Abstract
Visceral leishmaniasis (VL), caused by infection with the obligate intracellular protozoan parasite Leishmania infantum, is a fatal disease of dogs and humans. Protection against VL requires a T helper 1 (Th1) skewed CD4+ T response, but despite this knowledge, there are currently no approved-to-market vaccines for humans and only three veterinary-use vaccines globally. As VL progresses from asymptomatic to symptomatic, L. infantum–specific interferon gamma (IFNγ) driven- Th1 responses become dampened and a state of immune exhaustion established. T cell exhaustion and other immunoregulatory processes, starting during asymptomatic disease, are likely to hinder vaccine-induced responses if vaccine is administered to infected, but asymptomatic and seronegative, individuals. In this study we evaluated how immune exhaustion, shown previously by our group to worsen in concert with VL progression, effected the capacity of vaccine candidate antigen/toll-like receptor (TLR) agonist combinations to promote protective CD4+ T cell responses during progressive VL. In conjunction with Th1 responses, we also evaluated concomitant stimulation of immune-balanced IL-10 regulatory cytokine production by these vaccine products in progressive VL canine T cells. Vaccine antigen L111f in combination with TLR agonists significantly recovered CD4+ T cell IFN-γ intracellular production in T cells from asymptomatic VL dogs. Vaccine antigen NS with TLR agonists significantly recovered CD4+ T cell production in both endemic control and VL dogs. Combinations of TLR agonists and vaccine antigens overcame L. infantum induced cellular exhaustion, allowing robust Th1 CD4+ T cell responses from symptomatic dogs that previously had dampened responses to antigen alone. Antigen-agonist adjuvants can be utilized to promote more robust vaccine responses from infected hosts in endemic areas where vaccination of asymptomatic, L. infantum-infected animals is likely.
Keywords: Leishmania (L.) infantum, Toll-like receptor agonist, adjuvants, CD4+ T cells, cytokines
1. Introduction
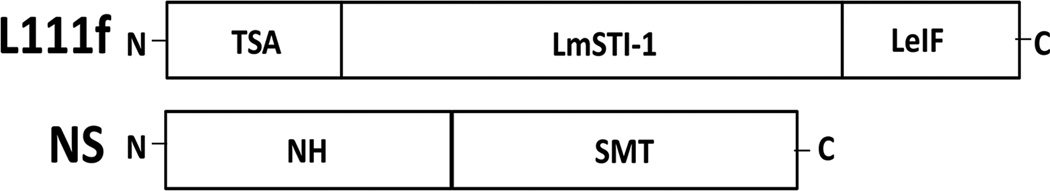
Leishmaniasis is a zoonotic disease that affects approximately 1.3 million individuals per year leading to 20–30,000 deaths [1, 2]. After malaria, Leishmania is the most common cause of parasite-induced mortality [3]. Visceral leishmaniasis (VL) is a severe disease form characterized by lymphadenopathy, splenomegaly and hepatomegaly that can be fatal without chemotherapy. Treatment is complicated by antimonial-resistant Leishmania strains [4], high drug toxicity [5], and treatment failure or relapse [6]. In North America and the Mediterranean basin, VL is caused by zoonotic L. infantum; both dogs and humans are affected. Vaccination as post-exposure immunoprophylaxis, or immunotherapy, in asymptomatic, infected individuals or animals would be an ideal and efficient method to mitigate the need for toxic, expensive, treatments and potentially minimize treatment challenges. Only two anti-Leishmania vaccines have been licensed in any country for use in humans and use to date has been limited. In Uzbekistan, a live parasite vaccine is used for prophylaxis [7]. In Brazil, a now discontinued vaccine, had been used for immune therapy [8]. L110f, L111f and KSAC are vaccine candidates currently in clinical trials and successfully promote a robust IFNγ immune response [9–14]. L111f along with a more recently developed subunit peptide vaccine candidate, NS, were used in this study to evaluate ex vivo responses in VL infected and non-infected dogs. L111f is a chimeric, three Leishmania gene fusion (TSA, LeIF and LmSTI1) [15]. NS is a two gene fusion of the Leishmania nucleoside hydrolase (NH) and a sterol 24-c-methyltransferase (SMT) genes (Fig. 1).
Fig. 1. Construction of anti-Leishmania vaccine antigens used for ex vivo stimulation of canine whole blood.
L111f is an 111kD fusion protein produced from a fusion of the L. major homologue of eukaryotic thiolspecific antioxidant (TSA), the L. major stress inducible protein-1 (LmSTI1) and the L. braziliensis elongation and initiation factor (LeIF) genes. NS is a 75kD fusion protein produced from the L. infantum nucleoside hydrolase (NH) and L. infantum sterol 24-c-methyltransferase (SMT) genes.
Dogs have similar VL disease progression to that of human patients from endemic areas, making them an ideal population to study vaccine responses [16, 17]. As VL is zoonotic, control of L. infantum infection and disease in dogs is critical for overall VL control. Two veterinary vaccines are currently available in Europe and Brazil, respectively. Vaccination of unowned dogs in endemic areas has been implemented using peptide formulations, DNA vaccination or modified-live parasites, with varying degrees of success [18–22].
While vaccine efficacy has been relatively high (~90%) when immunization has occurred in healthy, completely naïve, subjects, efficacy to prevent disease progression declined in infected, asymptomatic or symptomatic individuals [23]. Lower efficacy of vaccines in endemic settings has been observed in large field trials for malaria and tuberculosis vaccination, possibly due to the inclusion of asymptomatic infected individuals [24–26]. Control of Leishmania infection requires strong T helper 1 (Th1) responses marked by a robust CD4+ T cell proliferative response and IFNγ production. However, chronic Leishmania infection in murine models promotes regulatory responses involving production of classical CD25+, FoxP3+ regulatory T cells [27], IL-10 producing, regulatory, B cells [28] and immune exhaustion characterized by anti-inflammatory responses and an inability to proliferate in response to Leishmania-specific antigens [29]. In progressive VL, our data [29] and others [17] demonstrate that immune exhaustion starts prior to onset of clinical disease. Understanding how to stimulate an exhausted immune system to respond to vaccine antigens is essential for successful vaccination/immunotherapy of VL, and perhaps other infections, in endemic settings.
Our group has previously demonstrated that TLR agonist adjuvants protect against cutaneous leishmaniasis caused by L. major by recovering effector T cell responses during active infection in mice [10]. Different clinical presentations of Leishmania species lead to different responses to therapy. In these studies, we identify vaccine antigen/adjuvant pairings that mount a protective immune response ex vivo from naïve and L. infantum-infected dogs across the VL clinical spectrum.
2. Materials and Methods
2.1 Animals
A cohort of hunting dogs from the United States, naturally-infected with L. infantum and a long history of active VL surveillance was used in these studies [30]. Pen-matched dogs served as endemic controls. The University of Iowa Institutional Animal Care and Use Committee approved animal use, which ensure that the National Institutes of Health guide for the care and use of laboratory animals have been followed. Canine disease progression and VL classification was evaluated as in previous publications (Table 1) [16, 30, 31]. Dogs were examined by veterinarians for signs of leishmaniasis (lymphadenomegaly, palpable liver or spleen, poor hair coat, cachexia, epistaxis, arthrogryposis, alterations in hepatic or renal enzymes on serum chemistry). Dogs with >4 signs of disease were not included in this study. Parasite load was quantified by qPCR and immunofluorescence anti-Leishmania antibody testing (IFAT) as performed by the Center for Disease Control and Hygiene [32, 33]. Dogs were stratified into clinical groups: endemic controls (qPCR negative, 0 IFAT titer), asymptomatic (qPCR negative/borderline, >1/16 IFAT titer and ≤ 1/128), and symptomatic (qPCR positive/borderline, ≥1/256 IFAT titer, and ≥ 2 clinical signs) (Table 1).
Table 1.
