Abstract
There is an emerging interest to develop human vaccines against medically-important fungal pathogens and a need for a preclinical animal model to assess vaccine efficacies and protective correlates. HLA-DR4 (DRB1*0401 allele) transgenic mice express a human major histocompatibility complex class II (MHC II) receptor in such a way that CD4+ T-cell response is solely restricted by this human molecule. In this study HLA-DR4 transgenic mice were immunized with a live-attenuated vaccine (ΔT) and challenged by the intranasal route with 50-70 Coccidioides posadasii spores, a potentially lethal dose. The same vaccination regimen offers 100% survival for C57BL/6 mice. Conversely, ΔT-vaccinated HLA-DR4 mice displayed 3 distinct manifestations of Coccidioides infection including 40% fatal acute (FAD), 30% disseminated (DD) and 30% pulmonary disease (PD). The latter 2 groups of mice had reduced loss of body weight and survived to at least 50 days postchallenge (dpc). These results suggest that ΔT vaccinated HLA-DR4 mice activated heterogeneous immunity against pulmonary Coccidioides infection. Vaccinated HLA-DR4 mice displayed early expansion of Th1 and Th17 cells and recruitment of inflammatory innate cells into Coccidioides-infected lungs during the first 9 dpc. While contraction rates of Th cells and the inflammatory response during 14-35 dpc significantly differed among the 3 groups of vaccinated HLA-DR4 mice. The FAD group displayed a sharply reduced Th1 and Th17 response, while overwhelmingly recruiting neutrophils into lungs during 9-14 days. The FAD group approached moribund by 14 dpc. In contrast, vaccinated HLA-DR4 survivors gradually contracted Th cells and inflammatory response with the greatest rate in the PD group. While vaccinated HLA-DR4 mice are susceptible to Coccidioides infection, they are useful for evaluation of vaccine efficacy and identification of immunological correlates against this mycosis.
Keywords: Coccidioides, coccidioidomycosis, HLA-DR4 transgenic mice, fungal vaccine, innate and adaptive immunity
1. INTRODUCTION
Coccidioides immitis and Coccidioides posadasii are arid soil borne fungal pathogens and causative agents of coccidioidomycosis, a pulmonary disease of humans which is endemic to the Americas. Within the United States the most common regions of occurrence of this mycosis extends between West Texas and the San Joaquin Valley of Southern California [1]. Infection rates within the major endemic regions have continued to rise [2]. Combined with major growth of the population in southwestern U.S., these factors have underscored the need for a vaccine against coccidioidomycosis. To date, no FDA-approved vaccine against this respiratory disease exists.
Development of antifungal vaccines and characterization of vaccine immunity to fungal infections have been largely dependent on the use of animal models [3-6]. Mice are often employed for preclinical evaluation of vaccine efficacy and protection mechanisms due to low cost and the large numbers of genetically-modified mouse strains available to researchers. However, the use of conventional mouse strains to evaluate vaccine candidates may be problematic, given the differences in the MHC binding properties between murine and human antigen presenting cells.
Cell-mediated immunity plays a pivotal role in protection against coccidioidomycosis [5, 6]. T-helper 1 (Th1) and Th17-type cell-mediated immune responses contribute to protective immunity to Coccidioides infection [7, 8]. While high antibody titers to Coccidioides in patients typically correlate with poor clinical outcomes [9]. Central to CD4+ T cell-dependent immunity is the major histocompatibility class II receptor (MHC II). The latter is composed of two heterodimeric chains (α and β) forming a peptide-affinity cleft which binds and presents peptide antigens (epitopes) to CD4+ T cells. MHC II receptors are encoded by human leukocyte antigen (HLA) alleles. HLA-DR4 human haplotype occurs at high frequency amongst individuals of different racial backgrounds within North America [10, 11]. We have shown previously that antigen presenting cells isolated from HLA-DR4 mice are able to present peptide antigens of Coccidioides to autologous CD4+ T cells and to activate them to produce IFN-γ [12].
In this study, we used spores isolated from a live-attenuated (ΔT) strain as an established protective vaccine [13] to evaluate susceptibility and protective correlates induced by vaccination in HLA-DR4 transgenic mice against pulmonary Coccidioides infection. We have identified clinically relevant correlates of both protection and disease progression including physiological, immunological and histological features documented over 50 days postchallenge in vaccinated and non-vaccinated HLA-DR4 mice.
2. MATERIALS AND METHODS
2.1. Mouse strain
Inbred, HLA-DR4 (DRB1*0401) transgenic mice were obtained as a gift from Dr. Thomas Forsthuber at the University of Texas at San Antonio [14, 15]. Mice were housed in a specific-pathogen free animal facility at UTSA and handled according to guidelines approved by IACUC. At 7 to 10 weeks of age, sex-matched mice were relocated into an ABSL3 laboratory before experimentation.
2.2. Fungal growth conditions
Coccidioides posadasii (C735), a virulent clinical isolate was used to challenge mice. A genetically-engineered mutant strain (Δcts2/ard1/cts3) derived from the C735 isolate was used as a live, attenuated vaccine and referred to as “ΔT”. The virulent C735 inoculum and the ΔT vaccine were prepared in a BSL3 laboratory as previously described [13].
2.3. Vaccination, challenge, body weight measurements, survival and fungal burden
Mice were vaccinated in the abdominal region by the subcutaneous route with 5.0 × 104 viable ΔT spores suspended in 100 μl sterile PBS. A booster immunization of 2.5 × 104 spores was administered 2-week later. Non-vaccinated control mice were immunized with PBS. Four weeks after the booster immunization, mice were challenged by the intranasal route with approximately 50-70 spores of C. posadasii in 35 μl PBS. Mice immunized with the ΔT vaccine were identified by ear-punch and individual body weights were obtained by weighing mice at 24 hour intervals for 50 consecutive days postchallenge. The fungal burden in lungs and spleen was determined by assessing colony-forming units (CFUs) as previously described [13]. The number of CFUs were expressed on a log scale and presented as a box plot for each group of 8-13 animals.
2.4. Assays of selected cytokines produced by immune splenocytes
Spleens of non-vaccinated and ΔT-immunized mice were harvested at 3 weeks after second vaccination, separately pooled and macerated as reported [16]. Splenocytes obtained from age- and gender-matched naïve mice were used as a negative control. Splenocytes (5.0 × 106) were co-cultured in the presence of either Coccidioides T27K antigen (40μg/ml) or in medium alone [17]. Supernatants were collected after 48 h of incubation and assayed for amounts of selected cytokines using a Bio-Plex suspension array system (Bio-Rad).
