Abstract
Purpose
We have previously found that administration of erythropoietin (EPO) shortens the time course of recovery after experimental crush injury to the mouse sciatic nerve. The time course of recovery was more rapid than would be expected if EPO's effects were due to axonal regeneration, raising the question of whether recovery was instead due to promoting remyelination and/or preserving myelin on injured neurons. This study tests the hypothesis that EPO has a direct and local effect on myelination in vivo and in vitro.
Methods
Animals were treated with EPO after standard calibrated sciatic nerve crush injury and immunohistochemical analysis was performed to assay for myelinated axons. Combined in-vitro neuron-Schwann cell co-cultures were performed to directly assess EPO mediated effects on myelination and putative protective effects against oxidative stress. In-vivo local administration of EPO in a fibrin-glue carrier was used to demonstrate early local effects of EPO treatment well in advance of possible neuroregenerative effects.
Results
Systemic EPO administration maintained more in-vivo myelinated axons at the site of nerve crush injury. In vitro, EPO treatment promoted myelin formation and protected myelin from the effects of nitric oxide exposure in co-cultures of Schwann cells and dorsal root ganglion (DRG) neurons. In a novel, surgically applicable local treatment utilizing FDA-approved fibrin glue as a vehicle, EPO was as effective as systemic EPO administration at time points earlier than explainable using standard models of neuroregeneration.
Conclusions
In nerve-crush injury, EPO may be exerting a primary influence on myelin status to promote functional recovery.
Keywords: erythropoietin, functional recovery, peripheral nerve injury, regeneration
INTRODUCTION
Peripheral nerve injuries are frequently due to acute traumatic compression or crushingy. There are several mechanisms by which theseinjuries cause functional impairment including a loss of axonal integrity, destruction to myelin and denervation of muscle. Loss of function is variably reversible and currently there are no available treatments that accelerate recovery from these injuries. Current management is complicated by difficulties in both the diagnosis and treatment of these disorders, with little ability to predict the injuries in which enhanced recovery is even possible. As functional return must outpace motor end plate degeneration in order to prevent irreversible loss of function, enhancing this process could potentially prevent negative outcomes and allow partial or complete recovery in many settings.
One potential opportunity to enhance recovery in peripheral nerve injury is through the administration of erythropoietin (EPO). Although most commonly used to stimulate erythropoiesis, multiple studies have delineated the therapeutic and beneficial effects of EPO administration in multiple types of injuries and tissues1-5. In addition, EPO application ameliorates the loss of nerve conduction velocity in diabetic rats6, and accelerates functional recovery after peripheral nerve injury7-9.
While the cellular and molecular mechanisms by which EPO promotes repair in peripheral nerve injury are poorly understood, the speed of recovery observed in our previous studies 8 indicates that recovery is not likely due to regeneration of transected axons alone. While axonal regeneration following transection occurs at a rate of approximately 1mm/day, we observed accelerated improvement in the deficits of injured nerves within 7 days following systemic EPO treatment.
The purpose of this study was to elucidate the mechanism by which EPO promotes neuroregeneration and functional recovery in acute nerve injuries. We hypothesized that EPO has direct effects on myelination and that these effects are mediated by interactions between EPO and local Schwann cells. Behavioral, immunohistochemical, and biochemical evaluations were employed in vivo and in vitro to examine this hypothesis. We also sought to develop a system for local EPO delivery that would be as efficacious as systemic administration. We hypothesized that local EPO administration would enhance recovery of nerve function comparable to that seen with systemic EPO treatment.
MATERIALS AND METHODS
Mouse Model of Peripheral Nerve Injury
The experimental design and all procedures were approved by the University Committee on Animal Resources at our institution. Ten-week-old female C57BL/6 mice (n=25, 20 – 25 g) were used in this study. All mice received a standardized sciatic nerve crush injury, as previously described8. Briefly, mice were anesthetized using ketamine (60 mg/kg) and xylazine (4 mg/kg). Both hindlimbs were shaved, washed with 70% ethanol, and prepped with providone-iodine (Betadine). A gluteal-splitting approach was used for exposure of the left sciatic nerve immediately distal to the sciatic notch and proximal to the trifurcation. A smooth tipped needle driver (Miltex, Plainsboro, NJ) was then placed around the nerve and closed to the second notch for a duration of 30 seconds to create the acute crush injury. A sham surgery was performed on the contralateral limb of each mouse to serve as control. The same technique was used to expose the right sciatic nerve, but no manipulation or injury of the nerve was performed. A multi-layer closure was completed with simple interrupted 5-0 nylon sutures.
All mice tolerated the procedure well and returned to free cage activity post-operatively with unrestricted access to food and water. Buprenorphine (0.05 mg/kg) was given for postoperative analgesia immediately following surgery and every 12 hours thereafter for 3 days, at which time the mice were no longer exhibiting signs of pain.
Experimental Design
Mice were randomized into one of five groups separated into two separate study arms based on route of drug administration, with two groups in the systemically treated arm and three groups in the local delivery arm. Ten mice were randomized to receive either systemic erythropoietin (5000 U/kg)(n=5) or normal saline (as a control)(n=5). Recombinant human EPO (PROCRIT; Amgen, Thousand Oaks, CA) was administered as a single intraperitoneal dose immediately following the surgical procedure, with the dosage of 5000 U/kg selected based on previous studies in animals and humans 1,6,8-10.
Local Delivery of EPO through Fibrin Glue Matrix
If EPO is acting directly on Schwann cells themselves in contrast to stimulating a systemic healing response, then localized delivery of EPO could offer a means of harnessing the potential benefits of this protein while reducing the risk of possible adverse effects of systemic administration 11,12. To assess EPO's potential to improve functional recovery when administered locally on impaired nerves, we utilized a clinically applicable, FDA-approved delivery material, a fibrin sealant (Tisseel; Baxter Healthcare Corp., Westlake Village, CA), as the vehicle for local drug delivery 13,14. Fifteen mice were randomized to receive either fibrin glue (FG) vehicle (n=5), FG vehicle + EPO (n=5), or FG + saline (n=5). EPO was diluted in saline and added to thrombin and procoagulant protein, the two components of fibrin glue. EPO (0.5 U/mL) dosage was chosen based on the conversion of a clinically relevant dose (5000 U/kg/week) using the calculated ratio of body weight to sciatic nerve weight to derive a dosage appropriate for local administration. The final product was comprised of these components in a ratio of 1:1:4, respectively, to make 10 μL matrix at 25°C for local delivery onto the sciatic nerve. Implantation of the FG vehicle (+/− EPO or saline) was completed peri-operatively following the acute crush injury to the nerve, as described earlier.