Clinical groups by diagnostic status; qPCR and serology1
| Sex | Group (Infected) |
Group (Clinical) |
7/2013 IFAT2 |
7/2013 qPCR2 |
12/2013 IFAT3 |
12/2013 qPCR3 |
|---|---|---|---|---|---|---|
| F | Infected | Symptomatic | 512 | Positive | 256 | Positive |
| F | Infected | Symptomatic | 512 | Positive | 512 | Borderline |
| F | Infected | Symptomatic | 512 | Positive | 256 | Positive |
| M | Infected | Symptomatic | 128 | Positive | 512 | Positive |
| F | Infected | Symptomatic | 128 | Negative | 256 | Borderline |
| F | Infected | Symptomatic | 256 | Negative | 256 | Positive |
| F | Infected | Symptomatic | 256 | Negative | 512 | Borderline |
| M | Infected | Symptomatic | 0 | Positive | 512 | Positive |
| M | Infected | Asymptomatic | 128 | Negative | 32 | Negative |
| F | Infected | Asymptomatic | 64 | Negative | 16 | Negative |
| M | Infected | Asymptomatic | 128 | Negative | 64 | Negative |
| M | Infected | Asymptomatic | 0 | Negative | 128 | Negative |
| M | Infected | Asymptomatic | 32 | Negative | 16 | Negative |
| F | Infected | Asymptomatic | 16 | Negative | 0 | Borderline |
| F | Infected | Asymptomatic | 0 | Negative | 32 | Borderline |
| M | EC4 | Negative | 0 | Negative | 0 | Negative |
| F | EC | Negative | 0 | Negative | 0 | Negative |
| F | EC | Negative | 0 | Negative | 0 | Negative |
| F | EC | Negative | 0 | Negative | 0 | Negative |
| F | EC | Negative | 0 | Negative | 0 | Negative |
Serology by IFAT performed by the Centers for Disease Control, Atlanta, GA.
status 6 months prior to blood collection.
status at time of blood collection for vaccine antigen exposure.
EC- endemic control, pen-matched, dogs
2.2 Parasite DNA isolation, diagnostic qPCR, and IFAT serology
Parasite DNA isolation and qPCR was performed as previously described [29, 30, 34]. DNA was isolated from canine blood samples with the QIAamp DNA Blood Mini Kit per manufacturer’s instruction (QIAGEN, Valencia, CA). Ribosomal primer sequences F (5’-AAGCCACCCCAGAGGTAAAAA), and R (5’-GACGGGTCTGACCCTTGGTT) (Invitrogen, Life Technologies, Grand Island, NY), and probe: 5’ 6FAM-CGGTTCGGTGTGTGGCGCC-MGBNFQ (Applied Biosystems, Life Technologies, Grand Island, NY) were used as previously described [34]. Primers and probe were used at 10nM. Amplification was performed in duplicate using an ABI 7000 qPCR system (Applied Biosystems) with Super Mastermix (Rox) (Quanta Biosciences, Gaithersburg, MD) and cycling protocol as described previously [34]. Results were analyzed by ABI 7000 System SDS Software v1.2.3 (Applied Biosystems). Canine serum samples were stored at −20°C and sent to the Centers for Disease Control and Prevention for IFAT based on in vitro promastigote antigen culture as previously described [35, 36].
2.3 Stimulation of whole blood with vaccine antigens and TLR agonists ex vivo
Whole blood samples were collected as previously [30] and diluted 1:5 in RPMI. 150 µL of diluted whole blood was plated into 96 well plates. Vaccine antigens L111f [15] and NS [37] were added to wells at a final concentration of 1 µg/mL (Fig. 1). TLR agonists were added at final concentrations as described previously [10]: Glucopyranosyl Lipid Adjuvant (GLA) [TLR4; 1 µg/mL] [38], imiquimod (R837) [TLR7;10 µg/mL] [39], resiquimod (R848) [TLR7/8; 0.1 µg/mL] [39] (IDRI, Seattle, WA). Concanavalin A (ConA) (Sigma Aldrich, St. Louis, MO) [5 µg/mL] was used as a positive control mitogen. Media-only treated cells were used for baseline negative control. ConA treatment groups were cultured for 4 days; all other treatments were cultured for 7 days.
2.5 Flow cytometry
Flow cytometry was performed as previously described [40] and gating strategy demonstrated in Fig S1. Briefly, twenty-four hours prior to cell labeling, EdU (Life Technologies, Madison WI) was added to cells to assess proliferation per manufacturer’s directions [29]. Six hours prior to cell labling, 1× Brefeldin A (BD Biosciences, Franklin Lakes, NJ) was added to all cells. Cells were blocked with whole goat serum for 30 minutes, then labeled with anti-CD3 (CA17.2A12), CD4 (YKIX302.9), CD8 (YCATE55.9), (AbD Serotec/Bio-Rad, Hercules, California) IFNγ (142529), and IL-10 (138128) (R&D Systems, Minneapolis, MN) in 1× Perm/Wash Buffer (Life Technologies) per manufacturer’s directions. Cells were analyzed via an LSR II flow cytometer (BD Biosciences). 50,000 viable events were acquired and analyzed with FlowJo vX Software (Treestar, Ashland, CA).
2.6 SYBR Green Quantitative PCR and RNA extraction
SYBR green chemistry was utilized for qPCR assay using methods and primers as previously described [40, 41]. Briefly, the 2−ΔΔCT method was utilized for analysis. RNA was extracted using RNeasy extraction kit following manufacturer’s instruction (QIAGEN). RNA was quantified and standardized followed immediately by first strand cDNA synthesis via iScript cDNA Synthesis Kit (Bio-Rad). qPCR reactions were performed in duplicate.
2.7 Statistical Analysis
Differences between experimental group means were assessed using one-way ANOVA with Tukey’s post-hoc test via GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA). P values of ≤ 0.05 were considered statistically significant.
3. Results
3.1 CD4+ T cells from VL symptomatic dogs had reduced responses to vaccine antigens compared to those from asymptomatic dogs
Immune exhaustion, specifically a reduced ability to proliferate after exposure to antigen and reduced production of IFN-γ, was shown to occur in CD4+ and CD8+ T cells from dogs that had progressive VL [29]. CD4+ T cells are largely considered to be the predominant T cell subset for protective anti-Leishmania responses[42]; these cells were the primary focus of this analysis. Cells isolated from endemic control dogs, and VL asymptomatic and symptomatic-infected dogs were stimulated with a mitogen and Leishmania-specific vaccine antigens to determine if these cells exhibited signs of T cell exhaustion as demonstrated by quantitative PCR (Fig S2). The antigens used were L111f and NS. L111f has been safely administered and well-tolerated in subjects with and without evidence of prior Leishmania infection [15, 43, 44] and in combination with MPL-SE vaccine as an adjunct immunotherapy with standard chemotherapy for both cutaneous and mucocutaneous leishmaniasis [45]. NS was recently developed for vaccines targeting VL [37, 46], (Fig 1).
