Abstract
Clinical and histopathological characteristics of 16 dogs with nodal paracortical (T-zone) lymphoma (TZL) were evaluated. At initial examination, generalized lymphadenopathy was found in all dogs, and peripheral lymphocytosis was found in 10 of the 16 dogs. At initial diagnosis or during the disease course, 8 dogs (50%) were affected with demodicosis. Immunohistochemical analysis for CD3, CD20 and CD25 was performed for 6 dogs with TZL; the tumor cells were positive for CD3 and CD25 and negative for CD20. Median overall survival time was 938 days. A watchful waiting approach was adopted for 6 cases (38%), and 5 of the 6 dogs were still alive at the end of follow-up. The clinical course of TZL in dogs is generally indolent; however, many cases develop a variety of infectious and other neoplastic diseases after the diagnosis of TZL.
Keywords: CD25, dog, prognosis, treatment, t-zone lymphoma
Non-Hodgkin lymphomas are common in dogs, and their annual incidence has been estimated as 13–107 per 100,000 dogs per year [5, 6, 13]. In 2006, Valli et al. reported architectural features, immunophenotypes and molecular clonality findings for 66 cases of canine indolent lymphoma and proposed a new subtype named nodal paracortical (T-zone) lymphoma (TZL), which showed clinically indolent behavior with long survival despite generalized lymphadenopathy and limited clinical response to chemotherapy [27]. In the Kiel classification, the lymphoma subtype corresponding to TZL was shown to have cytological characteristics of small clear cell [8].
It was reported that the most common histopathological subtype in canine indolent lymphoma was TZL (61.7%), followed by marginal zone lymphoma (25%) in 60 dogs with indolent lymphoma [7]. Dogs with TZL had the longest median survival (622 days), although conceivably, they were diagnosed in later stages of the disease because of the lack of signs associated with progression [26]. Recently, immunophenotyping has been considered an important tool for classification of canine T-cell lymphoma (TCL), which is clinically and histologically heterogeneous. Seelig et al. demonstrated immunophenotyping assessment for dogs with TCL by flow cytometric analyses using several types of antibodies and reported that all cases with CD45− TCL could be classified as TZL [17].
In human medicine, WHO classification of lymphoid neoplasms has been used to distinguish the indolent subgroups from the major aggressive subgroups [22]. The clinical features of various lymphoma subtypes were found to be remarkably variable. Therapeutic trials focusing on these specific subgroups have been carried out to identify more effective treatment options [2, 22]. In veterinary medicine, a recent study showed the subtype categorization of 456 dogs with lymphoma and reported their prognoses after a variety of treatment protocols [26]. Further efforts to determine the most effective therapeutic strategy in each subtype of lymphoma are considered to be necessary for obtaining better prognoses and outcomes in canine lymphoma.
The aim of the present study is to understand the clinical and histopathological features in 16 dogs with TZL, which seems to have distinct clinical characteristics and disease course compared with the more common high-grade lymphomas in dogs.
MATERIALS AND METHODS
Criteria for selection of cases: A retrospective study was performed at the Veterinary Medical Center of the University of Tokyo (UTokyo-VMC) from May 2004 through November 2012. Medical records were reviewed to identify dogs with TZL that presented to the UTokyo-VMC during the period. Cases included in this study were those involving 25 dogs that were histologically diagnosed with multicentric TZL. Of the 25 cases, 9 were excluded for the following reasons: 6 cases for incomplete clinical data and 3 cases for inadequate biopsy procedures instead of the resection of a whole lymph node. All nodal biopsy specimens were reviewed by a single pathologist blinded to clinical information during the slide-review process. All cases were also required to have adequate follow-up information from initial diagnosis.
Immunological subtypes: To examine the clonal expansion of the cells and the cell lineage, polymerase chain reaction (PCR) for antigen receptor gene rearrangement (PARR) and immunohistochemistry were employed. For the detection of clonal rearrangement of IgH and TCRγ genes, PCR analyses were performed for 8 cases. DNA samples were extracted from primary lymphoma cells obtained by fine needle aspiration or biopsy. The primer sequences for IgH and TCRγ reported by Burnett et al. [4] and Valli et al. [27] are shown in Table 1. Samples for PCR were initially heated to 95°C for 15 min in both methods. The PCR condition using the primers reported by Burnett et al. was as follows: 94°C for 8 sec, 60°C for 10 sec and 72°C for 15 sec for 35 cycles without final extension procedure [4]. The PCR condition using the primers reported by Valli et al. was as follows: five cycles of 94°C for 30 sec and 72°C for 2 min, five cycles of 94°C for 30 sec and 70°C for 2 min and 35 cycles of 94°C for 20 sec and 68°C for 2 min. A final extension of 68°C for 10 min was performed. For the denaturation, the samples for the PCR were heated at 95°C for 10 min and then were allowed to reanneal at 4°C for 1 hr prior to polyacrylamide gel electrophoresis (heteroduplex analysis) [27]. All of the PCR analyses were run in duplicate.
