Abstract
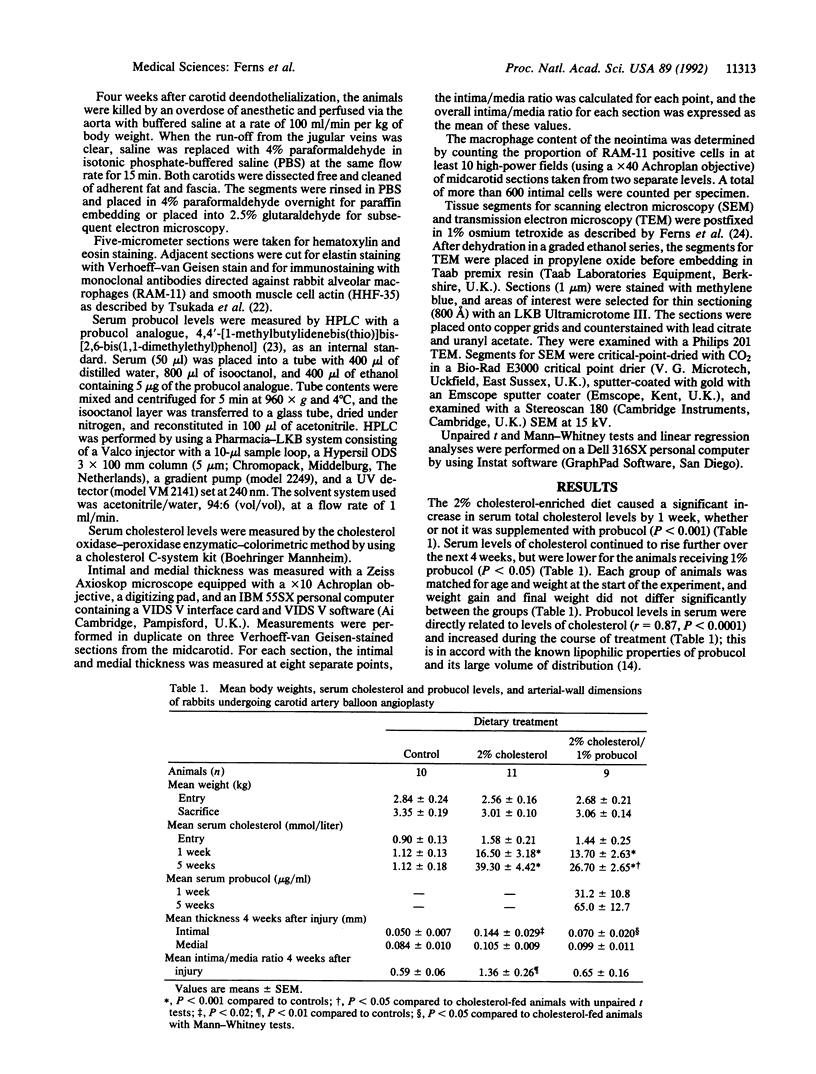
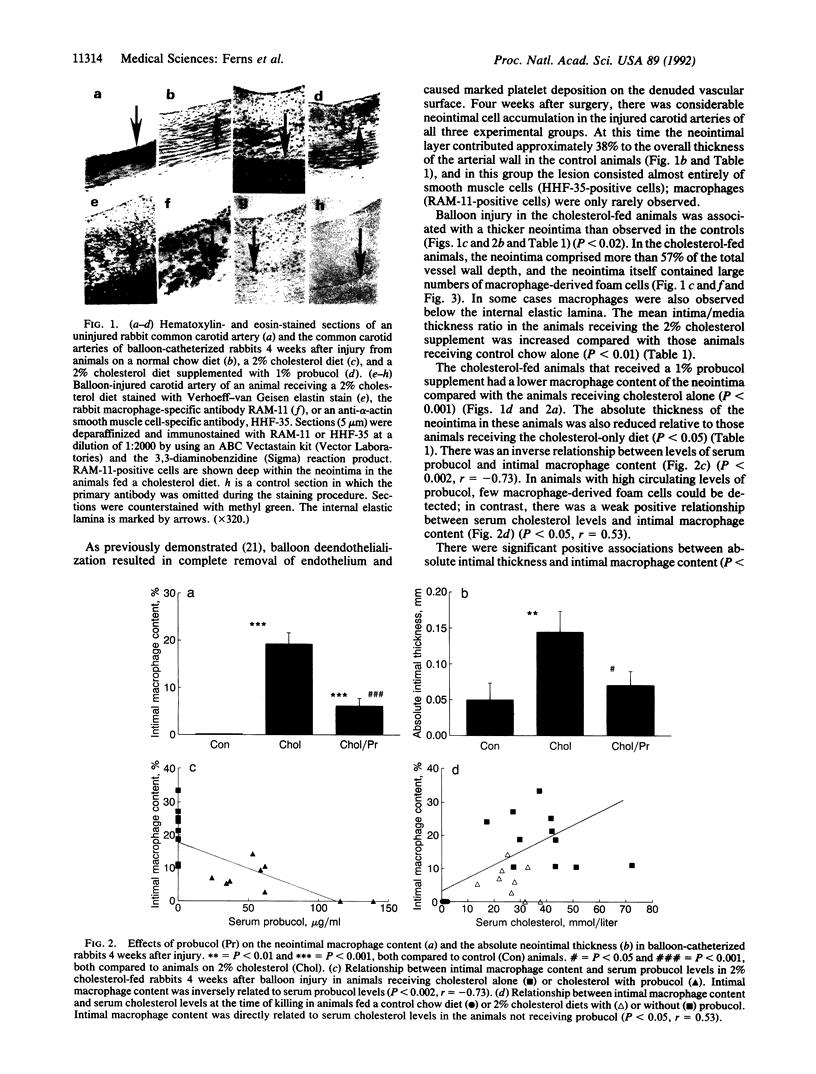
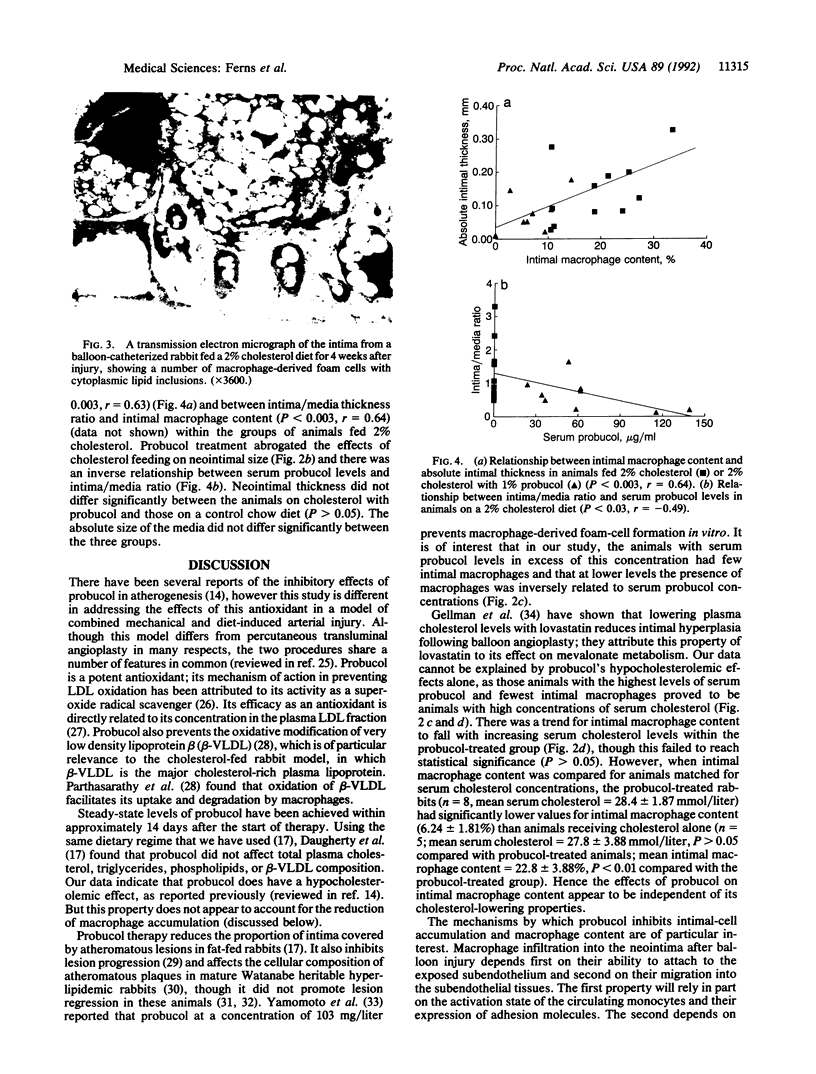
Restenosis is a frequent long-term complication after balloon angioplasty. Although smooth muscle cells form the major constituent of the occluding lesion, macrophage-derived foam cells are usually also present in high abundance. The latter have the potential to accelerate the rate of reocclusion because they elaborate many potent cytokines and growth factors, which may act to either recruit cells into the neointima or cause neointimal cell proliferation. Macrophage-derived foam-cell formation depends upon the uptake of modified low density lipoprotein via a scavenger receptor-mediated pathway. Foam-cell formation is accompanied by the release of smooth muscle cell mitogens and chemoattractants. We have examined the effects of probucol, a lipid-soluble antioxidant, in the balloon-catheterized carotid artery of the cholesterol-fed rabbit to evaluate the importance of oxidative processes in restenosis. After 5 weeks, serum cholesterol levels were 32% lower (P < 0.05) in rabbits fed 1% probucol with 2% cholesterol, compared with those receiving cholesterol alone. Probucol inhibited neointimal macrophage accumulation by 68% (P < 0.001), reduced absolute intimal size by 51% (P < 0.05), and reduced the intima/media thickness ratio by 51%. These inhibitory effects were directly related to serum probucol concentrations and appeared to be unrelated to probucol's hypocholesterolemic activity. These data suggest that reactive oxygen species may be involved in the intimal response to injury and that antioxidants, such as probucol, may be therapeutically useful as inhibitors of restenosis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akeson A. L., Woods C. W., Mosher L. B., Thomas C. E., Jackson R. L. Inhibition of IL-1 beta expression in THP-1 cells by probucol and tocopherol. Atherosclerosis. 1991 Feb;86(2-3):261–270. doi: 10.1016/0021-9150(91)90222-o. [DOI] [PubMed] [Google Scholar]
- Austin G. E., Ratliff N. B., Hollman J., Tabei S., Phillips D. F. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985 Aug;6(2):369–375. doi: 10.1016/s0735-1097(85)80174-1. [DOI] [PubMed] [Google Scholar]