2.5. Quantification of cell subpopulations by flow cytometry
Pulmonary leukocytes were isolated from non-vaccinated or mice vaccinated with the ΔT vaccine as well as naïve mice (untreated) as previously described [7]. The latter were used as negative control. Viable leukocytes were identified and counted by trypan blue exclusion using a hemocytometer. An aliquot of cells was stimulated with antibodies to CD3 and CD28 in the presence of GolgiStop in cRPMI 1640 for 4 h at 37°C. Cells were harvested and incubated with unlabeled anti-CD16/CD32 antibody to block non-specific binding and then permeabilized using a cytofix/cytoperm kit (BD Biosciences). Subsequently, the cells were stained with fluorochrome-conjugated antibodies to CD4, CD8 and a selected antibody to IFN-γ, IL-5 or IL-17A as previously described [7]. Percentages and numbers of cytokine-producing cells were determined as previously described [7].
A separate aliquot of pulmonary cells was stained for selected innate cells as previously described [7]. Gating strategies consisted of the following: for eosinophils (CD45+CD11c-SiglecF+), alveolar macrophages (CD45+CD11c−CD11bMed), dendritic cells (CD45+CD11c+CD11bHigh) and neutrophils (CD45+CD11b+/−Ly6G+). Fluorochrome-labeled cells were acquired using a FACSCalibr flow cytometer (BD Biosciences)) and results analyzed using a FlowJo software (FlowJo, Inc) as previously described [7].
2.6. ELISAs
To analyze the serum levels of IgG-total, IgG1, IgG2c and IgG3 ELISAs were performed with serum samples isolated from naïve, vaccinated and non-vaccinated mice against Coccidioides T27K antigen as previously described [18]. Sera samples were diluted to 1:50 and secondary horseradish peroxidase (HRP) conjugate antibodies were diluted to 1:4000 in PBS. The data are represented as the mean ± SEM.
2.7. Statistical analyses
Differences in survival between treatment groups were determined by the Kaplan-Meier method and Chi-square test. The Mann-Whitney U test was used to analyze CFUs and cell numbers as previously described [13]. The Student's t test was used to compare cytokine production and IgG antibody titers of non-vaccinated versus vaccinated mice at indicated time points. A P-value of <0.05 was considered statistically significant.
3. RESULTS
Vaccinated HLA-DR4 transgenic mice showed increased survival and enhanced clearance of Coccidioides
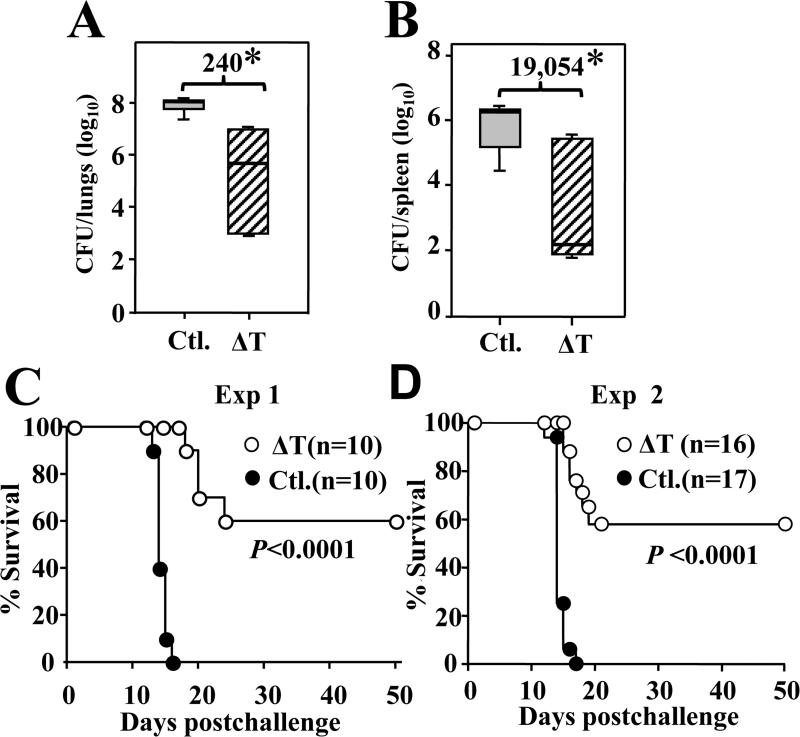
We first confirmed splenocytes isolated from HLA-DR4 transgenic mice expressed HLA-DR4 molecules but not murine MHC II-IAb (Supp. FIG. 1). At 14 dpc vaccinated HLA-DR4 mice revealed a significant 240-fold reduction of CFUs in the infected lungs compared to non-vaccinated mice (FIG. 1A). Fungal dissemination to the spleen at 14 dpc was also significantly reduced 19,054 folds in vaccinated mice compared to non-vaccinated mice (FIG. 1B). Likewise, vaccinated HLA-DR4 mice also showed a significantly greater percentage (60%) of survival to 50 dpc compared to non-vaccinated mice as shown in two independent experiments (P < 0.0001) (FIG. 1C, D).
FIG. 1. Vaccination of HLA-DR4 mice with the live, attenuated vaccine provides protection against coccidioidomycosis.
Colony-forming units (CFU) were determined by plate cultures of (A) lungs and (B) spleen homogenates of non-vaccinated and vaccinated mice at 14 dpc (n = 11/group). The data are presented as box plots of CFUs (log10) detected on plate cultures of lung or spleen homogenates. The boxes represent the 25th and 75th percentiles while the bars represent the 10th and 90th percentiles. The median values of the CFUs were indicated by horizontal lines inside each plot. Numbers shown are the relative reduction (folds) of CFUs in lungs (A) and spleen (B) of vaccinated versus non-vaccinated mice. Asterisks indicate statistically significant differences between CFU values of non-vaccinated and ΔT-vaccinated groups of mice (P < 0.01) as determined by the Mann-Whitney U test. The results are representative of two independent fungal burden assays. (C, D) Survival plots were determined for both non-vaccinated and vaccinated HLA-DR4 transgenic mice that were challenged with a potentially lethal inoculum of Coccidioides spores. Statistically significant differences between the non-vaccinated and vaccinated mice were determined by the Kaplan-Meier and Chi-square test (P < 0.0001).