We performed an experiment to determine the rate of local drug release from the FG delivery system to verify this method of delivery. EPO (3000 IU) was added to 10uL of FG matrix and put into 1000uL of PBS solution. The amount of EPO released into the solution was measured using an Erythropoietin (EPO) Human ELISA Kit (ab119522; Abcam, Cambridge, MA). Measurements were taken on days 1, 2, 4, and 6.
Sciatic Function Index (SFI) Determined by Walking Track Analysis
Walking track gait analysis was utilized for the assessment of motor functional recovery. This model has been described in detail previously 15-17. Briefly, the mice were allowed to walk freely down a 50-cm long corridor that was lined with white paper. The hindpaws were individually painted with ink (blue for the normal limb and black for the experimental limb), allowing footprints to be recorded on the white paper lining the floor of the walking track. Each mouse walked until clear print marks during a walking gait were obtained. (Two or three attempts were often required to obtain usable prints.)
Walking tracks were measured by two blinded observers only after randomization of all completed sample data to ensure proper blinding. Inked footprints were selected for measurement based on the clarity and morphology of the paw and anatomical structures. Three footprints were selected per limb and individually measured with digital calipers. Three parameters were obtained in both limbs for each mouse: (1) toe spread (TS) (first through fifth toes), (2) print length (PL), and (3) intermediate toe spread (IT) (second, third, and fourth toes). To assess gait, the SFI was calculated using the following formula:
where E represents the injured limb and N is the control limb, as in previous studies 17,18.
Walking track analysis was performed at days 1, 7, and 14 for the systemically treated groups (n=5 per group), and days 1, 2, 3, and 5 for the local delivery groups (n=5 per group). All walking tracks were measured and all parameters recorded in blinded fashion.
Primary Antibodies
The following primary antibodies were used: anti-myelin protein zero (P0), anti-neurofilament (NF) chicken monoclonal antibodies (Aves Labs, Tigard, OR); anti-myelin-basic protein (MBP) mouse monoclonal (Chemicon, Millipore, Billerica, MA); anti-Glutathione S-transferases (GST-Pi) monoclonal antibody (BD Biosciences, San Jose, CA); anti-gamma-Glutamylcysteine Synthetase (γ-GCS) rabbit polyclonal antibody (Abcam, Cambridge, MA); anti-thy1.1 (OX7) and anti-β-actin monoclonal antibodies (Santa Cruz Biotechnology, Dallas, TX); anti-fibronectin rabbit polyclonal antibody and rabbit complement (Sigma Aldrich, St. Louis, MO).
Immunohistochemistry and Morphometric Assessment
Sciatic nerves were harvested from contralateral and ipsilateral hindlimbs of mice at predetermined time-points (day 3 or day 7) following crush injury. All nerves were fixed in 4% paraformaldehyde solution for three hours and embedded in paraffin to evaluate cross sections. Slides were pretreated with 0.01 M citrate buffer (pH 6.0) for antigen retrieval. Nonspecific blocking was performed with 1:20 diluted goat serum. Sequential sectioned slides were incubated with anti-P0 antibody (1:1000 dilution) and anti-NF antibody (1:2000 dilution) separately in 2% goat serum overnight. Fluorescent secondary antibody incubation was performed after sections were washed in buffer to remove primary antibodies. Immunofluorescence was performed and antibodies to P0 and NF were used to evaluate and quantify myelination status and axonal integrity, respectively. Using fluorescent images captured with AxioVision software (Carl Zeiss Microscopy, Thornwood, NY), semi-automated analysis was performed with ImageJ (United States National Institutes of Health, Bethesda, MD, available at http://imagej.nih.gov/ij) to quantify P0 and NF19. Positive staining was first converted to a binary format for quantification, which was first verified by manual counts before the procedure was applied to all of the images. The areal fraction of fluorescent emission within the images was analyzed in a blinded fashion using semi-automated computational analysis.
Fifteen representative areas of 50 × 50 m from each section were chosen for the calculation of signal densities in a fashion consistent with other density quantification methods20,21. This method prevented inclusion of artifacts acquired during the nerve fixation process or areas of the image otherwise damaged, and controlled for variations in cross-sectional area of the sciatic nerve samples. The same image processing was performed on each image following the particle analysis procedure outlined by the user guide for ImageJ. Additional sections were also stained with hematoxylin-eosin to evaluate the nerve morphology with light microscopy.
Immunocytochemistry
Primary Schwann Cell Culture
Schwann cells were isolated from sciatic nerves (n=20) of 7-day-old Sprague-Dawley rats and prepared by modified Brockes’ method22,23. Cells were incubated on Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and treated with 5 μM anti-mitotic cytosine-β-D-arabinofuranoside (Ara-C) for 3 days to eliminate dividing fibroblasts. Complement killing using anti-Thy1.1 antibodies and rabbit complement was performed to further eliminate fibroblasts resulting in a typical yield of 106 purified Schwann cells 22. Purified Schwann cells were maintained in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 21 μg/ml bovine pituitary extract and incubated at 37°C, 5% CO2 24. Schwann cells were expanded in DMEM supplemented with forskolin (4 μM) to yield ~ 5 × 106 purifie Schwann cells for primary and co-cultures.
Dorsal Root Ganglia (DRG) Neuron Cell Culture
DRG neurons were isolated from 18 day-old embryos (E18) of Sprague-Dawley rats and seeded within laminin/poly-L-lysine-coated wells (48 wells/plate). Neuronal cultures were maintained in neurobasal media (Gibco, Life Technologies, Carlsbad, CA) supplemented with B27-containing antioxidants, 50 ng/mL NGF, and 1 μM Ara-C. Ara-C (1 μM) treatment was performed three times (for two days each time) following two days of fresh medium without Ara-C to eliminate fibroblasts. After two weeks, DRG neurons with extensive axons were seeded with Schwann cells for myelination analysis 25.
DRG Neuron and Schwann Cell Myelinating Co-cultures
30,000 Schwann cells were added to each well holding 5,000 DRG neurons. Co-cultures were maintained in differentiation medium (DMEM/F12 (1:1), 50 ng/mL NGF, N2 supplement) for one week. Co-cultures were then changed to myelination medium (MEM supplemented with 50 ng/mL NGF, 50 μg/mL ascorbic acid, 10% FBS) for two weeks. Medium was changed every two days 25,26.