Table 1. Primers used for the detection of clonal rearrangement of IgH and TCRγ genesa).
| Reaction number | Product | Primer specificity | Primer sequence (5′ −3′)b) |
|---|---|---|---|
| 1 | Cµ | Cµ | TTC CCC CTC ATC ACC TGT GA |
| Cµ | GGT TGT TGA TTG CAC TGA GG | ||
| 2 | IgH major | VH | CAG CCT GAG AGC CGA GGA CAC |
| JH | TGA GGA GAC GGT GAC CAG GGT | ||
| 3 | IgH minor | VH | CAG CCT GAG AGC CGA GGA CAC |
| JH | TGA GGA CAC AAA GAG TGA GG | ||
| 4 | TCRγ | JH | ACC CTG AGA ATT GTG CCA GG |
| JH | GTT ACT ATA AAC CTG GTA AC | ||
| VH | TCT GGG A/GTG TAC/T TAC TGT GCT GTC TGG | ||
| 5 | IgH | VH | GMC GVT TCA CCA TCT CCA RRG |
| JH | TGA RGA GAC RGT GAC CWG GGT | ||
| JH | GGA CAC GAA GAS TGA GGT GCC | ||
| 6 | TCRγ | Vγ | TGK TGC AGA ARC TGG AGA AGA |
| Jγ | GCA CTG TGC CAG GAC CAA ATA |
Immunohistochemistry for CD3 and CD20 was employed to determine the tumor phenotype for 16 cases in this study. Anti-CD3 (polyclonal rabbit anti-human CD3, A0452; DAKO, Glostrup, Denmark; 1:50 dilution) and anti-CD20 (polyclonal rabbit anti-human CD20, RB-9013-P; Thermo Fisher Scientific, Waltham, MA, U.S.A.; 1:400 dilution) antibodies were used as primary antibodies. Immunohistochemical analysis using anti-CD25 (monoclonal mouse anti-human CD25, clone 4C9; Thermo Fisher scientific; 1:40 dilution) antibody was performed for 6 dogs with TZL.
Routine protocols of immunohistochemistry were performed on 4-µm sections of formalin-fixed and paraffin-embedded tissues. These sections were dewaxed and rehydrated through graded alcohols to water, and antigen retrieval was performed in 1% citrate buffer solution (pH 6.0) in the autoclave at 120°C for 15 min. Endogenous peroxidase was inactivated by 1% hydrogen peroxide in methanol for 30 min, and blocking was processed by 8% skim milk in Tris-buffered saline (TBS) for 40 min. The slides then were incubated overnight at 4°C with a panel of primary antibodies directed to CD3, CD20 and CD25. Negative and positive control sections also were incubated at this stage. After all slides were washed for 15 min in TBS, EnVision + Dual Link System-HRP for CD3, CD20 and CD25 was applied for 45 min at room temperature. After the slides were washed for 15 min, binding of the antibody was visualized using 3,3′-diaminobenzidine (DAB) as a chromogen, and slides were counterstained in Mayer’s hematoxylin.
Clinicopathological characteristics: Medical records of the cases were reviewed to study clinicopathological characteristics. Age, sex, breed, physical examination features, the complete blood cell count profile at the time of diagnosis, concomitant illness and survival time were reviewed. Staging was based on the WHO clinical stage criteria for canine lymphomas [30]. In addition, dogs were assigned to substage categories “a” (without systemic signs of illness) or “b” (with systemic signs of illness) [30].
Sizes of the peripheral lymph nodes were measured with slide calipers, and the sum of the longest diameter for the target lesions (up to 5 nodes) was calculated [24]. All cases were divided into 4 groups according to treatment: dogs treated with (1) CHOP (cyclophosphamide, doxorubicin, vincristine and prednisolone)-based protocols; (2) MP (melphalan and prednisolone); (3) prednisolone alone; or (4) no chemotherapy. Owners or referring veterinarians were contacted when the information of the treatment or outcome in the medical record was incomplete.
Statistical analysis: Overall survival time (OST) and lymphoma-specific survival time (LSST) were calculated by Kaplan-Meier survival curves for 16 cases with TZL. For both OST and LSST calculations, dogs were censored if they were still alive or lost to follow up. OST was measured from the day on which dogs were newly diagnosed as having TZL to the date of death as a result of any cause. LSST was defined as the time from the day on which dogs were newly diagnosed with TZL to the date of death from TZL. Dogs that died due to causes not likely associated with lymphoma (e.g., progression of concomitant malignant tumors, cardiac disease) were censored.
RESULTS
Clinical conditions and complete blood cell count at diagnosis: Age, sex and breed for 16 dogs diagnosed with TZL are summarized in Table 2. All of the 16 dogs had generalized lymphadenopathy. Splenomegaly and/or hepatomegaly were found in 8 of the 16 dogs by diagnostic imaging. Cutaneous lesions were found in 6 dogs: 3 cases with demodicosis, 2 with pyoderma and 1 with otitis externa. Four dogs were found to have pale mucous membranes. Of the 16 cases, only 3 cases showed systemic symptoms, such as a reduction in activity and appetite (2 cases), and weight loss (1 case). In the WHO clinical staging, 10 cases (63%) were shown to have a large number of typical “clear cells” in peripheral blood and thus were classified into stage V. Of the 16 cases, 13 cases (81%) were assigned into substage “a”. Consequently, WHO clinical stages and substages of the 16 dogs were IIa (n=1), IIIa (n=3), IVa (n=1), IVb (n=1), Va (n=8) and Vb (n=2).
Table 2. Profiles of 16 dogs with T-zone lymphoma.
| Median | Range | ||
|---|---|---|---|
| Age (years) | 8 | 6–11 | |
| Body weight (kg) | 26.4 | 3.9–39.2 | |
| No of cases | % | ||
| Sex | |||
| Male castrated | 2 | 13 | |
| Female spayed | 4 | 25 | |
| Male intact | 7 | 44 | |
| Female intact | 3 | 19 | |
| Breed | |||
| Golden Retriever | 9 | 56 | |
| Shih Tzu | 3 | 19 | |
| Others | 4 | 25 | |
| Stage | |||
| I | 0 | 0 | |
| II | 1 | 6 | |
| III | 3 | 19 | |
| IV | 2 | 13 | |
| V | 10 | 63 | |
| Substage | |||
| a | 13 | 81 | |
| b | 3 | 19 | |
| Immunological phenotype | |||
| T | 15 | 94 | |
| T, B | 1 | 6 | |
| PARR | |||
| Clonal rearrangement for TCRγ gene | 4 | 25 | |
| Clonal rearrangement for IgH gene | 0 | 0 | |
| Clonal rearrangement for TCRγ & IgH genes | 1 | 6 | |
| Negative | 3 | 19 | |
| Not determined | 8 | 50 | |
| Enlarged lymph node | |||
| Systemic | 16 | 100 | |
| Local | 0 | 0 | |
| Clinical symptom | |||
| Enlargement of spleen or liver | 8 | 50 | |
| Cutaneous lesion | 6 | 38 | |
| Pale mucous membranes | 4 | 25 | |
| Reduction in activity and appetite | 2 | 13 | |
| Respiratory distress | 2 | 13 | |
| Hematological abnormality | |||
| Anemia | 2 | 13 | |
| Leukocytosis | 6 | 38 | |
| Lymphocytosis | 10 | 63 | |
| Thrombocytosis | 4 | 25 | |
| Chemotherapy | |||
| CHOP-based protocola) | 2 | 13 | |
| M-P protocolb) | 6 | 38 | |
| Prednisolone alone | 2 | 13 | |
| No treatment | 6 | 38 | |
a) CHOP-based protocol: cyclophosphamide, doxorubicin, vincristine and prednisolone. b) M-P protocol: melphalan and prednisolone.