Maintenance of body weight correlated with protection
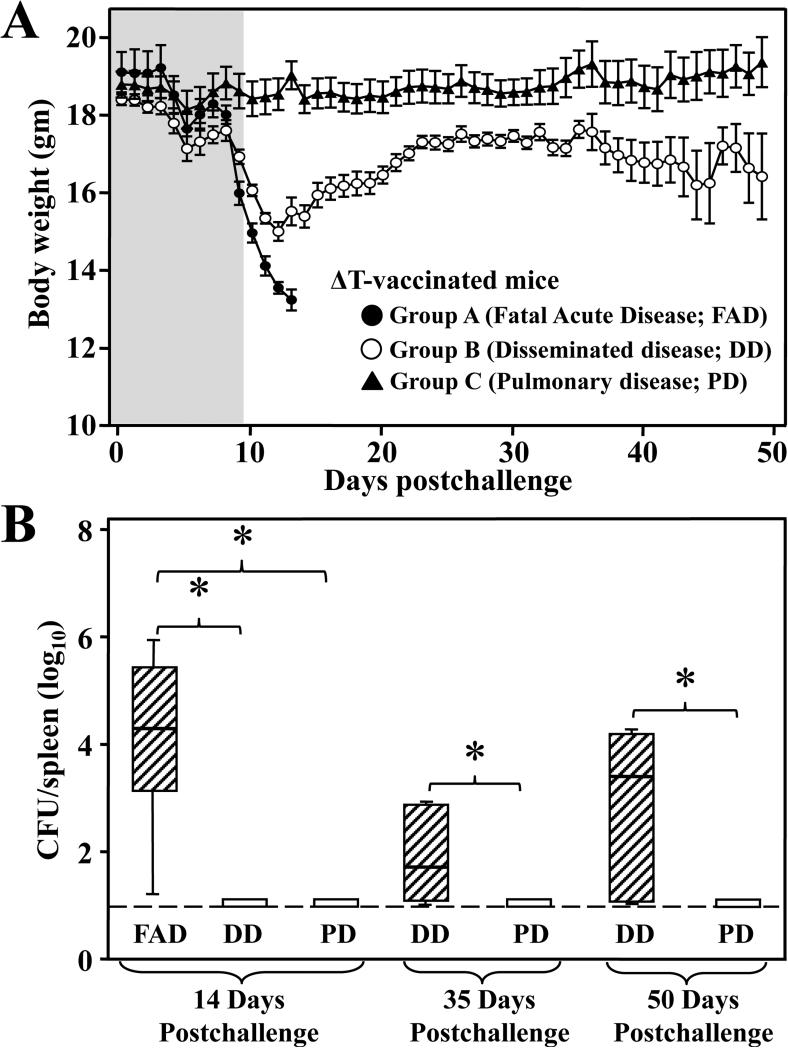
The vaccinated survivors could be separated into three distinct groups based on their daily change in body weight (FIG. 2A). Group A exhibited a precipitous loss of weight beginning at 9-10 dpc and they approached moribund by 14 dpc. Group B showed a similar drop in body weight during 9-12 dpc, but then were distinguished from Group A by their gradual increase in weight during the following 25 dpc. Group C was characterized by a stable average of 18.8 grams recorded over 50 dpc. All mice in Group B and C survived through 50 dpc. Prior to challenge, the initial body weights of mice which fell into Groups A, B, or C were 19.11 ± 0.52, 18.40 ± 0.14, and 18.79 ± 0.34 gms, respectively. There was no significant difference in body weights among these three groups of mice at the time of challenge suggesting that initial body weight did not predispose mice to a specific course of disease.
FIG. 2. Body weight profiles and fungal burden in spleen of ΔT-vaccinated mice over the course of 50 days postchallenge identified three distinct protective outcomes.
ΔT-vaccinated mice were intranasally challenged with approximate 50-70 spores, a potentially lethal dose and weighed daily for 50 dpc. (A) Individual mice (n = 8-10/group) were assigned to Group A, B, or C based on their profiles of body weight changes after 9 dpc. No significant body weight changes was observed for both the vaccinated and non-vaccinated mice for the first 9 days postchallenge (shaded time period). Data points represent the average daily body weight ± SEM for each disease group. The data presented are representative of three independent experiments. (B) Fungal dissemination from the lungs to the spleen was determined by CFU analysis of the 3 groups at 14, 35, and 50 dpc. Groups A-C were further classified as fetal acute disease (FAD), disseminated disease (DD) and pulmonary disease, respectively, based on mortality and fungal dissemination to spleen.. Asterisks identify statistically significant differences (Mann-Whitney U test, P < 0.01) between the indicated groups, and the dotted line represents the limit of detection.
We evaluated whether loss of body weight correlated with fungal dissemination to the spleen of mice assigned to Group A, B or C (FIG. 2B). At 14 dpc Group A displayed high CFUs in their spleen (4.29 ± 1.58) while mice in Groups B and C had no detectable CFUs. At 35 and 50 dpc Group C continued to show no detectable CFUs in their spleen. Group B revealed an increase in CFUs in the spleen between 35 and 50 dpc (1.78 ± 0.40 and 3.39 ± 0.53), respectively. On the basis of differences in survival and pathogen dissemination to spleen, Groups A, B and C mice were diagnosed as fatal acute disease (FAD), disseminated disease (DD) and localized, pulmonary disease (PD), respectively. Similarly, histopathological studies showed that lungs of the vaccinated mice with disseminated disease had visibly higher numbers of spherules in various stages of development compared to mice with pulmonary disease (Supp, FIG.2). These results suggested that increased fungal burdens in both lungs and spleen correlated with severity of this mycosis.
ΔT vaccine-primed splenocytes responded to Coccidioides antigen by production of high levels of Th1- and Th17-type cytokines
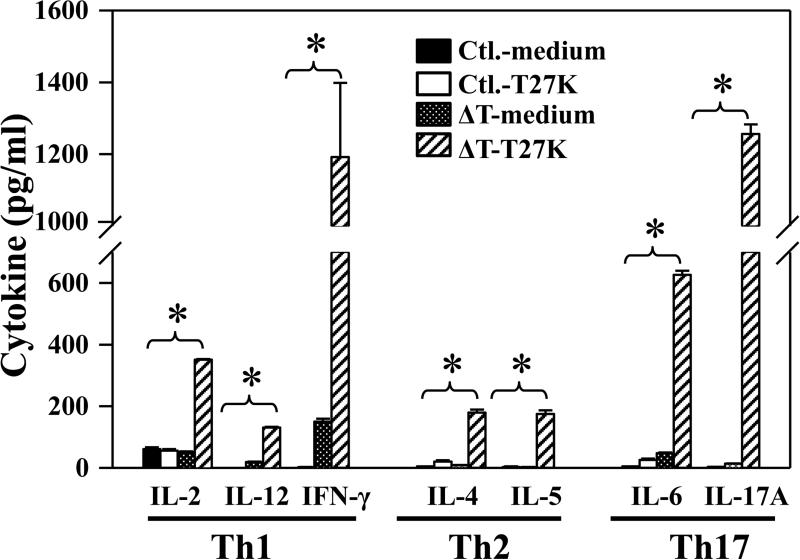
We employed a recall response assay to determine whether immune splenocytes secreted IL-2, IL-12, IFN-γ, IL-4, IL-5, IL-6 and IL-17A cytokines indicative of Th1, Th2 and Th17 response, respectively. T27K antigen was used to evaluate this ex vivo response as it has previously been shown to stimulate patient immune cells [19]. Significantly enhanced production of all examined cytokines by immune splenocytes cells was observed suggesting that clonal expansion of the Coccidioides-specific Th1, Th2 and Th17 cells had occurred (FIG.3). IFN-γ and IL-17A concentration were the highest among the examined cytokines, indicating that vaccinated HLA-DR4 mice developed a mixed Th1 and Th17 response to ΔT-vaccination (FIG.3).
