Abstract
Negative affective stimuli elicit behavioral and neural responses which vary on a continuum from adaptive to maladaptive, yet are typically investigated in a dichotomous manner (healthy controls vs. psychiatric diagnoses). This practice may limit our ability to fully capture variance from acute responses to negative affective stimuli to psychopathology at the extreme end. To address this, we conducted a functional magnetic resonance imaging study to examine the neural responses to negative valence/high arousal and neutral valence/low arousal images as a function of dysphoric mood and sex across individuals (n = 99) who represented traditional categories of healthy controls, major depressive disorder, bipolar psychosis, and schizophrenia. Observation of negative (vs. neutral) stimuli elicited blood oxygen‐level dependent responses in the following circuitry: periaqueductal gray, hypothalamus (HYPO), amygdala (AMYG), hippocampus (HIPP), orbitofrontal cortex (OFC), medial prefrontal cortex (mPFC), and greater connectivity between AMYG and mPFC. Across all subjects, severity of dysphoric mood was associated with hyperactivity of HYPO, and, among females, right (R) AMYG. Females also demonstrated inverse relationships between severity of dysphoric mood and connectivity between HYPO ‐ R OFC, R AMYG ‐ R OFC, and R AMYG ‐ R HIPP. Overall, our findings demonstrated sex‐dependent deficits in response to negative affective stimuli increasing as a function of dysphoric mood state. Females demonstrated greater inability to regulate arousal as mood became more dysphoric. These findings contribute to elucidating biosignatures associated with response to negative stimuli across disorders and suggest the importance of a sex‐dependent lens in determining these biosignatures. Hum Brain Mapp 37:3733–3744, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: dysphoric mood state, sex, functional magnetic resonance imaging, generalized psychophysiological interaction, negative affect, International Affective Picture System, Research Domain Criteria
INTRODUCTION
Maladaptive responses to negative affective stimuli are implicated in several major psychiatric disorders, including psychoses and major depressive disorder [Beauregard et al., 2006; Goldstein, 2006; Goldstein et al., 2015; Holsen et al., 2013; Monroe and Harkness, 2005; Myin‐Germeys et al., 2003; Rowland et al., 2013]. Even though behavioral and neural system responses to negative affective stimuli are typically investigated in a dichotomous manner comparing “healthy controls” to those with a psychiatric diagnosis, they vary on a continuum from adaptive to maladaptive. A focus on healthy versus psychopathological groups only may miss important variability in particular symptoms that are shared across populations and may provide clues to mechanisms underlying responses to negative experiences in life.
Circuitry associated with response to negative affective stimuli includes periaqueductal gray (PAG), hypothalamus (HYPO), amygdala (AMYG), hippocampus (HIPP), anterior cingulate cortex (ACC), medial prefrontal cortex (mPFC), and orbital prefrontal cortex (OFC) [Dougherty and Rauch, 1997; Goldstein et al., 2015, 2010; Holsen et al., 2013; Mayberg, 1997] regions that are among the most sexually dimorphic in the brain. Activity in these regions has been associated with cortisol response [Cunningham‐Bussel et al., 2009; Holsen et al., 2013; Kern et al., 2008; Liberzon et al., 2007; Urry et al., 2006; Veer et al., 2012], coupled with loss of parasympathetic cardiac response [Holsen et al., 2012], demonstrating neural and physiologic stress responses. Moreover, Wang et al. [2007] reported sex differences in the relationships between cortisol and brain response to psychological stress. HIPP, HYPO, AMYG, mPFC, and ACC are dense in sex steroid and glucocorticoid receptors [McEwen et al., 1986; Pacak et al., 1995; Tobet and Hanna, 1997]. We previously suggested that developmental alteration of normal sexual dimorphisms of this circuitry is associated with sex differences in abnormal neuroendocrine function and stress response circuitry function in adulthood and may predispose for sex differences in development of schizophrenia or depression [Goldstein, 2006; Goldstein et al., 2014b, 2014a]. Taken together, studies across depression and psychoses suggest there may be shared pathophysiology associated with dysregulation of circuitry associated with negative affective stimuli [Beauregard et al., 2006; Derntl et al., 2008; Domes et al., 2010; Goldstein, 2006; Goldstein et al., 2015; Monroe and Harkness, 2005; Myin‐Germeys et al., 2003; Rowland et al., 2013], contributing to sex differences across these disorders and possibly underlying variation in depressed mood across healthy and clinical populations.
In fact, this notion of shared traits across disorders has been underscored by the recent focus of NIMH on the Research Domain Criteria (RDoC) initiative [Insel et al., 2010]. Proponents of the RDoC approach argue that, for the development of more efficacious therapeutics, it is critical to identify genes, cells, and circuits associated with behavioral traits (i.e., neurobiologic signatures [Van Os and Kapur, 2009]) rather than diagnoses per se. Inspired by the RDoC framework, we used functional magnetic resonance imaging (fMRI) to investigate the impact of dysphoric mood state and sex on response to negative valence stimuli across healthy and clinical populations.
There is a long history to the finding that mood disorders are significantly more prevalent among women than men [Goldstein et al., 2014b; Kendler et al., 2006; Kessler, 2003; Seney and Sibille, 2014]. Animal studies demonstrated sex differences (greater in females than males) in a number of domains, including greater immobility in tasks associated with mood‐related phenotypic behavior [Alonso et al., 2000], increased ACTH, corticosterone, and glucocorticoid receptor binding [McCormick et al., 1995; Weinstock et al., 1992], and increased corticosterone sensitivity [Rhodes and Rubin, 1999]. A recent neuroimaging study in humans reported sex differences in fronto‐limbic connectivity with women having more affective and men more evaluative responses to negative affective stimuli [Lungu et al., 2015]. In the study reported here, we hypothesized that hyperactivity in subcortical arousal regions and hypoactivity in cortical regions and HIPP would be associated with severity of dysphoric mood, and that these deficits in ability to regulate neural response to negative affective stimuli will be greater among women than men.
