | 3.0 (2.0, 4.2) | 43 | 3.4 (1.9, 5.0) | 0.3924 |
| Control | 61 | 2.6 (1.8, 3.7) | 49 | 2.0 (1.7, 2.9) | 0.4134 |
| Postoperative ICU LOS, hours | |||||
| Active | 50 | 72.3 (47.6, 101.1) | 43 | 80.9 (46.4, 120.5) | 0.3924 |
| Control | 61 | 62.0 (43.4, 89.5) | 49 | 48.5 (41.4, 69.0) | 0.4134 |
| Time to extubation, hours | |||||
| Active | 50 | 23.5 (12.9, 31.8) | 43 | 5.7 (3.2, 13.7) | <.0001 |
| Control | 61 | 19.8 (12.4, 33.3) | 49 | 16.0 (7.3, 22.7) | 0.0910 |
| Postoperative critical care LOS, hours | |||||
| Active | 49 | 62.1 (40.4, 74.3) | 42 | 70.6 (43.9, 115.6) | 0.1811 |
| Control | 61 | 51.8 (40.5, 87.0) | 49 | 46.0 (41.2, 69.0) | 0.5657 |
| Time to continuous sedation/analgesia, hours | |||||
| Active | 32 | 36.2 (23.5, 46.8) | 27 | 20.1 (8.9, 41.7) | 0.0239 |
| Time to first introduction of enteral feeds, hours | |||||
| Active | 36 | 34.1 (26.9, 48.4) | 35 | 24.5 (15.6, 42.5) | 0.0043 |
| Average FLACC (pain) score, where at least three F | |||||
| Active | 41 | 0.8 (0.0, 2.2) | 27 | 1.5 (0.6, 2.7) | 0.0648 |
| Cumulative opioid, mg/kg* | |||||
| Active | 50 | 0.6 (0.3, 1.3) | 41 | 0.5 (0.3, 0.8) | 0.1466 |
| Cumulative benzodiazepine, mg/kg | |||||
| Active | 27 | 0.2 (0.1, 2.1) | 22 | 0.2 (0.1, 0.6) | 0.7861 |
| Cumulative dose of dex /kg | |||||
| Active | 10 | 1.8 (0.7, 14.5) | 21 | 4.3 (1.5, 6.6) | 0.9495 |
Abbreviations: dex= dexmedetomidine, ICU = intensive care unit, LOS = length of stay
Mg of morphine (converted to equianalgesic dose for other opioids)
Mg of midazolam
Active sites do not include one Model Site
We also sought to measure CPG compliance. For most elements of the CPG, compliance exceeded 85% (Table 5), with only two areas of low compliance: extubation readiness assessment and adequate pain assessment. The CPG defined “extubation readiness” criteria and goal blood gas parameters for those subjects extubated in the ICU using 9 criteria, related to both clinical assessment as well as blood gas criteria. The “extubation readiness” criterion for which compliance was lowest was the parameter of pH > 7.3, for which the compliance rate was 81.3%. In other words, 18.7% of patients extubated in the ICU between 1 and 6 hours after surgery had a pH lower than 7.3 at the time of extubation. For the purposes of this CPG, we required hourly FLACC scores for the first 12 hours, and centers were only 81.0% compliant with this component.
Table 5.