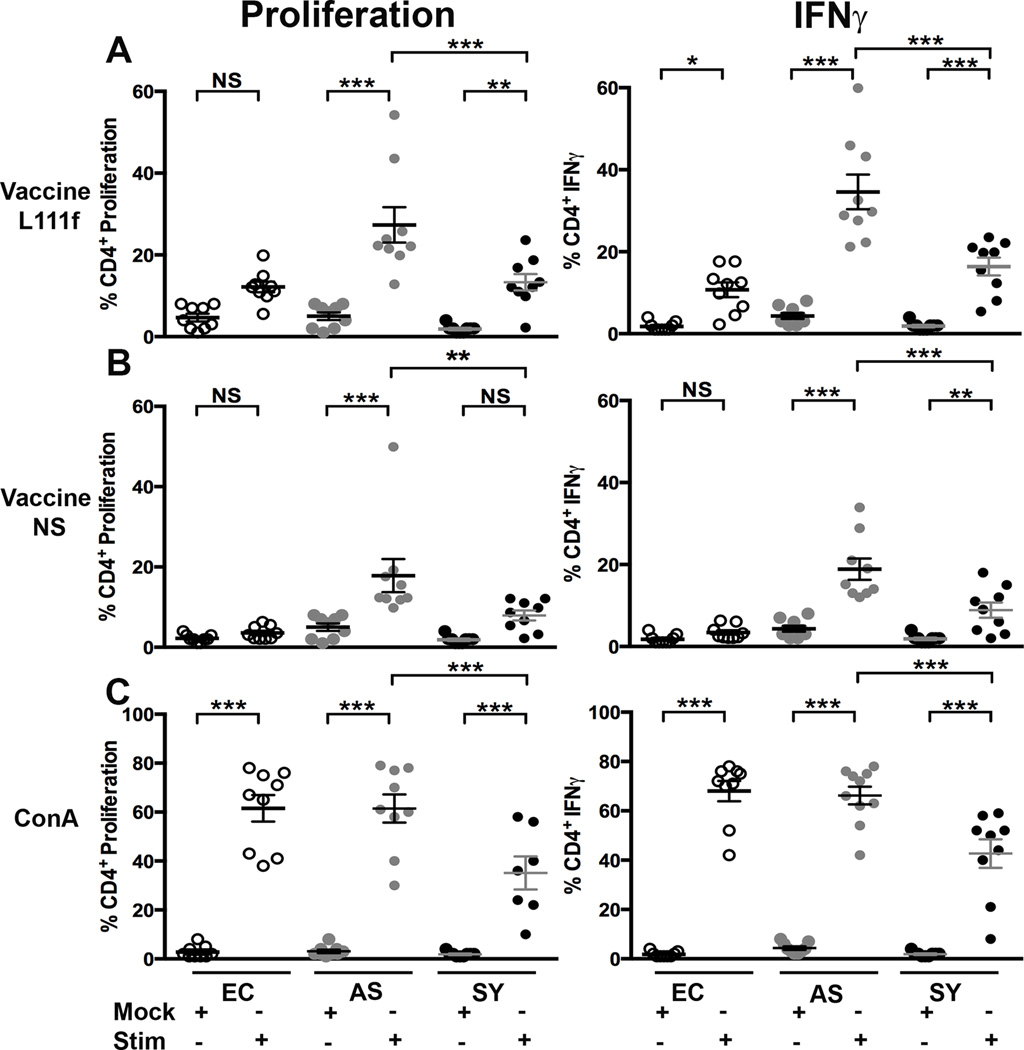
Exposure to the vaccine antigen L111f stimulated significant 5.4- and 7.1-fold increases in percent of CD4+ T cells undergoing proliferation from asymptomatic (p <0.001) and symptomatic (p <0.01) dogs, respectively compared to mock-treated cells. L111f exposure induced significant (p< 0.001) 8.0- and 8.7-fold increases in percentage of CD4+ T cells with intracellular IFNγ using the same comparisons as proliferation (Fig. 2A). NS stimulated less, yet still significant increase in percent CD4+ T cell proliferation; 3.6-fold (p <0.001) in cells from asymptomatic animals. Similarly there were significant increases in the percentage of CD4+ T cells producing IFNγ of 4.4- (p <0.001) and 4.7-fold (p <0.01) in samples from asymptomatic and symptomatic dogs (Fig. 2B). Similar trends were observed in vaccine antigen responses from CD8+ T cells by clinical group (Fig. S3).
Fig. 2. VL clinical status altered CD4+ T cell response to recombinant Leishmania protein vaccine stimulation ex vivo.
Whole blood from dogs across the VL clinical spectrum was stimulated ("stim") with 1 µg vaccine L111f (A) or 1 µg vaccine NS (B) or 1 µg ConA (C), or left untreated (“mock”). Each dot represents an individual dog. EC = endemic control (open), AS = asympatomatic, L. infantum positive (grey), SY = symptomatic, L. infantum positive (black). Error bars are ± SEM. N=7–10 per group. * p<0.05, **p<0.01, ***p<0.001 via one way-ANOVA.
CD4+ T cells from all groups responded significantly (p <0.001) to mitogen (ConA). IFNγ production levels also significantly (p <0.001) increased (Fig. 2C). The percent of CD4+ T cells positive for both IFNγ production and proliferation from VL-symptomatic dogs underwent significant (p <0.001), two-fold, reduction relative to populations of cells from asymptomatic dogs (Fig 2C). This likely indicates a global T cell exhaustion (Fig S2), rather than simply antigen-specific exhaustion, similar to previous observations [29, 30].
3.2 TLR agonist/L111f vaccine antigen modestly increased CD4+ T cell IFNγ responses
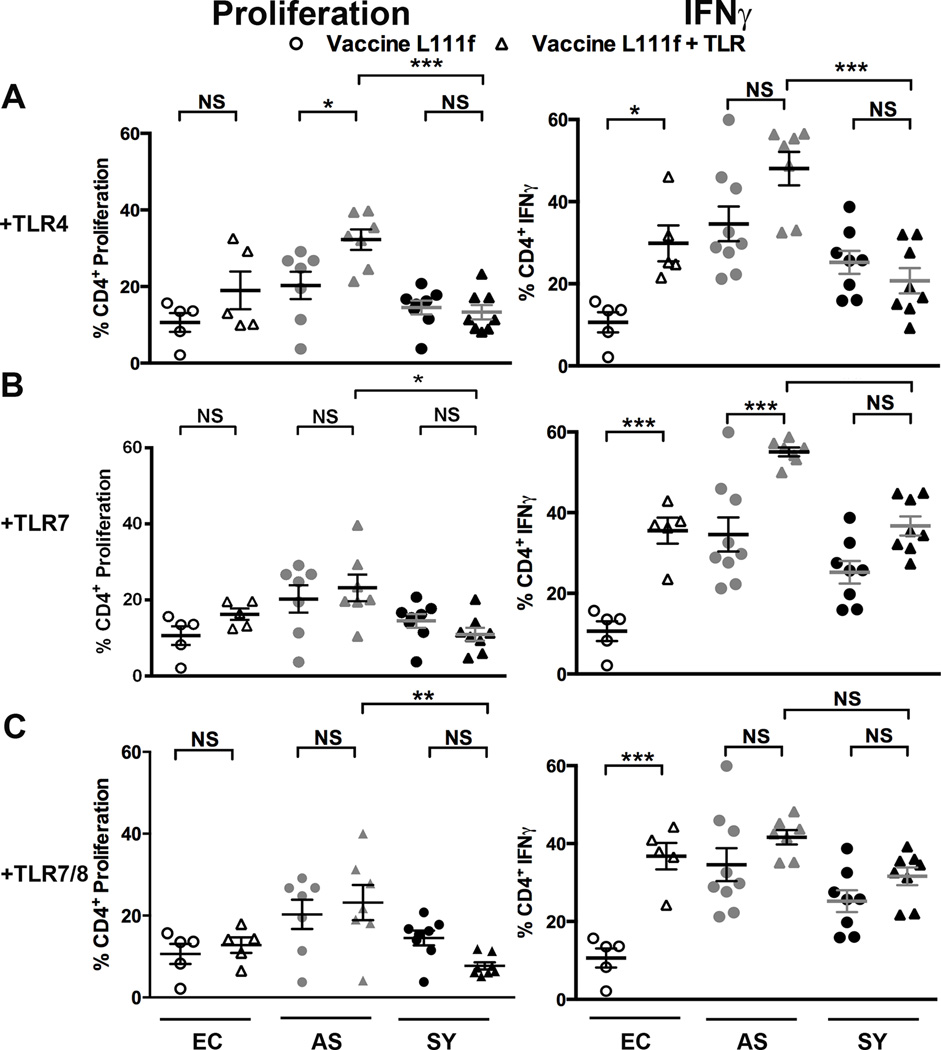
Vaccines incorporating multiple TLR agonists significantly stimulated murine CD4+ T cell IFNγ responses during experimental L. major infection. We examined if ex vivo incubation with Leishmania vaccine antigens and TLR agonists could recover effector functions from populations of exhausted symptomatic dog CD4+ T cells. Glucopyranosyl Lipid Adjuvant (TLR4 agonist) (triangles, Fig. 3A) with L111f modestly (p <0.05) increased CD4+ T cell proliferation 1.6-fold in VL-asymptomatic dogs and IFNγ responses 2.8-fold in CD4+ T cell populations from endemic control dogs, compared to L111f alone in cells from either clinical group (circles, Fig. 3A). Relative to L111f alone, this combination did not enhance recovery of exhausted CD4+ T cell function in VL-symptomatic dogs (Fig. 3A). Addition of imiquimod (TLR7 agonist) or resiquimod (TLR7/8 agonist) did not increase CD4+ T cell proliferation in CD4+ T cells from any clinical group, compared to treatment with L111f alone (Fig. 3B, C). Addition of TLR7 agonist to CD4+ T cells from endemic control dogs compared to cells exposed to L111f alone significantly increased (p <0.01) the percentage of CD4+ T cells producing IFNγ 3.5-fold (Fig. 3B). TLR7/8 agonist addition increased the percentage of endemic control CD4+ T cells producing IFNγ 1.6-fold (Fig. 3C). TLR agonists did not improve CD8+ T cell responses to L111f (Fig. S4). Addition of exogenous TLR agonists to L111f produced significant increases in CD4+ T cell responses from uninfected dogs, likely due to the TLR2 ligating potential of this vaccine antigen, but gave modest or no improvement in L111f-specific responses from cells of VL-asymptomatic or symptomatic dogs.
