Abstract
Cardiovascular diseases (CVDs) are among the leading causes of morbidity and mortality in both the developed and developing world. Rhizoma coptidis (RC), known as Huang Lian in China, is the dried rhizome of medicinal plants from the family Ranunculaceae, such as Coptis chinensis Franch, C. deltoidea C.Y. Cheng et Hsiao, and C. teeta Wall which has been used by Chinese medicinal physicians for more than 2000 years. In China, RC is a common component in traditional medicines used to treat CVD associated problems including obesity, diabetes mellitus, hyperlipidemia, hyperglycemia and disorders of lipid metabolism. In recent years, numerous scientific studies have sought to investigate the biological properties of RC to provide scientific evidence for its traditional medical uses. RC has been found to exert significant beneficial effects on major risk factors for CVDs including anti-atherosclerotic effect, lipid-lowering effect, anti-obesity effect and anti-hepatic steatosis effect. It also has myocardioprotective effect as it provides protection from myocardial ischemia-reperfusion injury. These properties have been attributed to the presence of bioactive compounds contained in RC such as berberine, coptisine, palmatine, epiberberine, jatrorrhizine, and magnoflorine; all of which have been demonstrated to have cardioprotective effects on the various parameters contributing to the occurrence of CVD through a variety of pathways. The evidence available in the published literature indicates that RC is a herb with tremendous potential to reduce the risks of CVDs, and this review aims to summarize the cardioprotective properties of RC with reference to the published literature which overall indicates that RC is a herb with remarkable potential to reduce the risks and damage caused by CVDs.
Keywords: coptis root, Huang Lian, Coptis chinensis Franch, cardiovascular diseases, ethnopharmacology
Introduction
Cardiovascular diseases (CVDs) appears set to continue as the largest cause of death and disease burden across the globe. They include a wide spectrum of life-threatening disorders such as coronary heart disease (CHD), cerebrovascular disease and peripheral arterial disease, all of which result from impairment to the heart and blood vessels (Wallace, 2011). Among the risk factors strongly associated with these disorders are high levels of low-density lipoprotein (LDL) cholesterol, hypertension, diabetes, and abdominal obesity (Walden and Tomlinson, 2011).
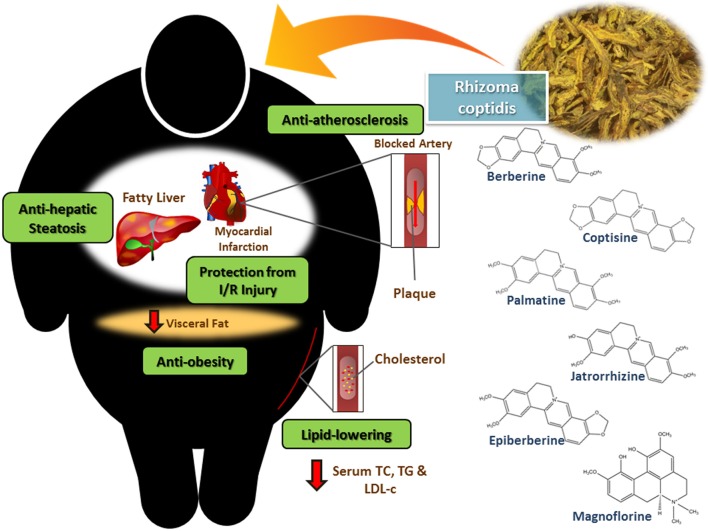
Rhizoma coptidis (RC), known as Huang Lian in China, is the dried rhizome of medicinal plants from the family Ranunculaceae, including Coptis chinensis Franch, C. deltoidea C.Y. Cheng et Hsiao, and C. teeta Wall (Chen et al., 2008; Ma et al., 2012). It is a well-known herb in traditional Chinese medicine and has a long history with its pharmacological uses first mentioned in the Shen Nong Ben Cao Jing (a compilation of information regarding Chinese herbs dating back to 2800 BC) in the Eastern Han Dynasty (Yi et al., 2013). Ancient beliefs state that it is “cold” in nature and is able to remove damp heat, fire or toxicity (Wang et al., 2014). For over 2000 years, Chinese medicinal physicians have used RC as a food additive and herbal medicine for its antibacterial, antiviral, anti-inflammatory, anti-hyperglycemic and hypolipidemic activities (Kou et al., 2016). Today, RC is still widely utilized in herbal medicine for the treatment of various conditions. This is evident based on a survey of patented medicines in China which reveals that RC is commonly used as one of the ingredients in preparations to treat obesity, diabetes mellitus, hyperlipidemia, hyperglycemia and lipid metabolism disorders (Chen, 2009; Ye et al., 2009; Guo, 2012; Li et al., 2015; Wang, 2015). Given the potential benefits in seeking new approaches to treating and preventing CVDs, there has been tremendous interest among the scientific community in exploring the biological properties of RC and providing scientific evidence for its traditional medical uses. At the same time, it is also crucial to investigate the phytoconstituents that are responsible for the biological properties (Moghadamtousi et al., 2013; Tan et al., 2015). Based on current knowledge, the major bioactive compounds contributing to RC's bioactive properties are berberine, coptisine, palmatine, epiberberine, jatorrhizine and magnoflorine, as illustrated in Figure 1 (Hung et al., 2007a; Kou et al., 2016). There is a large of body of work suggesting that RC has protective properties against several major risk factors and damage caused by CVDs. This review aims to summarize the currently available evidence of RC's cardioprotective properties—both in vitro and in vivo studies were included and are summarized in Table 1.
Figure 1.
Rhizoma coptidis which contains alkaloids such as berberine, coptisine, palmatine, epiberberine, jatrorrhizine and magnoflorine exerts cardioprotective activity through its anti-atherosclerotic effect, protection from myocardial ischemia-reperfusion injury, lipid-lowering effect, anti-obesity effect and anti-hepatic steatosis effect.
Table 1.
Summary of cardioprotective activity of Rhizoma coptidis.