For the investigations performed with Schwann cells and Schwann cell/DRG co-cultures, each experimental condition was done in triplicate wells and all cell culture experiments were repeated 4 times on independent days. Overall, one plate was created with primary Schwann cell culture (30,000 Schwann cells/well in 48 wells/plate) and two plates were created with DRG Neuron-Schwann cell co-cultures (5,000 DRG neurons + 30,000 Schwann cells co-cultured per well in 48 wells/plate) for use in the relevant investigations.
EPO Administration
To determine whether EPO exposure might alter Schwann cell function in a manner potentially relevant to our in vivo results, we examined effects of EPO on cultures of pure Schwann cells and on co-cultures of Schwann cells with DRG neurons in conditions that induce myelination. Co-cultures of Schwann cells and DRG neurons were exposed to various concentrations of EPO (1, 10, 100 U/mL of EPO and 0 U/mL for a control). EPO was administered at the beginning of the myelination induction phase, which correlated to three pre-determined time points: day 5 of differentiating stage and days 1 and 3 of the myelinating stage (corresponding to day 5, 7, 9 of evaluation).
As NO exposure is known to contribute to initiation and progression of demyelination and neurodegeneration in both CNS and PNS 23,27,28, we sought to examine EPO's potential in modifying these NO-induced injurious effects to myelin and related structures in vitro. Although it has proved difficult to determine the absolute levels of NO in vivo29-30, some studies have characterized the concentrations of NO anticipated to be present at a site of inflammation (~ 1 – 10 uM)30-33. These micromolar concentrations of NO are orders of magnitude higher than those within the physiological range and demonstrate similar findings of conduction block and axonal degeneration without significant cell death in both in vivo31,34 and in vitro23,32,35 studies. Preliminary tests with different experimental concentrations of the NO donor, S-Nitroso-N-acetyl-DL-penicillamine (SNAP), done in our lab found that 100uM is the most suitable concentration of SNAP to create the pathological state and NO-rich environment expected in vivo. Primary cultures of myelinating Schwann cells were also exposed to SNAP in the presence or absence of EPO (100 U/ml) to evaluate EPO's potential to overcome the NO-driven myelin inhibition. Wells holding saline-treated cells were created to serve as controls in experiments quantifying myelin density.
Western Blot
The cell culture samples were collected and lysed in RAPI buffer. Samples were resolved on SDS-PAGE gels and transferred to PVDF membranes (PerkinElmer Life Science, Wellesley, MA). After being blocked in 5% bovine serum albumin in PBS containing 0.1% Tween 20, membranes were incubated with a primary antibody, followed by incubation with a HRP-conjugated secondary antibody (Santa Cruz Biotechnology, Inc., Dallas, TX). Membranes were visualized using Western Blotting Luminol Reagent (Santa Cruz Biotechnology, Inc., Dallas, TX) and imaging system. Primary antibodies were used to detect Glutathione S-transferases (GST-Pi) and gamma-Glutamylcysteine Synthetase (γ-GCS), markers of cellular antioxidant and detoxification capabilities. Anti-β-actin was used for as internal control.
Myelin Quantification
To quantify the extent of myelination in Schwann cell cultures and co-cultures, the total number of MBP+ myelin segments were counted in each whole culture well and normalized by the total cell number (DAPI+). Cell nuclei were stained with DAPI. These MBP+ myelin segments were labelled and counted manually with computer guided-assistance using the ImageJ software (NIH).
Statistical Analysis
The sample size of mice needed for the study was determined based on a standard power analysis. With a significance level (α) set to 0.05 and the power (β) set to 0.8, we found that 5 animals per treatment group would be sufficiently powerful to perform our desired analysis. An equal number of samples and data points were obtained per group and time point in each of the experiments throughout our study. All data are expressed as mean ± SEM and were analyzed using the unpaired Student t-test. Differences were considered significant if P<0.05, with P<0.01 noted if present. All statistics were reviewed by an institution statistician to confirm appropriateness of analyses.
RESULTS
Systemic EPO Administration
SFI
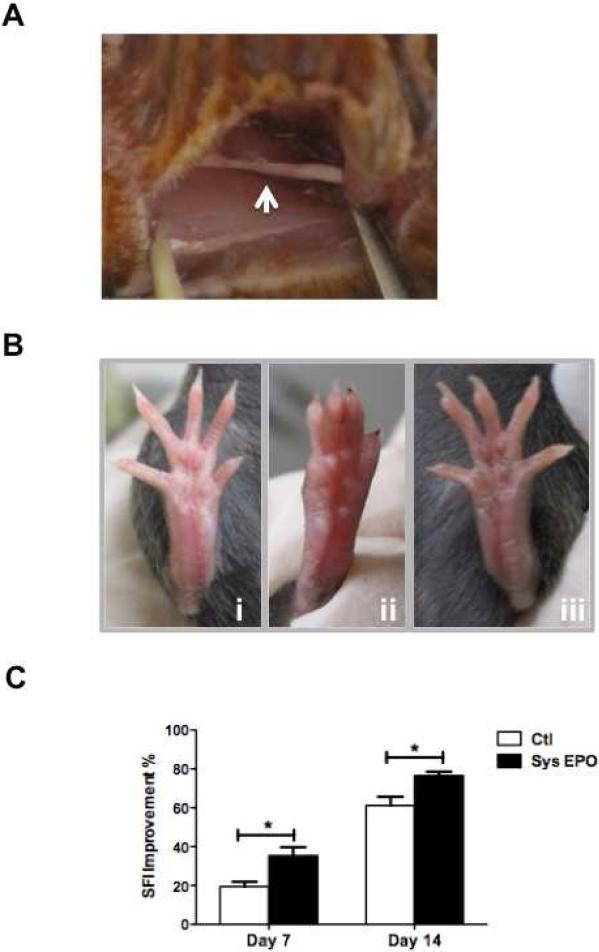
We confirmed our previous studies using crush injuries and found that recovery was enhanced by EPO administration. Systemic EPO administration accelerated functional recovery as measured by improvement in sciatic function index (SFI) at 7 and 14 days post-injury (P<0.05)(Figure 1C). Although the difference in SFI was not significant between groups when calculated 7 days after injury (P=0.08), the functional improvement observed on day 7 was greater in mice that received EPO post-operatively than those that received saline (P<0.05)(Table 1). On day 14 after injury, there was substantial improvement in both groups, but mice treated with EPO still showed a significantly greater recovery than untreated mice (P<0.05). Crushed nerves showed a reduced diameter at the point of crush (Figure 1A) while mice receiving these injuries showed a marked reduction in normal toe spread on day 7 as seen by visual inspection (Figure 1Bi-ii). In contrast, the toe spread on mice receiving EPO treatment was normal in its appearance (Figure 1Biii).