In complete blood cell count at initial diagnosis, of the 16 dogs, 2 (13%) had mild anemia (hematocrit [Hct] 0.30 and 0.34). Ten cases (63%) had lymphocytosis (5,230–19,200/µl) containing lymphoid cells characterized as “clear cells” in the peripheral blood [8]. Four cases had thrombocytosis (511,000–845,000/µl) above the laboratory’s reference range.
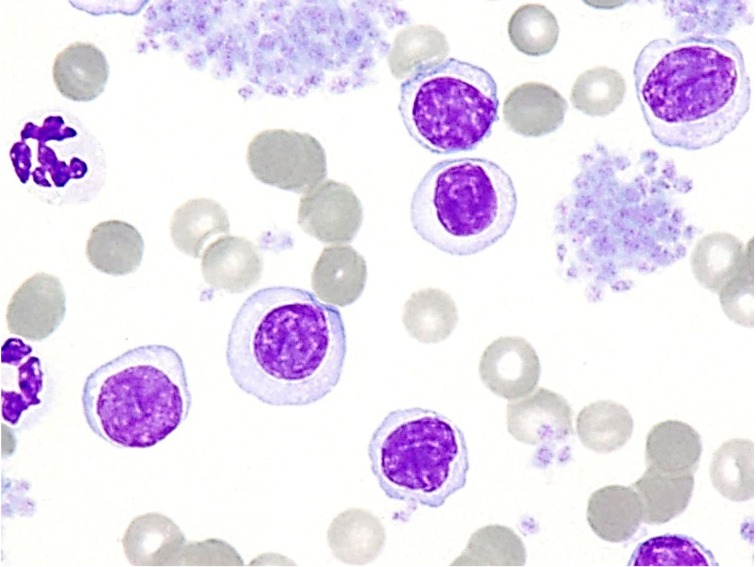
Cytological and histopathological characteristics: The mature lymphoid cells similar to “clear cells” that increased in the peripheral blood in 10 cases had small- to medium-sized nuclei (an average size of 1 to 1.5 red blood cells) showing densely stained chromatin and relatively abundant clear cytoplasm (Fig. 1). Such lymphoid cells were also found in the peripheral blood, even in cases without lymphocytosis, although these cases were not assigned to stage V in the WHO clinical staging system.
Fig. 1.
Neoplastic cells in peripheral blood. TZL cells are small or intermediate lymphocytes, and the cytoplasm is more abundant than that in normal lymphocytes and very lightly stained. Wright-Giemsa stain, × 1,000.
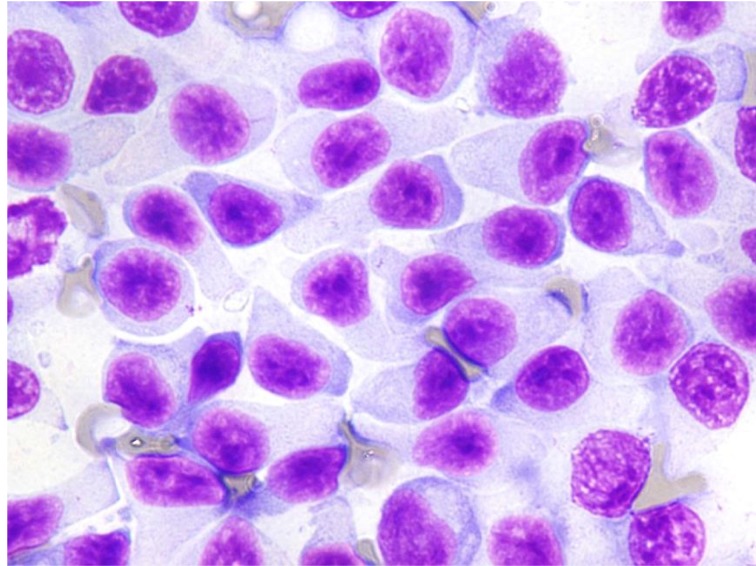
In the cytological specimens obtained from enlarged peripheral lymph nodes, TZL was composed of small- to medium-sized lymphocytes with clear cytoplasm sometimes showing extension to form hand-mirror or tear-drop image (Fig. 2). The nuclei had little internal detail and were small to medium in size, showed densely stained chromatin and sometimes had sharp shallow indentations. Mitotic figures were rare.
Fig. 2.
Neoplastic cells in lymph node. Cells with round to oval nuclei and abundant clear extended cytoplasm, resulting in hand mirror or tear-drop image. Mitoses are not present in most fields. Wright-Giemsa stain, × 1,000.