| Effect | Extract/compound (s) | Experiment | Effect | Possible mechanism | References |
|---|---|---|---|---|---|
| Anti-atherosclerotic effect | Berberine | In vitro | Inhibition of foam cell formation and reduced cholesterol accumulation in macrophages. Synergistic effect with atorvastatin in preventing atherosclerotic processes | Activation of AMPK-SIRT1-PPARγ pathway, decreased uptake of ox-LDL | Chi et al., 2014 |
| Ger-Gen-Chyn-Lian-Tang (Puerariae radix, scutellariae radix, coptidis -rhizoma and glycyrrhizae radix) | In vitro and in vivo | Decreased serum levels of TC, LDL, atherosclerotic lesions and collagen expression in atheroma plaques. Decreased VSMCs migration and lipid accumulation in hepatocytes | Activation of AMPK signaling | Ho et al., 2012 | |
| Berberine | In vitro | Inhibition of generation of ROS and subsequent mitochondrial membrane potential collapse, chromosome condensation, cytochrome C release, and caspase-3 activation induced by ox-LDL in HUVECs | Suppression of ROS overproduction, through modulation of expression or activity of ROS generating enzyme and ROS scavenging enzyme | Hsieh et al., 2007 | |
| Berberine | In vitro | Reduced expression of MMP-9 and EMMPRIN in PMA-induced macrophages | Suppression of activation of p38 pathway | Huang et al., 2011 | |
| Magnoflorine | In vitro | Inhibitory effect against Cu2+-induced lipid peroxidation of HDL and inhibition of generation of TBARS | Antioxidant action of magnoflorine | Hung et al., 2007a | |
| Magnoflorine | In vitro | Inhibitory effect against Cu2+-induced lipid peroxidation of LDL, glycated LDL and glycoxidated LDL. Inhibition of generation of TBARS | Antioxidant action of magnoflorine | Hung et al., 2007b | |
| Rhizoma coptidis extract and berberine | In vitro | Inhibition of LPS-induced MCP-1 production in murine macrophages | Inhibited activation of the transcription factors AP-1 and NFκB | Remppis et al., 2010 | |
| Berberine | In vitro and in vivo | Reduced aortic lesions, oxidative stress and expression of adhesion molecules in aorta of ApoE−/− mice. Increased UCP2 mRNA and protein expression in HUVECs | AMPK-dependent upregulation of UCP2 expression | Wang et al., 2011 | |
| Coptisine | In vitro and in vivo | Inhibition of LPS stimulated expression of inflammation-associated genes in mouse, human macrophages and in carrageenan-induced rats paw edema | Inhibition of activation of NFκB, MAPK, and PI3K/Akt activation | Wu et al., 2016 | |
| Lipid-lowering effect | Rhizoma coptidis alkaloids extract | In vivo | Decreased serum TC, TG, LDL-c, increased bile acid level in the liver of hyperlipidemic rats | Increased cholesterol conversion into bile acids by up-regulating gene expression of CYP7A1, increased its activity in the liver due to positive regulation of PPARα and negative modulation of FXR | Cao et al., 2012 |
| Coptisine | In vitro | Decreased TC and LDL-c in HepG2 cells | Up-regulated mRNA and protein expressions of LDLR and CYP7A1, down-regulated mRNA and protein expressions of HMGCR | Chen et al., 2015 | |
| Rhizoma coptidis total alkaloids, berberine, coptisine, palmatine, epiberberine and jatrorrhizine | In vivo | Decreased serum TC, TG, LDL-c and increased HDL-c, TBA levels in feces of hyperlipidemic hamsters | Down-regulated expression of HMGCR and up-regulated expression of LDLR and CYP7A1 as well as promoting the excretion of TBA in the feces | He et al., 2015a | |
| Coptisine | In vivo | Decreased serum TC, TG, LDL-c, increased HDL-c, TBA levels in feces of hyperlipidemic hamsters | Inhibited cholesterol synthesis through suppressing the HMGCR expression and promoting usage and excretion of cholesterol via up-regulating LDLR and CYP7A1 expression | He et al., 2015b | |
| Rhizoma coptidis total alkaloids, berberine, coptisine, palmatine, epiberberine and jatrorrhizine | In vitro and in vivo | Reduced lipid and cholesterol accumulation in HepG2 cells; decreased serum TC, TG, LDL-c and increased HDL-c, TC, TBA levels in feces of hypercholesterolemic hamsters | Up-regulation of LDL receptor and CYP7A1, as well as HMGCR downregulation | Kou et al., 2016 | |
| JiuHuangLian (Rhizoma coptidis steamed with rice wine) | In vivo | Decreased serum TC, TG, and free fatty acid levels in diabetic rats | Regulatory effect on glucose-lipid metabolism | Li et al., 2013 | |
| Berberine, coptisine, palmatine, epiberberine and Jatrorrhizine | In vivo | Decreased serum TC, TG by palmatine and jatrorrhizine, increased HDL-c by berberine, palmatine and jatrorrhizine in diabetic mice | – | Ma et al., 2016 | |
| Palmatine | In vivo | Decreased serum TC, TG, LDL-c, increased TC and TBA levels in feces of hyperlipidemic hamsters | Up-regulated LDLR and CYP7A1 mRNA and protein expression, down-regulated ASBT mRNA and protein expression, enhanced fecal excretion of TC and TBA | Ning et al., 2015 | |
| Berberine | In vivo | Decreased serum TC, TG, LDL-c, increased HDL-c and NO level in diabetic rats | – | Tang et al., 2006 | |
| Jatrorrhizine | In vivo | Decreased serum TC, TG, LDL-c, increased HDL-c and TBA levels in feces of hamsters | Improved utilization and excretion of cholesterol by up-regulating mRNA and protein expression of LDLR and CYP7A1 | Wu et al., 2014a | |
| Berberine, coptisine, palmatine, epiberberine and Jatrorrhizine | In vitro and in vivo | Decreased serum TC, TG, LDL-c, increased HDL-c in hamsters. Increased mRNA expression of LDLR in liver and HepG2 cells | Up-regulated LDLR mRNA expression | Wu et al., 2014c | |
| Rhizoma coptidis extract | In vivo | Decreased serum TC, LDL and oxidized LDL in rats | Reduction of cholesterol synthesis | Yokozawa et al., 2003 | |
| Berberine, jatrorrhizine, columbamine, berberrubine and demethyleneberberine | In vitro | Inhibition of lipid accumulation in Hep G2 cells | Up-regulated LDLR mRNA and protein expression | Zhou et al., 2014 | |
| Epiberberine | In vivo | Decreased serum TC, LDL-c, TBA, increased TC and TBA levels in feces of hyperlipidemic hamsters | Inhibited cholesterol synthesis through suppressing the HMGCR expression and promoting usage and excretion of cholesterol via up-regulating LDLR and CYP7A1 expression | Zou et al., 2016 | |
| Anti-obesity | Rhizoma coptidis methanol extract, berberine, epiberberine, coptisine, palmatine, and magnoflorine | In vitro | Reduction of intracellular triglyceride contents and lipid accumulation in 3T3-L1 cells | Down-regulated expression of major adipogenic transcription activator (PPARγ and C/EBP- α) proteins of the adipogenesis pathway | Choi et al., 2014 |
| Rhizoma coptidis total alkaloids, coptisine, berberine and palmatine | In vivo | Reduced body weight gain, TC, TG, LDL-C, TBA and lipopolysaccharide, liver fat deposition and epididymal adipose cell size in hyperlipidemic mice. Increased abundance of Sporobacter termitidis, Alcaligenes faecalis, Akkermansia muciniphila in the gut of mice, decreased abundance of Escherichia coli, Desulfovibrio C21 _c20, Parabacteroides distasonis | Modulation of gut microbiota and lipid metabolism by acting as agonists of FXR and TGR5, activators for SREBP2, LDLR, UCP2 and CYP7A1, inhibitors of HMGCR, TXNIP, TLR4 and JNK | He et al., 2016 | |