METHODS AND MATERIALS
Participants
Ninety‐nine adults (51 males) participated in this study. The majority were offspring of women who took part in the large scale (n = 17 741) Boston and Providence Collaborative Perinatal Project ([CPP], adult offspring whom we have been following for about 20 years in the New England Family Studies [NEFS] [Goldstein et al., 2014a; Niswander and Gordon, 1972]. Approximately 14% were non‐NEFS subjects but recruited using the same criteria, from the same community catchment area and not different on any sociodemographic or clinical characteristic than the rest of the sample. Fifteen individuals were diagnosed with non‐affective psychosis including schizophrenia, schizoaffective‐depressed type, and psychosis not otherwise specified (SCZ; 9 males), 16 individuals with affective psychosis including bipolar disorder with psychosis and schizoaffective disorder‐bipolar type (BP; 7 males), 27 with recurrent major depressive disorder without psychosis (MDD; 13 males), and 41 were healthy controls (CTRL; 22 males). These PSY classifications have a long history and validation in family studies and have been used in our population‐level NEFS studies over many years [Goldstein et al., 2010a]. Individuals with either MDD or SCZ were not currently in an episode (no clinically‐significant depressive symptoms in the MDD group; no active psychosis in the SCZ group).
As described in Jacobs et al. [2015], women were scanned on two occasions ‐ “low E2” and “high E2” conditions, categorized based on the relative change in 17β‐estradiol (E2) between two scanning visits (during early follicular and late follicular/midcycle). In our previous work with this task, we demonstrated more substantial sex differences in brain activity in our circuitry of interest for women during late follicular/midcyle versus men in comparison to the same women scanned during the early follicular menstrual phases versus men [Goldstein et al., 2010b]. This is consistent with others [Ossewaarde et al., 2010] who demonstrated that sensitivity to negative affect differed across menstrual cycle phase. Thus, in the analyses here, we included data from women scanned under “high E2” only, thus eliminating a potential confound among the women with respect to brain activity in AMYG, HIPP, and HYPO in response to negative affective stimuli. The mean baseline levels of estradiol were 102.88 pg/mL (SD = 74.95 pg/mL), which did not differ after exposure to the negative affective images.
Demographics and clinical characteristics of the sample are in Table 1. All participants provided written informed consent to a protocol approved by Harvard University, Brown University, Partners Healthcare system, and local psychiatric facilities. There were no differences between men and women in ethnicity, handedness, age, body mass index, parental SES, educational level, WAIS‐R vocabulary scores, rate of DSM diagnoses, or use of psychotropic medications. Compared to women, men scored higher on the WAIS block design (F (2,93)=4.29, P = 0.02; CTRL > PSY: P = 0.02) and had a higher rate of substance abuse disorders ( N = 99)=21.80, P < 0.0001).
Table 1.
Demographics and clinical characteristics of the sample
| Males (n = 51) | Females (n = 48) | Between group comparison | |
|---|---|---|---|
| Ethnicity | |||
| % Caucasian (n) | 68.63% (35/51) | 52.08% (25/48) | No difference |
| % Other (n) | 3.92% (2/51) | 14.58% (7/48) | |
| % Missing (n) | 27.45% (14/51) | 33.33% (16/48) | |
| Handedness | |||
| % Right‐handed (n) | 90.20% (46/51) | 93.75% (45/48) | No difference |
| Age | |||
| in years; M (SD) | 45.02 (3.88) | 44.33 (4.77) | No difference |
| BMI | |||
| M (SD) | 29.17 (5.15) | 29.86 (6.89) | No difference |
| Parental SESa | |||
| % Lowest SES quartile (n) | 23.53% (12/51) | 20.83% (10/48) | No difference |
| % Lower middle SES quartile (n) | 23.53% (12/51) | 18.75% (9/48) | |
| % Higher middle SES quartile (n) | 21.57% (11/51) | 25.00% (12/48) | |
| % Highest SES quartile (n) | 19.61% (10/51) | 20.83% (10/48) | |
| % Missing (n) | 11.76% (6/51) | 12.50% (6/48) | |
| Education level | |||
| % Without completed high school (n) | 9.8% (5/51) | 4.17% (2/48) | No difference |
| % Completed high school (n) | 21.57% (11/51) | 14.58% (7/48) | |
| % More than high school (n) | 66.67% (34/51) | 77.08% (37/48) | |
| % Missing (n) | 1.96% (1/51) | 4.17% (2/48) | |
| WAIS vocabulary, age‐scaled | |||
| M (SD) | 11.18 (3.35) | 10.09 (3.08) | No difference |
| WAIS block design, age‐scaled | |||
| M (SD) | 11.67 (3.35) | 9.74 (2.98) | Males > Females |
| DSM‐based diagnosis | |||
| % MDD in remission (n) | 25.49% (13/51) | 29.17% (14/48) | No difference |
| % Psychosis (n) | 31.37% (16/51) | 31.25% (15/48) | |
| % Healthy controls (n) | 43.14% (22/51) | 39.58% (19/48) | |
| Psychotropic medication | |||
| % on psychotropic medication (n) | 35.29% (18/51) | 31.25% (15/48) | No difference |
| Substance use disorder | |||
| % with any substance use disorder (n) | 64.72% (33/51) | 16.67% (8/48) | Males > Females |
Parental socioeconomic status (SES) was assigned a single, continuous score for education, occupation, and family income according to the system used for the United States Bureau of the Census (Myrianthopoulos and French, 1968). This composite index ranged from 0.0 (low) to 9.5 (high).
Assessment of Mood and Anxiety
Profile of Mood States (POMS) [McNair et al., 1992] and State‐Trait Anxiety Inventory (STAI) [Spielberger et al., 1983], self‐report questionnaires, were administered before the MRI scan to assess mood and anxiety. The POMS measured the degree to which each of 40 affective adjectives applied to current mood state (rated on a Likert‐type scale from 0 to 4). The STAI measured anxiety‐related symptoms using a 1 to 4 Likert‐type scale, with two versions that differentiate “state” (How do you feel right now) and “trait” (How do you generally feel) anxiety.
Dysphoric Mood State
To create a composite measure of mood and anxiety current state and traits, the five POMS subscales (tension/anxiety [TA], depression/dejection [DD], anger/hostility [AH], fatigue/inertia [FI], vigor/activity [VA], confusion/bewilderment [CB]), and two STAI subscales (Anxiety State, Anxiety Trait) were factor analyzed using the maximum likelihood factor method and Varimax rotation. The primary factor explaining the highest portion of variance reflected a clinical state of dysphoric feelings that we traditionally associate with depressed mood (see Supporting Information). We called this composite measure dysphoric mood state, to distinguish it from depression itself, given that we are measuring this clinical state across populations. In subsequent fMRI analyses, it is used in associations with blood oxygen‐level dependent (BOLD) brain activity responses and connectivity in our circuitry of interest.