Figure 1.
EPO improves SFI in mice after crush injury. A Representative pictures of crushed sciatic nerve in mice. B i) Representative photographs of the foot position of the sham-surgery group, ii) saline-treated mouse, iii) and systemic EPO-treated mouse 7 days after the surgical crush injury. C Effect of systemic EPO administration on improvement in SFI days 7 and 14 following acute crush injury (N=5 per group). Improvement (%) measured as the percent change in SFI from post-injury day 1, a time point of maximum injury shown by worst value of SFI (−100 to −110). * P < 0.05.
Table 1.
Walking Track Analysis and Functional Recovery with Systemic EPO
| PID1 | PID7 | PID14 | |||
|---|---|---|---|---|---|
| GROUP | SFI | SFI | Improvement | SFI | Improvement |
| Saline | −106.06 ± 1.45 | −81.68 ± 0.88 | 23.0% | −41.31 ± 5.59 | 61.1% |
| EPO | −111.94 ± 3.09 | −72.25 ± 3.76 | 35.5% | −26.73 ± 2.55 | 76.1% |
| P-value | >0.05 | >0.05 | <0.05 | <0.05 | <0.05 |
PID = Post-injury day. SFI = Sciatic Function Index. EPO = Erythropoietin.
Histomorphometry
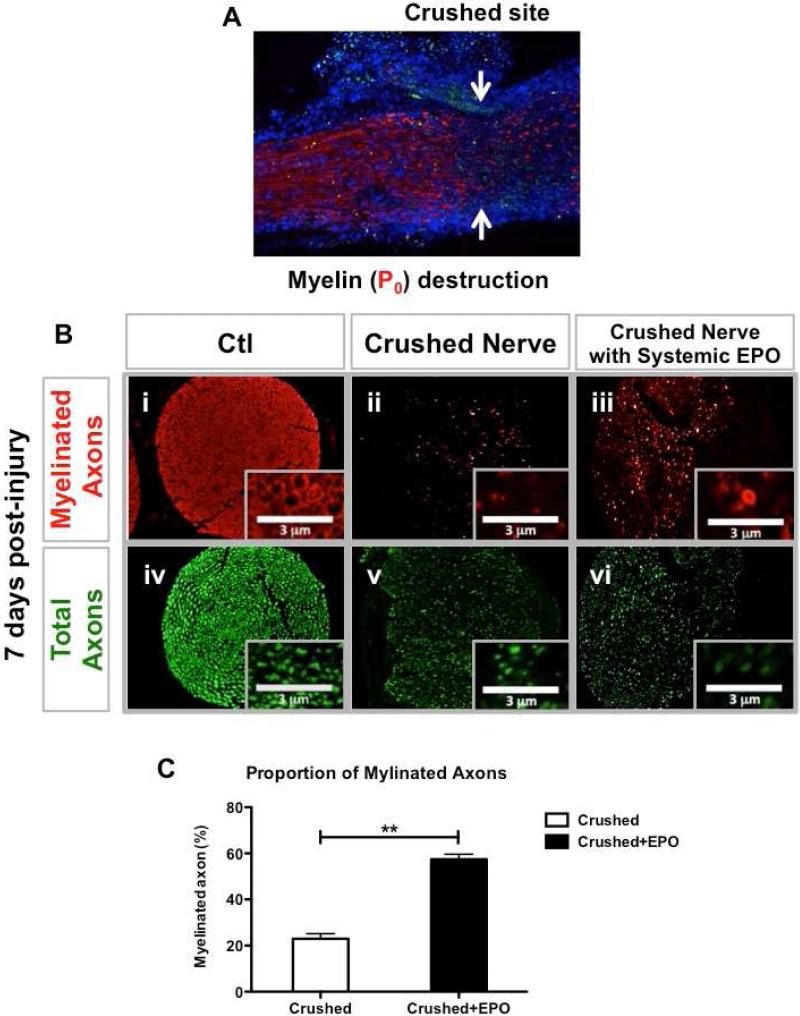
As the speed of recovery was too rapid to be accounted for by axonal regeneration alone (1 mm/day regrowth following Wallerian degeneration), we tested the hypothesis that EPO might be protecting or restoring myelination on spared axons, which could also restore function. There was a visually marked decrease in peripheral myelin at the site of crush injury (Figure 2A). Cytological analysis of sections cut at the level of injury are shown in Figure 2B. There were comparable reductions in the total number of axons in both treated and untreated nerves (P>0.05), but a greater number of these axons were myelinated in the EPO-treated mice (Table 2). In control mice, only 22.95 +/− 2.19% of surviving axons (defined by neurofilament expression) were myelinated, while 57.40 +/− 2.26% of the axons were myelinated in mice treated with EPO (P<0.01) (Figure 2C).
Figure 2.
EPO supported preservation of myelin within crushed nerve. A Labeling with anti-P0 antibodies reveals loss of myelin at the lesion site 7 days post-injury. (Red: P0; Blue: DPAI) B Analysis with anti-P0 (red; i-iii) and anti-NF (green; iv-vi) antibodies reveals that crush injury is associated with a reduction in numbers of myelinated axons (ii versus iii) seen in cross sections of sham control, crushed and crushed + EPO sciatic nerves 7 days post-injury. Representative images of the cross sections of the sciatic nerves (i, iv: healthy nerve; ii, iii, v, vi: crushed nerve). C EPO administration causes an ~3-fold increase in the percentage of NF-expressing axons surrounded by P0-expressing Schwann cells (N=3 per group). ** P < 0.01.
Table 2.
Histomorphometric Results
| Saline | EPO | P-value | ||||
|---|---|---|---|---|---|---|
| AXON | Sham | Crush | Sham | Crush | Sham | Crush |
| Myelinated (#) | 2337.50 ± 22.50 | 696.00 ± 86.51 | 2437.67 ± 93.24 | 1783.67 ± 15.51 | 0.40 | <0.001 |
| Total(#) | 2965.00 ± 229.00 | 3017.67 ± 83.38 | 3435.67 ± 373.73 | 3118.67 ± 142.59 | 0.36 | 0.57 |
| Ratio (P0/NF) | 79.25 ± 5.36 | 22.95 ± 2.19 | 72.06 ± 5.09 | 57.40 ± 2.26 | 0.41 | <0.001 |
P0 = Protein 0. NF = Neurofilament. EPO = Erythropoietin.
