Abstract
This study sought to elucidate the mechanisms underlying the anti-inflammatory effect of mango (Mangifera Indica L.) polyphenolics containing gallic acid and gallotanins, and the role of the miR-126/PI3K/AKT/mTOR signaling axis in vitro and in vivo. Polyphenolics extracted from mango (var. Keitt) were investigated in lipopolysaccharide (LPS)-treated CCD-18Co cells. Rats received either a beverage with mango polyphenolics or a control beverage, and were exposed to three cycles of 3% dextran sodium sulfate (DSS) followed by a 2-wk recovery period. The mango extract (10 mg GAE/L) suppressed the protein expression of NF-κB, p-NF-κB, PI3K (p85β), HIF-1α, p70S6K1, and RPS6 in LPS-treated CCD-18Co cells. LPS reduced miR-126 expression, whereas, the mango extract induced miR-126 expression in a dose-dependent manner. The relationship between miR-126 and its target, PI3K (p85β), was confirmed by treating cells with miR-126 antagomiR where mango polyphenols reversed the effects of the antagomiR. In vivo, mango beverage protected against DSS-induced colonic inflammation (47%, P = 0.05) and decreased the Ki-67 labeling index in the central and basal regions compared to the control. Mango beverage significantly attenuated the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, and iNOS at the mRNA and protein level. Moreover, the expression of PI3K, AKT, and mTOR was reduced, whereas, miR-126 was upregulated by the mango treatment. These results suggest that mango polyphenols attenuated inflammatory response by modulating the PI3K/AKT/mTOR pathway at least in part through upregulation of miRNA-126 expression both in vitro and in vivo; thus, mango polyphenolics might be relevant as preventive agents in ulcerative colitis.
Keywords: mango, rat, colitis, mTOR, miR-126
INTRODUCTION
Ulcerative colitis (UC) is a form of inflammatory bowel disease (IBD). The development of UC is affected by several factors, including genetic susceptibility, composition of the intestinal microbiota, immune dysregulation, and environmental factors including nutrition [1]. Imbalance of the immune system may cause the overproduction of reactive oxidative stress (ROS) and pro-inflammatory cytokines, such as IL-1β, IL-6, IL-8, and TNF-α through NF-κB activation, and lead to chronic inflammation and mucosal damage in the large intestine [2]. Although colitis is rarely lethal by itself, it is closely associated with human colorectal cancer [3]. Long-term chronic inflammation produces high levels of pro-inflammatory cytokines and inflammatory molecules, which can increase the risk of cancer [4]. Approximately, one-fifth of IBD patients develop colon cancer within 30 yr of disease onset [5], and UC-associated cancer has a higher mortality risk than sporadic colon cancer [6]. Anti-inflammatory drugs play an important role in the prevention and treatment of colitis, but after often characterized by serious side effects [7]. As a consequence, several studies have focused on developing new preventive agents for IBD, including natural anti-inflammatory products from dietary sources [8].
Polyphenolics derived from fruits and vegetables are considered natural anti-inflammatory compounds acting through the inhibition of NF-κB activation and induction of antioxidant defense systems [9]. Mango is rich in polyphenols, including gallic acid, galloyl derivatives, flavonol glycosides, and benzophenone, that exhibit antioxidant and anti-inflammatory properties in several cell types [10–13]. Gallic acid, the most abundant polyphenol in mango, has shown its chemopreventive effects by reducing colon carcinogenesis via the inhibition of oxidative stress and NF-κB activation [14,15]. In addition, mango polyphenolics were reported to have anti-oxidant [16] and anti-inflammatory activities through inhibition of iNOS, COX2, and TNF-α in in vitro and in vivo [17,18]. We previously demonstrated that mango extract inhibited the growth of several cancer cells, particularly colon cancer cells, through induction of apoptosis and reduction of reactive oxygen species (ROS) generation [13]. However, the underlying mechanisms relevant to the preventive effects of mango polyphenolics in colitis have not been well investigated.
The mammalian target of rapamycin (mTOR) pathway plays a central role in the regulation of cell growth and cell proliferation via downstream transduction of proliferative signals, such as p70S6K1 and RPS6 [19]. Activation of mTOR signaling occurred in bacterial-induced colitis in mice [20]. mTOR inhibitors are effective as anti-inflammatory drugs in colitis by inhibiting NF-κB activation and T-cell function [21–23]. Targeted suppression of these activated pathways during colitis by natural compounds may provide an important strategy in the prevention of colitis.
MicroRNAs (miRNAs) are emerging as a potential link between inflammation and cancer by inducing mRNA degradation or blocking translation of key molecular targets involved in IBD [24]. Moreover, polyphenolics are known to influence the post-transcriptional regulation of miRNAs [25], with the mTOR pathway serving as a promising target for the anti-cancer properties of miRNAs [26]. For example, miR-126 targets phosphatidylinositol 3-kinase regulatory subunit beta (PI3Kp85β), an upstream regulator of the mTOR pathway [27,28]. Accordingly, miR-126 may be a potential chemopreventive and anti-inflammatory target via the inhibition of angiogenesis, vascular integrity, inflammation, and proliferation [28]. Induction of miR-126 provides a possible mechanistic basis for the prevention and treatment of colitis.
The current investigation sought to understand the molecular targets involved in anti-inflammatory effects of mango polyphenolics in vitro and in vivo. We hypothesized that the anti-inflammatory activity of mango polyphenolics is due, at least in part, to targeting the miR-126/PI3K/AKT/mTOR axis. We report, for the first time, that mango polyphenolics modulate inflammation by regulating the expression of miR-126.
MATERIALS AND METHODS
Mango Chemistry
Mango fruit (cv. Keitt) was sourced from Mexico and received from the National Mango Board (Orlando, FL). The mango extract was prepared as described previously [13,29]. In brief, mango pulp was briefly blended with a solvent mixture (ethanol/acetone/methanol = 1:1:1). The mixture was incubated for 30 min, then filtered through a filtration cloth. The remaining solids were re-extracted thrice in total. Final filtration was performed with Whatman No 1. filter paper. The combined solvents were evaporated at 40°C with Rotavapor RII (Buchi, Switzerland). The residue was centrifuged at 2000g to remove insoluble materials. Polyphenols from the mango extract were partitioned onto a C18 Sep Pack cartridge (Waters, Milford, MA) then eluted with 100% methanol. Phenolic acids not adsorbed to the cartridge were partitioned into ethyl acetate with a separatory funnel. Solvent elutes were combined, and the solvent was evaporated. Separation and characterization of polyphenolics was performed on a Thermo Finnigan Surveyor HPLC and LCQ Deca XP Max mass spectrometer equipped with an ESI ion source as described previously (Thermo Fisher, San Jose, CA) (Figure 1) [13,29]. To prepare the experimental beverage, ripe mango was peeled to remove the skin and seed, the pulp was homogenized in a blender, and then heated with cellulase and pectinase enzymes to break down fiber. After heating at 55°C for 1 h, the puree was centrifuged and the supernatant was stored at −20°C. Mango is a good source of dietary fiber (pectin and cellulose), vitamin C, ß-carotene, calcium, iron, and phenolic compounds [30]. In this study, “mango beverage” contained most of the nutrients from the fruit such as sugar, vitamin C, and polyphenolics except fiber, whereas, “mango extract” had only total polyphenolics from mango. The total phenolic content in the mango extract and beverage were measured spectrophotometrically by the folinciocalteu assay against an external standard of gallic acid.