Fig. 3. Vaccine L111f and TLR agonists moderately increased Th1-responses in CD4+ T cells from asymptomatic dogs.
Whole blood was stimulated with either 1 µg Vaccine L111f (circles) or Vaccine L111f and TLR agonists (triangles). TLR agonists were added at 1µg for Glucopyranosyl Lipid Adjuvant [TLR4] (A), 0.1 µg for imiquimod [TLR7] (B) or 10 µg for resiquimod [TLR7/8] (C). Each dot represent an individual dog. EC = endemic control (open), AS = asympatomatic, L. infantum positive (grey), SY = symptomatic, L. infantum positive (black). Error bars are ± SEM. N=5–9 per group. * p<0.05, **p<0.01, ***p<0.001. Statistical analysis was performed via one way-ANOVA.
3.3 TLR agonists with NS significantly increased all CD4+ T cell responses
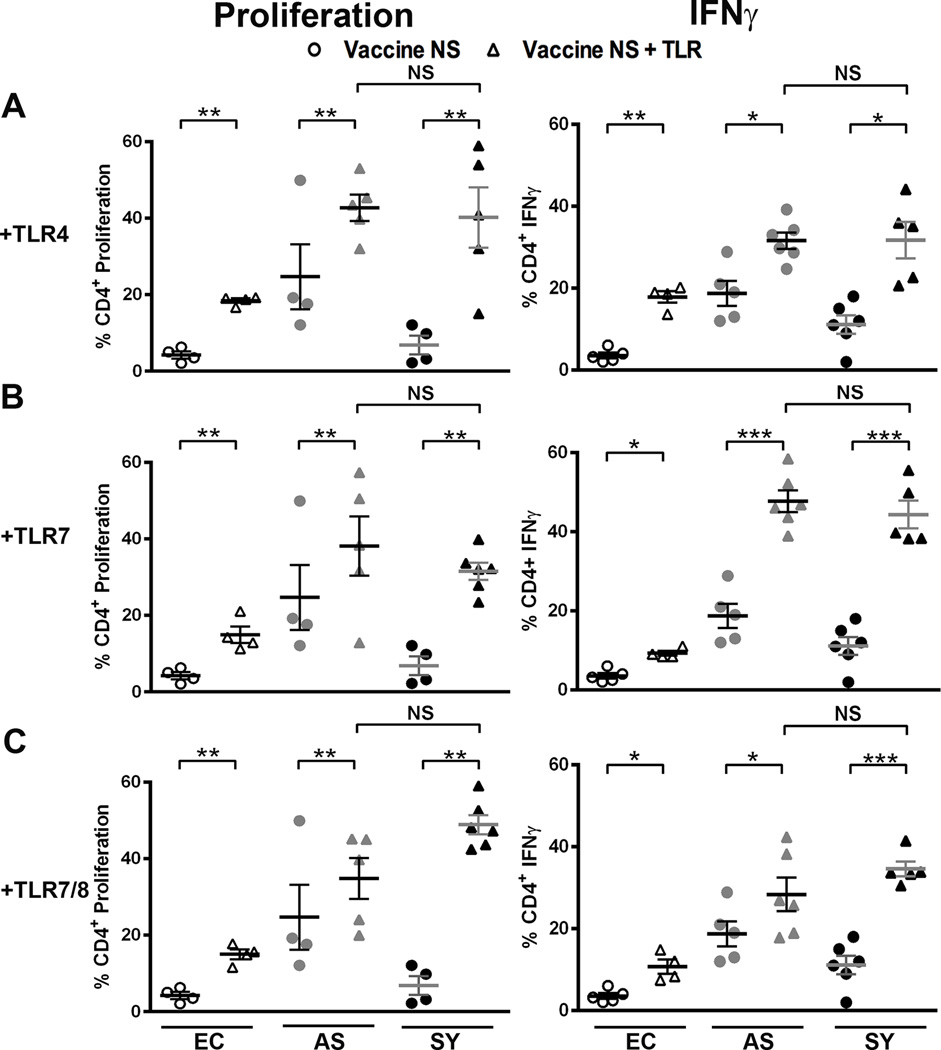
As NS is a more recently developed vaccine antigen [37, 46], we did not know how NS would perform with other TLR agonists when exposed to canine cells. While NS alone did not induce responses in uninfected dogs, NS with TLR4 agonist stimulated significant (p <0.01) increases in the population of proliferative CD4+ T cells, 4.3-, 1.7-, and 5.8-fold in cells from endemic control, VL-asymptomatic, and symptomatic dogs, respectively (Fig. 4A). Similar significant increases in CD4+ T cell populations were seen for IFNγ production (Fig. 4A; 5.0-, 1.7-, and 2.8-fold). NS with TLR7 or TLR7/8 agonists elicited similar percent population responses across clinical groups, with the most significant alterations in the L. infantum-infected dog CD4+ T cell populations producing IFNγ. Symptomatic dog CD4+ T cell percent proliferation increased 4.6-fold and 7.2-fold for TLR7 and TLR7/8 agonists respectively compared to vaccine alone (Fig. 4B and C, left, black triangles). Combination NS and TLR agonist stimulation similarly increased the CD4+ T cell population responses in both asymptomatic and symptomatic dogs. T cells from symptomatic dogs were significantly less responsive to NS alone (Fig. 2), underscoring dramatic improvement of these exhausted T cells from VL symptomatic animals (Fig. 4B and C). CD8+ T cells did not have altered NS vaccine antigen responses after addition of TLR4 agonist in cells from endemic control or symptomatic dogs. CD8+ T cells from VL asymptomatic dogs had decreased populations of proliferative and IFNγ-producing cells after addition of TLR7 or TLR7/8 agonists (Fig. S5), perhaps due to the earlier shift to non-recoverable exhaustion observed in CD8+ T cells vs. CD4+ T cells during progressive VL [17, 29]. In fact, our studies did demonstrate an increase in gene transcription of exhaustion makers LAG3, CLTA4, PD1 and TIM3 as well as secreted IL-27 (Fig S2), which in addition to IL-10 will drive immunoregulatory responses during visceral leishmanaisis [47]. CD4+ T cells from VL-symptomatic dogs were able to recover significant effector function with addition of TLR7 agonist to NS to populations equivalent to those seen from CD4+ T cells from healthy VL-asymptomatic dogs.