| Berberine | In vitro | Inhibition of differentiation and mitotic clonal expansion of 3T3-L1 preadipocytes | Inhibition of mRNA and protein expression of adipogenesis related transcription factors PPARγ and C/EBPα and their upstream regulator, C/EBPβ | Huang et al., 2006 | |
| Rhizoma coptidis ethanol extract, berberine | In vitro and in vivo | Reduced body visceral adipose weights, lipid levels, degradation of dietary polysaccharides, proportions of fecal Firmicutes and Bacteroidetes to total bacteria in high-fat diet-fed mice; inhibited growth of Lactobacillus under anaerobic conditions | Decreased degradation of dietary polysaccharides, lowered potential calorie intake, activation of Fiaf protein and related gene (AMPK, PCG1α, UCP2, CPT1α and Hadhb) expressions of mitochondrial energy metabolism in visceral adipose tissues | Xie et al., 2011 | |
| Anti-hepatic steatosis | Sam-Hwang-Sa-Sim-Tang (Rhizoma coptidis, Scutellariae radix and Rhei rhizoma) | In vivo | Reduced lipid accumulation in the liver of mice | Inhibition of mRNA expression of key hepatic molecules such as SREBP2, LXR, LDLR, and HMG-CoA | Ahn et al., 2013 |
| Rhizoma coptidis alkaloids extract, berberine, coptisine, palmatine, epiberberine, jatrorrhizine, columbamine and magnoflorine | In vitro | Attenuated triglyceride accumulation in HepG2 cells | – | Fan et al., 2013 | |
| Berberine | In vitro and in vivo | Attenuated hepatic steatosis, reduced expression of ACC and FAS in mice; reduced fat deposition in hepatocytes | Anti-inflammatory action of berberine | Guo et al., 2016 | |
| Jiao Tai Wan (Rhizoma coptidis and Cortex cinnamomi) | In vivo | Attenuated hepatic lipid accumulation in diabetic rats, down-regulation of ACC and FAS protein expressions, up-regulation of AMPK and pACC protein expressions in the liver tissues; reduced TG content in patients' livers | Inhibition of lipogenic gene expression in the liver | Huang et al., 2013 | |
| Berberine | In vivo | Attenuated liver steatosis in high-fat diet-induced steatotic rats | Reversal of alteration of hepatic gene expression that occurred in steatotic liver | Yuan et al., 2015 | |
| Berberine | In vitro and in vivo | Attenuated liver steatosis in mice and decreased free fatty acid-induced lipid accumulation in cultured hepatocytes | Reduction of endoplasmic reticulum stress | Zhang et al., 2016 | |
| Protection from Myocardial Ischemia-reperfusion | Berberine | In vivo | Decreased infarct size, duration and incidence of arrhythmias. Reduced AMPK concentration, ratio of ADP/ATP and AMP/ATP in the myocardial risk areas; increased AMPK concentration, ratio of ADP/ATP and AMP/ATP in the non-ischemic areas. | Regulation of AMPK activity in non-ischemic and risk areas of the heart | Chang et al., 2012 |
| Berberine | In vivo | Decreased infarct size and attenuated arrhythmias. Increased AMPK activity, ratio of AMP/ATP in the non-ischemic areas | Activation of AMPK, AKT phosphorylation, and GSK3β inhibition in the non-ischemic areas of the diabetic heart | Chang et al., 2016 | |
| Berberine | In vitro and in vivo | Improved recovery of cardiac systolic/diastolic function and reduced myocardial apoptosis in diabetic rats subjected to myocardial I/R; Reduced hypoxia/reoxygenation-induced myocardial apoptosis of neonatal rat cardiomyocytes | Activation of AMPK and PI3K–Akt–eNOS signaling | Chen et al., 2014 | |
| Coptisine | In vivo | Reduced infarct size and improved cardiac function after I/R injury in rats | Suppression of myocardial apoptosis and inflammation, through inhibition of Rho/ROCK pathway | Guo et al., 2013 | |
| Berberine | In vitro and in vivo | Enhanced H/R-induced cell viability, reduced myocardial infarct size, improved cardiac function of mouse hearts; decreased p-AMPK and p-mTORC2 in H9c2 myocytes | Inhibition of autophagy activation, through decreased expression of autophagy-related proteins such as SIRT1, BNIP3, and Beclin-1 | Huang et al., 2015 | |
| Palmatine | In vitro and in vivo | Improved I/R-induced myocardial dysfunction, inhibited increased LDH, CK and MDA contents in I/R rats serum, inhibited declined activity of SOD and catalase in I/R cardiac tissues, reduced COX-2 and iNOS expression in I/R myocardium; increased HO-1 induction in human aortic endothelial cells | Reduction of oxidative stress and modulation of inflammatory mediators | Kim et al., 2009 | |
| San-Huang-Xie-Xin-Tang (Coptidis rhizome, Scutellariae radix and Rhei rhizome) | In vivo | Reduced plasma levels of cardiac enzymes, arrhythmia scores, mortality rate of rats with I/R. Reduced infarct size and apoptosis induced by I/R | Increased myocardial eNOS expression, plasma nitrite and decreased activation of ERK1/2, p38 and JNK | Liou et al., 2011 | |
| Rhizoma coptidis extract | In vitro and in vivo | Decreased ST-T in ECG, serum levels of CK, LDH, MDA and improved SOD in rats; decreased level of LDH in neonatal rat cardiomyocytes | Improved oxidative damage of acute myocardial ischemia | Liu et al., 2010 | |
| Berberine | In vivo | Decreased myocardial infarction area, decreased serum levels of CK isozyme (CK-MB), LDH and cTnl, upregulated expression of Bcl-2 and mitochondrial cytochrome c and downregulated expression of Bax and cytosolic cytochrome c | Attenuation of myocardial apoptosis and improved mitochondrial dysfunction | Wang et al., 2015 | |
| Berberine | In vitro and in vivo | Improved cardiac function recovery, decreased myocardial apoptosis, infarct size, serum CK, LDH; attenuation of I/R-induced myocardial apoptosis of cultured cardiomyocytes | Modulation of Notch1/Hes1-PTEN/Akt signaling pathway | Yu et al., 2015 | |
| Berberine | In vitro and in vivo | Reduced I/R-induced myocardial infarct size, improved cardiac function, suppressed myocardial apoptosis, oxidative damage and I/R-induced ER stress; Reduced cell apoptosis, oxidative stress and ER stress of cultured cardiomyocytes | Activation of JAK2/STAT3 signaling pathway and attenuation of ER stress-induced apoptosis | Zhao et al., 2016b | |
| Berberine | In vivo | Attenuation of I/R-induced incidence of ventricular arrhythmia and amelioration of myocardial histological changes | Inhibited activation of PI3K/AKT signaling and subsequent reduced expression of IL-6, IL-1β and TNF-α | Zhu and Li, 2016 |
Cardioprotective activities of Rhizoma Coptidis
Anti-atherosclerotic effect
Atherosclerosis is one of the most important causative factors of certain CVDs such as myocardial infarction (MI), heart failure, stroke and claudication (Frostegård, 2013). Atherosclerosis refers to a specific form of arteriosclerosis in which an artery-wall thickens due to invasion and accumulation of lipid laden white blood cells (foam cells) and proliferation of intimal-smooth-muscle cell forming a fibrofatty plaque (Lucas and Greaves, 2001). There have also been studies supporting the anti-atherosclerotic effects of RC. For example, a study on the anti-atherosclerotic potential of Ger-Gen-Chyn-Lian-Tang (a traditional mixture which contains berberine as one of its major active components) has demonstrated reduced atherosclerotic lesions and collagen expression within the atheroma plaques in mice. In addition, in vitro work has also shown a reduction in migration of vascular smooth muscle cells, which is significant as pathological migration of these cells represents a key step in the pathogenesis of atherosclerosis (Ho et al., 2012).