Figure 1.
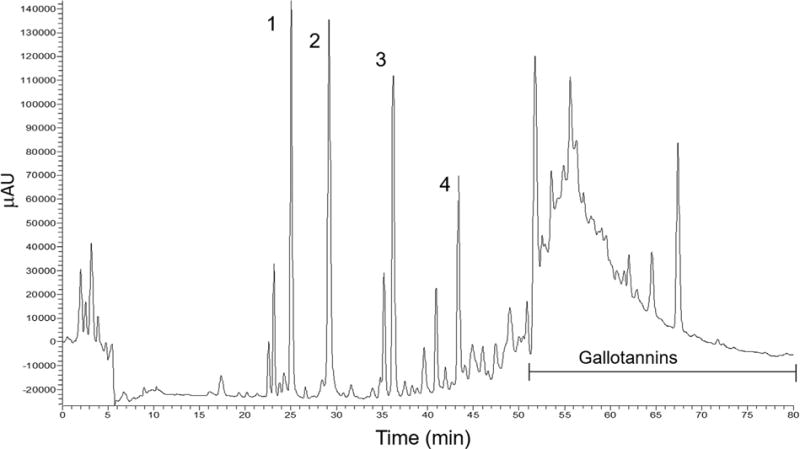
Representative chromatographic profile at 280 nm of phenolic compounds in mango extract (Keitt). Tentative peak assignments showing the presence of: (1) Monogalloyl Glucoside, (2) Gallic acid, (3) p-Hydroxybenzoic acid glycoside, (4) Dihydrophaseic acid glucoside, and gallotannins.
Cell Culture
Human colon CCD-18Co myofibroblastic and HT-29 adenocarcinoma cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA) and cultured as recommended by the ATCC. Cells were seeded for cell viability assay onto a 24-well plate (15,000 cells/well), for RNA extractions onto a 12-well plate (100,000 cells/well), and for the protein analysis onto a 6-well plate (200,000 cells/well). After 24 h, cells were exposed to mango extract (0–10 mg GAE/L) without lipopolysaccharide (LPS). The extract was administered again after 1 h with 1 μg/ml LPS. Cells were harvested at 4 h for RNA extraction, at 24 h for determination of protein levels by immunoblotting, and at 48 h for cell viability assays using a Z2™ Beckman coulter instrument (Fullerton, CA).
Transfection With AntagomiRs
Cells were seeded into 12-well plates and transfected with 20 nM antagomiR of miR-126 (Dharmacon, Lafayette, CO), using Lipofectamine 2000 (Invitrogen, Grand Island, NY) according the manufacturer’s protocol. A nonspecific oligonucleotide was used as controls. After transfection for 4 h, the transfection mix was replaced with LPS-free media containing 10 mg GAE/L of mango extract, and 1 h later with media containing mango extract plus 1 μg/ml LPS for 24 h, as previously described [31].
Animal Treatment and Tissue Sampling
The in vivo protocol was approved by the Institutional Animal Care and Use Committee of Texas A&M University. The DSS model is an excellent preclinical model of colitis that exhibits many phenotypic characteristics relevant to the human disease [32]. Treatment with DSS causes injury to the colonic epithelial barrier, which results in an increase in colonic mucosal permeability, causes destruction of the crypts, and produces pro-inflammatory cytokines [33]. Ten-wk-old Sprague–Dawley male rats (371–420 g) supplied from Harlan Teklad (Houston, TX) were acclimated to individual cages for 1 wk, and were randomly assigned to the mango and control treatment groups. In the latter case, for calorie and pH adjustment, 15.7 g sugar and 0.05 g citric acid were added to 100 ml distilled water and designated as “control beverage.” Mango and control beverage were provided ad libitum, before, during and after DSS exposure, with liquid intake measured on a daily basis. Rats were administered 3% (w/v) DSS (MP Biomedicals, Solon, OH) over three cycles, with a 14-d separation (n = 10 rats per group). Rats were killed 2 wk after the third and final DSS administration, and colon tissues were collected (Figure 3A). One centimeter sections were cut from the distal end of each rat colon and fixed in 4% paraformaldehyde for embedding in paraffin. The remaining colon was gently scraped for collecting protein and RNA samples [34].
Figure 3.
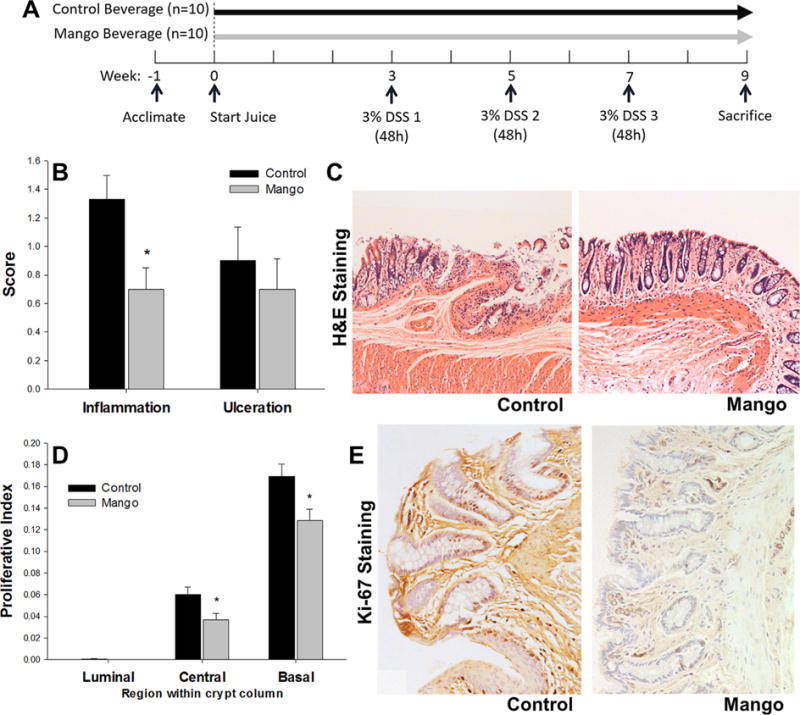
Experimental design and effects of mango beverage on colonic inflammation and ulceration and colonocyte proliferation in DSS-treated rats. (A) Experimental design of the in vivo rat study. (B) The inflammation score was decreased by the mango beverage intake compared to the control beverage intake in DSS-treated rats (P = 0.05). Colonic inflammation and ulceration were blindly assessed by a veterinary pathologist (C.P.). The scores were assessed on a scale of 0–3. (C) Representative images of H&E-stained colon sections in the control and mango groups. (D) The mango beverage significantly suppressed colonocyte proliferation in the central and basal regions compared to control beverage in DSS-treated rats. The measurement of Ki-67 was used to determine cell proliferation. (E) Ki-67 immunohistochemistry in rat colon mucosa in control and mango groups. Values are the mean±SEM (n = 10 per group). *P < 0.05.