FIG. 3. Concentrations of representative Th1-, Th2-, and Th17-type cytokines detected in culture supernatants of splenocytes isolated from ΔT-vaccinated HLA-DR4 mice (ΔT) or nonvaccinated animals (Ctl.).
Ex vivo stimulation of both immune and naïve splenocytes was conducted using the T27K antigen. Cells incubated with medium alone served as a negative control. The asterisks indicate significantly higher concentrations of selected cytokines in vaccinated mice compared to non-vaccinated mice (P≤0.01). Each sample was analyzed in triplicate. The results are presented as mean values ± SEM.
Kinetic changes of Th1 and Th17 cell subpopulations in lungs of vaccinated mice correlated with protection
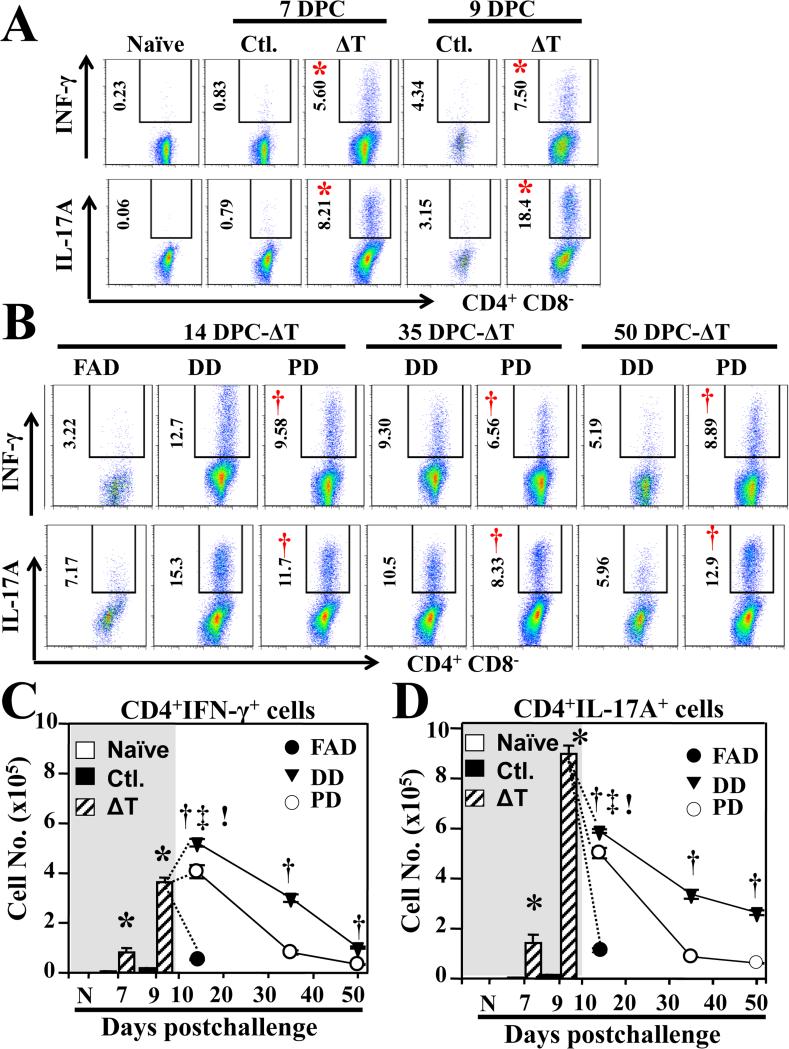
We first measured numbers of activated T cells that expressed high level of CD44 in lung homogenates by flow cytometry. Significantly higher numbers of both activated CD4+ and CD8+ T cells were observed in vaccinated mice (Supp. FIG. 3). Based on splenocyte recall data, we focused on the measurement of Th1 and Th17 response that have been previously shown to correlate with vaccine protection for C57BL/6 mice. Both percentages and numbers of Th1 and Th17 cells were significantly elevated in lungs of the vaccinated HLA-DR4 mice during the first 9 dpc (FIG. 4A and shaded time period in FIG. 4C, 4D) compared to nonvaccinated mice. Expansion of Th1 and Th17 subpopulations in lungs of vaccinated mice concurred with increase production of IFN-γ and IL-17A by ΔT-immune splenocytes that were restimulated with T27K antigen ex vivo (FIG.3). During the following 14-50 dpc significant differences in contraction rate of Th cell subpopulations were observed among the three distinct disease forms (FIG. 4B-D). FAD mice showed a sharp reduction in percentages and numbers of Th1 and Th17 cells during 9-14 dpc as the animals approached moribund condition. In contrast, both DD and PD mice showed reduced contraction rate of Th17 subpopulation during 9-14 dpc compared to FAD mice. While PD mice showed higher percentages of Th1 and Th17 cells at 50 dpc, they had significantly reduced numbers of both Th1 and Th17 subpopulations. Taken together, elevated contraction rate of Th1 and Th17 response during 14-50 dpc of the PD group suggested that they had dampened both adaptive and inflammatory responses and controlled the infection.
FIG. 4. Kinetic analysis of Th1 and Th17 cell types correlated with vaccine-induced protection.
(A) Percentages of Th1 (IFN-γ+) and Th17 (IL-17A+) cells within the gated Th cells (CD4+CD8−) population in the lungs of naïve, non-vaccinated (Ctl.) versus vaccinated (ΔT) mice were shown at 7 and 9 dpc. As an extension of this analysis, the percentages of these two T-cell subsets were examined in fetal acute disease (FAD) group at 14 dpc and in disseminated (DD) and pulmonary disease (PD) groups at 14, 35, and 50 dpc (B). Numbers left to the gates indicate the mean percentages of the gated Th1 and Th17 subsets. (C, D) The cell numbers of gated Th1 and Th17 per lungs were determined by intracellular cytokine staining and mean cell values ± SEM were plotted for each group of mice at 7, 9, 14, 35 and 50 dpc. Asterisks indicate significant differences in cell numbers between non-vaccinated (Ctl.) and ΔT-vaccinated groups at 7 or 9 dpc (Mann-Whitney U test; P < 0.05). The single dagger indicates significant differences between the PD and the DD groups (†, P < 0.05), exclamation mark indicates significant differences between the FAD and the DD groups (!, P < 0.05), while the double dagger indicates significant differences between acute and pulmonary disease groups (‡, P < 0.05) at 14, 35, or 50 d. The data presented are representative of two independent experiments.