Fig. 4. Vaccine NS and TLR agonists increase Th1-responses in CD4+ T cells from asymptomatic and symptomatic dogs.
Whole blood was stimulated with either 1 µg vaccine NS (circles) or vaccine NS and TLR agonists (triangles). TLR agonists were added as in figure 3. Each dot represents an individual dog. EC = endemic control (open), AS = asympatomatic, L. infantum positive (grey), SY = symptomatic, L. infantum positive (black). Error bars are ± SEM. N=4–6 per group. * p<0.05, **p<0.01, ***p<0.001. Statistical analysis was performed via one way-ANOVA.
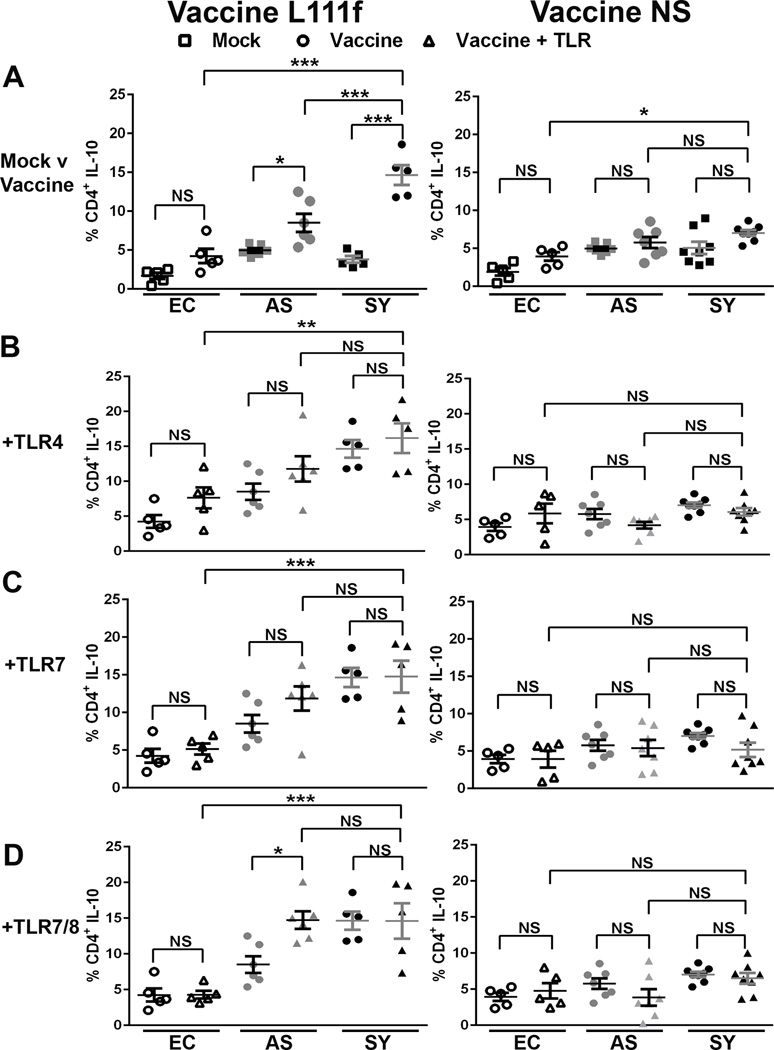
3.4 Percent of CD4+ T cell IL-10 positive population significantly increased after L111f exposure
IL-10, a regulatory cytokine, can dampen harmful effects of unchecked pro-inflammatory responses, but excess IL-10 can suppress leishmanicidal responses leading to progressive disease [48–50]. The percent of CD4+ T cells producing IL-10 significantly increased 3.9-fold in VL-symptomatic dog cells (p < 0.001), but only 1.7-fold in asymptomatic dog cells (p < 0.05) after stimulation with L111f alone compared to mock-treated cells (Fig. 5A, L111f). This increase in symptomatic dog cells was also a significantly (p < 0.001) 2.5-fold larger percent population than observed in endemic control CD4+ T cells following treatment with L111f (Fig. 5A, L111f). Further addition of TLR agonists did not significantly increase the percent population of IL-10 producing CD4+ T cells in endemic control or symptomatic dog cells compared to L111f alone, but there was a significant (p< 0.05) 1.7-fold increase in response to L111f and TLR 7/8 in asymptomatic dog CD4+ T cells. The lack of increase in IL-10 observed from symptomatic cells treated with L111f- TLR agonist combinations may be due to the fact that L111f alone causes significant IL-10 production in these cells. IL-10 production to L. infantum antigen is not unheralded, as canine CD4+ T cells from symptomatic dogs have previously been shown to respond to freeze-thawed L. infantum antigen stimulation with robust IL-10 production [29, 30]. However, when combination treatments were evaluated the percent CD4+ T cell IL-10 population was significantly higher in symptomatic dogs compared to similarly treated endemic controls at 2.1-, 2.9-, and 3.4-fold respectively for TLR4 (p < 0.01), TLR7 (p < 0.001), and TLR7/8 (p < 0.001) agonists (Fig 5B–D, L111f). There was no significant difference between symptomatic and asymptomatic cells treated with combination treatments. This suggests that L. infantum infection, regardless of disease progression, has altered the ability of CD4+ T cells to produce IL-10. CD8+ T cells from symptomatic dogs showed similar IL-10 population responses after exposure to L111f compared to mock-treated control CD8+ T cells (Fig S6 A, L111f). IL-10-producing CD8+ T cell populations significantly decreased from symptomatic dogs after exposure to L111f with TLR4 agonist (p < 0.01), but in asymptomatic dogs this addition of TLR agonists significantly increased IL-10 production. CD8+ T cell IL-10 populations increased 7.5-fold for TLR4 (p < 0.001) and 4.7-fold respectively for both TLR7 (p < 0.05) and TLR7/8 (p < 0.001) agonists addition (Fig 5B–D, L111f). Concomitantly with the Th1 response, L111f induces a balanced Il-10 regulatory response.
Fig. 5. Vaccine L111f increased IL-10 secretion from CD4+ T cells of symptomatic dogs.
Whole blood was stimulated (A) 1 µg vaccine (circles), or left untreated “mock” (squares) for 7 days. Vaccine, L111f or NS, used for stimulation is indicated in each column. In B to D whole blood was stimulated with either 1 µg vaccine L111f or NS alone (circles) or vaccine with TLR agonists (triangles). TLR agonists were added at 1 µg for TLR4 (B), 0.1 µg for TLR7 (C) or 10 µg for TLR7/8 (D). Each dot represents an individual dog. EC = endemic control (open), AS = asympatomatic, L. infantum positive (grey),SY = symptomatic, L. infantum positive (black). Error bars are ± SEM. * p<0.05, **p<0.01, ***p<0.001. Statistical analysis was performed via one way-ANOVA.
In contrast to L111f, stimulation with NS alone or in combination with TLR agonists had minimal, non-significant effects on CD4+ T cell IL-10 populations from control, asymptomatic, or symptomatic dogs (Fig. 5, NS). CD8+ T cell IL-10 populations were not affected by NS with or without additional exposure to TLR agonists in control or symptomatic dogs. (Fig. S6, NS). CD8+ T cell IL-10 populations from asymptomatic dogs had mild significant decreases (p<0.05) of 2.4- and 2.9-fold in response to stimulation with TLR4 and TLR7, respectively, in combination with NS. In parallel with production of robust Th1 responses, NS with TLR agonist exposure did not result in major decreases in IL-10 producing populations, particularly in control and symptomatic dog cells.