Acquisition of fMRI Data
fMRI data were acquired on a Siemens Tim Trio 3T MRI scanner with a 12‐channel head coil. A total of 180 volumes were acquired using a spin echo, T2*‐weighted sequence (TR = 2,000 ms, TE = 40 ms, FOV = 200 × 200 mm, matrix 64 × 64, in‐plane resolution 3.125 mm, slice thickness 5 mm, 23 contiguous slices aligned to AC‐PC plane). The fMRI task consisted of presentation of negative valence/high arousal (e.g., snake, car accident, burial, gun, bomb, tornado), neutral valence/low arousal (e.g., plant, umbrella, office, mushrooms, stool, bus), and fixation images (Fourier transformations of the neutral valence/low arousal images), that we adapted for fMRI use [Goldstein et al., 2005, 2010b] based on quantitative psychophysiologic ratings from the International Affective Picture System (IAPS) [NIMH Center for Emotion and Attention, 1999]. The IAPS is a well‐known set of images with demonstrated reliability and validity, known to invoke brain circuitry associated with negative affective stimuli, HPA hormone responses, and behavior [Bradley and Lang, 1994; Goldstein et al., 2005, 2010b; Holsen et al., 2013; Lang et al., 1997]. Our fMRI‐adapted IAPS task lasted approximately 18 min (3 runs, 6 min each). Each run contained a total of 72 images ordered in non‐randomized blocks of fixation, negative and neutral images. Each block of stimuli consisted of six images, each presented for 5 sec. (The specific IAPS images are provided in Supporting Information.) The mean normative valence and arousal of the negative valence/high arousal images was 2.19 (SD = 0.48) and 6.35 (SD = 0.52), respectively. The mean normative valence and arousal of the neutral valence/low arousal images was 4.90 (SD = 0.37) and 2.91 (SD = 0.40), respectively. To ensure attention, participants pressed a button when each new image appeared. No cognitive task was required to respond to these images, thus responses were unconfounded by cognitive capacities. After the fMRI session, participants saw eight neutral and eight negative images, chosen from those presented during the fMRI task, and reported their subjective evaluation of image valence and arousal using the Self‐assessment Manikin (SAM) [Bradley and Lang, 1994]. For details on the experimental task, see [Goldstein et al., 2005, 2010b; Holsen et al., 2013].
Analysis of fMRI Data
Data were preprocessed and analyzed using SPM8 [The FIL Methods Group, 2013]. Non‐linear volume‐based normalization used the MNI152 brain template and spatial smoothing with 6 mm FWHM Gaussian filter, which was then re‐sampled to 3 mm isotropic. Outliers in global mean image time series (threshold: 3.5 SDs from the mean) and movement (threshold: 0.7 mm, measured as scan‐to‐scan movement, separately for translation and rotation) were detected using an artifact detection toolbox [NITRC, 2011] and entered as nuisance regressors in the first‐level, single‐subject general linear model (GLM). Masks excluding voxels outside the brain were applied to ensure that voxels in regions with high inter‐participant variability in signal drop‐out (e.g., OFC) were not arbitrarily excluded. Comparisons of interest (negative > neutral) from the first‐level, single‐subject analyses were tested using linear contrasts and SPM t‐maps. Outputs from the first‐level, single‐subject analyses were submitted to second‐level random effects analysis (see below).
BOLD‐Response Analysis
One‐sample t‐test examined the BOLD signal responses to negative > neutral stimuli across the full sample (n = 99), with a whole‐brain, voxel‐wise family‐wise error (FWE)‐corrected threshold of P < 0.05. Next, an intersection analysis performed using MarsBaR [Brett et al., 2002], identified clusters with whole‐brain, voxel‐wise FWE‐corrected P < 0.05 threshold that were conjointly located within anatomic boundaries of PAG, HYPO, AMYG, HIPP, OFC, ACC, and mPFC (anatomical masks defined using a manually segmented MNI‐152 brain template). Given the small volumes and midline location of the PAG and HYPO, single regions of interest (ROIs) combining right and left hemispheres were used for each of these two regions; remaining ROI masks were bilateral. Average parameter estimates (percent signal change values) within each ROI were extracted for each participant using REX [REX Software, 2009] and exported into JMP (SAS Institute, Cary, NC), which was used for all remaining BOLD response analyses. Activity of the negative affective circuitry, operationalized as parameter estimates identified and extracted as described above, was then explored as a function of Dysphoric Mood State using regression analysis to identify main effects and interactions with sex, significant at P < 0.05 level. Finally, although not the main focus of this article, regression models were re‐run with the inclusion of diagnostic case status (no history of MDD/SCZ; history of MDD/SCZ) and psychotropic medication status (no current psychotropic medication; current use of psychotropic medication) in the models to explore the potential impact of these factors on BOLD activity.
Functional Connectivity Analysis
Similar to BOLD response analyses above, we assessed task‐related connectivity using generalized psychophysiological interaction [McLaren et al., 2012]. Time courses from seed ROIs (defined as above: clusters from the BOLD‐anatomic ROI intersection analyses in MarsBaR) were extracted and added to two additional PPI regressors (interaction of the seed time course with regressors for negative and neutral content) to individual subject‐level GLMs. These interaction regressors were orthogonal to the task and seed regressors, ensuring that the seed ROI activation and PPI connectivity were independent [McLaren et al., 2012]. Connectivity was measured at single‐subject level by estimating the difference between the interaction of the seed timecourse with the regressor for negative compared with neutral stimuli, conducted separately for each ROI. Results of single‐subject analysis were entered into second‐level random effects analysis to probe group‐level changes in connectivity during negative versus neutral condition.
First, task‐related connectivity was assessed across the whole sample, using clusters from subcortical arousal regions (PAG, HYPO, AMYG) as seeds. These were chosen based on known neuroanatomical connections between these regions and cortical ROIs. Next, connectivity was explored as a function of Dysphoric Mood State, mirroring BOLD‐response analyses and with seed regions chosen from subcortical arousal ROIs that emerged as significant within each parallel analysis at the BOLD‐level response. Analyses were then repeated to detect interactions with sex.