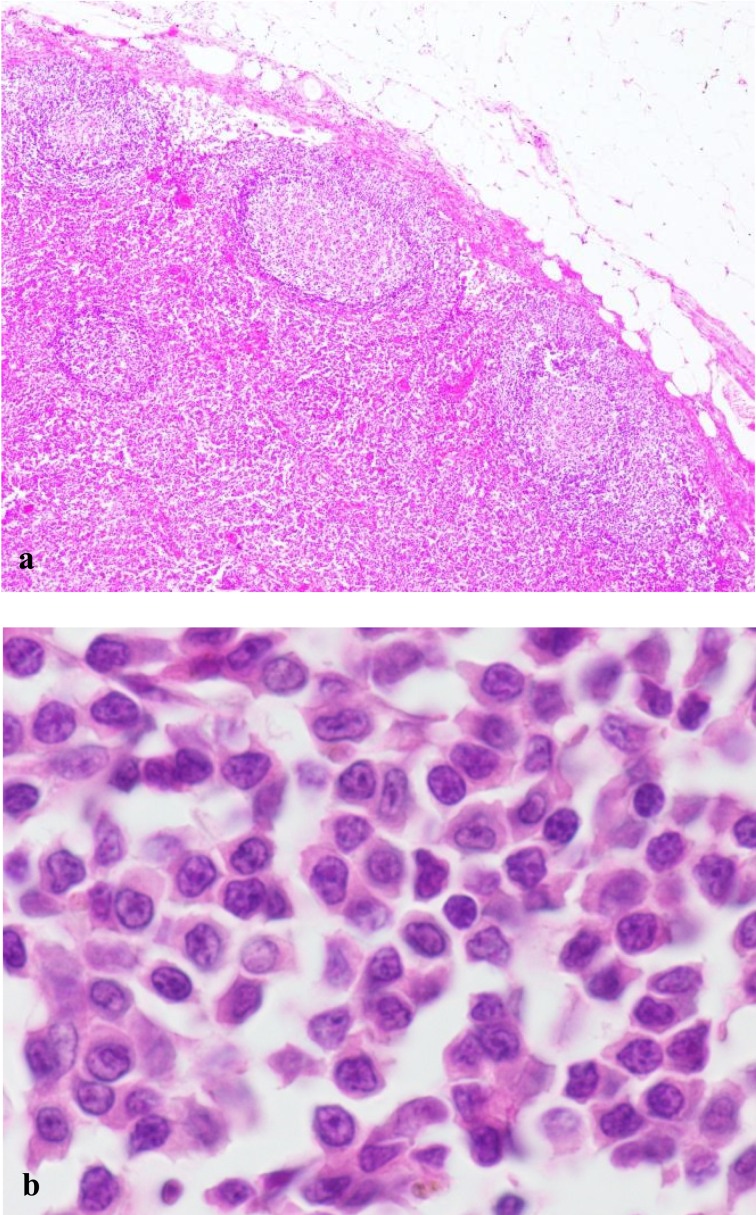
Histopathologically, neoplastic cells were small to intermediate in size, and they expanded from the paracortex to the cortex and medullary cords. The capsule of the lymph node was at least focally thinned, although perinodal tissue was not involved. Fading follicles and germinal centers were found and tended to be peripheralized by the expanding population of neoplastic T cells (Fig. 3a and 3b).
Fig. 3.
The histological features of canine TZL. (a) Neoplastic lymphocytes expand in the paracortex and are peripheralizing the fading lymphoid follicles. Hematoxylin and eosin (H&E) stain. × 40. (b) Neoplastic cells are small to intermediate in size. The nuclei are small and sometimes have sharp shallow indentations. Some nuclei have small central nucleoli. H&E stain. × 1,000.
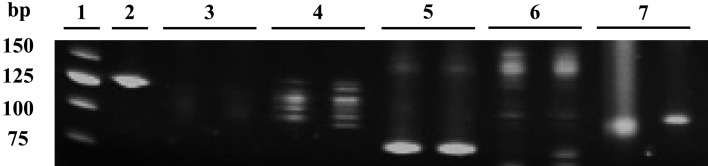
PARR and immunohistochemistry: Of 16 dogs, 8 underwent genetic clonality analyses. Four of the 8 dogs with T-zone lymphoma showed monoclonal rearrangement of TCRγ (Fig. 4). One dog showed clonal rearrangement of both IgH and TCRγ. In the other 3 dogs, clonal rearrangement of IgH or TCRγ was not detected by PARR.
Fig. 4.
PARR results in a dog with TZL. Lanes 2–5: primer sets reported by Burnett et al. [4]. Lanes: 6, 7: primer sets reported by Valli et al. [27]. Lane 1: 25 bp ladder, Lane 2: Cµ (positive control), Lane 3: IgH major, Lane 4: IgH minor, Lane 5: TCRγ, Lane 6: IgH,Lane 7: TCRγ. Of the lanes 3–7, only lane 5 (TCRγ) shows the clonal band. PCR analyses were run in duplicate.
Immunohistochemical analysis for CD3 and CD20 was performed for 16 dogs. In 15 dogs (94%), the majority of the lymphoid cells on the nodes were immunopositive for CD3. In 1 dog (6%), the tumor cells were double-positive for CD3 and CD20. The results regarding immunohistological phenotype and PARR are presented in Table 2.