Quantifying Inflammation, Cell Proliferation, and Apoptosis Indices
For histopathology and immunohistochemistry analysis, colon tissues were dehydrated, embedded in paraffin, and serially sectioned at 4 μm thickness. Hematoxylin and eosin stained slides were blindly examined by a veterinary pathologist (C.P.) to assess the degree of colitis. Inflammation was scored as follows: 0 = no inflammation observed; 1 = mild colitis; 2 = moderate colitis; 3 = severe colitis. Ulceration was scored as follows: 0 = no ulcers; 1 = ulceration; 2 = moderate ulceration; 3 = several minimal sites with ulceration or one large ulcer. For Ki-67 immunohistochemical staining, primary antibody against Ki-67 (dilution 1:50, BD Pharmingen, San Jose, CA) was followed by incubation with biotinylated anti-mouse IgG from the Vectastain ABC Elite kit (Vector Lab, Burlingame, CA). Twenty-five crypt columns per rat were selected for quantitative analysis. The number and position of labeled cells were recorded. The proliferative index was calculated as the number of labeled cells divided by the total number of cells in the luminal, central, and basal regions of the crypt column (n = 10).
Quantitative RT-PCR
In addition to total RNA, miRNA from cultured cells and colon mucosal scrapings was isolated using the mirVana miRNA Isolation Kit (Applied Biosciences, Foster City, CA) according to the manufacturer’s protocol. RNA (1 μg) was converted to cDNA using a reverse transcription kit (Invitrogen). Real-time PCR reactions were performed with 2 μl of cDNA in a Reverse Transcription Kit (Invitrogen). The SYBR Green PCR master Mix (Applied Biosystems) was used for qPCR analyses on a 7900HT Fast Real-Time PCR system (Applied Biosystems). The sequence of primers was designed using Primer3, an online primer design tool, and were obtained from Integrated DNA Technologies (Coralville, IA). Quantification of miRNAs was measured using the Taqman MicroRNA reverse Transcription kit (Applied Biosystems) and the Taqman 2 × Universal PCR master mix (No AmpErase UNG, Applied Biosystems, Foster City, CA) was used for the qRT-PCR reaction, with GAPDH as control, or miR-4.5S and miR-NU6B as endogenous controls for miRNA expression [35].
Western Blotting
After a 24 h mango extract treatment, cells were lyzed in RIPA buffer, a 1% protease and proteinase inhibitor cocktail were added (Pierce/Thermo Scientific, Rockford, IL) and centrifuged [36]. Mucosal scrapings were homogenized in a buffer (500 mM Tris–HCL, 1M Sucrose, 200 mM EDTA, 100 mM EGTA, 0.4M NaF, 10% Triton X–100, 10 mM Sodium Orthovanadate, and Protease Inhibitor Cocktail) then centrifuged at 15,000g for 10 min at 4°C, and the supernatant was stored at −80°C. Fifty microgram of protein was loaded onto the gel, followed by transfer onto PVDF membrane. Membranes were incubated with primary antibodies against NF-κB, p-NF-κB, COX-2, cleaved caspase-3, PARP-1 (Cell Signaling Technology, Beverly, MA), iNOS (Cayman Chemical, Ann Arbor, MI), PI3K (p85β), HIF-1α (Abcam, Cambridge, MA), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA).
Multiplex Bead Assay
Protein extracts from cultured cells and tissues were used to determine the relative abundance of cytokines (TNFα, IL-1β, IL-6), and the phosphorylation status of AKT/mTOR signaling intermediates (AKT, GSK3β, GSK3α, IGF1R, IR, IRS1, mTOR, p70 S6 kinase, PTEN, RPS6, and TSC2) using multiplex kits (Millipore, Billerica, MA) according to the manufacturer’s protocol. Multiplex analysis was performed on a Luminex L200 instrument (Luminex, Austin, TX) and data were analyzed by Luminex xPONENT software.
Statistical Analyses
Quantitative data represent mean±standard error, with paired comparisons analyzed by the Student t-test, and group comparisons via one-way ANOVA with Tukey’s post-hoc test (P < 0.05, SAS version 9, SAS Institute, Inc., Cary, NC).
RESULTS
Polyphenolics in Mango Extract
Using HPLC-MS analysis of mango extract the following compounds were identified: monogalloyl glucoside (Peak 1), gallic acid (Peak 2), p-hydroxybenzoic acid glycoside (Peak 3), and dihydrophaseic acid glucoside (Peak 4), with peak absorption at 280 nm (Figure 1). Other, unresolved gallotannins also were present in the mango extract.
In Vitro Evaluation of Inflammation and mTOR Biomarkers
The anti-inflammatory activities of mango polyphenolics were examined in prior studies using CCD-18Co colon-myofibroblastic cell lines [13]. However, the underlying molecular mechanisms were not pursued in detail. In the current investigation, mango extract did not affect overall cell viability in CCD-18Co cells, but reduced significantly the cell viability in HT-29 colon cancer cells at 10–20 mg GAE/L (Figure 2A). In LPS-treated CCD-18Co cells, mango extract at 10 mg GAE/L suppressed the mRNA expression of NF-κB by 19% and iNOS by 39% (Figure 2B). This was accompanied by reduced expression of NF-κB and pNF-κB protein (Figure 2C). In addition, mango extract at 10 mg GAE/L modulated the mTOR pathway. Specifically, mRNA expression was reduced by 31% for PI3K (p85β), by 43% for mTOR, and by 23% for p70S6K1 (Figure 2D), and the ratio of phosphorylated/total p70S6K1 and RPS6 were also attenuated by 25% and 35%, respectively by mango extract (Figure 2E). Protein levels of PI3K (p85β) and HIF1α also were suppressed at 10 mg GAE/L of mango extract in LPS-treated CCD-18Co cells (Figure 2C).
Figure 2.