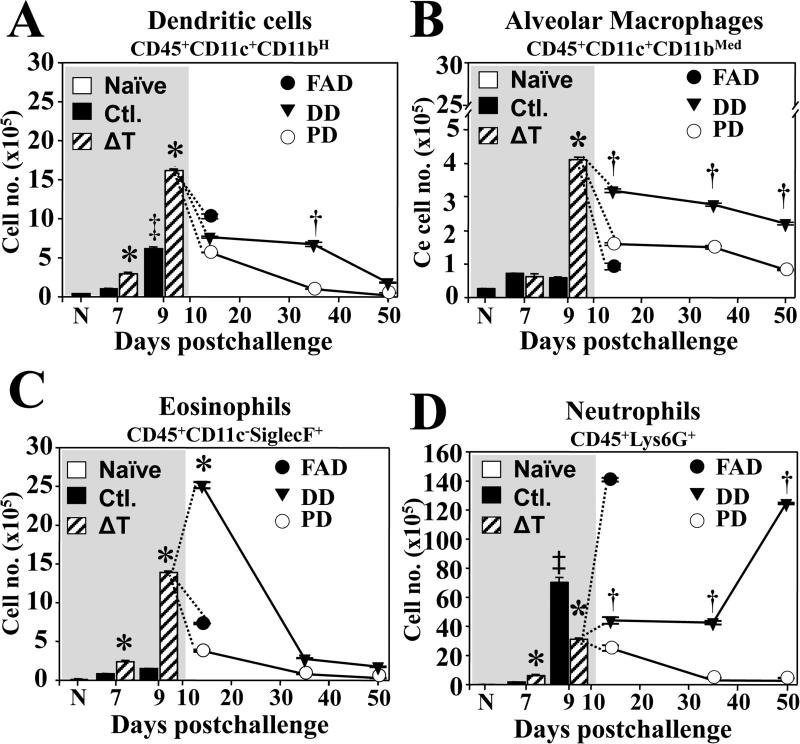
Recruitment of inflammatory innate cells during the first 9 days correlated with protection
At 7 and 9 dpc our results revealed a significant increase in numbers of dendritic cells, alveolar macrophages, eosinophils and neutrophils in lungs of both vaccinated and nonvaccinated mice compared to naïve mice, while they were significantly higher in the vaccinated animals, with the exception of neutrophils at 9 dpc (FIG. 5A-D). There was a dramatically sharp increase of neutrophils in lungs of non-vaccinated mice between 7-9 dpc, compared to vaccinated animals (FIG. 5D). We extended our analysis of these 4 types of innate cells for the mice with DD and PD disease conditions to 50 dpc. Dendritic cell, alveolar macrophage and eosinophil populations were all rapidly reduced during 14-50 dpc with an elevated rate in PD group compared to DD animals (FIG.5A-C). In contrast, numbers of neutrophils were continuously increased in lungs of the vaccinated mice with FAD and DD disease states and peaked at 14 and 50 dpc, respectively (FIG. 5D). Similarly, histopathological studies showed that vaccinated mice with disseminated disease had coalescing abscesses with concentrated inflammatory cells, mainly neutrophils, juxtaposed to large numbers of spherules (Supp, FIG.2). These results suggested that animals with disseminated disease displayed persistently inflammatory response to infection, which in combination with high fungal burden, exacerbated the damage to host tissue.
FIG. 5. Early infiltration of inflammatory innate cells in the first 9 dpc and followed by contraction after 14 dpc correlated with vaccine protection.
The lung infiltration of dendritic cells, alveolar macrophages, eosinophils and neutrophils of vaccinated versus non-vaccinated mice was first analyzed at 7 and 9 dpc. As an extension of this analysis, the infiltration of the same innate cells was examined in acute disease group at 14 dpc and in disseminated, and pulmonary disease groups at 14, 35, and 50 dpc. The absolute numbers of (A) dendritic cells (B) alveolar macrophages, (C) eosinophils, and (D) neutrophils per lungs were determined by flow cytometry. The results are presented as mean values ± SEM for each group of mice (n=4) at each time point postchallenge. Asterisks indicate significant differences in absolute numbers between innate cells of each respective group at 7 and 9 d (*, P < 0.05). The single dagger indicates significant differences between the PD and DD groups (†, P < 0.05), exclamation mark indicates significant differences between the FAD versus the DD groups (!, P < 0.05), and the double dagger indicates significant differences between the FAD and the PD groups (‡, P < 0.05) at 14, 35, or 50 d. The data presented are representative of two independent experiments.
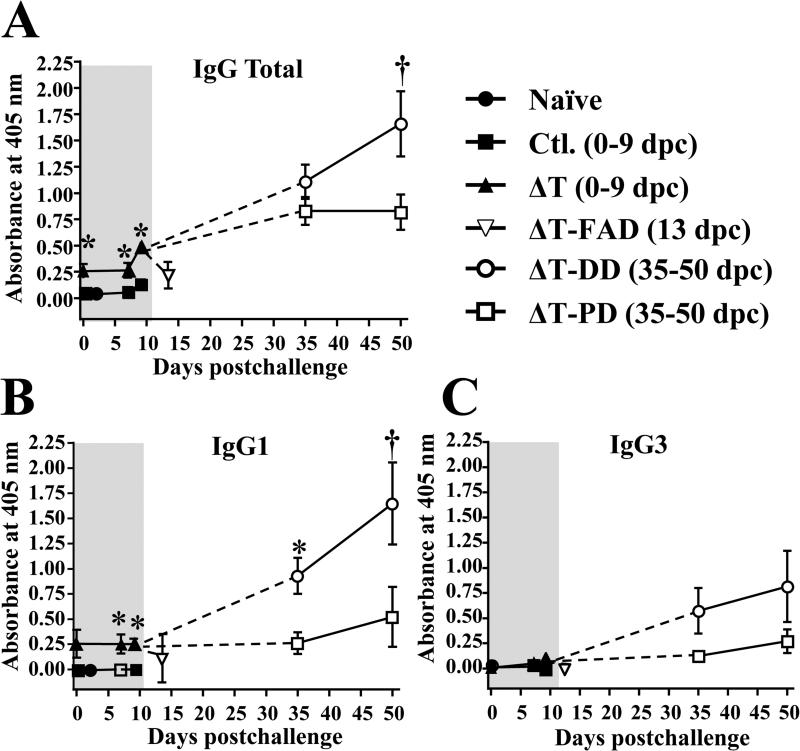
Persistently elevated antibody response correlated with disease severity
We evaluated anti-T27K titers for IgG total and selected isotypes (IgG1, IgG2c and IgG3) in sera isolated from vaccinated versus non-vaccinated mice prior to and after challenge at 0, 7 and 9 dpc. Vaccinated mice showed significantly elevated levels of IgG total and IgG1 reactivity during the first 9 dpc compared to non-vaccinated mice (FIG.6A, B), while no detectable IgG2c. At first glance, these results suggested that antibody response correlated with protection. In contrast, further analysis revealed that mice with disseminated disease showed a trend of higher IgG total, IgG1 and IgG3 antibody responses compared to mice with pulmonary disease during 35-50 dpc (FIG.6A-C). Furthermore, significant increase in IgG total and IgG1 titers were recorded at 50 dpc for mice with disseminated disease. Together, these data suggest that continuously rising anti-Coccidioides antibody production during 35-50 dpc in the vaccinated mice was indicative of poor protection outcome.