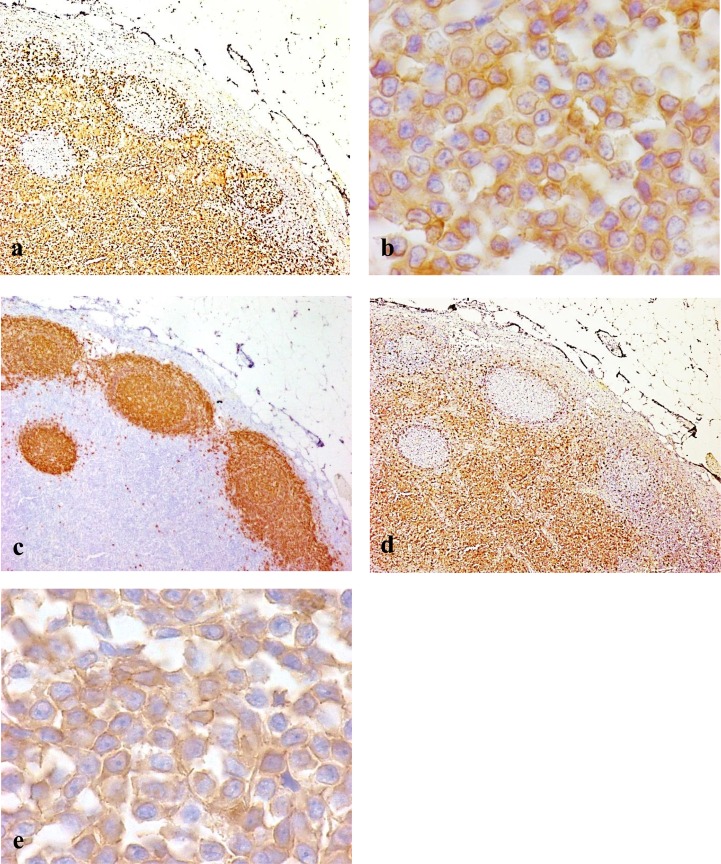
Immunohistochemical analysis for the CD25 antigen was performed for 6 dogs with TZL that were positive for CD3, as confirmed by immunohistochemistry. In all 6 cases, CD25-positive cells were found in accordance with CD3-positive and CD20-negative neoplastic cellular localization. Among the expanding population of neoplastic cells in the paracortical regions of nodes, almost all the cells were strongly immunopositive for CD25 (Fig. 5a−e).
Fig. 5.
The immunohistochemical features of canine TZL. (a) Neoplastic T cells in the paracortex are uniformly immunopositive for CD3. Immunolabeling with anti-CD3. × 40. (b) Intense labeling of neoplastic T cells with CD3. The neoplastic T cells are small or intermediate in size. Immunolabeling with anti-CD3. × 1,000. (c) B cells in fading lymphoid follicles are immunopositive for CD20. Follicular structures are pressed against the outer sinus by the expanding population of neoplastic T cells. Immunolabeling with anti-CD20. × 40. (d) Neoplastic cells in the paracortex are strongly immunopositive for CD25. B cells in the lymphoid follicles are negative for CD25. Immunolabeling with anti-CD25. × 40. (e) CD25-positive neoplastic cells are small to medium in size. Some cells have sharp shallow nuclear indentations and abundant cytoplasm. Immunolabeling with anti-CD25. × 1,000.
Concurrent diseases observed in the 16 dogs with TZL: Eleven (69%) of 16 cases had concurrent medical problems during the disease course by follow-up (181–1,662 days; median, 841 days): demodicosis (8 cases), cystitis (4 cases), pyoderma (4 cases), otitis externa (3 cases), corneal ulcer (2 cases), conjunctivitis (2 cases), epulis (2 cases), mast cell tumor (2 cases), sebaceous carcinoma (1 case), pyelonephritis (1 case), chronic renal failure (1 case), melanoma (1 case), hemangiosarcoma (1 case) and meningioma (1 case). Two cases (13%) had benign cutaneous tumors (papilloma and plasmacytoma), and there were no concurrent medical problems observed in 5 cases (31%) (Table 3).
Table 3. Concurrent diseases that occurred during the observation period in 16 dogs with TZL.
| No of cases | % | |
|---|---|---|
| Demodicosis | 8 | 50 |
| Cystitis | 4 | 25 |
| Pyoderma | 4 | 25 |
| Otitis externa | 3 | 19 |
| Corneal ulcer | 2 | 13 |
| Conjunctivitis | 2 | 13 |
| Epulis | 2 | 13 |
| Mast cell tumor | 2 | 13 |
| Sebaceous carcinoma | 1 | 6 |
| Pyelonephritis | 1 | 6 |
| Chronic renal failure | 1 | 6 |
| Melanoma | 1 | 6 |
| Hemangiosarcoma | 1 | 6 |
| Meningioma | 1 | 6 |
| IMHAa) | 1 | 6 |
| IMTb) | 1 | 6 |
| Congestive heart failure | 1 | 6 |
| Benign cutanesous tumorsc) | 2 | 13 |
a) IMHA: immune-mediated hemolytic anemia. b) IMT: immune-mediated thrombocytopenia. c) Benign cutaneous tumors: papilloma and plasmacytoma.
Treatment and response to treatment: All cases were divided into 4 groups according to the treatment: dogs treated with a CHOP-based protocol (cyclophosphamide, doxorubicin, vincristine and prednisolone) [9] (2 cases); MP (melphalan and prednisolone) [1] (6 cases); prednisolone alone (2 cases); and no chemotherapy (6 cases) (Table 2).
Chemotherapy with a CHOP-based protocol was conducted for 2 cases, because these dogs were found at the initial visit to have respiratory distress due to the lymphadenopathy revealed by radiography. After the treatment, 1 case attained a partial remission (PR); however, the other case did not respond to CHOP-based protocol.
Treatment with MP protocol was conducted for 6 cases, because of respiratory distress from enlargement of nodes (2 cases) and progressive lymphocytosis in the peripheral blood (4 cases). Of the 6 cases, 5 attained PR, and 1 achieved CR. Prednisolone alone was administered for 2 cases. One case achieved PR, but the other did not show any response.
Watchful waiting was selected for 6 cases, because they did not show any symptoms caused by lymphoma during the follow-up period. Although these dogs did not receive any chemotherapy, the lymph node sizes were found to wax and wane for the observation period (10.7–37.1 months, median 26.2 months).