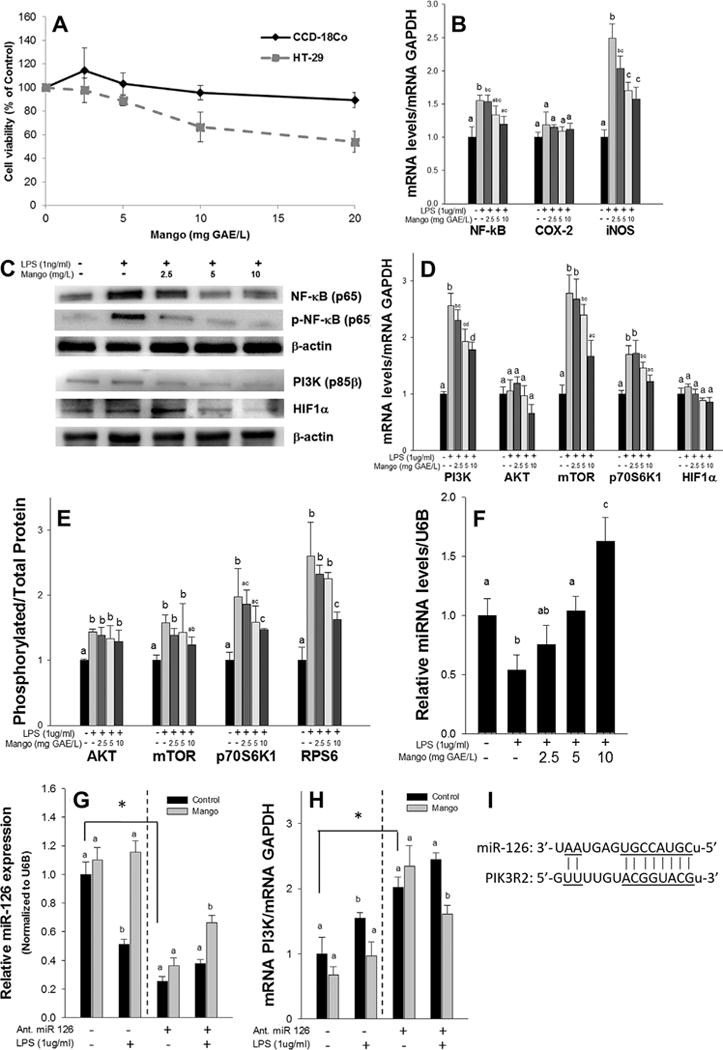
Effects of mango extract on the inflammatory and the mTOR signaling pathway in LPS-treated CCD-18Co cells. (A) The mango extract did not affect cell viability in CCD-18Co cells, but reduced HT-29 cell viability at 10–20 mg GAE/L. (B) The mango extract suppressed the expression of NF-κb and iNOS mRNA and (C) the expression of NF-κB, pNF-κB, PI3K (p85β), and HIF1α protein in LPS-treated CCD-18Co cells. (D) The mango extract suppressed the expression of PI3K (p85β), mTOR, and p70S6K1 mRNA and (E) the ratio of phosphorylated/total protein of p70S6K1 and RPS6 in LPS treated CCD-18Co cells. (F) The mango extract induced the expression of miR-126 in LPS-treated CCD-18Co cells in dose dependent manners. (G) The mango extract reversed the effect of miR-126 antagomiR on transfected cells, and (H) the reversed expression of miR-126 was accompanied by a decreased expression of PI3K (p85β) mRNA. CCD-18Co cells were transfected with 20 nmol/ml of miR-126 antagomiR. After that, the cells were treated with the mango extract (10 mg GAE/L) and LPS (1 μg/ml) for 24 h. miR-126 was analyzed as a ratio to the miR-NU6B endogenous control, and PI3K was assayed as ratio to GAPDH. (I) The miRNA/mRNA pairing regions between miR-126 and the target Pi3k2r. All of the experiments were performed at least three times and the results were expressed as the mean±SEM (n = 3). The different letters indicate significance at P < 0.05. *P < 0.05.
Mango extract (0–10 mg GAE/L) produced a corresponding increase in miR-126, in a dose-dependent manner, in LPS-treated CCD-18Co cells, and this was statistically significantly at 5–10 mg GAE/L of mango extract compared to controls (Figure 2F). miR-126 is known to target PI3K (p85β), which regulates signaling through PI3K/AKT [26,27] (Figure 2I). In order to determine the involvement of miR-126 and its target gene, PI3K (p85b) in the anti-inflammatory activities of mango, we investigated treatment of cells with the antagomiR of miR-126. After the transfection of miR-126 antagomiR, cells were treated with the mango extract (10 mg GAE/L) and 1 μg/ml LPS. AntagomiR treatment reduced the expression of miR-126 by 75%, but the treatment with mango extract partially reversed the effect of the miR-126 antagomiR in the transfected cells (260%, P < 0.05) (Figure 2G). In addition, a decrease in the expression of PI3K (p85β) mRNA (by 20%, P < 0.05) was seen after mango treatment (Figure 2H). Thus, miR-126 is responsible, at least in part, for mango polyphenol-dependent modulation of PI3K signaling.
Beverage Consumed by DSS-Treated Rats
The concentration of total soluble phenolics in mango beverage was 475.8 mg gallic acid equivalents (GAE)/L. Rats in the mango group (445.4 g final body weight) consumed 84.17 ml of mango beverage (475.8 mg GAE/L) (Supplement Table S1). The selected dose was equivalent to 89.74 mg GAE/kg/d for rats. The human equivalent amount of the mango beverage that was consumed by rats was calculated using the body surface area normalization method [37,38], and the dose was 14.55 mg GAE/kg/d for human. The 70 kg human equivalent dose of mango beverage was around 1 g GAE/d of mango polyphenolics. There were no adverse symptoms such as extreme diarrhea, blood in the stool, or severe inflammation immediately after the DSS treatment in both groups.
Mango Reduced the Inflammation Score and Decreased Cell Proliferation in DSS-Treated Rats
The DSS-induced murine colitis model resembles the pathogenesis of human ulcerative colitis with respect to changes in epithelial cell permeability and acute inflammation in the colon [39]. The model has been widely used to test the efficacy of preventive and therapeutic agents in IBD [39]. In the current investigation, three cycles of 3% DSS were administered for 48 h and this was sufficient to induce inflammation, without severe ulceration [34,38,40]. Histologic evaluation was performed to assess the extent of colonic inflammation and ulceration in the different treatment groups. Mango beverage reduced morphological signs of cell inflammation, and the inflammation score was decreased by 47% compared to the control group (P = 0.05) (Figure 3B and C). There was no statistically significant difference in the ulceration score between groups (Figure 3B). As expected, Ki-67-positive cells were prominent in the basal region of the colonic crypt, and were detected in the central region, but rarely in the luminal region (Figure 3D and E). A significant reduction in Ki-67 labeling by the mango treatment occurred in the central and basal regions compared to the control group, where the reduced rates of labeling were 39% and 23%, respectively (P < 0.05, Figure 3D).