FIG. 6. Analysis of humoral immune response to Coccidioides infection revealed that the ΔT-vaccinated mice with disseminated disease had continuously rising antibody titers during the course of Coccidioides infection.
IgG total and subclass of IgG1, IgG2c and IgG3 responses to the T27K antigen by vaccinated (ΔT) and non-vaccinated mice (Ctl.) prior to and after challenge at 0, 7 and 9 dpc were determined by ELISA. IgG2c reactivity was not detected for all test samples. Sera obtained from ΔT-vaccinated mice with fatal acute disease (FAD) were also assayed at 13 dpc and for mice with pulmonary (PD) and disseminated disease (DD) at 35 and 50 dpc. Sera samples were diluted 1 to 50. The results are expressed as absorbance at 405 nm and presented as means ± SEM. The asterisks indicate statistically significant differences (P < 0.05) between vaccinated mice versus non-vaccinated control animals and the dagger indicates significant difference between the pulmonary versus disseminated groups.
4. DISCUSSION
Identification of immune correlates of protection after vaccination is important for both rational design of new vaccines that elicit tailored protective immunity and informal estimation of protective efficacy. Here we report the use of HLA-DR4 mice to evaluate protective efficacy and immune correlates of a live, attenuated (ΔT) vaccine against pulmonary coccidioidomycosis. HLA-DR4 mice are engineered on C57BL/6 mouse genetic background and express a chimeric MHC II receptor that has the same peptide binding specificity as human molecule [14, 20, 21]. We have previously compared C57BL/6 and HLA-DR4 transgenic mice for their CD4+ T-cell response to synthetic peptides containing predicted promiscuous human MHC II-binding epitopes derived from cell wall-associated proteins of Coccidioides posadasii [12, 22]. Indeed differences in MHC II-restricted epitope recognition of Coccidioides peptides were disclosed between these two strains of mice [12]. In the current study ΔT-vaccinated HLA-DR4 mice showed significantly prolonged survival and reduced fungal burden that were associated with activation of Th1 and Th17 cells, recruitment of inflammatory innate cells and elevated IgG antibody reactivity during the first 9 dpc. Our results suggest that HLA-DR4 mice can be employed for preclinical evaluation of candidate vaccines against Coccidioides infection.
We have observed reduced protective efficacy of the ΔT vaccine in HLA-DR4 mice in this study, when retrospectively compared to C57BL/6 animals [13]. The vaccination regimens and challenge protocol were the same as that used in our previous evaluation of protective efficacy of the ΔT vaccine in a C57BL/6 mouse model of pulmonary Coccidioides infection [7, 13]. All ΔT-vaccinated C57BL/6 mice survived to at least 75 days after a potentially lethal challenge, while only 60% of the vaccinated HLA-DR4 mice survived to 50 dpc. The precise mechanisms underlying such phenotypic variation between these two strains of mice are still elusive. One potential factor is that murine MHC-II and human HLA-DR4 molecules recognize and present different sets of inlaid epitopes in ΔT vaccine that affects robustness and durability of protective immunity against coccidioidomycosis. Inbred HLA-DR4 mice are presumed to be virtually isogenic, while they display phenotypic variability of vaccination outcome among inbred littermates against pulmonary Coccidioides infection. Variability is likely to be reflected in, and potentially driven by, epigenetic differences of individual HLA-DR4 mice. Epigenetic variability has contributed to differences in phenotypic traits determined in embryotic or mature stages of inbred mice [23, 24]. For example, inbred Avy mice carrying the agouti viable yellow allele show a range of coat colors associated with diverse DNA methylation states at the locus, but not difference in genetic DNA sequences [23]. The methylation state of the locus within an individual is conserved across tissue types suggesting establishment very early in embryonic development [23]. Difference in epigenetic DNA methylation can also occur in mature individual mice during differentiation and development of immune cells against microbial infections [24]. A whole genome analysis of changes in DNA methylation pattern during differentiation of naïve CD4+ T cells into variable subsets has also shown that Th17 cells display high demethylation of 7 signature loci compared to Th1 cells [24]. Even though DNA methylation status of the individual HLA-DR4 and C57BL/6 mice used in our experiments is unknown, non-vaccinated HLA-DR4 and C57BL/6 mice appear to have similar susceptibility to pulmonary Coccidioides infection [13, 25]. These data argue that susceptibility of naïve mice of these two strains is not likely a factor that contributes to the observed difference in protective efficacy against Coccidioides infection. On the other hand, epigenetic variability in vaccine-induced immune response of individual mice may be a potential underlying factor.
We have identified body weight as a protective correlate, which have allowed us to further dissect immunological markers that can be used to predict the outcome of vaccination. Both clinical and laboratory studies have shown that body weight loss in patients and experimental animals is a commonly assessable marker of disease severity and a correlate of vaccination efficacy against microbial infections [26-30]. Daily monitoring of body weight of Coccidioides-infected mice allowed us to identify three distinct body weight profiles that correlated with fatal acute, disseminated, and pulmonary disease forms of coccidioidomycosis. Vaccinated mice with pulmonary disease showed early recruitment of Th1 and Th17 cells plus inflammatory innate cells that gradually dampened between 14-50 dpc. Lessened inflammatory response during this period postchallenge correlated with well-formed granulomas, minimal tissue damage, and absence of intact fungal cells. Thus we have revealed that early activation of T cells, expansion of Th1 and Th17 subpopulations and moderate recruitment of inflammatory innate cells are protective correlates of vaccination against pulmonary coccidioidomycosis for HLA-DR4 mice and they are the same essential immune response for susceptible C57BL/6 mice[7, 8, 31-33].