- Autio I., Jaakkola O., Solakivi T., Nikkari T. Oxidized low-density lipoprotein is chemotactic for arterial smooth muscle cells in culture. FEBS Lett. 1990 Dec 17;277(1-2):247–249. doi: 10.1016/0014-5793(90)80857-f. [DOI] [PubMed] [Google Scholar]
- Barnhart R. L., Busch S. J., Jackson R. L. Concentration-dependent antioxidant activity of probucol in low density lipoproteins in vitro: probucol degradation precedes lipoprotein oxidation. J Lipid Res. 1989 Nov;30(11):1703–1710. [PubMed] [Google Scholar]
- Bridges A. B., Scott N. A., Belch J. J. Probucol, a superoxide free radical scavenger in vitro. Atherosclerosis. 1991 Aug;89(2-3):263–265. doi: 10.1016/0021-9150(91)90068-e. [DOI] [PubMed] [Google Scholar]
- Buckley M. M., Goa K. L., Price A. H., Brogden R. N. Probucol. A reappraisal of its pharmacological properties and therapeutic use in hypercholesterolaemia. Drugs. 1989 Jun;37(6):761–800. doi: 10.2165/00003495-198937060-00002. [DOI] [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clagett G. P., Robinowitz M., Youkey J. R., Fisher D. F., Jr, Fry R. E., Myers S. I., Lee E. L., Collins G. J., Jr, Virmani R. Morphogenesis and clinicopathologic characteristics of recurrent carotid disease. J Vasc Surg. 1986 Jan;3(1):10–23. doi: 10.1067/mva.1986.avs0030010. [DOI] [PubMed] [Google Scholar]
- Cushing S. D., Fogelman A. M. Monocytes may amplify their recruitment into inflammatory lesions by inducing monocyte chemotactic protein. Arterioscler Thromb. 1992 Jan;12(1):78–82. doi: 10.1161/01.atv.12.1.78. [DOI] [PubMed] [Google Scholar]
- Daugherty A., Zweifel B. S., Schonfeld G. Probucol attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Br J Pharmacol. 1989 Oct;98(2):612–618. doi: 10.1111/j.1476-5381.1989.tb12635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty A., Zweifel B. S., Schonfeld G. The effects of probucol on the progression of atherosclerosis in mature Watanabe heritable hyperlipidaemic rabbits. Br J Pharmacol. 1991 May;103(1):1013–1018. doi: 10.1111/j.1476-5381.1991.tb12293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns G. A., Reidy M. A., Ross R. Balloon catheter de-endothelialization of the nude rat carotid. Response to injury in the absence of functional T lymphocytes. Am J Pathol. 1991 Apr;138(4):1045–1057. [PMC free article] [PubMed] [Google Scholar]