4. Discussion
Vaccine-induced responses have been studied in depth under experimental Leishmania conditions [48, 51–60], but little is known regarding how these vaccines would serve as a post-exposure prophylaxis or immunotherapy to boost existing responses in already infected animals. This study used dogs, a natural host of Leishmania infantum, to demonstrate possible outcomes of vaccination during subclinical and clinical VL, approximating vaccine responses within an endemic population where vaccination could or would often occur after infection. Prophylactic vaccination against Leishmania and subsequent protection against experimental infection is enhanced by appropriate formulation with TLR agonists [9, 10]. Our data demonstrate that the inclusion of TLR agonists enhanced IFNγ production and proliferation of CD4+ T cells isolated from VL-infected dogs after stimulation with Leishmania vaccine antigens.
The vaccine antigens used in this study are proprietary chimeric fusion proteins currently in (human) clinical trials [9, 13, 37]. Previous studies of L111f demonstrated an ability to stimulate murine CD4+ T cells to produce IFN-γ and TNF-α and when used prophylactically in conjunction with TLR4 agonist MPL-SE, prevented clinical progression of canine VL [14, 55]. Controlling disease in domestic animal reservoirs is important for overall VL control. Provided the close association of human and canine VL, dogs are a commonly targeted vaccine population in L. infantum endemic areas. In our U.S. canine cohort NS with TLR-adjuvant supplementation induced greater CD4+ T cell proliferative and IFNγ responses across L. infantum-infected groups compared to vaccine L111f. Interestingly, L111f contains a LeIF, a TLR2 agonist glycoprotein, as one of its antigenic components. As TLR2 when paired with TLR6 as a heterodimer can lead to Th2 skewed responses, this TLR agonist effect may dampen antigen-specific responses to produce a less robust CD4+ T cell population responses, as is suggested in this study. Consistent with antigen specific dampening properties, L111f, with or without TLR agonist, significantly increased IL-10 in symptomatic dogs. We hypothesize that this IL-10 increase represents and immune balancing effect, serving to buffer pro-inflammatory Th1 responses induced by the vaccine and/or agonists to prevent immunopathology [61]. IL-10 production has also been demonstrated as being an important prognosticator of disease severity and immunological outcomes [62, 63], nonetheless is an important factor to be observed in response to cellular stimulation. Interestingly, we did not observe elevations in IL-10 responses to NS upon the addition of TLR4, TLR7 or TLR7/8 agonists, suggesting that the TLR2 activating component of L111f may contribute to this response.
Vaccination has been previously studied as an immunotherapy for leishmaniasis in Brazil, with conflicting results. These studies demonstrated success following TLR agonist supplementation [64] and contrarily that inclusion of a TLR4 agonist was not successful in halting disease progression [65, 66]. Vaccine dosage, scheduling, or route of infection, could account for these discrepancies. Perhaps more importantly, infected animals progressing to clinical VL have immune exhaustion [29], which our data identify does interfere with vaccine/immunotherapeutic responses. VL symptomatic dog CD4+ T cells had reduced proliferative and IFNγ-production responses to vaccine antigens and mitogen compared to those from asymptomatic dogs (Fig. 2A). While these data are encouraging for use as a post-exposure prophylactic to prevent Leishmania infection progressing to disease in yet asymptomatic hosts, it is imperative to use targeted vaccine formulation able to recover exhausted T cell responses before consideration as a treatment of VL.
5. Conclusion
The foremost challenge in development of VL vaccines is successfully mounting protective immunity in populations with parasite exposure; not naïve hosts. Appropriate pairing of TLR agonists with vaccine antigens may be a way to overcome this challenge as the right pairing(s) were able to ameliorate T cell exhaustion in cells from VL asymptomatic and symptomatic dogs and promote a productive CD4+ T cell response. In vivo trials in naturally infected dogs to assess the ability of vaccine constructs with TLR agonist adjuvants may be merited.
Supplementary Material
Highlights.
Vaccine-specific T cell responses limited during clinical VL.
TLR agonists recovered T cell effector functions in cells from Leishmania-infected dogs.
Rational vaccine selection to improve antigen-fatigued responses crucial for endemic areas.
Acknowledgments
The authors would like to thank the hound owners for generously providing the samples for this study. The authors would also like to thank Ben Scott, Hailie Fowler, and Dr. Mandy Larson for their help in collecting blood samples and Ian Lamb for his help in sample processing. The data presented herein were obtained at the Flow Cytometry Facility, which is a Carver College of Medicine / Holden Comprehensive Cancer Center core research facility at the University of Iowa. The Facility is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veteran's Administration Medical Center.
Funding
This work was funded by the Morris Animal Foundation grant DC-501A and start-up funds from the University of Iowa, College of Public Health, and Department of Epidemiology. IDRI’s leishmaniasis vaccine program is funded by grants from the Bill and Melinda Gates Foundation (631 and 39129) and the National Institutes of Health (AI25038).
Abbreviations
- VL
visceral leishmaniasis
- IFNγ
interferon gamma
- Th1
T helper 1
- TLR
toll like receptor
- IFAT
immunofluorescence anti-Leishmania antibody testing
- IDRI
Infectious Disease Research Institute
- Con A
concanavalin A
- TNFα
tumor necrosis factor alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The vaccine adjuvants utilized in this study are propriety to the Infectious Disease Research Institute. The authors have no competing interests.
Contributors
RGS designed and performed experiments, analyzed data, wrote and revised the manuscript. TLG performed experiments, analyzed data, wrote and revised the manuscript. KJE designed and performed experiments. AJT performed experiments, wrote and revised the manuscript. MSD, RFH, and SGR supplied reagents and revised the manuscript. CAP designed experiments, analyzed data, and wrote and revised the manuscript.