The nature of a protective outcome depends on the amount of host damage that results from interplay between microbial pathogenesis factors and host immune response [34]. Both extremely weak and overly exuberant host immune responses to Coccidioides infection can cause severe tissue damage as seen in non-vaccinated and vaccinated mice displaying acute fatal disease, respectively. The sudden diminishment of a protective T cell response displayed by vaccinated HLA-DR4 mice with fatal acute disease concomitantly occurring with a sharp increase of neutrophils during 9-14 dpc is likely the result of a negative influence of an exuberant inflammatory response. Inflammation induced hypoxia occurring in lung microenvironment may affect vigorous changes of T cell metabolism that is required for activation, expansion and effector function. Similarly, during the contraction phase of an immune response, metabolic regulation is crucial for restraining inflammatory responses and long-term maintenance of memory T cells [35]. In support of this observation, DBA/2 mice that are naturally resistant to Coccidioides infection express elevated levels of hypoxia-inducible factor 1 compared to susceptible C57BL/6 mice [36].
IgG subclass switch is T-cell dependent. IgG1 and IgG3 are induced by Th17 cells, while IgG2c by IFN-γ and Th1 cells [37-39]. Increased IgG1 titers to Coccidioides T27K antigen concurs with expansion of protective Th17 cells in lungs of vaccinated HLA-DR4 mice during the first 9 dpc, suggesting antibody response correlated with protection. On the contrary, antibody activities continuously rose and were significantly elevated in mice with disseminated disease compared to animals displaying pulmonary disease during 35-50 dpc. These data suggested that antibody played a minor role and was not essential for vaccine immunity during this period of time. Similarly, elevated titers of complement-fixing (CF) antibody are diagnostic of coccidioidal infection and rising CF titers is an indicator of disease prognosis [40]. Though these data do not rule out the existence of protective antibodies against Coccidioides infection, they are in agreement with the clinical finding that high antibody reactivity with Coccidioides antigens correlates with prognosis of chronic coccidioidomycosis.
In conclusion, we have demonstrated that maintenance of body weight, early expansion of Th1, Th17 and innate inflammatory cells including macrophages, dendritic cells, neutrophils and eosinophils in lungs of vaccinated HLA-DR4 mice during the first 9 days post Coccidioides challenge correlates with protection. In contrast, overwhelming recruitment of neutrophils, sustaining strong Th1 and Th17 response and continuously rising high antibody titers againstCoccidioides after 14 days postchallenge correlate with severity of this mycosis. We also suggest that HLA-DR4 transgenic mouse model of coccidioidomycosis is a practical animal model for preclinical evaluation of protective efficacy of vaccine candidates, especially subunit vaccines containing human epitopes against Coccidioides infection.
Supplementary Material
Highlights.
HLA-DR4 mice can be used to evaluate vaccine efficacies against Valley Fever
Maintenance of body weight correlates with vaccine protection
Early expansion of Th1 and Th17 cells before 9 dpc correlate with protection
Persistence of adaptive immunity after 35 dpc associates with poor protection
ACKNOWLEDGEMENTS
This work was supported by research grants from the National Institutes of Health (R01 AI-071118; R21 AI114762). Additional support was provided by Coccidioides research funds donated by the Valley Fever of the Americas Foundation and community supporters.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Brown J, Benedict K, Park BJ, Thompson GR., 3rd Coccidioidomycosis: epidemiology. Clin Epidemiol. 2013;5:185–197. doi: 10.2147/CLEP.S34434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guevara RE, Motala T, Terashita D. The Changing Epidemiology of Coccidioidomycosis in Los Angeles (LA) County, California, 1973-2011. PLoS One. 2015;10:e0136753. doi: 10.1371/journal.pone.0136753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steele C, Wormley FL., Jr Immunology of fungal infections: lessons learned from animal models. Curr Opin Microbiol. 2012;15:413–419. doi: 10.1016/j.mib.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Clemons KV, Capilla J, Stevens DA. Experimental animal models of coccidioidomycosis. Ann N Y Acad Sci. 2007;1111:208–224. doi: 10.1196/annals.1406.029. [DOI] [PubMed] [Google Scholar]
- 5.Cole GT, Xue JM, Okeke CN, Tarcha EJ, Basrur V, Schaller RA, et al. A vaccine against coccidioidomycosis is justified and attainable. Med Mycol. 2004;42:189–216. doi: 10.1080/13693780410001687349. [DOI] [PubMed] [Google Scholar]
- 6.Cox RA, Magee DM. Coccidioidomycosis: host response and vaccine development. Clin Microbiol Rev. 2004;17:804–839. doi: 10.1128/CMR.17.4.804-839.2004. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung CY, Gonzalez A, Wuthrich M, Klein BS, Cole GT. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17). Infect Immun. 2011;79:4511–4522. doi: 10.1128/IAI.05726-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest. 2011;121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oldfield EC, 3rd, Bone WD, Martin CR, Gray GC, Olson P, Schillaci RF. Prediction of relapse after treatment of coccidioidomycosis. Clin Infect Dis. 1997;25:1205–1210. doi: 10.1086/516115. [DOI] [PubMed] [Google Scholar]
- 10.Mori M, Beatty PG, Graves M, Boucher KM, Milford EL. HLA gene and haplotype frequencies in the North American population: the National Marrow Donor Program Donor Registry. Transplantation. 1997;64:1017–1027. doi: 10.1097/00007890-199710150-00014. [DOI] [PubMed] [Google Scholar]
- 11.Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, et al. Several common HLA-DR types share largely overlapping peptide binding repertoires. J Immunol. 1998;160:3363–3373. [PubMed] [Google Scholar]
- 12.Tarcha EJ, Basrur V, Hung CY, Gardner MJ, Cole GT. Multivalent recombinant protein vaccine against coccidioidomycosis. Infect Immun. 2006;74:5802–5813. doi: 10.1128/IAI.00961-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xue J, Chen X, Selby D, Hung CY, Yu JJ, Cole GT. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect Immun. 2009;77:3196–3208. doi: 10.1128/IAI.00459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito K, Bian HJ, Molina M, Han J, Magram J, Saar E, et al. HLA-DR4-IE chimeric class II transgenic, murine class II-deficient mice are susceptible to experimental allergic encephalomyelitis. J Exp Med. 1996;183:2635–2644. doi: 10.1084/jem.183.6.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madsen LS, Andersson EC, Jansson L, krogsgaard M, Andersen CB, Engberg J, et al. A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nat Genet. 1999;23:343–347. doi: 10.1038/15525. [DOI] [PubMed] [Google Scholar]