Compliance Rates for Elements of Clinical Practice Guideline
| Compliance measure | Compliant N (%) |
|---|---|
| 1) Documentation of an anesthetic plan to allow early extubation | 101/121 (83.5%) |
| 1a) Communication of a pre-op anesthetic plan | 105/121 (86.8%) |
| 1b) Communication for a post-op sedation plan | 103/121 (85.1%) |
| 2) Use of an anesthetic plan for early extubation | 112/121 (92.6%) |
| 3) Handoff with adequate communication of anesthetic sedation plan | 99/121 (81.8%) |
| 3a) Communication that the ICU staff was informed | 100/121 (82.6%) |
| 3b) Communication from the anesthesiologist with ICU care team | 101/121 (83.5%) |
| 4) Extubation readiness assessment | 64/81 (79.0%) |
| 4a) Use of Vasoactive drugs | 53/53 (100.0%) |
| 4b) Arrhythmia requiring therapy | 51/53 (96.2%) |
| 4c) Bleeding requiring reoperation | 53/53 (100.0%) |
| 4d) Spontaneous respiratory rate | 42/48 (87.5%) |
| 4e) pH greater than 7.3 | 39/48 (81.3%) |
| 4f) paCO2 less than 55mmHg | 46/48 (95.8%) |
| 4g) SaO2 greater than 90% | 43/48 (89.6%) |
| 4h) Base value between −8 and 8 | 47/48 (97.9%) |
| 4i) Lactic Acid | 48/48 (100.0%) |
| 5) Adequate pain assessment | 98/121 (81.0%) |
| 6) Close Monitoring for up to 6 hours post arrival in ICU | 121/121 (100.0%) |
| 6a) Initial vital sign assessment | 121/121 (100.0%) |
| 6b) 4th complete vital sign assessment | 121/121 (100.0%) |
| 6c) Documentation of monitoring | 121/121 (100.0%) |
Discussion
This study has demonstrated that the introduction of a CPG developed through a collaborative learning process was associated with a significant increase in the rate of early extubation without a change in reintubation rates in infants following reparative cardiac surgery.
The post-operative care process after congenital heart surgery is complex, and many aspects are not standardized. There are several key milestones in the “progression” of a patient in the post-operative phase. These milestones include hemostasis, weaning from vasoactive support, extubation, removal of central catheters and drains, advancement of enteral feeds, discharge from the intensive care unit, and increase in mobility/physical activity. Our collaborative team chose to focus on post-operative intubation. For many cardiac operations, the physiology and hemodynamics are more stable after surgery than before. This is true with corrective surgery such as repair of tetralogy of Fallot or repair of coarctation of the aorta. Following these operations, intracardiac shunts are essentially eliminated and hypoxemia resolved. Moreover, early extubation is considered a quality benchmark in the adult cardiac surgery scorecard.7
Our study found that more than 70% of infants following repair of tetralogy of Fallot could be extubated early. The rate of early extubation was slightly lower in the coarctation cohort. This may be due to the fact that over 50% of the subjects in this coarctation cohort were <28 days of age, and many were < 1 week of age. The CPG allowed centers to use more than one approach to early extubation. For many centers, the ideal setting for early extubation was in the operating room immediately on completion of the procedure. The advantage of this strategy is that it is a controlled setting familiar to anesthesiologists. However, some centers have favored extubation in the ICU before or after hand-off of the patient, thereby preventing delay in turnover of the operating room and allowing respiratory therapists to play a greater role in the process.
Early extubation following congenital heart surgery has previously been described in the literature using various anesthetic approaches.8–11 Some institutions have favored the use of epidural anesthesia.8,12 This approach can allow for lower doses of opioids; however, it does carry some—albeit small—risk of infection and bleeding complications. Our CPG did not employ epidural anesthesia. Rather our approach was to use low dose opioid in the form of fentanyl paired with a volatile anesthetic. Some anesthesiologists have suggested that very high dose fentanyl, i.e. > 100 mcg/kg, can blunt the stress response to cardiac surgery.13,14 However, this association has not fully been analyzed and the impact of the stress response on clinical outcomes remains to be determined. Dexmedetomidine, an intravenous alpha-agonist which combines effective sedation with some analgesic properties, while preserving respiratory drive, served as a useful adjunct for the majority of subjects in the post-implementation phase of this study.
There has been some hesitation in adopting an early extubation strategy in young children following cardiac surgery. Concerns have been raised that caregivers may need to withhold analgesia in order to successfully execute an early extubation protocol, which might cause distress to the child or hemodynamic instability. The present analysis provides important data to address these issues. Our analysis did demonstrate a slightly higher FLACC score in the post-implementation phase. However, the median FLACC score in the post-implementation phase in the first 12 hours following surgery was only 2.1. This is still in the “mild discomfort range” and would not generally merit added bolus analgesia.15 We also tracked hypertension following surgery for coarctation of the aorta and were encouraged that this was not different in the post-implementation phase.