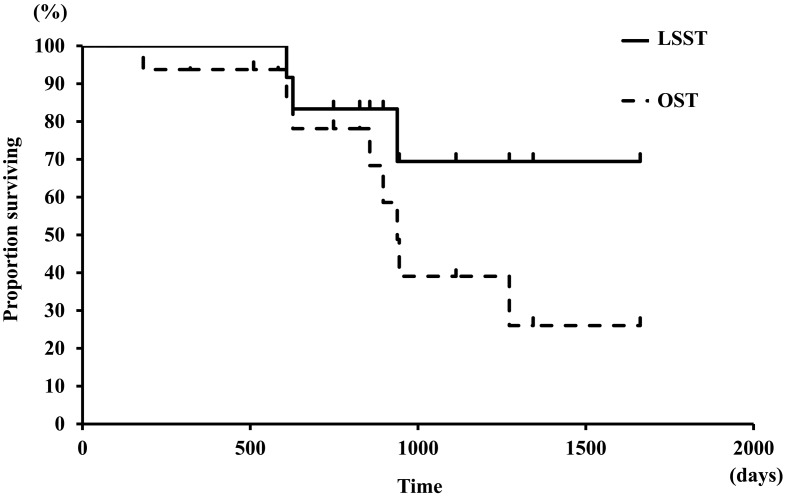
Prognostic analyses: The median OST was 938 days, and the median LSST was not calculated because lymphoma-related death was found in only 3 dogs, and the number did not exceed half (Fig. 6). Of the 16 dogs with TZL, 8 dogs died of any reason by the end of the follow-up duration, and none of the 8 dogs was euthanized. One of the 3 dogs had respiratory distress from enlargement of nodes, and the other 2 dogs had disseminated intravascular coagulation (DIC) associated with progression of lymphoma. All 3 dogs that died of the progression of lymphoma received chemotherapy with CHOP-based protocol or MP protocol. Cause of death other than lymphoma included metastasis of sebaceous carcinoma (1 dog), hemangiosarcoma (1), IMHA plus IMT (1), congestive heart failure (1) and melanoma (1).
Fig. 6.
Survival curves for overall survival and lymphoma-specific survival in 16 dogs with TZL. The median OST was 938 days, and the median LSST was not reached.
DISCUSSION
At the initial diagnosis or during the disease course, many cases were found to be affected with demodicosis or experienced various infectious and neoplastic diseases. The peripheral lymph nodes biopsied from the 16 cases in this study showed characteristic histological appearances as reported by Valli et al. [18, 28]. Moreover, immunohistochemistry revealed that the tumor cells of canine TZL were strongly positive for CD25.
Golden Retrievers and Shih Tzu accounted for three-fourths of all breeds evaluated, and other breeds included in the study were Cavalier King Charles Spaniel, Maltese, Pembroke Welsh Corgi and mixed breed. It might be possible that TZL occurs with elevated frequency in Golden retrievers and Shih Tzu, as described in the previous reports [7, 17]. In this study, the median age at the time of diagnosis was 8 years (range: 6–11years). A previous study showed that the median age of 10 dogs with TZL was 8.9 years [27]. Age of the dogs that developed TZL is conceivably similar or slightly older than that of dogs with lymphomas in general [26].
At the initial visit, generalized lymphadenopathies in the peripheral lymph nodes were found in all of the 16 cases in this study. Demodicosis was found frequently, occurring in 8 cases (50%) throughout the observation period. A previous report showed that almost 10% of canine cases with TZL had demodex infection at diagnosis [7]; however, many more cases with TZL in the present study developed an infection of demodex at time of initial diagnosis (3 cases, 19%) and during the treatment (8 cases, 50%). Demodex canis is normally present in dog skin, but it has been assumed that immune dysfunctions and genetic disorders cause overgrowth of demodex in canine patients [3, 16]. Humans undergoing chemotherapy have been shown to have an increased incidence of demodex infestation [10, 18]. Of the 8 dogs with demodicosis, 2 dogs developed the disease after initiation of chemotherapy (1 dog with CHOP-based protocol and 1 dog with MP protocol), but 6 dogs were found to have demodicosis without application of any chemotherapy. TZL could be one of the underlying diseases for the development of demodicosis in dogs. However, immunosuppression due to chemotherapy might be also associated with the development of demodicosis in some dogs. The immune abnormalities might be associated with the development of TZL and lead to a frequent occurrence of demodicosis. When peripheral lymphadenopathy is found in the presence of dermatologic problems, differentiation between hyperplastic/inflammatory and neoplastic changes should be made. Diagnosis as TZL should be made not just by cytology but by histopathological examination in order to discriminate accurately between neoplastic proliferation and hyperplasia by cutaneous lesions. PARR also could be used for the demonstration of the clonal expansion of the lymphoid cells; however, the sensitivity for TCRγ genes might be low. A study by Valli et al. reported that clonal band was detected in five of eight dogs (63%) with T-zone lymphoma [27]. Flow cytometric analyses would be also useful for the diagnosis of canine TZL, because the tumor cells of TZL have a characteristic immunological phenotype including CD45-negative status [17].
Immunohistochemically, the neoplastic cells of 6 cases with TZL examined were intensely positive for CD25. It has been known that heterotrimerization of interleukin-2 receptor α, β and γ chains leads to high-affinity binding for IL-2 and IL-2/IL-2R complex and has an important role in controlling proliferation and differentiation of immune system cells [12]. In humans, previous reports have shown that CD25 expression is found in several types of leukemia or malignant lymphoma, including Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL), which express CD25 antigen in a large number of cases [14, 21]. The complete biological role of IL-2Rα expression in tumors is unclear, although IL-2Rα can stimulate tumor cell proliferation [15, 20, 23]. It has been reported that particular hematological tumors highly express the membranous CD25 forming an important phenotypic trait that could be a therapeutic target molecule [11, 19, 29]. A recent study revealed that canine TZL was associated with loss of CD45 expression, and CD45-negative cells expressed CD25 by flow cytometry analysis [17]. A large amount of CD25 expression on canine TZL cells might have some influence on proliferation of lymphoma cells or status of host immunity that leads to demodicosis in the dogs with TZL. It is important to learn more about aberrant phenotypes in canine lymphomas, not only for accurate diagnostic information but also for the study of the pathophysiology of canine lymphoma.