Reactive oxygen species (ROS) play a crucial role in the development and progression of atherosclerotic lesions because the formations of lesions are related to several ROS-regulated events. It was found that uncoupling protein 2 (UCP-2), the mitochondrial inner membrane protein can provide antioxidant mediated defense by negatively regulating ROS production (Moukdar et al., 2009). Utilizing a mouse model, Wang et al. (2011) reported that chronic administration of berberine resulted in significant reduction of aortic lesions, diminished oxidative stress and lowered expression of adhesion molecules in the aorta; this anti-atherogenic activity was due to AMPK-dependent ROS suppression through enhanced expression of UCP-2. Oxidative modification of LDL is also believed to be one of the main risk factors leading to atherosclerosis (Itabe, 2009); berberine—one of the major bioactive components of RC—has been reported to inhibit oxidation of LDL by virtue of its significant antioxidant activity (Hsieh et al., 2007). Berberine also appears to protect against oxidized-LDL induced apoptosis as demonstrated by reduced levels of mitochondrial cytochrome C, cleaved apoptotic effectors, caspase 3 and poly(ADP-ribose) polymerase in human umbilical vein endothelial cells treated with this compound (Hsieh et al., 2007). Magnoflorine, another alkaloid isolated from RC extract also possesses similarly beneficial effects; an effective antioxidant, it prevents the oxidation of various forms of LDL, including native, glycated and glycoxidated LDL (Hung et al., 2007a). Aside from this, magnoflorine also significantly inhibits Cu2+ and thermo-labile radical initiator (AAPH)-induced lipid peroxidation of high density lipoprotein (HDL); thus resulting in HDL retaining its ability to protect LDL from oxidation (Hung et al., 2007b).
Chronic inflammatory processes are a key additional factor leading to the development of atherosclerotic lesions in the vessel wall (Remppis et al., 2010). Hence, substances with anti-inflammatory property may have the potential to prevent atherosclerosis. Remppis et al. (2010) studied the effect of RC extract and berberine on lipopolysaccharide (LPS)-induced inflammatory activity in a murine macrophage cell line. The results of this study demonstrated that RC extract and berberine inhibit the production of monocyte chemoattractant protein 1 (MCP-1/CCL2) in macrophages, likely via inhibition of the transcription factors activator protein 1 (AP-1) and nuclear factor-kappaB (NFκB). Coptisine, another of the bioactive compounds in RC, also possesses demonstrable anti-inflammatory property. It resulted in decreased nitric oxide (NO) production in LPS-stimulated macrophages via inhibited protein and mRNA expression of inducible nitro oxide synthase (iNOS); additionally, the expression of pro-inflammatory cytokines interleukin (IL)-1β and IL-6 was inhibited at transcriptional level. The proposed underlying mechanism for these observations was blockade of NFκB, mitogen-activated protein kinases (MAPK), and phosphoinositide-3-kinase (PI3K)/Akt activation, which are the intracellular inflammation signaling pathways in macrophages (Supriady et al., 2015; Wu et al., 2016).
The accumulation of lipid-laden foam cells is also an important step in the progression of atherosclerosis (Chi et al., 2014). The authors demonstrated that berberine effectively inhibited oxidized-LDL-induced foam cell formation through the activation of adenosine 5′-monophosphate-activated protein kinase (AMPK)-sirtuin 1-peroxisome proliferator-activated receptor gamma (PPAR-γ) pathway as well as through reduced uptake of oxidized LDL by foam cells. Furthermore, the same study showed that combination therapy of berberine and atorvastatin (a well-established anti-atherosclerotic drug) was more effective in preventing atherosclerotic processes compared to administration of atorvastatin alone.
Clinically, the major concern when artherosclerosis is present is the occurrence of plaque disruption which may then lead to unstable angina, or an outright MI. Plaque progression and destabilization is due to the over-expression of matrix metalloproteinases-9 (MMP-9) and extracellular matrix metalloproteinase inducer (EMMPRIN) in monocytes/macrophages (Cao et al., 2014). Berberine inhibits the up-regulation of MMP-9 and EMMPRIN in phorbol 12-myristate 13-acetate-induced macrophages by suppressing the activation of p38 pathway, showing its ability to stabilize atherosclerotic plaque (Huang et al., 2011).
RC seems to have multiple active components that target varying stages of the development of atherosclerosis through a variety of pathways, thus making it an effective anti-atherosclerotic agent.
Lipid-lowering effect
Hyperlipidemia or dyslipidemia, which is characterized by increased plasma triglyceride concentration and lowered HDL cholesterol, is one of the major risk factor contributing to the progression of CVDs such as CHD and peripheral artery disease. Hence, maintenance of total cholesterol (TC) level within the normal range can be an effective strategy to lower the risk of cardiovascular events (Zou et al., 2016). The liver is the main site of cholesterol homeostasis maintenance, which involves several distinct processes including biosynthesis via the activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR), uptake by low density lipoprotein receptors (LDLR), release of lipoprotein in the blood, storage, degradation and conversion into bile acids (Trapani et al., 2012). RC possesses lipid-lowering effects as shown by the lowered cholesterol levels in different animal models treated with RC extract (Yokozawa et al., 2003; Cao et al., 2012; Kou et al., 2016). Studies have indicated that the five main bioactive compounds in RC (berberine, coptisine, palmatine, epiberberine, and jatrorrhizine) exert their lipid-lowering effects through distinct mechanisms. Berberine's hypolipidemic effect was the result of up-regulation of LDLR mRNA and protein expression (Zhou et al., 2014). Coptisine treatment also resulted in increased expression of LDLR, and additionally was associated with up-regulated expression of cholesterol 7-alpha-hydroxylase (CYP7A1) and down-regulated expression of HMGCR (Chen et al., 2015; He et al., 2015b). In addition to up-regulated expression of LDLR and CYP7A1, treatment with palmatine and epiberberine also caused down-regulation of apical sodium-dependent bile salt transporter (ASBT) mRNA and protein expression, as well as enhanced fecal excretion of TC and total bile acids (Ning et al., 2015; Zou et al., 2016). Jatrorrhizine has been linked to up-regulated mRNA and protein expression of LDLR and CYP7A1 but it has no effect on the expression of ASBT and HMGCR (Wu et al., 2014a). Comparative studies have been done on the lipid-lowering effect of individual alkaloids and among those tested, coptisine was reported to have the highest lipid-lowering effect (Wu et al., 2014c). More recently, research involving combined treatment with the alkaloids in RC extract has also been carried out. While each of the compounds administered in isolation did demonstrate lipid-lowering effect, the administration of a combination of these alkaloids resulted in synergistic effects, with combination therapy resulting in a significantly greater lipid-lowering effect compared to treatment with a single alkaloid. The main mechanisms associated with this effect are thought to be the retardation of cholesterol synthesis and accelerated clearance of cholesterol (He et al., 2015a; Kou et al., 2016).