Mango Suppressed Markers of Inflammation in DSS-Treated Rats
Gallic acid is a major polyphenolic compound in mango, known to target key players involved in inflammation [11]. Based on this information, we predicted that administration of mango might reduce inflammation in DSS-induced colitis. To further quantify the activities of mango polyphenolics on inflammatory markers in vivo, we examined inflammatory cytokines, NF-κB, iNOS, and COX-2. Mango decreased the expression of TNF-α and IL-1β mRNA (by 56% and 60%, respectively, Figure 4A), as well as TNF-α, IL-1β, and IL-6 protein levels in the intestinal mucosa of rats (by 53%, 74%, and 70%, respectively, Figure 4B). Mango also reduced the expression of iNOS and COX-2 mRNA (by 70% and 74%, respectively, Figure 4C) and, in some animals, NF-κB(p65) and iNOS protein (Figure 4D) compared to controls.
Figure 4.
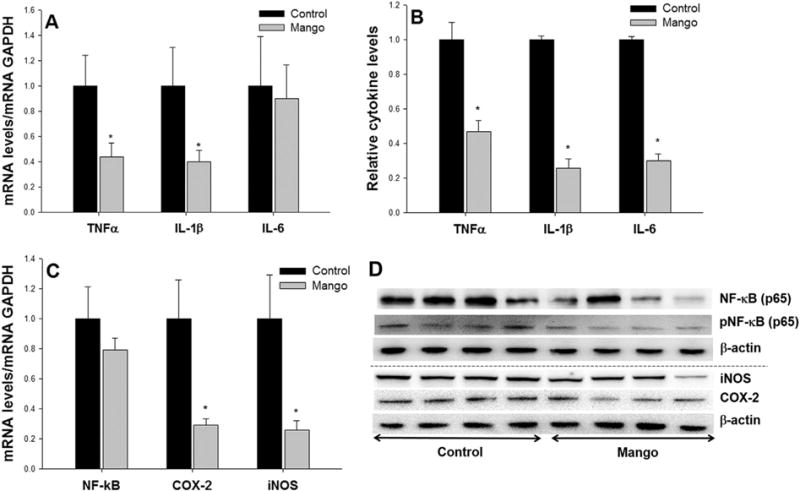
Effects of mango beverage on pro-inflammatory cytokines, NF-κb, COX-2 and iNOS in DSS-treated rats. The mango beverage decreased the expressions of (A) TNFα and IL-1β mRNA, (B) TNFα, IL-1β, and IL-6 cytokine levels in the intestinal mucosa of rats. (C) The mango beverage suppressed the expression of COX-2 and iNOS mRNA, and (D) NF-κB (p65) and iNOS protein in DSS-treated rats. Values are the mean±SEM (n = 10 per group). *P < 0.05.
Mango modulated the miR-126/mTOR Axis in DSS-Treated Rats
The mTOR pathway that is activated by PI3K/AKT signaling plays a central role in the regulation of cell growth, cell proliferation, and inflammation through downstream transduction of proliferative signals, for example via p70S6 K, RPS6, and HIF-1α [19]. Several reports indicate that suppression of the PI3K/AKT/mTOR pathway may be of clinical relevance for treating colitis [21]. The intake of mango decreased the expression of PI3K (by 48%), mTOR (by 62%), p70S6K (by 64%), and HIF-1α (by 42%) mRNA (P < 0.05) in DSS-treated rats (Figure 5A). In addition, mango intake in DSS-treated rats suppressed the protein expression of PI3K (p85β) (Figure 5B). This was accompanied by decreased expression of total and phosphorylated AKT (by 27% and 78%, respectively) and mTOR (by 48% and 74%, respectively), as well as p70S6K phosphorylated protein (by 72%), RPS6 total protein (by 35%) (Figure 5C and D), and HIF-1α (Figure 5B).
Figure 5.
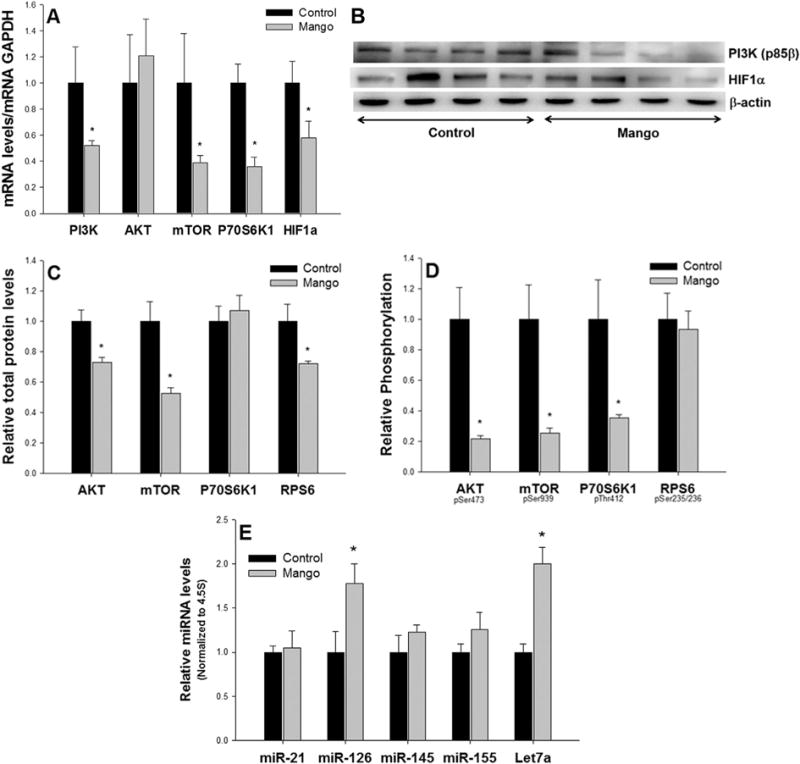
Effects of mango beverage on the mTOR signaling pathway in DSS-treated rats. (A) The mango beverage decreased the expression of PI3K, mTOR, p70S6K1, and HIF1α mRNA in DSS-treated rats. (B) The mango beverage suppressed the expression of PI3K (p85β) and HIF1α protein, (C) the total protein of AKT, mTOR and RPS6, and (D) the phosphorylated protein of AKT, mTOR and p70S6K1 in DSS-treated rats. (E) The mango beverage induced the expression of miR-126 and Let-7a compared to the control beverage in DSS-treated rats. Values are the mean±SEM (n = 10 per group). *P < 0.05.
Various miRNAs are involved in the regulation of inflammatory responses, including those that target the mTOR pathway [26]. We screened several miRNAs implicated in mTOR signaling, and observed induction of miR-126 and Let-7a by mango treatment, compared with controls (Figure 5E). No changes were detected in the levels of miR-21, miR-145, and miR-155. These in vivo findings support the results from the in vitro studies. Previously, we demonstrated that pomegranate polyphenolics reduced inflammation in carcinogen-treated rats through modulation of the miR-126/PI3K axis [31]. In DSS-treated rats, upregulation of miR-126 by mango intake was accompanied by suppression of PI3K mRNA and protein expression (Figure 5A and B).