For these functional connectivity analyses, we used small volume correction (SVC) approach in SPM8, which limits voxel‐wise analyses to voxels within a priori hypothesized ROIs. Target ROIs (PAG, HYPO, AMYG, HIPP, OFC, ACC, mPFC) were defined as anatomical masks (manually segmented from MNI‐152 brain template, as described above) and implemented as overlays on the SPM8 canonical brain. False positives were controlled using FWE‐correction: within an anatomical ROI, significant results identified using SVC (initial voxel‐wise threshold P < 0.05 uncorrected) were reported as significant if they additionally met the peak‐level threshold of P < 0.05, FWE‐corrected. Additionally, for calculation of effect sizes and illustrative purposes in figures below, average connectivity values (beta weights of PPI regressors) in significant target clusters were extracted using REX [REX Software, 2009]. Furthermore, regression models were re‐run with the inclusion of diagnostic case status (no history of MDD/SCZ; history of MDD/SCZ) and psychotropic medication status (no current psychotropic medication; current use of psychotropic medication) in the models to explore the potential impact of these factors on functional connectivity.
RESULTS
Factor Analysis of Mood and Anxiety Symptoms
Factor analysis identified two main composite measures of mood and anxiety states and traits. The first factor, explaining 33% of variance, reflected mainly dysphoric mood state and thus we will refer to this factor as “Dysphoric Mood.” The second factor explained 22.7% of variance and reflected mainly anxiety‐related symptoms (see Supporting Information Table 1 for the exact rotated factor loadings). There was a main effect of sex on Factor 1 (F (5,68)=12.50, P < 0.0001, R 2 = 0.48), with men exhibiting worse dysphoric mood than women (t (73) = −7.78, P < 0.0001, Cohen's d = 1.72). Although this was initially unexpected, men had more substance abuse and dependence, and subjects with substance use disorders expressed more severe dysphoric feelings (t (73) = −3.14, P = 0.002), thus contributing to explaining the higher mean levels of dysphoric mood among the men. Means and standard deviations for the STAI scaled scores, POMS subscale scores, and composite factors by sex are provided in Supporting Information Table 2. Men and women did not differ on SAM ratings of valence and arousal for the IAPS stimuli (see Supporting Information Table 2).
fMRI Data: BOLD Response and Functional Connectivity
First, BOLD response to negative (>neutral) stimuli was examined at the whole‐brain analysis level (FWE P < 0.05) to establish whether the task elicited significant activity in the a priori ROIs. Whole brain analysis across all subjects revealed significantly increased BOLD response to negative (vs. neutral) stimuli in the PAG, HYPO, AMYG, HIPP, mPFC, and OFC (Fig. 1A). Additionally, connectivity analyses using PAG, HYPO, and AMYG as seeds showed that observation of negative (vs. neutral) stimuli was associated with increased right (R) AMYG – R mPFC connectivity (Fig. 1B).
Figure 1.
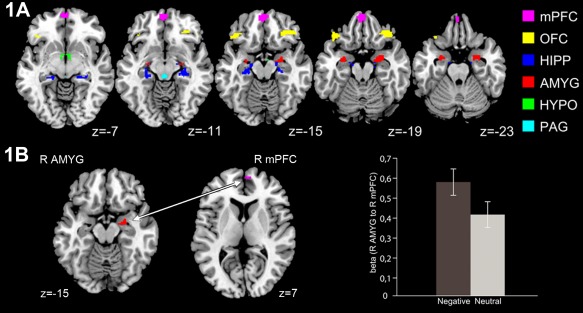
Task‐related BOLD activity and connectivity in response to negative affective stimuli. Observation of negative versus neutral images elicited response in 32 voxels in PAG, 57 voxels in HYPO, 255 voxels in left AMYG, 251 voxels in right AMYG, 140 voxels in left HIPP, 126 voxels in right HIPP, 2015 voxels in left mPFC, 120 voxels in right mPFC, 111 voxels in left OFC, 184 voxels in right OFC (A). These regions of interest are the outputs of intersection analysis (in MarsBaR) between (1) FWE‐corrected BOLD response in all the 99 participants to negative > neutral contrast, and (2) anatomically‐defined masks for the circuitry activated by negative affective stimuli (PAG, AMYG, HIPP, HYPO, ACC, mPFC, OFC, anterior insula). Observation of negative vs. neutral images increased AMYG – mPFC connectivity (B).
Next, the BOLD response to negative (vs. neutral) stimuli was examined across all subjects as a function of Dysphoric Mood, followed by analyses to test for an interaction between dysphoric mood and sex. Severity of dysphoric mood was positively associated with BOLD response in the HYPO and R AMYG (Table 2, Fig. 2). Sex‐dependent analyses revealed a significant interaction between dysphoric mood and sex in R AMYG (F (1,69) = 4.81, P = 0.03; Table 2, Fig. 2B). That is, there was a positive relationship between severity of dysphoric mood and BOLD response in the R AMYG in women (t (38) = 2.95, P = 0.006, R 2 = 0.19), but not in men (t (33) = 0.14, P = 0.71; Table 2).
Table 2.
Effects of dysphoric mood on task‐evoked (negative vs. neutral stimuli) increases in BOLD response
| Effect | ROI | t or F test | P | Effect direction and size |
|---|---|---|---|---|
| Dysphoric mood | HYPO | t (73)=2.31 | 0.02 | Positive relationship: R 2 = 0.07 |
| R AMYG | t (72)=2.33 | 0.02 | Positive relationship: R 2 = 0.07 | |
| Dysphoric mood × sex | R AMYG | F (1,69)=4.81 | 0.03 | Positive relationship in females: R 2 = 0.19 |
Figure 2.
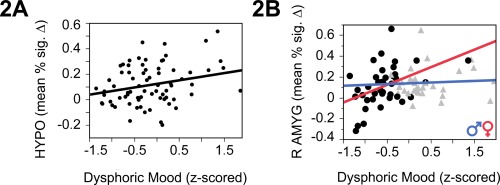
In females but not males, dysphoric mood predicted increased BOLD activity in hypothalamus (A) and amygdala in response to negative affective stimuli (B). [Color figure can be viewed at http://wileyonlinelibrary.com.]