- 16.Xue J, Hung C-Y, Yu J-J, Cole GT. Immune response of vaccinated and non-vaccinated mice to Coccidioides posadasii infection. Vaccine. 2005;23:3535–3544. doi: 10.1016/j.vaccine.2005.01.147. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann CR, Johnson SM, Martens GW, White AG, Zimmer BL, Pappagianis D. Protection against lethal murine coccidioidomycosis by a soluble vaccine from spherules. Infection & Immunity. 1998;66:2342–2345. doi: 10.1128/iai.66.5.2342-2345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung CY, Hurtgen BJ, Bellecourt M, Sanderson SD, Morgan EL, Cole GT. An agonist of human complement fragment C5a enhances vaccine immunity against Coccidioides infection. Vaccine. 2012;30:4681–4690. doi: 10.1016/j.vaccine.2012.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ampel NM, Kramer LA, Li L, Carroll DS, Kerekes KM, Johnson SM, et al. In vitro whole-blood analysis of cellular immunity in patients with active coccidioidomycosis by using the antigen preparation T27K. Clin & Diag Lab Immunol. 2002;9:1039–1043. doi: 10.1128/CDLI.9.5.1039-1043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snir O, Rieck M, Gebe JA, Yue BB, Rawlings CA, Nepom G, et al. Identification and functional characterization of T cells reactive to citrullinated vimentin in HLA-DRB1*0401-positive humanized mice and rheumatoid arthritis patients. Arthritis Rheum. 2011;63:2873–2883. doi: 10.1002/art.30445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Touloukian CE, Leitner WW, Topalian SL, Li YF, Robbins PF, Rosenberg SA, et al. Identification of a MHC class II-restricted human gp100 epitope using DR4-IE transgenic mice. J Immunol. 2000;164:3535–3542. doi: 10.4049/jimmunol.164.7.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarcha EJ, Basrur V, Hung CY, Gardner MJ, Cole GT. A recombinant aspartyl protease of Coccidioides posadasii induces protection against pulmonary coccidioidomycosis in mice. Infect Immun. 2006;74:516–527. doi: 10.1128/IAI.74.1.516-527.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oey H, Isbel L, Hickey P, Ebaid B, Whitelaw E. Genetic and epigenetic variation among inbred mouse littermates: identification of inter-individual differentially methylated regions. Epigenetics Chromatin. 2015;8:54. doi: 10.1186/s13072-015-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang BH, Floess S, Hagemann S, Deyneko IV, Groebe L, Pezoldt J, et al. Development of a unique epigenetic signature during in vivo Th17 differentiation. Nucleic Acids Res. 2015;43:1537–1548. doi: 10.1093/nar/gkv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurtgen BJ, Hung CY, Ostroff GR, Levitz SM, Cole GT. Construction and evaluation of a novel recombinant T cell epitope-based vaccine against coccidioidomycosis. Infect Immun. 2012;80:3960–3974. doi: 10.1128/IAI.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abuodeh RO, Shubitz LF, Siegel E, Snyder S, Peng T, Orsborn KI, et al. Resistance to Coccidioides immitis in mice after immunization with recombinant protein or a DNA vaccine of a proline-rich antigen. Infect Immun. 1999;67:2935–2940. doi: 10.1128/iai.67.6.2935-2940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clemons KV, Sobel RA, Williams PL, Pappagianis D, Stevens DA. Efficacy of intravenous liposomal amphotericin B (AmBisome) against coccidioidal meningitis in rabbits. Antimicrob Agents Chemother. 2002;46:2420–2426. doi: 10.1128/AAC.46.8.2420-2426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortez KJ, Walsh TJ, Bennett JE. Successful treatment of coccidioidal meningitis with voriconazole. Clin Infect Dis. 2003;36:1619–1622. doi: 10.1086/375235. [DOI] [PubMed] [Google Scholar]
- 29.Gibbs BT, Neff RT. A 22-year-old Army private with chest pain and weight loss. Mil Med. 2004;169:157–160. doi: 10.7205/milmed.169.2.157. [DOI] [PubMed] [Google Scholar]
- 30.Park DW, Sohn JW, Cheong HJ, Kim WJ, Kim MJ, Kim JH, et al. Combination therapy of disseminated coccidioidomycosis with caspofungin and fluconazole. BMC Infect Dis. 2006;6:26. doi: 10.1186/1471-2334-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung CY, Castro-Lopez N, Cole GT. Vaccinated C57BL/6 mice develop protective and memory T cell responses to Coccidioides posadasii infection in the absence of interleukin-10. Infect Immun. 2014;82:903–913. doi: 10.1128/IAI.01148-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung CY, Jimenez-Alzate Mdel P, Gonzalez A, Wuthrich M, Klein BS, Cole GT. Interleukin-1 receptor but not Toll-Like receptor 2 is essential for MyD88-dependent Th17 immunity to Coccidioides infection. Infect Immun. 2014;82:2106–2114. doi: 10.1128/IAI.01579-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, LeBert V, Hung CY, Galles K, Saijo S, Lin X, et al. C-type lectin receptors differentially induce Th17 cells and vaccine immunity to the endemic mycosis of North America. J Immunol. 2014;192:1107–1119. doi: 10.4049/jimmunol.1302314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pirofski LA, Casadevall A. The damage-response framework of microbial pathogenesis and infectious diseases. Adv Exp Med Biol. 2008;635:135–146. doi: 10.1007/978-0-387-09550-9_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Man K, Kallies A. Synchronizing transcriptional control of T cell metabolism and function. Nat Rev Immunol. 2015;15:574–584. doi: 10.1038/nri3874. [DOI] [PubMed] [Google Scholar]
- 36.Woelk CH, Zhang JX, Walls L, Viriyakosol S, Singhania A, Kirkland TN, et al. Factors regulated by interferon gamma and hypoxia-inducible factor 1A contribute to responses that protect mice from Coccidioides immitis infection. BMC Microbiol. 2012;12:218. doi: 10.1186/1471-2180-12-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker DC. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 38.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 39.Mitsdoerffer M, Lee Y, Jager A, Kim HJ, Korn T, Kolls JK, et al. Proinflammatory T helper type 17 cells are effective B-cell helpers. Proc Natl Acad Sci U S A. 2010;107:14292–14297. doi: 10.1073/pnas.1009234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pappagianis D, Zimmer BL. Serology of coccidioidomycosis. Clin Microbiol Rev. 1990;3:247–268. doi: 10.1128/cmr.3.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.