Type 2 diabetes is strongly associated with significant morbidity and mortality due to cardiovascular complications. This is in part due to the impaired utilization of carbohydrate that is part of the metabolic profile of diabetes, which results in accelerated lipolysis and thus hyperlipidemia or dyslipidemia (Vijayaraghavan, 2010; Ma et al., 2016). RC has been shown to have the ability to attenuate the impaired lipid metabolism associated with diabetes. JiuHuangLian (RC steamed with rice wine), a traditional Chinese medicine preparation has been reported to restore the disordered lipid metabolism of type 2 diabetic rats, by decreasing the TC, total glyceride and free fatty acid levels (Li et al., 2013). The anti-hyperlipidemic effect of the alkaloids isolated from RC have been further tested on diabetic mice. Palmatine and jatrorrhizine were shown to decrease the concentrations of serum TC and triglyceride; while elevated HDL cholesterol levels were found in diabetic mice administered with palmatine, jatrorrhizine and berberine (Ma et al., 2016). In another study, there were decreased serum TC, triglyceride, LDL cholesterol and increased HDL cholesterol in diabetic rats treated with berberine (Tang et al., 2006), showing the ability of RC to attenuate the impaired lipid metabolism in diabetic conditions.
In short, RC has remarkable lipid-lowering effects, mainly through inhibition of cholesterol synthesis, in combination with increased usage and excretion of cholesterol.
Anti-obesity effect
Obesity has become a global epidemic and is strongly associated with occurrence of CVDs including heart failure, CHD, sudden cardiac death and atrial fibrillation (Lavie et al., 2009). As the condition is characterized by accumulation of extra body fat into adipocytes, the typical feature of obesity is an increase in the number and size of adipocytes. The etiology of obesity is rooted in the process of differentiation of preadipocytes into adipocytes, known as adipogenesis (Kwak et al., 2013; Choi et al., 2014). The key transcription regulator genes involved in adipogenesis are PPAR-γ and CCAAT/enhancer binding protein alpha (C/EBP-α). The inhibition of these two transcription factors could represent an effective method of inhibiting adipogenesis (Choi et al., 2014). RC has potential as an anti-obesity agent as the extract of RC as well as its isolated alkaloids have demonstrated the ability to inhibit cellular triglyceride accumulation in 3T3-L1 adipocytes. Significant down-regulation of expression of PPAR-γ and C/EBP-α by the alkaloids was also demonstrated, which is consistent with the reported mechanism for the anti-adipogenic activity of berberine (Huang et al., 2006).
The anti-obesity potential of RC has also been demonstrated in in vivo studies. The administration of RC and berberine to mice fed a high fat diet has resulted in significantly lower body and visceral adipose weight. Interestingly, this effect has been related to the antimicrobial activities of both RC and berberine; with reduced fecal microbes resulting in decreased degradation of dietary polysaccharides, lower calorie intake and de novo lipogenesis. This was followed by activation of fasting-induced adipose factor (Fiaf) protein and related gene expressions of mitochondrial energy metabolism such as AMPK, PGC1α, UCP2, CPT1α, and Hadhb in visceral adipose tissue (Xie et al., 2011). Recently, the gut microbiota of obese mice treated with RC alkaloids has been further studied, demonstrating an increased abundance of microbial species negatively associated with obesity namely Sporobacter termitidis, Alcaligenes faecalis, and Akkermansia muciniphila, and a decreased abundance of the microbial species positively associated with obesity namely Escherichia coli, Desulfovibrio C21 _c20 and Parabacteroides distasonis. This indicates the ability of RC to prevent the development of obesity by restoring the balance of disrupted gut microflora associated with the obese condition (He et al., 2016). RC appears to be effective in preventing obesity as it is able to inhibit adipogenesis and modulate the gut microflora that plays important role for the development of obesity.
Anti-hepatic steatosis effect
Nonalcoholic fatty liver disease (NAFLD)—a common liver disease with a disease spectrum ranging from simple steatosis to steatohepatitis—is regarded as the hepatic manifestation of metabolic syndrome (Liu and Lu, 2014). Recently, hepatic steatosis has been identified as one of the associated risk factors closely related to the morbidity and mortality of CVDs. It has been reported that patients with NAFLD have higher risk of getting CHD compared to the general population (Pisto et al., 2014). There are several possible mechanisms by which hepatic steatosis may contribute to the risk of CVDs including oxidative stress, inflammation, dyslipidemia, ischemia reperfusion injury, visceral fat, low adiponectin, ectopic adipose tissue distribution, endothelial dysfunction and postprandial dyslipidemia (Liu and Lu, 2014). In spite of the many advances in modern medicine, there is as yet no effective treatment of NAFLD (Huang et al., 2013). Herbal medicine has been an area of interest for scientists seeking new modalities of treatment of hepatic steatosis due to the low incidence of side effects and promising therapeutic benefits (Xiao et al., 2013; Tan et al., 2016). For example, Jiao Tai Wan, a classic traditional Chinese prescription consisting of RC and Cortex Cinamomi was able to inhibit hepatic lipid accumulation in diabetic rats and humans, due to down-regulation of lipogenic gene expression and inhibition of lipogenesis in liver tissue (Huang et al., 2013). Sam-Hwang-Sa-Sim-Tang, a Korean traditional preparation which contains RC, Scutellaria radix and Rhei rhizoma also exhibited protective effect against hepatic steatosis by inhibiting the expression of hepatic molecules that modulate cholesterol metabolism (Ahn et al., 2013). While RC extract clearly does have significant potential as an anti-hepatic steatosis agent, there has been interest in identifying which of the extract's active components exert the greatest anti-hepatic steatosis effect. Using a free fatty-acid induced hepatic steatosis HepG2 cell assay, Fan et al. (2013) demonstrated that berberine and coptisine are the alkaloids with strongest inhibitory effect on triglyceride accumulation. This is consistent with the findings of other studies that proposed berberine as the effective compound against hepatic steatosis (Yuan et al., 2015; Guo et al., 2016; Zhang et al., 2016). In short, RC possesses anti-hepatic steatosis effect which is related to its ability to inhibit hepatic lipogenesis.
Protection from myocardial ischemia-reperfusion injury
Coronary artery disease resulting in compromised oxygen supply to the myocardium is a common cause of morbidity and mortality. Medical intervention now makes it possible to achieve rapid restoration of blood flow to the ischemic myocardium following an acute event, which should produce better clinical outcomes as prompt restoration of blood circulation helps to prevent further tissue injury. However, the restoration of blood flow or reperfusion, will also cause injury, which is known as myocardial ischemia reperfusion injury (I/R) (Yu et al., 2015). Apoptosis is an important cellular mechanism resulting in I/R injury, with oxidative stress representing one of the major contributing factors promoting apoptosis. The excessive production of ROS which are mainly produced by mitochondria will initiate apoptotic signaling (Chan et al., 2012, 2015). Hence, reducing oxidative stress may help inhibit apoptosis thus potentially providing an effective strategy to attenuate I/R injury (Fan et al., 2012). Traditional Chinese herbal treatment have been commonly used to treat MI for hundreds of years and recently, it has also been reported to exert protective effect against myocardial I/R injury (Liu et al., 2013; Li et al., 2014; Zhao et al., 2016a).