This interaction between dysphoric mood state and sex was further examined using connectivity analyses. Based on BOLD‐level findings reported above, the HYPO and R AMYG were selected as seeds. These analyses revealed an interaction between dysphoric mood and sex on connectivity, during observation of negative (> neutral) images, between (1) R AMYG – R HIPP (Fig. 3A), (2) R AMYG – R OFC (Fig. 3B), and HYPO – R OFC (Fig. 3C) (see Table 3). First, variation in severity of dysphoric mood was associated with R AMYG – R HIPP connectivity, which differed in directionality according to sex: negatively correlated in women (t (38) = −2.91, P = 0.006) and positively related in men (t (34) = 3.15, P = 0.003; see Fig. 3). Furthermore, in women but not men, the more severe the dysphoric mood, the lower the connectivity between R AMYG – R OFC (F: t (38) = −5.13, P < 0.0001; M: t (34) = 0.05, P = 0.96) and HYPO – R OFC (F: t (37) = −4.56, P < 0.0001; M: P = 0.43) (see Fig. 3). This remained significant among the women even after excluding potential outliers.
Figure 3.
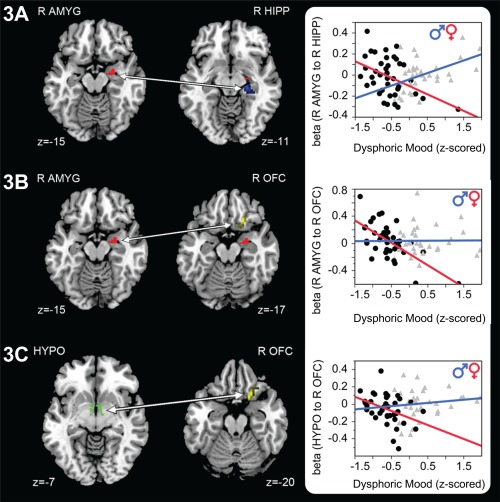
Sex‐dependent effects of dysphoric mood on connectivity in response to negative affective stimuli. Dysphoric mood state predicted decrease in R AMYG – R HIPP (A), R AMYG ‐ R OFC (B), and HYPO – R OFC (C) connectivity during observation of negative vs. neutral stimuli in females but not males.
Table 3.
Effects of Dysphoric Mood on task‐evoked (negative vs. neutral stimuli) increases in connectivity
| Effect | Seed ROI | Target ROI | Z‐value | P (FWE‐corrected) | Direction of effect and associated effect size | ||||
|---|---|---|---|---|---|---|---|---|---|
| Name | Voxels | Peak coordinate | |||||||
| x | y | z | |||||||
| Mean across full sample | R AMYG | R mPFC | 5 | 6 | 59 | 7 | 3.04 | 0.038 | |
| Dysphoric mood × sex | R AMYG | R HIPP | 34 | 24 | −25 | −11 | 3.76 | 0.008 | Negative relationship in females: R 2 = 0.19 |
| Positive relationship in males: R 2 = 0.23 | |||||||||
| R AMYG | R OFC | 30 | 21 | 23 | −17 | 3.62 | 0.038 | Negative relationship in females: R 2 = 0.42 | |
| HYPO | R OFC | 31 | 18 | 14 | −20 | 3.78 | 0.02 | Negative relationship in females: R 2 = 0.37 | |
The addition of diagnostic status and psychotropic medication status in the GLM did not change these results (data not shown), with the exception that psychotropic medication contributed to the variance in R AMYG BOLD (higher R AMYG BOLD as severity of dysphoric mood state increased in women on these medications but not men (F: t (38)=5.74, P = 0.02; M: t (33)=0.34, P = 0.56)). However, there was no simple main effect of psychotropic medication on R AMYG BOLD in females (t(36) = 0.37, P = 0.71) or males (t(47) = −0.13, P = 0.9) when analyzed alone (outside of the of the model with covariates). Finally, our findings on sex differences on the impact of dysphoric mood state on brain activity and connectivity were unaltered when substance use history was included in the model.
DISCUSSION
Based on increasing recognition of common pathways underlying symptom‐related pathogenesis across healthy and clinical populations, and the critical importance of sex differences therein, the primary aim of this investigation was to assess the relationship between a key emotional domain (dysphoric mood symptomatology) and brain activity and connectivity in response to negative affective stimuli in men and women with or without psychiatric illness. To that end, our results are threefold. First, observation of negative (vs. neutral) affective stimuli across all subjects elicited significant BOLD responses in the PAG, HYPO, AMYG, HIPP, OFC, and mPFC, and increased connectivity between the AMYG and mPFC. Second, greater severity of dysphoric mood state at baseline was associated with increased BOLD response in the HYPO and, in women, the right AMYG. Finally, dysphoric mood at baseline was strongly positively associated with connectivity between (1) HYPO and OFC, (2) AMYG and OFC, and (3) AMYG and HIPP in women but not men. Importantly, these findings remained overwhelmingly consistent after accounting for diagnostic status, psychotropic medication use, and substance use history. Taken together, our data provide evidence for the unique coupling between brain circuitry activity in response to negative affective stimuli and variation in dysphoric mood and the impact of sex on these relationships. At a broader level, results support the utility of integrative approaches for analyzing data across heterogeneous, transdiagnostic populations in the search for biomarkers underlying vulnerability toward mental illness.
With a substantial sample size (n = 99) and a priori selected ROI based on previous studies using this paradigm [Goldstein et al., 2005, 2010b; Holsen et al., 2013;], we found significant BOLD activity in response to our negative affect task at a strict threshold level (FWE‐corrected across whole‐brain). This provided evidence that our fMRI task robustly recruited subcortical and cortical regions known to be implicated in circuitry associated with response to negative affective stimuli and the stress response. The pattern of results is consistent with previous studies of emotional [Phelps and LeDoux, 2005] and social [Phelps, 2006] processes. Anatomical differences in this circuitry have been linked to negative affect [Phelps, 2006], and altered functional connectivity within this circuitry has been linked to anxiety [Holmes et al., 2012]. Furthermore, the finding of greater connectivity between AMYG and mPFC during negative vs. neutral affect mirrors recent evidence of enhanced AMYG – mPFC coupling following a psychosocial stressor, particularly in cortisol responders [Quaedflieg et al., 2015]. Although we are unable to determine causality in our study, we speculate, as have others, this may partially reflect the role of the mPFC in inhibiting the arousal response of AMYG during processing of negative emotional stimuli [Shin et al., 2005; Urry et al., 2006].