- Ferns G. A., Stewart-Lee A. L., Anggård E. E. Arterial response to mechanical injury: balloon catheter de-endothelialization. Atherosclerosis. 1992 Feb;92(2-3):89–104. doi: 10.1016/0021-9150(92)90268-l. [DOI] [PubMed] [Google Scholar]
- Ferns G., Reidy M., Ross R. Vascular effects of cyclosporine A in vivo and in vitro. Am J Pathol. 1990 Aug;137(2):403–413. [PMC free article] [PubMed] [Google Scholar]
- Frostegård J., Nilsson J., Haegerstrand A., Hamsten A., Wigzell H., Gidlund M. Oxidized low density lipoprotein induces differentiation and adhesion of human monocytes and the monocytic cell line U937. Proc Natl Acad Sci U S A. 1990 Feb;87(3):904–908. doi: 10.1073/pnas.87.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellman J., Ezekowitz M. D., Sarembock I. J., Azrin M. A., Nochomowitz L. E., Lerner E., Haudenschild C. C. Effect of lovastatin on intimal hyperplasia after balloon angioplasty: a study in an atherosclerotic hypercholesterolemic rabbit. J Am Coll Cardiol. 1991 Jan;17(1):251–259. doi: 10.1016/0735-1097(91)90735-r. [DOI] [PubMed] [Google Scholar]
- Glazier J. J., Williams M. G., Madden S., Rickards A. F. Clinical outcome following coronary balloon angioplasty in 100 consecutive patients with multivessel coronary artery disease. J R Coll Physicians Lond. 1990 Oct;24(4):292–294. [PMC free article] [PubMed] [Google Scholar]
- Gruntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet. 1978 Feb 4;1(8058):263–263. doi: 10.1016/s0140-6736(78)90500-7. [DOI] [PubMed] [Google Scholar]
- Hara S., Nagano Y., Sasada M., Kita T. Probucol pretreatment enhances the chemotaxis of mouse peritoneal macrophages. Arterioscler Thromb. 1992 May;12(5):593–600. doi: 10.1161/01.atv.12.5.593. [DOI] [PubMed] [Google Scholar]
- Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Narumiya S., Kawai C. Prevention of atherosclerotic progression in Watanabe rabbits by probucol. Am J Cardiol. 1988 Jul 25;62(3):13B–19B. doi: 10.1016/s0002-9149(88)80045-6. [DOI] [PubMed] [Google Scholar]