DISCUSSION
In this investigation, mango polyphenols reduced the inflammatory response associated with colitis in vitro and in vivo, through modulation of the mTOR pathway and associated post-translational mechanisms. Mango is a rich source of polyphenolics, including gallic acid and gallotannins, which have demonstrated antioxidant and anti-inflammatory activities in cancer cells [10,11,14,41]. It was previously shown that mango extracts act on inflammation-related proteins, including iNOS, COX-2, and TNF-α, in a rat colitis model [18]. However, the mechanisms underlying the anti-inflammatory activities of mango in colitis remains unclear.
The mango extract decreased NF-κB and iNOS mRNA levels, as well as NF-κB and p- NF-κB protein levels in vitro at 10 mg GAE/L. In DSS-treated rats, mango beverage decreased inflammatory scores, and suppressed the colonic cell proliferative index in the central and basal regions. In the early stages of colitis, signal transduction cascades can act via NF-κB and mTOR pathways [42] to induce cell proliferation [43] and promote the formation of colonic aberrant crypt foci [44]. Activation of NF-κB increases the expression of genes that encode for pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α), as well as enhancing COX-2 and iNOS in macrophages and epithelial cells [45]. Induction of COX-2 and iNOS expression elicits the production of further inflammatory mediators, such as oxygen free radicals, NO, and PGE2 [46]. The intake of mango reduced the expression of pro-inflammatory cytokines and cytokines, and inflammation-related markers in vivo.
The PI3K/AKT pathway has well-established upstream regulators of mTOR. In previous studies, gallic acid has been shown to have anti-inflammatory and anti-metastasis effects through the down-regulation of the PI3K/AKT pathway and NF-κB activity in prostate and gastric cancer cells [47,48]. The current investigation indicated that mango also decreased the mTOR pathway in vitro and in vivo via suppression of PI3K/AKT signaling. The mango extract suppressed PI3K (p85β) mRNA and protein levels, and mTOR transcript levels, which would counter the activation of p70S6 K [19] and induction of HIF-1α in CCD-18Co cells [49]. This was consistent with in vivo studies, where mango suppressed PI3K (p85β) mRNA and protein, and reduced the expression of total and phosphorylated protein of AKT and mTOR. Previously, it was reported in a DSS-induced colitis model that the induction of HIF-1α triggers an inflammatory response by inducing pro-inflammatory mediators including COX-2, iNOS, TNF-α, IL-6, and IL-1β [50]. In this investigation, downstream markers of the mTOR pathway such as p70S6 K, RPS6, and HIF-1α protein were down-regulated by mango intake, consistent with prior studies showing mTOR inhibitors to be effective in suppressing DSS-induced colitis [21,23]. Targeted suppression of mTOR pathways by natural compounds might provide a viable strategy for the prevention of colitis [51], and mango is a rich source of potential mTOR inhibitors that could be used to reduce and/or prevent the incidence of ulcerative colitis [26] (Figure 6).
Figure 6.
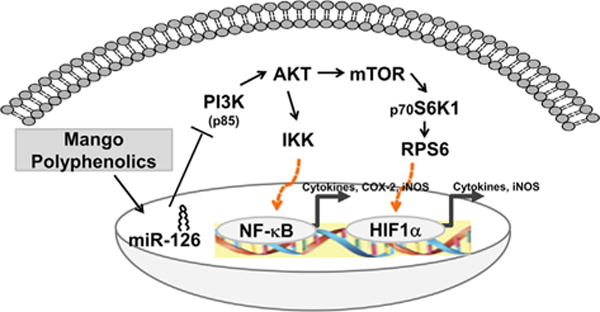
Schematic representation showing molecular mechanisms involved in ulcerative colitis treated with mango polyphenolics. Mango targeted miR-126/PI3K/AKT/mTOR axes.
PI3K is regulated by miR-126 through a seed sequence in the 3′-UTR region of the corresponding p85β mRNA [27]. Accordingly, we postulated that miR-126 may be a suitable target related to inflammation-associated pathways involved in colitis [28]. Our previous studies with red wine showed a significant suppression of inflammatory markers in LPS-treated CCD-18Co cells, and miR-126 was implicated in the underlying inhibitory mechanisms [52]. Also, pomegranate polyphenolics decreased inflammation via down-regulation of the PI3K/AKT pathways through interactions with miR-126 in AOM-treated rats, and in colon cancer cell lines [31]. In this study, mango decreased expression of the PI3K (p85β) mRNA and protein, which was accompanied by up-regulation of miR-126 in vitro and in vivo. To confirm the relationship between miR-126 and PI3K (p85β), cells were transfected with the antagomiR for miR-126. Treatment with mango polyphenolics partially reversed by the effect of the antagomiR, which was accompanied by reduced levels of PI3K (p85β) mRNA.
There are certain limitations of this experiment that warrant further study. The first limitation concerns the lack of experiments comparing active single compounds in mango (e.g., gallic acid) to total polyphenols. Our study focused on the overall, potentially synergistic effects of natural mango polyphenols, including gallotannins, on colitis and inflammation, as well as their mechanisms. While other studies have determined the effect of gallic acid on carcinogenesis and inflammation [14,53], the contribution of gallotannins to the anti-inflammatory efficacy of total mango polyphenols is uncertain as microbiota degrade these compounds into gallic acid in the colon. Due to this uncertainty, the combined effects of total mango polyphenols were investigated in this study. As with any study treatment that consists of a complete fruit, other compounds may have influenced the outcome. However, in vitro cells were treated with mango polyphenols as a physiological representation of the large intestines. The results confirmed the in vivo findings, indicating that the anti-inflammatory activities are due to the polyphenolic content. A further limitation is that the final body weights of animals in the mango group were lower by 6% than those in the control group (Supplement Table S1). While the total caloric intake was not significantly different overall, mango consumption was slightly lower than the liquid intake in the control group, whereas the solid food intake was slightly higher in the mango group. The reduction of bodyweight in the mango group may have contributed in part to the anti-inflammatory effects observed. In previous studies, it was reported that weight loss due to a low calorie diet reduced the levels of inflammation markers, TNF-α, IL-6, CRP, and ICAM-1 in overweight men [54]. However, in this study, there was no correlation between the body weight of rats and the levels of TNF-α and IL-6 in rat mucosa (R2 = 0.1527 and 0.2127). For this reason, the influence of weight loss caused by mango intake might not have been a critical contributor to the protective effects noted for mango. Finally, anticancer effects of mango extract on prostate cancer were associated in prior studies with induction of apoptosis [55]. An increase in cleaved caspase and PARP1 protein was detected after mango treatment in vitro (Supplement Figure S1A). However, no significant changes in PARP1 or Caspase 3 mRNA levels were detected in the intestinal mucosa of animals given mango in vivo (Supplement Figure S1B). Moreover, protein levels of PARP1 and Caspase 3 were decreased by mango intake in rats compared with the control group (Supplement Figure S1C). Further studies are in progress on the apoptotic mechanism triggered by mango.