In examining the relationship between dysphoric mood and BOLD activity, HYPO and AMYG demonstrated significant associations, suggesting activity of these regions in response to negative affective stimuli is partially potentiated by one's current mood state. The HYPO plays a critical role in the initiation of the response to stressful stimuli [Herman and Cullinan, 1997] and activity in this region during emotion processing has been shown to co‐vary with ratings of self‐relatedness [Northoff et al., 2009]. In our study, coupling between BOLD activity in AMYG and dysphoric mood was present exclusively in women. The AMYG is involved in regulation of vigilance and attention toward threat, initiation of the stress response via the hypothalamic pituitary adrenal (HPA) axis [Dedovic et al., 2009] and affect regulation [Mayberg, 1997; Price and Drevets, 2010], with previous evidence of an association between negative affect and increased AMYG volume in a large sample of healthy young adults [Holmes et al., 2012]. Current findings are in agreement with studies documenting elevated AMYG activity during fear conditioning in healthy women compared to men [Lebron‐Milad et al., 2012], and may be related to the impact of gonadal and adrenal hormones on brain circuitry in response to negative affective stimuli or the stress response [Holsen et al., 2013; Jacobs et al., 2015]. Overall, our findings support the relationship between dysphoric mood and hyperactivity in subcortical arousal regions of circuitry implicated in response to negative affective stimuli.
We extended previous work by demonstrating that connectivity between regions of this circuitry was dependent on one's sex. In addition to positive relationships between AMYG BOLD activity and dysphoric mood among women in our study, women with higher dysphoric mood at baseline showed decreased connectivity between HYPO and AMYG with OFC, and AMYG with HIPP. Anatomical and functional connectivity between the OFC (particularly the medial OFC [Zald et al., 2014], as found here) and subcortical limbic regions, such as the HYPO and AMYG, have been implicated in the processing of emotional stimuli and stress [Clewett et al., 2013]. The directionality of the current results (negative relationships between AMYG‐OFC and AMYG‐HIPP connectivity and dysphoric mood in women) is in agreement with recent evidence of stress‐induced decreases in AMYG‐OFC connectivity in healthy individuals [Clewett et al., 2013].
Nuclei in the HYPO and central medial AMYG are the most sexually dimorphic regions in the brain, and HIPP and OFC are also sexually dimorphic [Goldstein et al., 2001]. In fact, we previously demonstrated HYPO and AMYG abnormalities in developmental prenatal stress models of adult depressive and anxiety‐like behaviors in rats [Carbone and Handa, 2013] and mice [Stratton et al., 2014], particularly in females, thus suggesting deficits in sexually dimorphic regions here have sex‐dependent developmental roots. This is also suggested by our recent work in population‐level studies of the same prenatal cohort as subjects in the study presented here, in which we demonstrated prenatal immune exposures predicting sex differences in the risk for depression [Gilman et al., in press] and psychoses [Goldstein et al., 2014a]. Sex differences in AMYG connectivity have been also reported by others, for example, during rest [Kilpatrick et al., 2006], and substantial impact of circulating estradiol on functional connectivity between AMYG nuclei and regions of the default mode network during rest was suggested [Engman et al., 2016].
There are a few potential limitations of this study. Our measure of dysphoric mood reflected clinical state rather than as a trait, thus hindering generalization to long‐standing mood dysregulation. However, participants in our study with psychiatric illness were recurrent cases in remission and correlations between the POMS measures and Hamilton Depression Scale were high (see Supporting Information), suggesting findings may also reflect trait characteristics. Future studies will benefit from including trait‐related behavioral markers in relation to brain activity phenotyping. Another limitation of our study is the fact that we measured dysphoric mood only at baseline and thus our findings relate to baseline dysphoric mood and brain responses to negative affective stimuli. In future studies, it would be advantageous to assess changes in dysphoric mood induced by the negative affective stimuli.
The fact that we did not have the power to correct for the multiple comparisons related to the number of subsequently tested seeds or ROI is also a potential limitation. However, the initial whole brain analysis demonstrated significant (FWE‐corrected) activity in the hypothesized negative affective circuitry regions. Thus, the ROI in the subsequent BOLD and connectivity analyses were derived independently through a robust (n = 99), FWE‐corrected analysis, which reduced the odds of potential type 1 errors and supported the relevance of the results. In addition, given that we chose a hypothesis‐driven approach and limited the number of tested seeds and ROI to the hypothesized a priori selected ones, we cannot generalize to non‐hypothesized regions in the brain. However, in independent samples over the last 10 years, we have consistently activated these regions using this fMRI paradigm and demonstrated significant sex differences in brain activity. Further, findings here remained consistent after accounting for potential diagnostic, medication, or substance use history.
Finally, we unexpectedly found greater severity of dysphoric mood in men compared to women in our sample, in contrast to a large body of literature, including other studies of ours, demonstrating elevated dysphoric mood deficits among women. However, post hoc examination of the data revealed a higher rate of substance use among men than women, and subjects with substance use histories demonstrated greater severity of dysphoric mood state. This is consistent with other studies reporting high rates of mood dysregulation among individuals with substance use [Anthenelli, 2010; Schuler et al., 2015], and some studies demonstrating a sex difference in the link between depressed mood and self‐medication [Lo et al., 2015]. Therefore, sex differences in mood state detected in the study reported here were likely explained by higher substance use in men.
In conclusion, deficits in brain activity associated with response to negative affective stimuli were shared across individuals with and without psychiatric illness, and were dependent on one's sex as mood state became more dysphoric. It is important for future work to delineate the underlying physiological mechanisms and origins of these deficits to more fully identify the biosignature [Insel et al., 2010] associated with arousal and negative affect. A recent fMRI study combined brain activity measures with clinical measures with success at better predicting variance in treatment response [Doehrmann et al., 2013]. The ultimate goal of identifying the neural, physiologic and genetic signatures of shared traits is to discover new targets for drug discovery to enhance treatment efficacy. We demonstrated here that this entails a sex‐dependent lens on therapeutic development, particularly when considering mood states across the spectrum of healthy individuals and those with psychiatric disorders.
Supporting information
Supporting Information
Supporting Information Table 1.
Supporting Information Table 2.
ACKNOWLEDGMENTS
We would also like to thank Harlyn Aizley, Ed.M., Anne Remington, M.A., Jenn Walch, M.Ed., and Sara Cherkerzian, Sc.D. for their immense contributions to the collection and management of data from the original studies associated with the sample. We are also grateful to the anonymous donor who contributed to the financial support of this work as well. Financial Disclosures: We had no competing financial interests in relation to the work described, thus no conflict of interest for any author.