One example of the traditional Chinese medications is San-Huang-Xie-Xin-Tang (SHXT), which consists of three herbs: RC, Scutellariae radix and Rhei rhizome. Liou et al. (2011) showed that pre-treatment with SHXT was associated with significantly lower arrhythmia scores, reduced mortality rates and reduced levels of cardiac enzymes such as creatine kinase (CK), lactate dehydrogenase (LDH) and troponin I in rats with myocardial I/R injury. Additionally, there was reduced infarct size and decreased apoptosis in myocytes, partly due to modulation of endothelial nitric oxide synthase (eNOS) and MAPK pathways. While it is commonly used as a component of herbal mixtures, the extract of RC alone has been shown to possess cardioprotective effect against I/R damage. Administration of crude extract of RC for 7 days preceding acute myocardial injury in rats was associated with reduced myocardial injury as evidenced by decreased ST-T elevation on electrocardiogram (ECG) and biochemically by decreased levels of CK and LDH. Other beneficial effects were reduction in oxidative stress reflected by reduced levels of malondiealdehyde (MDA) and increased activity of superoxide dismutase (SOD) (Liu et al., 2010).
Several active compounds identified in RC have been suggested as possible candidates accounting for the cardioprotective activities against I/R injury as demonstrated. One of the compounds that appears to have cardioprotective activity is berberine. Although the cardioprotective activity of berberine is well recognized, the exact mechanism by which it protects from I/R injury remains unknown. Chang et al. (2012) found that pretreatment with berberine decreased infarct size and reduced the incidence as well as duration of arrhythmias in rats with I/R injury with demonstrable difference in AMPK activity in both non-ischemic areas (NIA) and myocardial areas at risk (AAR). Up-regulation of AMPK in the NIA helped to enhance cardiac function through increased uptake of glucose, glycolysis and oxidation of fatty acid whereas down-regulation of AMPK in the areas at risk helped to protect the cardiac myocytes from apoptosis. This protective effect of berberine was later specifically demonstrated in the CVD prone diabetic heart, as demonstrated by the regulation of myocardial energy metabolism by berberine during I/R injury (Chang et al., 2016). In myocardial I/R, there is always excessive autophagy which will lead to expansive degradation of cytosolic proteins and organelles, resulting in collapse of cellular functions. Huang et al. (2015) have reported the beneficial effect of berberine in lowering the cellular autophagy level in berberine-treated myocytes exposed to I/R injury which were found to have reduced expression of autophagy-related proteins, which then led to suppression of autophagy activation. Berberine's cardioprotective action may also be associated with its anti-apoptotic action. Based on both in vitro and in vivo results, berberine decreases I/R-induced myocardial apoptosis, as a result of modulation of the Notch1/ Hairy and enhancer of split 1(Hes1)- Phosphatase and tensin homolog deleted on chromosome 10 (PTEN)/Akt signaling pathway (Yu et al., 2015). In a diabetic rat model, berberine exerted anti-apoptotic activity through activation of AMPK and PI3K-Akt-eNOS signaling (Chen et al., 2014).
Mitochondrial dysfunction is an additional factor believed to be responsible for myocardial I/R injury. Treatment with berberine has been shown to improve mitochondrial dysfunction, as demonstrated by improved mitochondrial membrane potential, increased mitochondrial complex I activity and reduced release of cytochrome C from the mitochondria (Wang et al., 2015). Endoplasmic reticulum (ER) stress is also known to play an important part during I/R injury. Unfolded protein response (UPR) is initiated in the myocardium after I/R and the continuous UPR causes upregulation of proapoptotic proteins. Consequently, cellular apoptosis occurs, resulting in myocardial I/R injury (Wu et al., 2014b). The role of berberine in modulation of ER stress level during myocardial I/R injury has also been described. Pretreatment with berberine has resulted in suppressed myocardial I/R induced ER stress due to the activation of Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signaling (Zhao et al., 2016b) An additional mechanism by which berberine is able to ameliorate myocardial I/R injury is via repression of the inflammatory response. Lower levels of inflammatory markers, namely the cytokines tumor necrosis factor (TNF)-α, IL-6 and IL-β were found in rats pre-treated with berberine prior to induction of myocardial ischemia; this was believed to be mediated by downregulation of PI3K/AKT signaling thus preventing myocardial I/R injury (Zhu and Li, 2016).
Coptisine is one of the other compounds in RC that might have a role in prevention of I/R injury. In a rat model, administration of coptisine alleviated I/R-induced arrhythmias and attenuated the reduction in ejection fraction as well as fractional shortening on echocardiography. Treatment with coptisine was also associated with reduced infarct size, suppressed myocardial apoptosis and reduced proinflammatory cytokines. Based on the reduced expression of Rho, Rho-kinase 1 (ROCK1), ROCK2 and attenuation of myosin phosphatase targeting subunit-1 phosphorylation, it was speculated that inhibition of the Rho/ROCK pathway was likely to be coptisine's mechanism of cardioprotection (Guo et al., 2013).
Palmatine, another component contained in RC extract, is also recognized as a potential cardioprotective agent. The administration of palmatine to rats prior to myocardial I/R injury was associated with reduced I/R–induced myocardial dysfunction as evidenced by inhibition of the expected increase in LDH, CK, and MDA, as well as significant reduction in cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) expression. Interestingly, in vitro studies using human aortic endothelial cells resulted in increased heme-oxygenase-1 induction, indicating the strong antioxidant and anti-inflammatory action of palmatine (Kim et al., 2009). Based on the studies, RC is a potential protective agent against myocardial I/R injury through its active compounds which exert different protection actions including regulation of cellular energy metabolism, anti-apoptosis effect, protection against mitochondrial dysfunction and ER stress as well as antioxidant and anti-inflammatory action.
Conclusion
In conclusion, results of recent studies appear to support for the usage of RC for treatment of CVDs and related conditions. They have provided scientific evidence that RC's use in traditional Chinese medicine for the last 2000 years was likely to have truly resulted in desirable effects on CVDs via its effects on the various risk factors and biochemical pathways involved in CVDs pathogenesis. RC appears to have tremendous potential as a cardioprotective agent given its ability to improve a large number of parameters associated with increased risk of CVDs including anti-atherosclerotic effect, lipid-lowering effect, anti-obesity effect and anti-hepatic steatosis effect. Aside from reducing incidence, it also reduces the damage caused by CVDs as it confers protection from myocardial I/R injury. These properties are mainly attributed to its bioactive compounds: berberine, coptisine, palmatine, epiberberine, jatrorrhizine and magnoflorine. However, knowledge regarding the underlying mechanisms of action of these compounds is still limited. More research investigating the exact mechanisms of RC's cardioprotective activities is needed. This is to fully exploit this traditional remedy's potential to contribute to the development of new cardioprotective agents as this could be a new opportunity to reduce global prevalence of CVDs.