Local Delivery of EPO
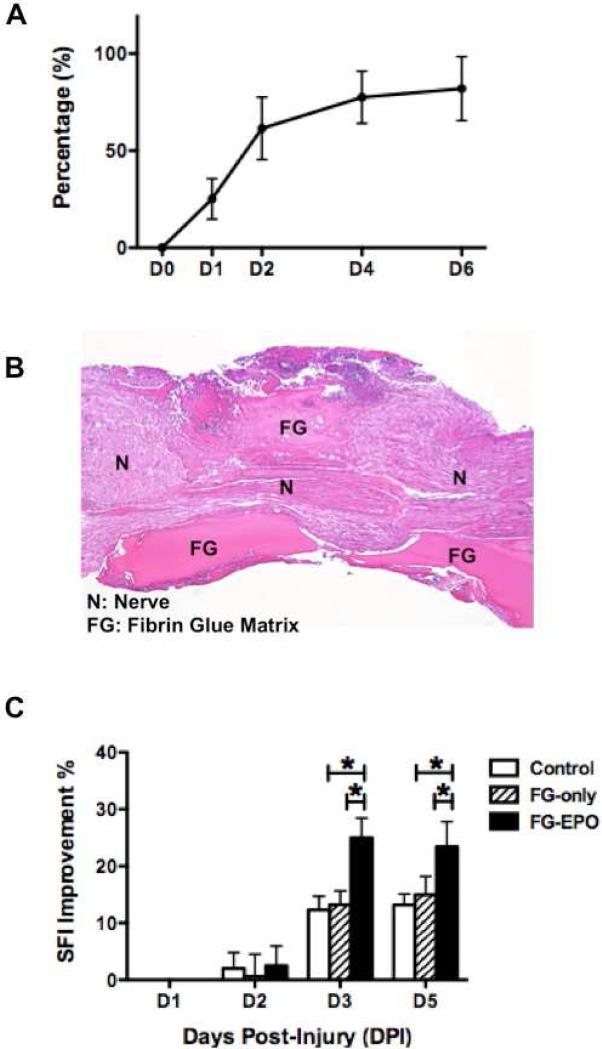
When placed directly on the nerve immediately following crush injury, extended release of the drug occurs with 90% of the EPO released from the material over a period of 6 days, as shown by in vitro pharmacokinetic analysis (Figure 3A). Mice treated by localized EPO delivery in fibrin glue immediately after injury showed enhanced functional recovery of crushed nerves when compared to control-treated mice (Table 3), which mimics that observed with systemic EPO treatment. Saline- and fibrin glue vehicle-treated control groups did not differ in SFI or percent recovery over the course of 5 days (P>0.05). However, local EPO-treated animals showed significantly greater functional improvements in SFI than either control groups at day 3 and day 5 post-injury (P<0.05)(Figure 3C).
Figure 3.
Local EPO treatment via fibrin glue vehicle improves motor function. A EPO is released from fibrin glue vehicle over a several day period in vitro (N=5 per group), shown as the percentage of EPO released from the local drug delivery system into PBS solution over time (concentration of EPO in PBS/concentration of EPO in fibrin glue vehicle x 100%). B Photomicrograph of crushed sciatic nerve to which fibrin glue matrix was applied, visualized with hematoxalin-eosin staining of nerve section as seen 3 days after surgery. C 0.5 U EPO-loaded fibrin glue implant improved SFI significantly over the course of 5 days when compared to saline or vehicle-only groups (N=5 per group). * P < 0.05.
Table 3.
Walking Track Analysis and Functional Recovery with Local Delivery of EPO
| PID1 | PID2 | PID3 | PID5 | ||||
|---|---|---|---|---|---|---|---|
| GROUP | SFI | SFI | Improvement | SFI | Improvement | SFI | Improvement |
| Control | −106.06 ± 1.30 | −103.90 ± 4.16 | 2.03 ± 2.79% | −92.88 ± 3.28 | 12.33 ± 2.39% | −92.16 ± 3.57 | 13.19 ± 1.90% |
| FG Only | −102.47 ± 1.72 | −104.19 ± 2.84 | 0.65 ± 3.86 % | −96.39 ± 4.58 | 13.21 ± 2.42 % | −87.10 ± 3.23 | 14.97 ± 3.24 % |
| FG-EPO | −102.76 ± 2.38 | −99.95 ± 4.11 | 2.51 ± 3.48 % | −77.04 ± 3.37 | 24.97 ± 3.46 % | −78.43 ± 3.64 | 23.46 ± 4.35 % |
| P-value | >0.05 | >0.05 | >0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
PID = Post-injury day. SFI = Sciatic function index. FG = Fibrin Glue. EPO = Erythropoietin.
In Vitro Studies
Immunohistochemical staining
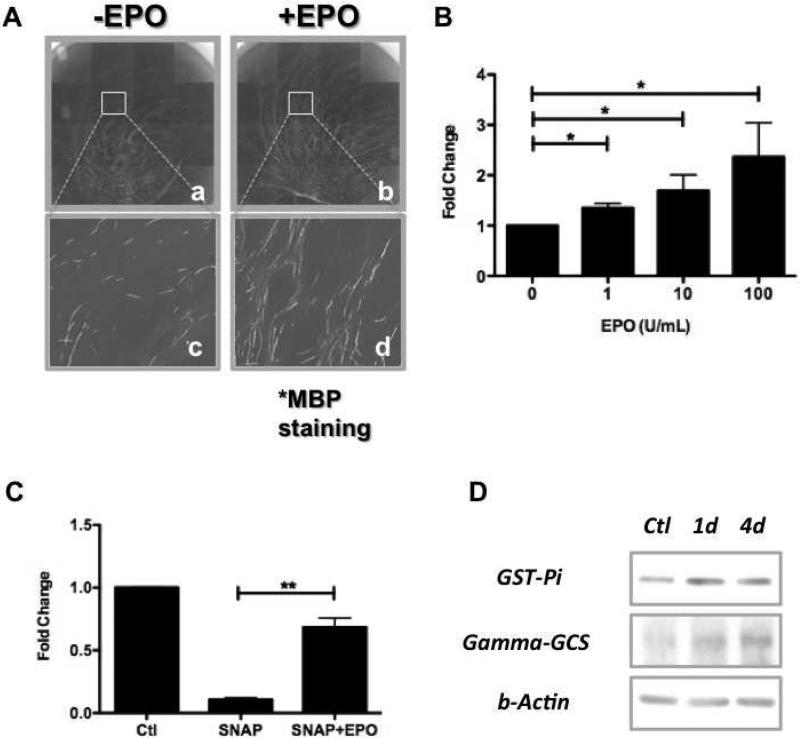
In Schwann cell-DRG neuron co-cultures exposed to various concentrations of EPO (1, 10, 100 U/mL), we found EPO exposure increased the total number of MBP-expressing Schwann cells in a dose-dependent manner (P<0.05) (Figure 4B). The baseline number of MBP+ Schwann cells that arose in pro-myelinating conditions was almost eradicated in cultures when exposed to 100 μM SNAP (although it did not cause a concomitant increase in cell death). This impact of SNAP exposure was minimized to almost negligible levels if cultures were also exposed to 100 U/ml of EPO (P<0.01)(Figure 4C). In addition, exposure of purified Schwann cell cultures to EPO caused increases in levels of GST-Pi and γ-GCS, as seen by Western Blot (Figure 4D), suggesting a direct effect of EPO on these cells in respect to expression of enzymes relevant to protection from oxidative stress, a known effect of exposure to NO 36,37.