REFERENCES
- Alonso SJ, Damas C, Navarro E (2000): Behavioral despair in mice after prenatal stress. J Physiol Biochem 56:77–82. [DOI] [PubMed] [Google Scholar]
- Anthenelli RM (2010): Focus on: Comorbid mental health disorders. Alcohol Res Health 33:109. [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Paquette V, Levesque J (2006): Dysfunction in the neural circuitry of emotional self‐regulation in major depressive disorder. Neuroreport 17:843–846. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ (1994): Measuring emotion: The self‐assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry 25:49–59. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J‐L, Valabregue R, Poline JB. Region of Interest Analysis Using an SPM Toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002.
- Carbone DL, Handa RJ (2013): Sex and stress hormone influences on the expression and activity of brain‐derived neurotrophic factor. Neuroscience 239:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett D, Schoeke A, Mather M (2013): Amygdala functional connectivity is reduced after the cold pressor task. Cogn Affect Behav Neurosci 13:501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham‐Bussel AC, Root JC, Butler T, Tuescher O, Pan H, Epstein J, Weisholtz DS, Pavony M, Silverman ME, Goldstein MS, Altemus M, Cloitre M, Ledoux J, McEwen B, Stern E, Silbersweig D (2009): Diurnal cortisol amplitude and fronto‐limbic activity in response to stressful stimuli. Psychoneuroendocrinology 34:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC (2009): The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. NeuroImage 47:864–871. [DOI] [PubMed] [Google Scholar]
- Derntl B, Kryspin‐Exner I, Fernbach E, Moser E, Habel U (2008): Emotion recognition accuracy in healthy young females is associated with cycle phase. Horm Behav 53:90–95. [DOI] [PubMed] [Google Scholar]
- Doehrmann O, Ghosh SS, Polli FE, Reynolds GO, Horn F, Keshavan A, Triantafyllou C, Saygin ZM, Whitfield‐Gabrieli S, Hofmann SG, Pollack M, Gabrieli JD (2013): Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA Psychiatry 70:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Schulze L, Bottger M, Grossmann A, Hauenstein K, Wirtz PH, Heinrichs M, Herpertz SC (2010): The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum Brain Mapp 31:758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty D, Rauch SL (1997): Neuroimaging and neurobiological models of depression. Harv Rev Psychiatry 5:138–159. [DOI] [PubMed] [Google Scholar]
- Engman J, Linnman C, Van Dijk KR, Milad MR (2016): Amygdala subnuclei resting‐state functional connectivity sex and estrogen differences. Psychoneuroendocrinology 63:34–42. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Cherkerzian S, Buka SL, Hahn J, Horing M, Goldstein M (2016). Prenatal immune programming of the sex‐dependent risk for major depression, Transl Psychiatry, doi: 10.1038/tp.2016.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM (2006): Sex, hormones and affective arousal circuitry dysfunction in schizophrenia. Horm Behav 50:612–622. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS Jr, Faraone SV, Tsuang MT (2001): Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex 11:490–497. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N (2005): Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci 25:9309–9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Buka SL, Seidman LJ, Tsuang MT (2010a): Specificity of familial transmission of schizophrenia psychosis spectrum and affective psychoses in the New England family study's high‐risk design. Arch Gen Psychiatry 67:458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield‐Gabrieli S, Makris N (2010b): Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci 30:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J, Cherkerzian S, Seidman L, Donatelli JA, Remington AG, Tsuang MT, Hornig M, Buka SL (2014): Prenatal maternal immune disruption and sex‐dependent risk for psychoses. Psychol Med 44:3249–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Holsen L, Handa R, Tobet S (2014): Fetal hormonal programming of sex differences in depression: Linking women's mental health with sex differences in the brain across the lifespan. Front Neurosci 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Lancaster K, Longenecker JM, Abbs B, Holsen LM, Cherkerzian S, Whitfield‐Gabrieli S, Makris N, Tsuang MT, Buka SL, Seidman LJ, Klibanski A (2015): Sex differences, hormones, and fMRI stress response circuitry deficits in psychoses. Psychiatry Res 232:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE (1997): Neurocircuitry of stress: Central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci 20:78–84. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Lee PH, Hollinshead MO, Bakst L, Roffman JL, Smoller JW, Buckner R (2012): Individual differences in amygdala‐medial prefrontal anatomy link negative affect, impaired social functioning, and polygenic depression risk. J. Neurosci 32:18087–18100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Lee JH, Spaeth SB, Ogden LA, Klibanski A, Whitfield‐Gabrieli S, Sloan RP, Goldstein JM (2012): Brain hypoactivation, autonomic nervous system dysregulation, and gonadal hormones in depression: A preliminary study. Neurosci Lett 514:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen L, Lancaster K, Klibanski A, Whitfield‐Gabrieli S, Cherkerzian S, Buka S, Goldstein JM (2013): HPA‐Axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience 250:733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN, Garvey MA, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P (2010): Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry 167:748–751. [DOI] [PubMed] [Google Scholar]
- Jacobs EG, Holsen LM, Lancaster K, Makris N, Whitfield‐Gabrieli S, Remington A, Weiss B, Buka S, Klibanski A, Goldstein JM (2015): 17β‐Estradiol differentially regulates stress circuitry activity in healthy and depressed women. Neuropsychopharmacology 40:566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K, Gatz M, Gardner C, Pedersen N (2006): A Swedish national twin study of lifetime major depression. Am J Psychiatry 163:109–114. [DOI] [PubMed] [Google Scholar]
- Kern S, Oakes TR, Stone CK, McAuliff EM, Kirschbaum C, Davidson RJ (2008): Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology 33:517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC (2003): Epidemiology of women and depression. J Affect Disord 74:5–13. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L, Zald D, Pardo J, Cahill L (2006): Sex‐related differences in amygdala functional connectivity during resting conditions. NeuroImage 30:452–461. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert MM (1997): Motivated attention: Affect, activation, and action In: Lang PJ, Simons RF, Balaban MT, editors. Attention and Orienting: Sensory and Motivational Processes. Hillsdale, NJ: Erlbaum; pp 97–135. [Google Scholar]