For the first time, this investigation has provided evidence that mango polyphenolics attenuate the levels of inflammatory markers via suppression of the PI3K/AKT/mTOR signaling pathway, in part through up-regulation of miRNA-126. Interaction of mango with the miR-126/PI3K/AKT/mTOR axis was identified as a key component of the anti-inflammatory mechanisms of mango polyphenolics relevant to colitis.
Supplementary Material
Acknowledgments
Grant sponsor: National Mango Board (Orlando, FL), RHD is currently supported by NIH grants CA090890 and P30 ES023512, the John S. Dunn Foundation, and a Chancellor’s Research Initiative from Texas A&M University
Abbreviations
- ATCC
American type culture collection
- DSS
dextran sodium sulfate
- GAE
gallic acid equivalent
- IBD
inflammatory bowel disease
- LPS
lipopolysaccharide
- miRNA
MicroRNA
- mTOR
mammalian target of rapamycin
- PI3K p85β
phosphatidylinositol 3-kinase regulatory subunit beta
- ROS
reactive oxidative stress
- UC
ulcerative colitis
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114 e2105. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 4.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 5.Lakatos PL, Lakatos L. Risk for colorectal cancer in ulcerative colitis: Changes, causes, and management strategies. World J Gastroenterol. 2008;14:3937–3947. doi: 10.3748/wjg.14.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munkholm P. Review article: The incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18:1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, DuBois RN. The role of anti-inflammatory drugs in colorectal cancer. Ann Rev Med. 2013;64:131–144. doi: 10.1146/annurev-med-112211-154330. [DOI] [PubMed] [Google Scholar]
- 8.Yuan G, Wahlqvist ML, He G, Yang M, Li D. Natural products and anti-inflammatory activity. Asia Pac J Clin Nutr. 2006;15:143–152. [PubMed] [Google Scholar]
- 9.Shapiro H, Singer P, Halpern Z, Bruck R. Polyphenols in the treatment of inflammatory bowel disease and acute pancreatitis. Gut. 2007;56:426–435. doi: 10.1136/gut.2006.094599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masibo M, He Q. Major mango polyphenols and their potential significance to human health. Compr Rev Food Sci F. 2008;7:309–319. doi: 10.1111/j.1541-4337.2008.00047.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Jun CD, Suk K, et al. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol Sci. 2006;91:123–131. doi: 10.1093/toxsci/kfj063. [DOI] [PubMed] [Google Scholar]
- 12.Erdelyi K, Kiss A, Bakondi E, et al. Gallotannin inhibits the expression of chemokines and inflammatory cytokines in A549 cells. Mol Pharmacol. 2005;68:895–904. doi: 10.1124/mol.105.012518. [DOI] [PubMed] [Google Scholar]
- 13.Noratto GD, Bertoldi MC, Krenek K, Talcott ST, Stringheta PC, Mertens-Talcott SU. Anticarcinogenic effects of polyphenolics from mango (Mangifera indica) varieties. J Agric Food Chem. 2010;58:4104–4112. doi: 10.1021/jf903161g. [DOI] [PubMed] [Google Scholar]
- 14.Giftson JS, Jayanthi S, Nalini N. Chemopreventive efficacy of gallic acid, an antioxidant and anticarcinogenic polyphenol, against 1,2-dimethyl hydrazine induced rat colon carcinogenesis. Invest New Drugs. 2010;28:251–259. doi: 10.1007/s10637-009-9241-9. [DOI] [PubMed] [Google Scholar]
- 15.Al-Halabi R, Bou Chedid M, Abou Merhi R, et al. Gallotannin inhibits NFkB signaling and growth of human colon cancer xenografts. Cancer Biol Ther. 2011;12:59–68. doi: 10.4161/cbt.12.1.15715. [DOI] [PubMed] [Google Scholar]
- 16.Martinez G, Delgado R, Perez G, Garrido G, Nunez Selles AJ, Leon OS. Evaluation of the in vitro antioxidant activity of Mangifera indica L. extract (Vimang) Phytother Res. 2000;14:424–427. doi: 10.1002/1099-1573(200009)14:6<424::aid-ptr643>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Garrido G, Gonzalez D, Lemus Y, et al. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VI-MANG) Pharmacol Res. 2004;50:143–149. doi: 10.1016/j.phrs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Marquez L, Perez-Nievas BG, Garate I, et al. Anti-inflammatory effects of Mangifera indica L. extract in a model of colitis. World J Gastroenterol. 2010;16:4922–4931. doi: 10.3748/wjg.v16.i39.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang YJ, Dai Q, Sun DF, et al. MTOR signaling pathway is a target for the treatment of colorectal cancer. Ann Surg Oncol. 2009;16:2617–2628. doi: 10.1245/s10434-009-0555-9. [DOI] [PubMed] [Google Scholar]
- 20.Sun X, Threadgill D, Jobin C. Campylobacter jejuni induces colitis through activation of mammalian target of rapamycin signaling. Gastroenterology. 2012;142:86–95 e85. doi: 10.1053/j.gastro.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhonde MR, Gupte RD, Dadarkar SD, et al. A novel mTOR inhibitor is efficacious in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1237–G1245. doi: 10.1152/ajpgi.90537.2008. [DOI] [PubMed] [Google Scholar]
- 22.Deore V, Yewalkar N, Bhatia D, et al. Synthesis and therapeutic evaluation of pyridyl based novel mTOR inhibitors. Bioorg Med Chem Lett. 2009;19:2949–2952. doi: 10.1016/j.bmcl.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 23.Farkas S, Hornung M, Sattler C, et al. Rapamycin decreases leukocyte migration in vivo and effectively reduces experimentally induced chronic colitis. Int J Colorectal Dis. 2006;21:747–753. doi: 10.1007/s00384-005-0793-7. [DOI] [PubMed] [Google Scholar]
- 24.Guo C, Sah FJ, B L, Willson JKV, Markowitz SD, Guda K. The non-coding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer. 2008;47:939–946. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Link A, Balaguer F, Goel A. Cancer chemoprevention by dietary polyphenols: Promising role for epigenetics. Biochem Pharmacol. 2010;80:1771–1792. doi: 10.1016/j.bcp.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alqurashi N, Hashimi SM, Wei MQ. Chemical inhibitors and microRNAs (miRNA) targeting the mammalian target of rapamycin (mTOR) pathway: Potential for novel anticancer therapeutics. Int J Mol Sci. 2013;14:3874–3900. doi: 10.3390/ijms14023874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo C, Sah JF, Beard L, Willson JK, Markowitz SD, Guda K. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer. 2008;47:939–946. doi: 10.1002/gcc.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meister J, Schmidt MH. MiR-126 and miR-126*: New players in cancer. Scientific World Journal. 2010;10:2090–2100. doi: 10.1100/tsw.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krenek KA, Barnes RC, Talcott ST. Phytochemical composition and effects of commercial enzymes on the hydrolysis of gallic acid glycosides in mango (Mangifera indica L. cv. ‘Keitt’) Pulp. J Agri Food Chem. 2014;62:9515–9521. doi: 10.1021/jf5031554. [DOI] [PubMed] [Google Scholar]