One of the theoretical benefits of early extubation relates to the throughput of the patients. We hypothesized that a high rate of early extubation would result in a shorter duration of ICU stay. However, early extubation was not associated with a lower ICU LOS. The likely explanation is that many factors other than the need for mechanical ventilation impact ICU LOS. Some patients may be extubated and receive supportive respiratory measures such as non-invasive positive pressure ventilation. Typically such adjunctive therapies are unsuitable for ICU transfer. Other important steps in post-operative care must be completed prior to transfer to a lower level of care. These steps include removal of central vascular lines and discontinuation of vasoactive agents. It is possible that with more experience with early extubation, centers might then turn their attention to accelerating these other processes. Importantly, we find that early extubation was associated with lower total doses and duration of continuous IV sedation or analgesia. A recent analysis of the Society of Thoracic Surgeons database suggests that an early extubation strategy may significantly impact length of stay after some operations and not others.16 Our subanalysis identified a non-significant trend toward shorter ICU LOS in the tetralogy of Fallot group with a median difference of 16 hours after CPG implementation. It is also important to highlight that there are significant changes in other key clinical outcomes following the implementation of the CPG. Early extubation was associated with a shorter time to first enteral feeds. This is a key clinical variable as feeding can provide valuable adjunct to analgesic management following cardiac surgery.
The CPG used in this project was developed largely based upon expert opinion and consensus amongst participating centers. As part of the CPG we developed extubation readiness criteria related to physiologic and laboratory data. In the assessment of CPG compliance, the extubation criteria were violated on occasion. Nonetheless, the clinical outcomes were excellent and reintubation rates were unchanged. This would suggest that refinement of the CPG might allow more lenient blood gas criteria for extubation. It is important to remember that some degree of hypercapnia may be common following extubation whether early or traditional, and after many different types of surgeries. However, this is generally well tolerated. One of the key points is that the criteria for extubation following general anesthesia for surgery differ from the criteria for extubation of a chronically ventilated child with intrinsic lung disease. This represented one of the important points of dissemination of the CPG.
There are a number of important limitations of this study. We hypothesized that the collaborative learning process that included site visits was essential to achieve a high rate of CPG compliance and hence early extubation. However, it is possible that an alternative streamlined approach without in-person meetings and site visits might have achieved the same results. Also it is possible that changes in extubation rates were not due to the collaborative learning CPG alone. The incorporation of control sites that were similar in size and patient distribution, however, suggest that there was not a secular trend toward early extubation during the time of the study.
The collaborative learning model is a promising strategy to transmit clinical practices from high performing centers to peer institutions. Collaborative learning appears well-suited to address complex inpatient care practice and can improve clinical outcomes. Using this approach, early extubation following infant heart surgery can be rapidly implemented at congenital heart centers. Early extubation can be performed safely in infants undergoing heart surgery with demonstrable benefits on some clinical measures.
Supplementary Material
Acknowledgments
This work was supported by HL068270, HL109777, HL109816, HL109818, HL109778, HL109743, HL109673, HL109741, HL109737, and HL109781 from the National Heart, Lung, and Blood Institute (NHLBI).
Footnotes
The views expressed in this manuscript are those of the authors and do not necessarily represent official positions of the NHLBI or NIH.”