- Lebron‐Milad K, Abbs B, Milad MR, Linnman C, Rougemount‐Bücking A, Zeidan MA, Holt DJ, Goldstein JM (2012): Sex differences in the neurobiology of fear conditioning and extinction: A preliminary fMRI study of shared sex differences with stress‐arousal circuitry. Biol Mood Anxiety Disord 2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, King AP, Britton JC,K, Luan Phan M, Abelson JL, Taylor SF (2007): Paralimbic and medial prefrontal cortical involvement in neuroendocrine responses to traumatic stimuli. Am J Psychiatry 164:1250–1258. [DOI] [PubMed] [Google Scholar]
- Lo CC, Cheng TC, de la Rosa IA (2015): Depression and substance use: A temporal‐ordered model. Subst Use Misuse 50:1274–1283. [DOI] [PubMed] [Google Scholar]
- Lungu O, Potvin S, Tikàsz A, Mendrek A (2015): Sex differences in effective fronto‐limbic connectivity during negative emotion processing. Psychoneuroendocrinology 62:180–188. [DOI] [PubMed] [Google Scholar]
- Mayberg HS (1997): Limbic‐cortical dysregulation: A proposed model of depression. J Neuropsychiatry Clin Neurosci 9:471–481. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ (1995): Sex‐specific effects of prenatal stress on hypothalamic‐pituitary‐adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res 84:55–61. [DOI] [PubMed] [Google Scholar]
- McEwen BS, De Kloet ER, Rostene W (1986): Adrenal steroid receptors and actions in the nervous system. Physiol Rev 66:1121–1188. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC (2012): A generalized form of context‐dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L (1992): Revised Manual for the Profile of Mood States, Vol. 731 San Diego, CA: Educational and Industrial Testing Services; pp 732–733. [Google Scholar]
- Monroe SM, Harkness KL (2005): Life stress, the “kindling” hypothesis, and the recurrence of depression: Considerations from a life stress perspective. Psychol Rev 112:417. [DOI] [PubMed] [Google Scholar]
- Myin‐Germeys I, Peeters F, Havermans R, Nicolson NA, DeVries MW, Delespaul P, Van Os J (2003): Emotional reactivity to daily life stress in psychosis and affective disorder: An experience sampling study. Acta Psychiatry Scand 107:124–131. [DOI] [PubMed] [Google Scholar]
- Myrianthopoulos NC, French KS (1968): An application of the US Bureau of the Census socioeconomic index to a large, diversified patient population. Soc Sci Med 2:283–299. [DOI] [PubMed] [Google Scholar]
- NIMH Center for Emotion and Attention (1999): International Affective Picture System [digitized photographs] [digitized photographs]. Gainesville, Florida: Center for Research in Psychophysiology, NIMH Center for Emotion & Attention, University of Florida. [Google Scholar]
- Niswander KR, Gordon M (1972): The Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke: The Women and Their Pregnancies. Washington, D.C: Government Printing Office, U.S. Department of Health, Education, and Welfare. [Google Scholar]
- NITRC (2011): Artifact Detection Tools (ART) [computer program]. Cambridge, MA. Release version 07/19/11.
- Northoff G, Schneider F, Rotte M, Matthiae C, Tempelmann C, Wiebking C, Bermpohl F, Heinzel A, Danos P, Heinze HJ, Bogerts B, Walter M, Panksepp J (2009): Differential parametric modulation of self‐relatedness and emotions in different brain regions. Hum Brain Mapp 30:369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Bäckström T, Fernández G (2010): Neural mechanisms underlying changes in stress‐sensitivity across the menstrual cycle. Psychoneuroendocrinology 35:47–55. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Kopin IJ, Goldstein DS (1995): Stress‐induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary‐adrenocortical and sympathoadrenal activity: In vivo microdialysis studies. Front Neuroendocrinol 16:89–150. [DOI] [PubMed] [Google Scholar]
- Phelps EA (2006): Emotion and cognition: Insights from studies of the human amygdala. Annu Rev Psychol 57:27–53. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE (2005): Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 48:175–187. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC (2010): Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedflieg C, van de Ven V, Meyer T, Siep N, Merckelbach H, Smeets T (2015): Temporal dynamics of stress‐induced alternations of intrinsic amygdala connectivity and neuroendocrine levels. PLoS One 10:e0124141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REX Software (2009): REX Software [computer program]. Cambridge, MA.
- Rhodes ME, Rubin RT (1999): Functional sex differences ('sexual diergism') of central nervous system cholinergic systems, vasopressin, and hypothalamic‐pituitary‐adrenal axis activity in mammals: A selective review. Brain Res Brain Res Rev 30:135–152. [DOI] [PubMed] [Google Scholar]
- Rowland JE, Hamilton MK, Lino BJ, Ly P, Denny K, Hwang EJ, Mitchell PB, Carr VJ, Green MJ (2013): Cognitive regulation of negative affect in schizophrenia and bipolar disorder. Psychiatry Res 208:21–28. [DOI] [PubMed] [Google Scholar]
- Schuler MS, Vasilenko SA, Lanza ST (2015): Age‐varying associations between substance use behaviors and depressive symptoms during adolescence and young adulthood. Drug Alcohol Depend 157:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Sibille E (2014): Sex differences in mood disorders: Perspectives from humans and rodent models. Biol Sex Differ 5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL (2005): A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 62:273–281. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983): Manual for the State‐Trait Anxiety Inventory Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stratton MS, Staros M, Budefeld T, Searcy BT, Nash C, Eitel C, Carbone D, Handa RJ, Majdic G, Tobet SA (2014): Embryonic GABA(B) receptor blockade alters cell migration, adult hypothalamic structure, and anxiety‐ and depression‐like behaviors sex specifically in mice. PLoS One 9:e106015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The FIL Methods Group (2013): Neuroimaging WTCf. SPM8 Manural. London, UK: Institute of Neuroimaging; 2013. [Google Scholar]
- Tobet SA, Hanna IK (1997): Ontogeny of sex differences in the mammalian hypothalamus and preoptic area. Cell Mol Neurobiol 17:565–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ (2006): Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci 26:4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Os J, Kapur S (2009): Schizophrenia. Lancet 374:635–645. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NY, Spinhoven P, van Buchem MA, Elzinga BM, Rombouts SA (2012): Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology 37:1039–1047. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS (1992): Prenatal stress selectively alters the reactivity of the hypothalamic‐pituitary adrenal system in the female rat. Brain Res 595:195–200. [DOI] [PubMed] [Google Scholar]
- Zald DH, McHugo M, Ray KL, Glahn DC, Eickhoff SB, Laird AR (2014): Meta‐analytic connectivity modeling reveals differential functional connectivity of the medial and lateral orbitofrontal cortex. Cereb Cortex 24:232–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information Table 1.
Supporting Information Table 2.