References
- 1.Ready PD. Epidemiology of visceral leishmaniasis. Clinical epidemiology. 2014;6:147–154. doi: 10.2147/CLEP.S44267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PloS one. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.den Boer M, Argaw D, Jannin J, Alvar J. Leishmaniasis impact and treatment access. Clin Microbiol Infect. 2011;17:1471–1477. doi: 10.1111/j.1469-0691.2011.03635.x. [DOI] [PubMed] [Google Scholar]
- 4.Decuypere S, Vanaerschot M, Brunker K, Imamura H, Muller S, Khanal B, et al. Molecular mechanisms of drug resistance in natural Leishmania populations vary with genetic background. PLoS Negl Trop Dis. 2012;6:e1514. doi: 10.1371/journal.pntd.0001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray HW. Clinical and experimental advances in treatment of visceral leishmaniasis. Antimicrob Agents Chemother. 2001;45:2185–2197. doi: 10.1128/AAC.45.8.2185-2197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanaerschot M, Dumetz F, Roy S, Ponte-Sucre A, Arevalo J, Dujardin JC. Treatment failure in leishmaniasis: drug-resistance or another (epi-) phenotype? Expert review of anti-infective therapy. 2014;12:937–946. doi: 10.1586/14787210.2014.916614. [DOI] [PubMed] [Google Scholar]
- 7.Palatnik-de-Sousa CB. Vaccines for leishmaniasis in the fore coming 25 years. Vaccine. 2008;26:1709–1724. doi: 10.1016/j.vaccine.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Genaro O, de Toledo VP, da Costa CA, Hermeto MV, Afonso LC, Mayrink W. Vaccine for prophylaxis and immunotherapy, Brazil. Clin Dermatol. 1996;14:503–512. doi: 10.1016/0738-081x(96)00040-5. [DOI] [PubMed] [Google Scholar]
- 9.Raman VS, Duthie MS, Fox CB, Matlashewski G, Reed SG. Adjuvants for Leishmania vaccines: from models to clinical application. Front Immunol. 2012;3:144. doi: 10.3389/fimmu.2012.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raman VS, Bhatia A, Picone A, Whittle J, Bailor HR, O'Donnell J, et al. Applying TLR synergy in immunotherapy: implications in cutaneous leishmaniasis. J Immunol. 2010;185:1701–1710. doi: 10.4049/jimmunol.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goto Y, Bhatia A, Raman VS, Vidal SE, Bertholet S, Coler RN, et al. Leishmania infantum sterol 24-c-methyltransferase formulated with MPL-SE induces cross-protection against L. major infection. Vaccine. 2009;27:2884–2890. doi: 10.1016/j.vaccine.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto Y, Bhatia A, Raman VS, Liang H, Mohamath R, Picone AF, et al. KSAC, the first defined polyprotein vaccine candidate for visceral leishmaniasis. Clin Vaccine Immunol. 2011;18:1118–1124. doi: 10.1128/CVI.05024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duthie MS, Raman VS, Piazza FM, Reed SG. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine. 2012;30:134–141. doi: 10.1016/j.vaccine.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coler RN, Goto Y, Bogatzki L, Raman V, Reed SG. Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD4+ T cells. Infect Immun. 2007;75:4648–4654. doi: 10.1128/IAI.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velez ID, Gilchrist K, Martinez S, Ramirez-Pineda JR, Ashman JA, Alves FP, et al. Safety and immunogenicity of a defined vaccine for the prevention of cutaneous leishmaniasis. Vaccine. 2009;28:329–337. doi: 10.1016/j.vaccine.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 16.Gibson-Corley KN, Hostetter JM, Hostetter SJ, Mullin K, Ramer-Tait AE, Boggiatto PM, et al. Disseminated Leishmania infantum infection in two sibling foxhounds due to possible vertical transmission. Can Vet J. 2008;49:1005–1008. [PMC free article] [PubMed] [Google Scholar]
- 17.Gautam S, Kumar R, Singh N, Singh AK, Rai M, Sacks D, et al. CD8 T cell exhaustion in human visceral leishmaniasis. J Infect Dis. 2014;209:290–299. doi: 10.1093/infdis/jit401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shahbazi M, Zahedifard F, Saljoughian N, Doroud D, Jamshidi S, Mahdavi N, et al. Immunological comparison of DNA vaccination using two delivery systems against canine leishmaniasis. Vet Parasitol. 2015;212:130–139. doi: 10.1016/j.vetpar.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro RA, Teixeira-Neto RG, Belo VS, Ferreira EC, Schallig HD, Silva ES. Ability of immunodiagnostic tests to differentiate between dogs naturally infected with Leishmania infantum and Leishmune((R))-vaccinated dogs. Vet Res Commun. 2015;39:87–95. doi: 10.1007/s11259-015-9625-6. [DOI] [PubMed] [Google Scholar]
- 20.Miura R, Kooriyama T, Yoneda M, Takenaka A, Doki M, Goto Y, et al. Efficacy of Recombinant Canine Distemper Virus Expressing Leishmania Antigen against Leishmania Challenge in Dogs. PLoS Negl Trop Dis. 2015;9:e0003914. doi: 10.1371/journal.pntd.0003914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martins VT, Duarte MC, Chavez-Fumagalli MA, Menezes-Souza D, Coelho CS, de Magalhaes-Soares DF, et al. A Leishmania-specific hypothetical protein expressed in both promastigote and amastigote stages of Leishmania infantum employed for the serodiagnosis of, and as a vaccine candidate against, visceral leishmaniasis. Parasit Vectors. 2015;8:363. doi: 10.1186/s13071-015-0964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiuza JA, Gannavaram S, Santiago HdaC, Selvapandiyan A, Souza DM, Passos LS, et al. Vaccination using live attenuated Leishmania donovani centrin deleted parasites induces protection in dogs against Leishmania infantum. Vaccine. 2015;33:280–288. doi: 10.1016/j.vaccine.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 23.Rezvan H, Moafi M. An overview on Leishmania vaccines: A narrative review article. Veterinary research forum : an international quarterly journal. 2015;6:1–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Olotu A, Lusingu J, Leach A, Lievens M, Vekemans J, Msham S, et al. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5–17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect Dis. 2011;11:102–109. doi: 10.1016/S1473-3099(10)70262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White MT, Verity R, Griffin JT, Asante KP, Owusu-Agyei S, Greenwood B, et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis. 2015;15:1450–1458. doi: 10.1016/S1473-3099(15)00239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moliva JI, Turner J, Torrelles JB. Prospects in Mycobacterium bovis Bacille Calmette et Guerin (BCG) vaccine diversity and delivery: why does BCG fail to protect against tuberculosis? Vaccine. 2015;33:5035–5041. doi: 10.1016/j.vaccine.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 28.Schaut RG, Lamb IM, Toepp AJ, Scott B, Mendes-Aguiar CO, Coutinho JF, et al. Regulatory IgDhi B Cells Suppress T Cell Function via IL-10 and PD-L1 during Progressive Visceral Leishmaniasis. Journal of immunology (Baltimore, Md 1950) 2016 doi: 10.4049/jimmunol.1502678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esch KJ, Juelsgaard R, Martinez PA, Jones DE, Petersen CA. Programmed death 1-mediated T cell exhaustion during visceral leishmaniasis impairs phagocyte function. J Immunol. 2013;191:5542–5550. doi: 10.4049/jimmunol.1301810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boggiatto PM, Ramer-Tait AE, Metz K, Kramer EE, Gibson-Corley K, Mullin K, et al. Immunologic indicators of clinical progression during canine Leishmania infantum infection. Clin Vaccine Immunol. 2010;17:267–273. doi: 10.1128/CVI.00456-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boggiatto PM, Gibson-Corley KN, Metz K, Gallup JM, Hostetter JM, Mullin K, et al. Transplacental transmission of Leishmania infantum as a means for continued disease incidence in North America. PLoS neglected tropical diseases. 2011;5:e1019. doi: 10.1371/journal.pntd.0001019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schantz PM, Steurer FJ, Duprey ZH, Kurpel KP, Barr SC, Jackson JE, et al. Autochthonous visceral leishmaniasis in dogs in North America. J Am Vet Med Assoc. 2005;226:1316–1322. doi: 10.2460/javma.2005.226.1316. [DOI] [PubMed] [Google Scholar]
- 33.Duprey ZH, Steurer FJ, Rooney JA, Kirchhoff LV, Jackson JE, Rowton ED, et al. Canine visceral leishmaniasis, United States and Canada, 2000–2003. Emerg Infect Dis. 2006;12:440–446. doi: 10.3201/eid1203.050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vida B, Toepp A, Schaut RG, Esch KJ, Juelsgaard R, Shimak RM, et al. Immunologic progression of canine leishmaniosis following vertical transmission in United States dogs. Veterinary immunology and immunopathology. 2016;169:34–38. doi: 10.1016/j.vetimm.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duprey ZH, Steurer FJ, Rooney JA, Kirchhoff LV, Jackson JE, Rowton ED, et al. Canine visceral leishmaniasis, United States and Canada, 2000–2003. Emerging Infectious Diseases. 2006;12:440–446. doi: 10.3201/eid1203.050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badaró R, Reed SG, Carvalho EM. Immunofluorescent antibody test in American visceral leishmaniasis: sensitivity and specificity of different morphological forms of two Leishmania species. The American journal of tropical medicine and hygiene. 1983;32:480–484. doi: 10.4269/ajtmh.1983.32.480. [DOI] [PubMed] [Google Scholar]