Copyright form disclosures: Dr. Mahle received support for article research from the National Institutes of Health (NIH). Dr. Nicolson received support for article research from the NIH. Dr. Hollenbeck-Pringle received support for article research from the NIH. Her institution received funding from the NIH. Dr. Witte received support for article research from the NIH. Her institution received funding from the Pediatric Heart Network. Dr. Lee received support for article research from the NIH. Her institution received funding from the National Science Foundation. Dr. Goldsworthy received support for article research from the NHLBI. Dr. Stark received support for article research from the NIH. His institution received funding from the NIH. Dr. Burns received support for article research from the NIH. Dr. Thiagarajan’s institution received funding from Bristol Myers Squibb (Events Adjudication Committee). Dr. Colan received support for article research from the NIH. His institution received funding from NIH HLBI. Dr. Schamberger received support for article research from the NIH. His institution received funding from the Pediatric Heart Network. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.O'Connor GT, Plume SK, Olmstead EM, et al. A regional prospective study of in-hospital mortality associated with coronary artery bypass grafting. The Northern New England Cardiovascular Disease Study Group. JAMA. 1991;266(6):803–809. [PubMed] [Google Scholar]
- 2.Prager RL, Armenti FR, Bassett JS, et al. Michigan Society of Thoracic and Cardiovascular Surgeons. Cardiac surgeons and the quality movement: the Michigan experience. Seminars in Thoracic and Cardiovascular Surgery. 2009;21(1):20–27. doi: 10.1053/j.semtcvs.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Johnson SH, Theurer PF, Bell GF, et al. A statewide quality collaborative for process improvement: internal mammary artery utilization. The Annals of Thoracic Surgery. 2010;90(4):1158–1164. doi: 10.1016/j.athoracsur.2010.05.047. discussion 1164. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs JP, He X, O'Brien SM, Welke KF, et al. Variation in ventilation time after coronary artery bypass grafting: an analysis from the society of thoracic surgeons adult cardiac surgery database. The Annals of Thoracic Surgery. 2013;96(3):757–762. doi: 10.1016/j.athoracsur.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 5.Wolf MJ, Lee EK, Nicolson SC, Pearson GD, et al. for the Pediatric Heart Network Investigators. Rationale and Methodology of a Collaborative Learning Project in Congenital Cardiac Care. American Heart Journal. doi: 10.1016/j.ahj.2016.01.013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatric Nursing. 1997;23(3):293–297. [PubMed] [Google Scholar]
- 7.Society of Thoracic Surgeons Quality Performance Measures. [Accessed 12/30/15]; http://www.sts.org/quality-research-patient-safety/quality/quality-performance-measures.
- 8.Mittnacht AJ, Thanjan M, Srivastava S, et al. Extubation in the operating room after congenital heart surgery in children. The Journal of Thoracic and Cardiovascular Surgery. 2008;136(1):88–93. doi: 10.1016/j.jtcvs.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 9.Heinle JS, Fox LS. Early extubation of neonates and young infants after cardiac surgery. Seminars in Thoracic and Cardiovascular surgery. Pediatric Cardiac Surgery annual. 1998;1:103–108. doi: 10.1016/s1092-9126(98)70013-4. [DOI] [PubMed] [Google Scholar]
- 10.Abuchaim DC, Bervanger S, Medeiros SA, et al. Early extubation in the operating room in children after cardiac heart surgery. Revista brasileira de cirurgia cardiovascular : orgao oficial da Sociedade Brasileira de Cirurgia Cardiovascular. 2010;25(1):103–108. doi: 10.1590/s0102-76382010000100020. [DOI] [PubMed] [Google Scholar]
- 11.Garg R, Rao S, John C, et al. Extubation in the operating room after cardiac surgery in children: a prospective observational study with multidisciplinary coordinated approach. Journal of Cardiothoracic and Vascular Anesthesia. 2014;28(3):479–487. doi: 10.1053/j.jvca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Leyvi G, Taylor DG, Reith E, et al. Caudal anesthesia in pediatric cardiac surgery: does it affect outcome? Journal of Cardiothoracic and Vascular Anesthesia. 2005;19(6):734–738. doi: 10.1053/j.jvca.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Duncan HP, Cloote A, Weir PM, et al. Reducing stress responses in the pre-bypass phase of open heart surgery in infants and young children: a comparison of different fentanyl doses. British Journal of Anaesthesia. 2000;84(5):556–564. doi: 10.1093/bja/84.5.556. [DOI] [PubMed] [Google Scholar]
- 14.Naguib AN, Tobias JD, Hall MW, et al. The role of different anesthetic techniques in altering the stress response during cardiac surgery in children: a prospective, double-blinded, and randomized study. Pediatric Critical Care Medicine. 2013;14(5):481–490. doi: 10.1097/PCC.0b013e31828a742c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crellin DJ, Harrison D, Santamaria N, et al. Systematic review of the Face, Legs, Activity, Cry and Consolability scale for assessing pain in infants and children: is it reliable, valid, and feasible for use? Pain. 2015;156(11):2132–2151. doi: 10.1097/j.pain.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 16.Mahle WT, Jacobs JP, Jacobs ML, et al. Early Extubation Following Repair of Tetralogy of Fallot and the Fontan Procedure: An Analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. The Annals of Thoracic Surgery. doi: 10.1016/j.athoracsur.2016.03.013. published online May 9 2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