Author contributions
HT and BG contributed to the literature database search, data collection, data extraction, and writing of the manuscript. PP, SS, AD, TM, KC, LL, and BG contributed vital insight and proofread on the writing. The research topic was conceptualized by BG.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was inspired by Monash Pharmacy Degree Course, Unit PAC2412 which entitled “Integrated therapeutics: Introduction and cardiovascular” and financially supported by the MONASH PVC Award Grant (Project Q7 No. PVC-ECR-2016), Monash University Malaysia ECR Grant (5140077-000-00), External Industry Grant (Biotek Abadi Vote No. GBA-808813), Fundamental Research Grant Scheme (FRGS/1/2014/SKK01/MUSM/03/2) of Malaysia Ministry of Higher Education, MOSTI eScience Funds (02-02-10-SF0215 and 06-02-10-SF0300), University of Malaya for High Impact Research Grant (UM-MOHE HIR Nature Microbiome Grant No. H-50001-A000027 and No. A000001-50001).
Glossary
Abbreviations
- ACC
acetyl coenzyme A carboxylase
- ADP
adenosine monophosphate
- AMPK
adenosine 5′-monophosphate (AMP)-activated protein kinase
- AP-1
activator protein 1
- ASBT
apical sodium-dependent bile salt transporter
- ATP
adenosine triphosphate
- Bax
Bcl-2-associated X protein
- Bcl-2
B cell lymphoma 2
- BNIP3
BCL2/adenovirus E1B 19kDa interacting protein 3
- C/EBP-α
CCAAT/enhancer-binding protein- α
- CK
creatine kinase
- COX-2
cyclooxygenase-2
- CPT1α
carnitine palmitoyl transferase 1-alpha
- cTn1
cardiac troponin I
- CYP7A1
cholesterol 7-alpha-hydroxylase
- EMMPRIN
extracellular matrix metalloproteinase inducer
- eNOS
endothelial nitric oxide synthase
- FAS
fatty acid synthase
- FXR
farnesoid X receptor
- GSK3β
glycogen synthase kinase 3β
- HDL
high density lipoprotein
- HDL-c
high density lipoprotein cholesterol
- Hes1
Hairy and enhancer of split 1
- HMG-CoA
3-hydroxy-3methylglutary-CoA
- HMGCR
3-Hydroxy-3-methylglutaryl CoA reductase
- HO-1
heme-oxygenase-1
- HUVECs
human umbilical vein endothelial cells
- IL
Interleukin
- I/R
ischemia/reperfusion
- iNOS
inducible nitric oxide synthase
- JAK
Janus kinase
- JNK
c-Jun N-terminal kinase
- LDH
lactate dehydrogenase
- LDL
low density lipoprotein
- LDL-c
low density lipoprotein cholesterol
- LDLR
low-density lipoprotein receptor
- LPS
Lipopolysaccharide
- LXR
liver X receptor
- MAPK
p38 mitogen-activated protein kinase
- MCP-1
monocyte chemoattractant protein-1
- MDA
malondialdehyde
- MMP
matrix metalloproteinase
- mTORC2
mammalian target of rapamycin complex 1
- NFκB
Nuclear factor-kappaB
- Ox-LDL
oxidized low density lipoprotein
- pACC
phosphorylated-ACC
- PGC1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PI3K
phosphoinositide 3-kinase
- PMA
phorbol 12-myristate 13-acetate
- PPAR
proliferator-activated receptor
- PTEN
Phosphatase and tensin homolog deleted on chromosome 10
- ROCK
Rho-kinase
- ROS
reactive oxygen species
- SIRT1
Sirtuin 1
- SOD
superoxide dismutase
- SREBP2
Sterol regulatory element binding transcription factor 2
- STAT3
signal transducer and activator of transcription 3
- TBA
total bile acids
- TBARS
thiobarbituric acid reactive substances
- TC
total cholesterol
- TG
triglyceride
- TGR5
G-protein coupled bile acid receptor
- TLR4
toll-like receptor 4
- TNF
tumor necrosis factor
- TXNIP
thioredoxin-interacting protein
- UCP 2
uncoupling protein 2
- VMSCs
vascular smooth muscle cells.
References
- Ahn T. G., Lee J. Y., Cheon S. Y., An H. J., Kook Y. B. (2013). Protective effect of Sam-Hwang-Sa-Sim-Tang against hepatic steatosis in mice fed a high-cholesterol diet. BMC Complement. Altern. Med. 13:366. 10.1186/1472-6882-13-366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Han Z., Tian L., Chen K., Fan Y., Ye B., et al. (2014). Curcumin inhibits EMMPRIN and MMP-9 expression through AMPK-MAPK and PKC signaling in PMA induced macrophages. J. Transl. Med. 12:266. 10.1186/s12967-014-0266-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Bei W., Hu Y., Cao L., Huang L., Wang L., et al. (2012). Hypocholesterolemia of Rhizoma Coptidis alkaloids is related to the bile acid by up-regulated CYP7A1 in hyperlipidemic rats. Phytomedicine 19, 686–692. 10.1016/j.phymed.2012.03.011 [DOI] [PubMed] [Google Scholar]
- Chan C. K., Goh B. H., Kamarudin M. N. A., Kadir H. A. (2012). Aqueous fraction of Nephelium ramboutan-ake rind induces mitochondrial-mediated apoptosis in HT-29 human colorectal adenocarcinoma cells. Molecules 17, 6633–6657. 10.3390/molecules17066633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. K., Supriady H., Goh B. H., Kadir H. A. (2015). Elephantopus scaber induces apoptosis through ROS-dependent mitochondrial signaling pathway in HCT116 human colorectal carcinoma cells. J. Ethnopharm. 168, 291–304. 10.1016/j.jep.2015.03.072 [DOI] [PubMed] [Google Scholar]
- Chang W., Li K., Guan F., Yao F., Yu Y., Zhang M., et al. (2016). Berberine pretreatment confers cardioprotection against ischemia-reperfusion injury in a rat model of type 2 diabetes. J. Cardiovasc. Pharmacol. Ther. 21, 486–494. 10.1177/1074248415627873 [DOI] [PubMed] [Google Scholar]
- Chang W., Zhang M., Li J., Meng Z., Xiao D., Wei S., et al. (2012). Berberine attenuates ischemia-reperfusion injury via regulation of adenosine-5′-monophosphate kinase activity in both non-ischemic and ischemic areas of the rat heart. Cardiovasc. Drugs Ther. 26, 467–478. 10.1007/s10557-012-6422-0 [DOI] [PubMed] [Google Scholar]
- Chen B., Xue D. F., Han B., Kou S. M., Ye X. L., Li X. G. (2015). Regulatory effect of coptisine on key genes involved in cholesterol metabolism. Zhongguo Zhong Yao Za Zhi 40, 1548–1553. 10.4268/cjcmm20150823 [DOI] [PubMed] [Google Scholar]
- Chen J., Zhao H., Wang X., Lee F. S.-C., Yang H., Zheng L. (2008). Analysis of major alkaloids in Rhizoma coptidis by capillary electrophoresis-electrospray-time of flight mass spectrometry with different background electrolytes. Electrophoresis 29, 2135–2147. 10.1002/elps.200700797 [DOI] [PubMed] [Google Scholar]
- Chen K., Li G., Geng F., Zhang Z., Li J., Yang M., et al. (2014). Berberine reduces ischemia/reperfusion-induced myocardial apoptosis via activating AMPK and PI3K–Akt signaling in diabetic rats. Apoptosis 19, 946–957. 10.1007/s10495-014-0977-0 [DOI] [PubMed] [Google Scholar]
- Chen X. (2009). Medical Composition Containing Dioscorea Opposite, Coptis Chinensis, Vitamins, Major Elements and Trace Elements for Lowering Blood Sugar and Blood Lipid, and Diabetes Mellitus, and Its Formulation. CN. Patent No 101579464 Beijing: State Intellectual Property Office of the P.R.C.