- 30.Masibo M, He Q. Mango bioactive compounds and related nutraceutical properties—A review. Food Rev Int. 2009;25:346–370. [Google Scholar]
- 31.Banerjee N, Kim H, Talcott S, Mertens-Talcott S. Pomegranate polyphenolics suppressed azoxymethane-induced colorectal aberrant crypt foci (ACF) and Inflammation: Possible role of miR-126/VCAM-1 and miR-126/PI3K/AKT/mTOR. Carcinogenesis. 2013 doi: 10.1093/carcin/bgt295. [DOI] [PubMed] [Google Scholar]
- 32.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in humans and animal models. Carcinogenesis. 2003;24:353–362. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 33.Solomon L, Mansor S, Mallon P, et al. The dextran sulphate sodium (DSS) model of colitis: An overview. Comp Clin Pathol. 2010;19:235–239. [Google Scholar]
- 34.Hong MY, Bancroft LK, Turner ND, et al. Fish oil decreases oxidative DNA damage by enhancing apoptosis in rat colon. Nutr Cancer. 2005;52:166–175. doi: 10.1207/s15327914nc5202_7. [DOI] [PubMed] [Google Scholar]
- 35.Banerjee N, Talcott S, Safe S, Mertens-Talcott SU. Cytotoxicity of pomegranate polyphenolics in breast cancer cells in vitro and vivo: Potential role of miRNA-27a and miRNA-155 in cell survival and inflammation. Breast Cancer Res Treat. 2012;136:21–34. doi: 10.1007/s10549-012-2224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mertens-Talcott SU, Noratto GD, Li X, Angel-Morales G, Bertoldi MC, Safe S. Betulinic acid decreases ER-negative breast cancer cell growth in vitro and in vivo: Role of Sp transcription factors and microRNA-27a:ZBTB10. Mol Carcinog. 2013;52:591–602. doi: 10.1002/mc.21893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 38.Cui X, Jin Y, Hofseth AB, et al. Resveratrol suppresses colitis and colon cancer associated with colitis. Cancer Prev Res (Phila) 2010;3:549–559. doi: 10.1158/1940-6207.CAPR-09-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaudio E, Taddei G, Vetuschi A, et al. Dextran sulfate sodium (DSS) colitis in rats: Clinical, structural, and ultrastructural aspects. Dig Dis Sci. 1999;44:1458–1475. doi: 10.1023/a:1026620322859. [DOI] [PubMed] [Google Scholar]
- 40.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 41.Rocha Ribeiro SM, Queiroz JH, Lopes Ribeiro de Queiroz ME, Campos FM, Pinheiro Sant’ana HM. Antioxidant in mango (Mangifera indica L. pulp. Plant Foods Hum Nutr. 2007;62:13–17. doi: 10.1007/s11130-006-0035-3. [DOI] [PubMed] [Google Scholar]
- 42.Dos Santos S, Delattre AI, De Longueville F, Bult H, Raes M. Gene expression profiling of LPS-stimulated murine macrophages and role of the NF-kappaB and PI3K/mTOR signaling pathways. Ann N Y Acad Sci. 2007;1096:70–77. doi: 10.1196/annals.1397.071. [DOI] [PubMed] [Google Scholar]
- 43.Vetuschi A, Latella G, Sferra R, Caprilli R, Gaudio E. Increased proliferation and apoptosis of colonic epithelial cells in dextran sulfate sodium-induced colitis in rats. Dig Dis Sci. 2002;47:1447–1457. doi: 10.1023/a:1015931128583. [DOI] [PubMed] [Google Scholar]
- 44.Kukitsu T, Takayama T, Miyanishi K, et al. Aberrant crypt foci as precursors of the dysplasia-carcinoma sequence in patients with ulcerative colitis. Clin Cancer Res. 2008;14:48–54. doi: 10.1158/1078-0432.CCR-07-1835. [DOI] [PubMed] [Google Scholar]
- 45.Schottelius AJ, Baldwin AS., Jr A role for transcription factor NF-kappa B in intestinal inflammation. Int J Colorectal Dis. 1999;14:18–28. doi: 10.1007/s003840050178. [DOI] [PubMed] [Google Scholar]
- 46.Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306. doi: 10.1016/s0016-5085(98)70196-9. [DOI] [PubMed] [Google Scholar]
- 47.Ho HH, Chang CS, Ho WC, Liao SY, Wu CH, Wang CJ. Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-kappaB activity and downregulation of PI3 K/AKT/small GTPase signals. Food Chem Toxicol. 2010;48:2508–2516. doi: 10.1016/j.fct.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 48.Liu K-C, Huang A-C, Wu P-P, et al. Gallic acid suppresses the migration and invasion of PC-3 human prostate cancer cells via inhibition of matrix metalloproteinase-2 and-9 signaling pathways. Oncol Rep. 2011;26:177. doi: 10.3892/or.2011.1264. [DOI] [PubMed] [Google Scholar]
- 49.Frede S, Berchner-Pfannschmidt U, Fandrey J. Regulation of hypoxia-inducible factors during inflammation. Methods Enzymol. 2007;435:405–419. doi: 10.1016/S0076-6879(07)35021-0. [DOI] [PubMed] [Google Scholar]
- 50.Shah YM, Ito S, Morimura K, et al. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134:2036–2048. 2048 e2031–2033. doi: 10.1053/j.gastro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson SM, Gulhati P, Arrieta I, et al. Curcumin inhibits proliferation of colorectal carcinoma by modulating Akt/mTOR signaling. Anticancer Res. 2009;29:3185–3190. [PMC free article] [PubMed] [Google Scholar]
- 52.Angel-Morales G, Noratto G, Mertens-Talcott S. Red wine polyphenolics reduce the expression of inflammation markers in human colon-derived CCD-18Co myofibroblast cells: Potential role of microRNA-126. Food Funct. 2012;3:745–752. doi: 10.1039/c2fo10271d. [DOI] [PubMed] [Google Scholar]
- 53.Kim S-H, Jun C-D, Suk K, et al. Gallic acid inhibits histamine release and pro-inflammatory cytokine production in mast cells. Toxicol Sci. 2006;91:123–131. doi: 10.1093/toxsci/kfj063. [DOI] [PubMed] [Google Scholar]
- 54.Sharman MJ, Volek JS. Weight loss leads to reductions in inflammatory biomarkers after a very-low-carbohydrate diet and a low-fat diet in overweight men. Clin Sci (Lond) 2004;107:365–370. doi: 10.1042/CS20040111. [DOI] [PubMed] [Google Scholar]
- 55.Prasad S, Kalra N, Shukla Y. Induction of apoptosis by lupeol and mango extract in mouse prostate and LNCaP cells. Nutr Cancer. 2007;60:120–130. doi: 10.1080/01635580701613772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