- Kita T., Nagano Y., Yokode M., Ishii K., Kume N., Ooshima A., Yoshida H., Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritchevsky D., Kim H. K., Tepper S. A. Influence of 4,4'-(isopropylidenedithio)bis(2,6-di-t-butylphenol) (DH-581) on experimental atherosclerosis in rabbits. Proc Soc Exp Biol Med. 1971 Apr;136(4):1216–1221. doi: 10.3181/00379727-136-35461. [DOI] [PubMed] [Google Scholar]
- Ku G., Doherty N. S., Schmidt L. F., Jackson R. L., Dinerstein R. J. Ex vivo lipopolysaccharide-induced interleukin-1 secretion from murine peritoneal macrophages inhibited by probucol, a hypocholesterolemic agent with antioxidant properties. FASEB J. 1990 Apr 1;4(6):1645–1653. doi: 10.1096/fasebj.4.6.2318380. [DOI] [PubMed] [Google Scholar]
- Ku G., Doherty N. S., Wolos J. A., Jackson R. L. Inhibition by probucol of interleukin 1 secretion and its implication in atherosclerosis. Am J Cardiol. 1988 Jul 25;62(3):77B–81B. doi: 10.1016/s0002-9149(88)80057-2. [DOI] [PubMed] [Google Scholar]
- Leimgruber P. P., Roubin G. S., Hollman J., Cotsonis G. A., Meier B., Douglas J. S., King S. B., Jr, Gruentzig A. R. Restenosis after successful coronary angioplasty in patients with single-vessel disease. Circulation. 1986 Apr;73(4):710–717. doi: 10.1161/01.cir.73.4.710. [DOI] [PubMed] [Google Scholar]
- Mao S. J., Yates M. T., Rechtin A. E., Jackson R. L., Van Sickle W. A. Antioxidant activity of probucol and its analogues in hypercholesterolemic Watanabe rabbits. J Med Chem. 1991 Jan;34(1):298–302. doi: 10.1021/jm00105a046. [DOI] [PubMed] [Google Scholar]
- Meier B. Long-term results of coronary balloon angioplasty. Annu Rev Med. 1991;42:47–59. doi: 10.1146/annurev.me.42.020191.000403. [DOI] [PubMed] [Google Scholar]
- Nagano Y., Nakamura T., Matsuzawa Y., Cho M., Ueda Y., Kita T. Probucol and atherosclerosis in the Watanabe heritable hyperlipidemic rabbit--long-term antiatherogenic effect and effects on established plaques. Atherosclerosis. 1992 Feb;92(2-3):131–140. doi: 10.1016/0021-9150(92)90272-i. [DOI] [PubMed] [Google Scholar]