In our study, of the 16 dogs, only 4 dogs died of lymphoma by the end of the follow-up duration. In 3 of the 4 dogs, typical small clear cells were observed in the FNA samples of peripheral lymph nodes obtained at the stages showing their progressive enlargement, indicating the clinical progression of TZL. One of the 4 dogs developed DLBCL during the disease course of TZL and died of the progression of DLBCL revealed by the histopathological examination. The case was included in the analysis for the OST and censored at the onset of DLBCL in the prognostic analyses. All of them were treated with CHOP-based chemotherapy or MP protocol. Although the number of dogs treated with chemotherapy was small, response rates for dogs treated with CHOP-based chemotherapy or MP protocol were 50% and 100%, respectively. In dogs treated with prednisolone alone or those who did not undergo chemotherapy, there were no cases of death from progression of TZL. A previous report concluded that combination chemotherapy seemed to be less effective in dogs with TZL, compared with that of more aggressive lymphomas [25]. It was also reported that systemic chemotherapy, such as CHOP-based chemotherapy, did not make a difference in improvement of outcome, and thus, watchful waiting may be a reasonable approach in the early phase of the disease [7]. The findings from this study also concluded that therapeutic intervention based on aggressive chemotherapy was not necessarily required for all dogs with TZL. Moreover, MP protocol could be an effective and reasonable approach in terms of response rate and fewer side effects, if chemotherapy is required.
Clinical features associated with TZL were characterized in most cases by systemic generalized lymphadenopathy, splenomegaly and/or hepatomegaly, or lymphocytosis. There have been no criteria for initiation of treatment and therapy protocols, and therefore, chemotherapy was started for the cases with conceivable disease-related symptoms, such as extreme fatigue, occurrence of anemia and/or thrombocytopenia, progressive lymphocytosis, or massive or progressive splenomegaly or lymphadenopathy in this study. This study included only a small number of cases. Therefore, further studies are required to clarify the optimal timing for the initiation of chemotherapy for canine TZL.
A watchful waiting approach was made for 6 (38%) of 16 cases in the study. During the clinical course, most cases retained a good health performance with repeated regression and swelling of lymph nodes for a long time, and no cases experienced the lymphoma-related death by the end of follow-up. It could be suggested that watchful waiting may be a reasonable approach for a proportion of dogs with TZL, especially for dogs without problems in general conditions and blood cytopenias.
In conclusion, TZL is a recognized subgroup of lymphoma in dogs. Dogs with TZL show an indolent clinical course; however, it may be difficult to achieve a complete remission even if using chemotherapy. Although TZL has an indolent feature, there seems to be aberrant immune function in dog patients suffered from this disease as revealed by the frequent development of demodicosis.
Acknowledgments
This study was supported by Japan Society for the Promotion of Science, KAKENHI 23380182 and 26292158.
REFERENCES
- 1.Alberts D. S., Chen H. G., Benz D., Mason N. L.1981. Effect of renal dysfunction in dogs on the disposition and marrow toxicity of melphalan. Br. J. Cancer 43: 330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage J. O.2012. The aggressive peripheral T-cell lymphomas: 2012 update on diagnosis, risk stratification, and management. Am. J. Hematol. 87: 511–519. [DOI] [PubMed] [Google Scholar]
- 3.Barriga O. O., al-Khalidi N. W., Martin S., Wyman M.1992. Evidence of immunosuppression by Demodex canis. Vet. Immunol. Immunopathol. 32: 37–46. [DOI] [PubMed] [Google Scholar]
- 4.Burnett R. C., Vernau W., Modiano J. F., Olver C. S., Moore P. F., Avery A. C.2003. Diagnosis of canine lymphoid neoplasia using clonal rearrangements of antigen receptor genes. Vet. Pathol. 40: 32–41. [DOI] [PubMed] [Google Scholar]
- 5.Dobson J. M., Samuel S., Milstein H., Rogers K., Wood J. L.2002. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 43: 240–246. [DOI] [PubMed] [Google Scholar]
- 6.Dorn C. R., Taylor D. O., Schneider R., Hibbard H. H., Klauber M. R.1968. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J. Natl. Cancer Inst. 40: 307–318. [PubMed] [Google Scholar]
- 7.Flood-Knapik K. E., Durham A. C., Gregor T. P., Sánchez M. D., Durney M. E., Sorenmo K. U.2013. Clinical, histopathological and immunohistochemical characterization of canine indolent lymphoma. Vet. Comp. Oncol. 11: 272–286. [DOI] [PubMed] [Google Scholar]
- 8.Fournel-Fleury C., Magnol J. P., Bricaire P., Marchal T., Chabanne L., Delverdier A., Bryon P. A., Felman P.1997. Cytohistological and immunological classification of canine malignant lymphomas: comparison with human non-Hodgkin’s lymphomas. J. Comp. Pathol. 117: 35–59. [DOI] [PubMed] [Google Scholar]
- 9.Garrett L. D., Thamm D. H., Chun R., Dudley R., Vail D. M.2002. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. J. Vet. Intern. Med. 16: 704–709. [DOI] [PubMed] [Google Scholar]