Figure 4.
EPO exposure enhances Schwann cell myelination and protects against NO-induced reductions in myelination in vitro. A Immunocytochemical staining of Schwann cell/DRG neuron co-cultures with anti-MBP antibodies shows a marked increase in MBP+ Schwann cells. B Quantitative analysis of the number of MBP-expressing Schwann cells in the presence of different concentrations of EPO shows a dose-dependent increase (N=4 per group). * P < 0.05. C Exposure to 100 μM of SNAP during the myelination-induction process almost eliminates Schwann cell expression of MBP, whereas this SNAP-induced inhibition can be rescued by treatment with 100 U/mL EPO (N=4 per group). ** P < 0.01. D Levels of glutathione S-transferase (GST-Pi) and gamma-glutamylcycteine synthetase (gamma-GCS) are increased in Schwann cells exposed to 100 U/mL EPO, as detected by Western blot analysis.
DISCUSSION
We and others previously found that systemic EPO treatment promotes functional recovery after peripheral nerve injuries 8,38, and diabetic neuropathy 6. Initially, we repeated our previous observation that systemic administration of EPO can accelerate motor function measured by sciatic function index following acute nerve crush8. Improvement can be observed within several days after EPO administration, a time course too rapid to be accounted for by axonal regeneration. Our present studies suggest that this benefit may be mediated by preservation and/or promotion of Schwann cell myelination. Importantly, we demonstrated that EPO doesn’t necessarily require systemic administration to produce these neuroregenerative effects. Instead, localized EPO delivery may provide comparable results and a safer means of harnessing these benefits for eventual therapeutic application.
Previous studies have shown that EPO prevents apoptosis in neurons and glial cells in both CNS and PNS 39,40 and is neuroprotective in a wide variety of injury paradigms 41,42. Our current study suggests that EPO may also promote Schwann cell myelination. The marked increase we observed in the proportion of axons that are myelinated 7 days after crush injury in EPO-treated mice could be due to preservation of myelin and/or to promotion of remyelination after injury. The fact that EPO also enhances recovery even if administration is delayed for a week after injury 8 suggests that there are effects that extend beyond the prevention of apoptosis and preservation of myelin immediately after injury. It has been suggested that EPO also exerts neuroprotective effects to prevent axonal degeneration39,43 as well as regenerative effects to promote neurite sprouting and regrowth to restore axonal continuity9,44-46.
The success of localized EPO administration was particularly important in considering how we might best apply these findings in the clinical arena. EPO has a number of undesirable side effects that have been described, including thrombosis, hypertension, and decrease of survival rate of cancer patients 2,11. The severity of these side effects is such that systemic EPO therapy may not be a clinically viable option for treatment of peripheral nerve injuries in certain patients and clinical settings. Interestingly, we were able to obtain benefits similar to those seen with systemic delivery by using an FDA-approved fibrin glue matrix loaded with EPO and implanted at the injury site. This novel approach of promoting recovery from peripheral nerve injury may help facilitate the transition of EPO's neuroregenerative effects into benefits that are clinically relevant and usable.
Previous studies have reported that EPO can also protect against oxidative stress 47-49, which can be induced by NO exposure following injury 36,37. Our findings from in vitro studies agree with this neuroprotective role of EPO. We found that EPO protected against adverse effects of NO production and caused increased expression in levels of enzymes relevant to protection against oxidative stress.
Although neurons and Schwann cells express EPO receptors 24,50,51, it has not been clear whether beneficial effects of EPO administration are due to effects locally at the lesion site or whether systemic effects of EPO are required in order to improve functional recovery. We found that EPO administration promotes MBP expression in Schwann cell-DRG co-cultures and that localized slow-release delivery of EPO provides equivalent levels of benefit as systemic delivery. Taken together, these findings suggest that local effects on cells are important in the mechanism by which EPO administration confers benefit.
The extent of the motor improvement observed is of considerable potential clinical relevance. The ability to promote more rapid recovery after peripheral nerve injury is important because nerve recovery must outpace the degradation of the denervated motor endplate 52. This interplay between a recovering nerve and the time envelope for a significant recovery is an even greater concern in the elderly where little recovery is often seen after complex nerve injury. This situation is further complicated by the difficulty in distinguishing partial and complete nerve injuries as both leave patients without any function, creating a diagnostic dilemma without operative exploration and assessment. The improved function associated with localized EPO administration in the period following injury is therefore of potential importance in improving outcomes after nerve crush injury.
There are limitations of this study that should be noted. First, we did not perform any testing to assess the extent of systemic absorption with our local delivery system. As one of the main benefits of local administration of EPO may be minimizing the deleterious side effects that commonly occur with systemic therapy, it is imperative that any systemic impact of local EPO be defined and quantified. We do believe that the local EPO described in this study would likely result in negligible systemic effects as the dose of EPO used in the local delivery system is markedly less than that given systemically. Any concentration of EPO found in systemic circulation with the local delivery would be minimal and far less than that expected to produce measureable side effects. Second, our study was strictly evaluating acute traumatic nerve injuries, which are less common than chronic nerve compression (CNC) injuries. As CNC injuries are due to local damage to myelin with relative preservation of axons, we believe that EPO would likely provide comparable therapeutic effects in this chronic setting if we extrapolate our current findings to any situation of myelin damage. This is something worthy of further investigation, which is currently being undertaken in our lab. Last, local concentration of NO in the vicinity of peripheral nerve is currently undetermined in both physiologic and pathologic states. Lack of a reproducible and standardized method of quantification leaves this as a topic of interest with no consistent findings in the literature to date. We were unable to find any literature on the predicted [NO] in pathologic situations of peripheral nerve injury, as most of the investigations have been revolved around CNS pathology or inflammatory neuropathies, such as seen in systemic conditions. This is an area that would be useful to investigate further to elucidate a value of [NO] that could be used as a reference standard.