- 37.Coler RN, Duthie MS, Hofmeyer KA, Guderian J, Jayashankar L, Vergara J, et al. From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clin Transl Immunology. 2015;4:e35. doi: 10.1038/cti.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PloS one. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 40.Schaut RG, Lamb IM, Toepp AJ, Scott B, Mendes-Aguiar CO, Coutinho JF, et al. Regulatory IgDhi B Cells Suppress T Cell Function via IL-10 and PD-L1 during Progressive Visceral Leishmaniasis. J Immunol. 2016;196:4100–4109. doi: 10.4049/jimmunol.1502678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esch KJ, Schaut RG, Lamb IM, Clay G, Morais Lima AL, do Nascimento PR, et al. Activation of Autophagy and NLR Family, Pyrin Domain Containing 3 Inflammasome during Leishmania infantum-Associated Glomerulonephritis. Am J Pathol. 2015 doi: 10.1016/j.ajpath.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 43.Duthie MS, Raman VS, Piazza FM, Reed SG. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine. 2011 doi: 10.1016/j.vaccine.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chakravarty J, Kumar S, Trivedi S, Rai VK, Singh A, Ashman JA, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine for use in the prevention of visceral leishmaniasis. Vaccine. 2011;29:3531–3537. doi: 10.1016/j.vaccine.2011.02.096. [DOI] [PubMed] [Google Scholar]
- 45.Llanos-Cuentas A, Calderon W, Cruz M, Ashman JA, Alves FP, Coler RN, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1+MPL-SE vaccine when used in combination with sodium stibogluconate for the treatment of mucosal leishmaniasis. Vaccine. 2010;28:7427–7435. doi: 10.1016/j.vaccine.2010.08.092. [DOI] [PubMed] [Google Scholar]
- 46.Duthie MS, Favila M, Hofmeyer KA, Tutterrow YL, Reed SJ, Laurance JD, et al. Strategic evaluation of vaccine candidate antigens for the prevention of Visceral Leishmaniasis. Vaccine. 2016 doi: 10.1016/j.vaccine.2016.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ansari NA, Kumar R, Gautam S, Nylen S, Singh OP, Sundar S, et al. IL-27 and IL-21 are associated with T cell IL-10 responses in human visceral leishmaniasis. J Immunol. 2011;186:3977–3985. doi: 10.4049/jimmunol.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin V, Vouldoukis I, Moreno J, McGahie D, Gueguen S, Cuisinier AM. The protective immune response produced in dogs after primary vaccination with the LiESP/QA-21 vaccine (CaniLeish(R)) remains effective against an experimental challenge one year later. Vet Res. 2014;45:69. doi: 10.1186/1297-9716-45-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nylen S, Maurya R, Eidsmo L, Manandhar KD, Sundar S, Sacks D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med. 2007;204:805–817. doi: 10.1084/jem.20061141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gautam S, Kumar R, Maurya R, Nylen S, Ansari N, Rai M, et al. IL-10 neutralization promotes parasite clearance in splenic aspirate cells from patients with visceral leishmaniasis. J Infect Dis. 2011;204:1134–1137. doi: 10.1093/infdis/jir461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soto M, Corvo L, Garde E, Ramirez L, Iniesta V, Bonay P, et al. Coadministration of the Three Antigenic Leishmania infantum Poly (A) Binding Proteins as a DNA Vaccine Induces Protection against Leishmania major Infection in BALB/c Mice. PLoS Negl Trop Dis. 2015;9:e0003751. doi: 10.1371/journal.pntd.0003751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saljoughian N, Taheri T, Zahedifard F, Taslimi Y, Doustdari F, Bolhassani A, et al. Development of novel prime-boost strategies based on a tri-gene fusion recombinant L. tarentolae vaccine against experimental murine visceral leishmaniasis. PLoS Negl Trop Dis. 2013;7:e2174. doi: 10.1371/journal.pntd.0002174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta R, Kumar V, Kushawaha PK, Tripathi CP, Joshi S, Sahasrabuddhe AA, et al. Characterization of glycolytic enzymes--rAldolase and rEnolase of Leishmania donovani, identified as Th1 stimulatory proteins, for their immunogenicity and immunoprophylactic efficacies against experimental visceral leishmaniasis. PloS one. 2014;9:e86073. doi: 10.1371/journal.pone.0086073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carneiro MB, de Andrade e Sousa LM, Vaz LG, Dos Santos LM, Vilela L, de Souza CC, et al. Short-term protection conferred by Leishvacin(R) against experimental Leishmania amazonensis infection in C57BL/6 mice. Parasitol Int. 2014;63:826–834. doi: 10.1016/j.parint.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Trigo J, Abbehusen M, Netto EM, Nakatani M, Pedral-Sampaio G, de Jesus RS, et al. Treatment of canine visceral leishmaniasis by the vaccine Leish-111f+MPL-SE. Vaccine. 2010;28:3333–3340. doi: 10.1016/j.vaccine.2010.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santos FN, Borja-Cabrera GP, Miyashiro LM, Grechi J, Reis AB, Moreira MA, et al. Immunotherapy against experimental canine visceral leishmaniasis with the saponin enriched-Leishmune vaccine. Vaccine. 2007;25:6176–6190. doi: 10.1016/j.vaccine.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neogy AB, Vouldoukis I, da Costa JM, Monjour L. Exploitation of parasite-derived antigen in therapeutic success against canine visceral leishmaniosis. Veterinary Group of Lupino. Vet Parasitol. 1994;54:367–373. doi: 10.1016/0304-4017(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 58.Borja-Cabrera GP, Santos FN, Santos FB, Trivellato FA, Kawasaki JK, Costa AC, et al. Immunotherapy with the saponin enriched-Leishmune vaccine versus immunochemotherapy in dogs with natural canine visceral leishmaniasis. Vaccine. 2010;28:597–603. doi: 10.1016/j.vaccine.2009.09.071. [DOI] [PubMed] [Google Scholar]
- 59.Borja-Cabrera GP, Cruz Mendes A, Paraguai de Souza E, Hashimoto Okada LY, de ATFA, Kawasaki JK, et al. Effective immunotherapy against canine visceral leishmaniasis with the FML-vaccine. Vaccine. 2004;22:2234–2243. doi: 10.1016/j.vaccine.2003.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferreira JHL, dos Santos Silva L, Longo-Maugéri IM, Katz S, Barbiéri CL. Use of a recombinant cysteine proteinase from Leishmania (Leishmania) infantum chagasi for the immunotherapy of canine visceral leishmaniasis. PLoS Negl Trop Dis. 2014;8(3):e2729. doi: 10.1371/journal.pntd.0002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Belkaid Y, Sun CM, Bouladoux N. Parasites and immunoregulatory T cells. Curr Opin Immunol. 2006;18:406–412. doi: 10.1016/j.coi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 62.Schwarz T, Remer KA, Nahrendorf W, Masic A, Siewe L, Muller W, et al. T cell-derived IL-10 determines leishmaniasis disease outcome and is suppressed by a dendritic cell based vaccine. PLoS Pathog. 2013;9:e1003476. doi: 10.1371/journal.ppat.1003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar R, Nylen S. Immunobiology of visceral leishmaniasis. Front Immunol. 2012;3:251. doi: 10.3389/fimmu.2012.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miret J, Nascimento E, Sampaio W, Franca JC, Fujiwara RT, Vale A, et al. Evaluation of an immunochemotherapeutic protocol constituted of N-methyl meglumine antimoniate (Glucantime) and the recombinant Leish-110f + MPL-SE vaccine to treat canine visceral leishmaniasis. Vaccine. 2008;26:1585–1594. doi: 10.1016/j.vaccine.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreno J, Nieto J, Masina S, Canavate C, Cruz I, Chicharro C, et al. Immunization with H1, HASPB1 and MML Leishmania proteins in a vaccine trial against experimental canine leishmaniasis. Vaccine. 2007;25:5290–5300. doi: 10.1016/j.vaccine.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gradoni L, Foglia Manzillo V, Pagano A, Piantedosi D, De Luna R, Gramiccia M, et al. Failure of a multi-subunit recombinant leishmanial vaccine (MML) to protect dogs from Leishmania infantum infection and to prevent disease progression in infected animals. Vaccine. 2005;23:5245–5251. doi: 10.1016/j.vaccine.2005.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.