- O'Brien K., Nagano Y., Gown A., Kita T., Chait A. Probucol treatment affects the cellular composition but not anti-oxidized low density lipoprotein immunoreactivity of plaques from Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb. 1991 May-Jun;11(3):751–759. doi: 10.1161/01.atv.11.3.751. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Quinn M. T., Schwenke D. C., Carew T. E., Steinberg D. Oxidative modification of beta-very low density lipoprotein. Potential role in monocyte recruitment and foam cell formation. Arteriosclerosis. 1989 May-Jun;9(3):398–404. doi: 10.1161/01.atv.9.3.398. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Young S. G., Witztum J. L., Pittman R. C., Steinberg D. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest. 1986 Feb;77(2):641–644. doi: 10.1172/JCI112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Rao G. N., Berk B. C. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992 Mar;70(3):593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Tawara K., Ishihara M., Ogawa H., Tomikawa M. Effect of probucol, pantethine and their combinations on serum lipoprotein metabolism and on the incidence of atheromatous lesions in the rabbit. Jpn J Pharmacol. 1986 Jun;41(2):211–222. doi: 10.1254/jjp.41.211. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Rosenfeld M., Ross R., Gown A. M. Immunocytochemical analysis of cellular components in atherosclerotic lesions. Use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Arteriosclerosis. 1986 Nov-Dec;6(6):601–613. doi: 10.1161/01.atv.6.6.601. [DOI] [PubMed] [Google Scholar]
- Waller B. F., Pinkerton C. A., Orr C. M., Slack J. D., VanTassel J. W., Peters T. Morphological observations late (greater than 30 days) after clinically successful coronary balloon angioplasty. Circulation. 1991 Feb;83(2 Suppl):I28–I41. [PubMed] [Google Scholar]
- Yamamoto A., Takaichi S., Hara H., Nishikawa O., Yokoyama S., Yamamura T., Yamaguchi T. Probucol prevents lipid storage in macrophages. Atherosclerosis. 1986 Dec;62(3):209–217. doi: 10.1016/0021-9150(86)90095-x. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Lipton B. A., Rosenfeld M. E., Särkioja T., Yoshimura T., Leonard E. J., Witztum J. L., Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]