In summary, our results suggest that the functional benefit of EPO administration in peripheral nerve crush injury is due to Schwann cell re-myelination. Based on our current findings that localized EPO administration is sufficient to generate beneficial outcomes after nerve crush injuries, we provide a possible new avenue of using EPO therapeutically by avoiding the several risks that have been associated with systemic EPO administration 2,11.
Clinical Relevance.
Mixed injury to myelin and axons may allow the opportunity for the repurposing of EPO for use as a myeloprotective agent where injuries spare a requisite number of axons to allow early functional recovery.
Acknowledgements
This work was supported by National Institutes of Health (NIH) K08 Clinical Investigator Award number 1 K08 AR060164-01A. In addition, the work was supported by the Hand Surgeon Scientist Award from the American Society for Surgery of the Hand and startup funding from The University of Rochester. We would also like to thank Marylou Milazzo for help in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests
REFERENCES
- 1.Juul S. Erythropoietin in the central nervous system, and its use to prevent hypoxic-ischemic brain damage. Acta Paediatr. Suppl. 2002;91(438):36–42. doi: 10.1111/j.1651-2227.2002.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith KJ, Bleyer AJ, Little WC, Sane DC. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003;59(3):538–548. doi: 10.1016/s0008-6363(03)00468-1. [DOI] [PubMed] [Google Scholar]
- 3.Facio F, Jr., Burnett AL. Penile rehabilitation and neuromodulation. ScientificWorldJournal. 2009;9:652–664. doi: 10.1100/tsw.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho YK, Kim G, Park S, et al. Erythropoietin promotes oligodendrogenesis and myelin repair following lysolecithin-induced injury in spinal cord slice culture. Biochem Biophys Res Commun. 2012;417(2):753–759. doi: 10.1016/j.bbrc.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Gobe GC, Morais C, Vesey DA, Johnson DW. Use of high-dose erythropoietin for repair after injury: A comparison of outcomes in heart and kidney. J Nephropathol. 2013;2(3):154–165. doi: 10.12860/JNP.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchi R, Buyukakilli B, Brines M, et al. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci U S A. 2004;101(3):823–828. doi: 10.1073/pnas.0307823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campana WM, Myers RR. Exogenous erythropoietin protects against dorsal root ganglion apoptosis and pain following peripheral nerve injury. Eur J Neurosci. 2003;18(6):1497–1506. doi: 10.1046/j.1460-9568.2003.02875.x. [DOI] [PubMed] [Google Scholar]
- 8.Elfar JC, Jacobson JA, Puzas JE, Rosier RN, Zuscik MJ. Erythropoietin accelerates functional recovery after peripheral nerve injury. J Bone Joint Surg Am. 2008;90(8):1644–1653. doi: 10.2106/JBJS.G.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin ZS, Zhang H, Bo W, Gao W. Erythropoietin promotes functional recovery and enhances nerve regeneration after peripheral nerve injury in rats. AJNR Am J Neuroradiol. 2010;31(3):509–515. doi: 10.3174/ajnr.A1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Kordi A, Radyushkin K, Ehrenreich H. Erythropoietin improves operant conditioning and stability of cognitive performance in mice. BMC Biol. 2009;7:37. doi: 10.1186/1741-7007-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khuri FR. Weighing the hazards of erythropoiesis stimulation in patients with cancer. N. Engl. J. Med. 2007;356(24):2445–2448. doi: 10.1056/NEJMp078101. [DOI] [PubMed] [Google Scholar]
- 12.Berardi D, Agati L. Cardiovascular adverse reactions after the administration of recombinant human erythropoietin: light and shade. Minerva Cardioangiol. 2012;60(2):227–236. [PubMed] [Google Scholar]
- 13.Spicer PP, Mikos AG. Fibrin glue as a drug delivery system. J. Control. Release. 2010;148(1):49–55. doi: 10.1016/j.jconrel.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stendel R, Scheurer L, Schlatterer K, Gminski R, Mohler H. Taurolidine-Fibrin-Sealant-Matrix using spray application for local treatment of brain tumors. Anticancer research. 2004;24(2B):631–638. [PubMed] [Google Scholar]
- 15.de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77(3):634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- 16.Inserra MM, Bloch DA, Terris DJ. Functional indices for sciatic, peroneal, and posterior tibial nerve lesions in the mouse. Microsurgery. 1998;18(2):119–124. doi: 10.1002/(sici)1098-2752(1998)18:2<119::aid-micr10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83(1):129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Gladman SJ, Huang W, Lim SN, et al. Improved outcome after peripheral nerve injury in mice with increased levels of endogenous omega-3 polyunsaturated fatty acids. J. Neurosci. 2012;32(2):563–571. doi: 10.1523/JNEUROSCI.3371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geuna S. The revolution of counting “tops”: two decades of the disector principle in morphological research. Microsc Res Tech. 2005;66(5):270–274. doi: 10.1002/jemt.20167. [DOI] [PubMed] [Google Scholar]
- 21.Geuna S, Tos P, Battiston B, Guglielmone R. Verification of the two-dimensional disector, a method for the unbiased estimation of density and number of myelinated nerve fibers in peripheral nerves. Ann Anat. 2000;182(1):23–34. doi: 10.1016/S0940-9602(00)80117-X. [DOI] [PubMed] [Google Scholar]
- 22.Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165(1):105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann HC, Kohne A, Meyer zu Horste G, et al. Role of nitric oxide as mediator of nerve injury in inflammatory neuropathies. J Neuropathol Exp Neurol. 2007;66(4):305–312. doi: 10.1097/nen.0b013e3180408daa. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Gonias SL, Campana WM. Schwann cells express erythropoietin receptor and represent a major target for Epo in peripheral nerve injury. Glia. 2005;51(4):254–265. doi: 10.1002/glia.20202. [DOI] [PubMed] [Google Scholar]