- 10.Herron M. D., O’reilly M. A., Vanderhooft S. L.2005. Refractory Demodex folliculitis in five children with acute lymphoblastic leukemia. Pediatr. Dermatol. 22: 407–411. [DOI] [PubMed] [Google Scholar]
- 11.Horiuchi S., Koyanagi Y., Tanaka Y., Waki M., Matsumoto A., Zhou Y. W., Yamamoto M., Yamamoto N.1997. Altered interleukin-2 receptor alpha-chain is expressed in human T-cell leukaemia virus type-I-infected T-cell lines and human peripheral blood mononuclear cells of adult T-cell leukaemia patients through an alternative splicing mechanism. Immunology 91: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowenthal J. W., Zubler R. H., Nabholz M., MacDonald H. R.1985. Similarities between interleukin-2 receptor number and affinity on activated B and T lymphocytes. Nature 315: 669–672. [DOI] [PubMed] [Google Scholar]
- 13.Mellanby R. J., Herrtage M. E., Dobson J. M.2003. Owners’ assessments of their dog’s quality of life during palliative chemotherapy for lymphoma. J. Small Anim. Pract. 44: 100–103. [DOI] [PubMed] [Google Scholar]
- 14.Nakase K., Kita K., Nasu K., Ueda T., Tanaka I., Shirakawa S., Tsudo M.1994. Differential expression of interleukin-2 receptors (alpha and beta chain) in mature lymphoid neoplasms. Am. J. Hematol. 46: 179–183. [DOI] [PubMed] [Google Scholar]
- 15.Peuchmaur M., Emilie D., Crevon M. C., Solal-Celigny P., Maillot M. C., Lemaigre G., Galanaud P.1990. IL-2 mRNA expression in Tac-positive malignant lymphomas. Am. J. Pathol. 136: 383–390. [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkar P., Mukherjee J., Ghosh A., Bhattacharjee M., Mahato S., Chakraborty A., Mondal M., Banerjee C., Chaudhuri S.2004. A comparative analysis of immunorestoration and recovery with conventional and immunotherapeutic protocols in canine generalized demodicosis: a newer insight of immunotherapeutic efficacy of T11TS. Immunol. Invest. 33: 453–468. [DOI] [PubMed] [Google Scholar]
- 17.Seelig D. M., Avery P., Webb T., Yoshimoto J., Bromberek J., Ehrhart E. J., Avery A. C.2014. Canine T-zone lymphoma: unique immunophenotypic features, outcome, and population characteristics. J. Vet. Intern. Med. 28: 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seyhan M. E., Karincaoğlu Y., Bayram N., Aycan O., Kuku I.2004. Density of Demodex folliculorum in haematological malignancies. J. Int. Med. Res. 32: 411–415. [DOI] [PubMed] [Google Scholar]
- 19.Sheibani K., Winberg C. D., van de Velde S., Blayney D. W., Rappaport H.1987. Distribution of lymphocytes with interleukin-2 receptors (TAC antigens) in reactive lymphoproliferative processes, Hodgkin’s disease, and non-Hodgkin’s lymphomas. An immunohistologic study of 300 cases. Am. J. Pathol. 127: 27–37. [PMC free article] [PubMed] [Google Scholar]
- 20.Strauchen J. A., Breakstone B. A.1987. IL-2 receptor expression in human lymphoid lesions. Immunohistochemical study of 166 cases. Am. J. Pathol. 126: 506–512. [PMC free article] [PubMed] [Google Scholar]
- 21.Tesch H., Günther A., Abts H., Jücker M., Klein S., Krueger G. R., Diehl V.1993. Expression of interleukin-2R alpha and interleukin-2R beta in Hodgkin’s disease. Am. J. Pathol. 142: 1714–1720. [PMC free article] [PubMed] [Google Scholar]
- 22.The Non-Hodgkin’s Lymphoma Classification Project1997. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood 89: 3909–3918. [PubMed] [Google Scholar]
- 23.Tsilivakos V., Tsapis A., Kakolyris S., Iliakis P., Perraki M., Georgoulias V.1994. Characterization of interleukin 2 receptors on B-cell chronic lymphocytic leukemia cells. Leukemia 8: 1571–1578. [PubMed] [Google Scholar]
- 24.Vail D. M., Michels G. M., Khanna C., Selting K. A., London C. A., Veterinary Cooperative Oncology Group2010. Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)—a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 8: 28–37. [DOI] [PubMed] [Google Scholar]
- 25.Valli V. E.2007. Mature (peripheral) nodal T-cell (T-zone) lymphoma. pp. 294–302. In: Veterinary Comparative Hematopathology 1st ed., Blackwell, Ames. [Google Scholar]
- 26.Valli V. E., Kass P. H., San Myint M., Scott F.2013. Canine lymphomas: association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet. Pathol. 50: 738–748. [DOI] [PubMed] [Google Scholar]
- 27.Valli V. E., Vernau W., de Lorimier L. P., Graham P. S., Moore P. F.2006. Canine indolent nodular lymphoma. Vet. Pathol. 43: 241–256. [DOI] [PubMed] [Google Scholar]
- 28.Valli V. E., San Myint M., Barthel A., Bienzle D., Caswell J., Colbatzky F., Durham A., Ehrhart E. J., Johnson Y., Jones C., Kiupel M., Labelle P., Lester S., Miller M., Moore P., Moroff S., Roccabianca P., Ramos-Vara J., Ross A., Scase T., Tvedten H., Vernau W.2011. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet. Pathol. 48: 198–211. [DOI] [PubMed] [Google Scholar]
- 29.Waldmann T. A., White J. D., Goldman C. K., Top L., Grant A., Bamford R., Roessler E., Horak I. D., Zaknoen S., Kasten-Sportes C., England R., Horak E., Mishra B., Dipre M., Hale P., Fleisher T. A., Junghans R. P., Jaffe E. S., Nelson D. L.1993. The interleukin-2 receptor: a target for monoclonal antibody treatment of human T-cell lymphotrophic virus I-induced adult T-cell leukemia. Blood 82: 1701–1712. [PubMed] [Google Scholar]
- 30.Withrow S. J., Vail D. M.2007. Withrow and MacEwen’s Small Animal Clinical Oncology, 4th ed., Elsevier Saunders, Edinburgh. [Google Scholar]