- 25.Syed N, Reddy K, Yang DP, et al. Soluble neuregulin-1 has bifunctional, concentration-dependent effects on Schwann cell myelination. J. Neurosci. 2010;30(17):6122–6131. doi: 10.1523/JNEUROSCI.1681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banker G, Goslin K. Culturing nerve cells. 2nd ed. MIT Press; Cambridge, Mass: 1998. [Google Scholar]
- 27.Bizzozero OA, DeJesus G, Howard TA. Exposure of rat optic nerves to nitric oxide causes protein S-nitrosation and myelin decompaction. Neurochemical research. 2004;29(9):1675–1685. doi: 10.1023/b:nere.0000035802.27087.16. [DOI] [PubMed] [Google Scholar]
- 28.Conti G, Rostami A, Scarpini E, et al. Inducible nitric oxide synthase (iNOS) in immune-mediated demyelination and Wallerian degeneration of the rat peripheral nervous system. Exp Neurol. 2004;187(2):350–358. doi: 10.1016/j.expneurol.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Hall CN, Garthwaite J. What is the real physiological NO concentration in vivo? Nitric Oxide. 2009;21(2):92–103. doi: 10.1016/j.niox.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9(1):69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redford EJ, Kapoor R, Smith KJ. Nitric oxide donors reversibly block axonal conduction: demyelinated axons are especially susceptible. Brain. 1997;120(Pt 12):2149–2157. doi: 10.1093/brain/120.12.2149. [DOI] [PubMed] [Google Scholar]
- 32.Shrager P, Custer AW, Kazarinova K, Rasband MN, Mattson D. Nerve conduction block by nitric oxide that is mediated by the axonal environment. J Neurophysiol. 1998;79(2):529–536. doi: 10.1152/jn.1998.79.2.529. [DOI] [PubMed] [Google Scholar]
- 33.Smith KJ, Kapoor R, Hall SM, Davies M. Electrically active axons degenerate when exposed to nitric oxide. Ann Neurol. 2001;49(4):470–476. [PubMed] [Google Scholar]
- 34.Sunico CR, Portillo F, Gonzalez-Forero D, Kasparov S, Moreno-Lopez B. Evidence for a detrimental role of nitric oxide synthesized by endothelial nitric oxide synthase after peripheral nerve injury. Neuroscience. 2008;157(1):40–51. doi: 10.1016/j.neuroscience.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Custer AW, Mattson D, Shrager P. Reversible conduction block of rat sciatic nerve by nitric oxide generating compounds (abstract) 1996:1793. Located at: Neurosci Abstr. [Google Scholar]
- 36.Rajasekaran M, Hellstrom WJ, Sikka SC. Nitric oxide induces oxidative stress and mediates cytotoxicity to human cavernosal cells in culture. J. Androl. 2001;22(1):34–39. [PubMed] [Google Scholar]
- 37.Zhao K, Huang Z, Lu H, Zhou J, Wei T. Induction of inducible nitric oxide synthase increases the production of reactive oxygen species in RAW264.7 macrophages. Biosci. Rep. 2010;30(4):233–241. doi: 10.1042/BSR20090048. [DOI] [PubMed] [Google Scholar]
- 38.Jehle T, Meschede W, Dersch R, Feltgen N, Bach M, Lagreze WA. [Erythropoietin protects retinal ganglion cells and visual function after ocular ischemia and optic nerve compression]. Ophthalmologe. 2010;107(4):347–353. doi: 10.1007/s00347-009-2030-1. [DOI] [PubMed] [Google Scholar]
- 39.Campana WM, Li X, Shubayev VI, Angert M, Cai K, Myers RR. Erythropoietin reduces Schwann cell TNF-alpha, Wallerian degeneration and pain-related behaviors after peripheral nerve injury. Eur J Neurosci. 2006;23(3):617–626. doi: 10.1111/j.1460-9568.2006.04606.x. [DOI] [PubMed] [Google Scholar]
- 40.Iwai M, Stetler RA, Xing J, et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke; a journal of cerebral circulation. 2010;41(5):1032–1037. doi: 10.1161/STROKEAHA.109.570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel NS, Nandra KK, Thiemermann C. Bench-to-bedside review: Erythropoietin and its derivatives as therapies in critical care. Crit. Care. 2012;16(4):229. doi: 10.1186/cc11315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H. Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best Pract Res Clin Anaesthesiol. 2010;24(4):573–594. doi: 10.1016/j.bpa.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Keswani SC, Buldanlioglu U, Fischer A, et al. A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann Neurol. 2004;56(6):815–826. doi: 10.1002/ana.20285. [DOI] [PubMed] [Google Scholar]
- 44.Ostrowski D, Ehrenreich H, Heinrich R. Erythropoietin promotes survival and regeneration of insect neurons in vivo and in vitro. Neuroscience. 2011;188:95–108. doi: 10.1016/j.neuroscience.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 45.Lykissas MG, Sakellariou E, Vekris MD, et al. Axonal regeneration stimulated by erythropoietin: an experimental study in rats. J Neurosci Methods. 2007;164(1):107–115. doi: 10.1016/j.jneumeth.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Berkingali N, Warnecke A, Gomes P, et al. Neurite outgrowth on cultured spiral ganglion neurons induced by erythropoietin. Hear Res. 2008;243(1-2):121–126. doi: 10.1016/j.heares.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Amer J, Dana M, Fibach E. The antioxidant effect of erythropoietin on thalassemic blood cells. Anemia. 2010;2010:978710. doi: 10.1155/2010/978710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Y, Shang Y, Sun S, Liu R. Antioxidant effect of erythropoietin on 1-methyl-4-phenylpyridinium-induced neurotoxicity in PC12 cells. Eur. J. Pharmacol. 2007;564(1-3):47–56. doi: 10.1016/j.ejphar.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Maiese K, Chong ZZ, Hou J, Shang YC. Erythropoietin and oxidative stress. Curr. Neurovasc. Res. 2008;5(2):125–142. doi: 10.2174/156720208784310231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campana WM, Myers RR. Erythropoietin and erythropoietin receptors in the peripheral nervous system: changes after nerve injury. FASEB J. 2001;15(10):1804–1806. doi: 10.1096/fj.00-0857fje. [DOI] [PubMed] [Google Scholar]
- 51.Grasso G, Sfacteria A, Passalacqua M, et al. Erythropoietin and erythropoietin receptor expression after experimental spinal cord injury encourages therapy by exogenous erythropoietin. Neurosurgery. 2005;56(4):821–827. doi: 10.1227/01.neu.0000156493.00904.7e. discussion 821-827. [DOI] [PubMed] [Google Scholar]
- 52.Kang JR, Zamorano DP, Gupta R. Limb salvage with major nerve injury: current management and future directions. J. Am. Acad. Orthop. Surg. 2011;19(Suppl 1):S28–34. doi: 10.5435/00124635-201102001-00006. [DOI] [PubMed] [Google Scholar]