- Chi L., Peng L., Pan N., Hu X., Zhang Y. (2014). The anti-atherogenic effects of berberine on foam cell formation are mediated through the upregulation of sirtuin 1. Int. J. Mol. Med. 34, 1087–1093. 10.3892/ijmm.2014.1868 [DOI] [PubMed] [Google Scholar]
- Choi J. S., Kim J. H., Ali M. Y., Min B. S., Kim G. D., Jung H. A. (2014). Coptis chinensis alkaloids exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBP-α and PPAR-γ. Fitoterapia 98, 199–208. 10.1016/j.fitote.2014.08.006 [DOI] [PubMed] [Google Scholar]
- Fan H., Chen Y. Y., Bei W. J., Wang L. Y., Chen B. T., Guo J. (2013). In vitro screening for antihepatic steatosis active components within Coptidis Rhizoma Alkaloids Extract using liver cell extraction with HPLC analysis and a free fatty acid-induced hepatic steatosis HepG2 cell assay. Evid. Based Complement. Alternat. Med. 2013:459390. 10.1155/2013/459390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Yang L., Fu F., Xu H., Meng Q., Zhu H., et al. (2012). Cardioprotective effects of salvianolic acid A on myocardial ischemia-reperfusion injury in vivo and in vitro. Evid. Based Complement. Alternat. Med. 2012:508938. 10.1155/2012/508938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frostegård J. (2013). Immunity, atherosclerosis and cardiovascular disease. BMC Med. 11:117. 10.1186/1741-7015-11-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J. (2012). Chinese Medicinal Composition Containing Saponins of Panax and Alkaloids of Coptidis for Preventing and Treating Lipid Metabolism Disorder. WO. Patent No 2012100440 Geneva: World Intellectual Property Organization.
- Guo J., Wang S. B., Yuan T. Y., Wu Y. J., Yan Y., Li L., et al. (2013). Coptisine protects rat heart against myocardial ischemia/reperfusion injury by suppressing myocardial apoptosis and inflammation. Atherosclerosis 231, 384–391. 10.1016/j.atherosclerosis.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Guo T., Woo S. L., Guo X., Li H., Zheng J., Botchlett R., et al. (2016). Berberine ameliorates hepatic steatosis and suppresses liver and adipose tissue inflammation in mice with diet-induced obesity. Sci. Rep. 6:22612. 10.1038/srep22612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K., Hu Y., Ma H., Zou Z., Xiao Y., Yang Y., et al. (2016). Rhizoma coptidis alkaloids alleviate hyperlipidemia in B6 mice by modulating gut microbiota and bile acid pathways. BBA Mol. Basis Dis. 1862, 1696–1709. 10.1016/j.bbadis.2016.06.006 [DOI] [PubMed] [Google Scholar]
- He K., Kou S., Zou Z., Hu Y., Feng M., Han B., et al. (2015a). Hypolipidemic effects of alkaloids from Rhizoma coptidis in diet-induced hyperlipidemic hamsters. Planta Med. 82, 690–697. 10.1055/s-0035-1568261 [DOI] [PubMed] [Google Scholar]
- He K., Ye X., Wu H., Wang Y., Zou Z., Ning N., et al. (2015b). The safety and anti-hypercholesterolemic effect of coptisine in Syrian golden hamsters. Lipids 50, 185–194. 10.1007/s11745-014-3983-7 [DOI] [PubMed] [Google Scholar]
- Ho F. M., Liao Y. H., Yang A. J., Lee Chao P. D., Hou Y. C., Huang C. T., et al. (2012). Anti-atherosclerotic action of Ger-Gen-Chyn-Lian-Tang and AMPK-dependent lipid lowering effect in hepatocytes. J. Ethnopharmacol. 142, 175–187. 10.1016/j.jep.2012.04.034 [DOI] [PubMed] [Google Scholar]
- Hsieh Y. S., Kuo W. H., Lin T. W., Chang H. R., Lin T. H., Chen P. N., et al. (2007). Protective effects of berberine against low-density lipoprotein (LDL) oxidation and oxidized LDL-induced cytotoxicity on endothelial cells. J. Agric. Food Chem. 55, 10437–10445. 10.1021/jf071868c [DOI] [PubMed] [Google Scholar]
- Huang C., Zhang Y., Gong Z., Sheng X., Li Z., Zhang W., et al. (2006). Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARγ pathway. Biochem. Biophys. Res. Commun. 348, 571–578. 10.1016/j.bbrc.2006.07.095 [DOI] [PubMed] [Google Scholar]
- Huang Z., Han Z., Ye B., Dai Z., Shan P., Lu Z., et al. (2015). Berberine alleviates cardiac ischemia/reperfusion injury by inhibiting excessive autophagy in cardiomyocytes. Eur. J. Pharmacol. 762, 1–10. 10.1016/j.ejphar.2015.05.028 [DOI] [PubMed] [Google Scholar]
- Huang Z., Wang L., Meng S., Wang Y., Chen T., Wang C. (2011). Berberine reduces both MMP-9 and EMMPRIN expression through prevention of p38 pathway activation in PMA-induced macrophages. Int. J. Cardiol. 146, 153–158. 10.1016/j.ijcard.2009.06.023 [DOI] [PubMed] [Google Scholar]
- Huang Z., Xu X., Lu F., Wang N., Chen G., Zhao Y., et al. (2013). Jiao tai wan attenuates hepatic lipid accumulation in type 2 diabetes mellitus. Evid. Based Complement. Alternat. Med. 2013:567045. 10.1155/2013/567045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T. M., Lee J. P., Min B. S., Choi J. S., Na M., Zhang X., et al. (2007a). Magnoflorine from Coptidis Rhizoma protects high density lipoprotein during oxidant stress. Biol. Pharm. Bull. 30, 1157–1160. 10.1248/bpb.30.1157 [DOI] [PubMed] [Google Scholar]
- Hung T. M., Na M., Min B. S., Zhang X., Lee I., Ngoc T. M., et al. (2007b). Protective effect of magnoflorine isolated from coptidis rhizoma on Cu2+-induced oxidation of human low density lipoprotein. Planta Med. 73, 1281–1284. 10.1055/s-2007-981615 [DOI] [PubMed] [Google Scholar]
- Itabe H. (2009). Oxidative modification of LDL: its pathological role in atherosclerosis. Clin. Rev. Allergy Immunol. 37, 4–11. 10.1007/s12016-008-8095-9 [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Ha Y. M., Jin Y. C., Shi L. Y., Lee Y. S., Kim H. J., et al. (2009). Palmatine from Coptidis rhizoma reduces ischemia-reperfusion-mediated acute myocardial injury in the rat. Food Chem. Toxicol. 47, 2097–2102. 10.1016/j.fct.2009.05.031 [DOI] [PubMed] [Google Scholar]
- Kou S., Han B., Wang Y., Huang T., He K., Han Y., et al. (2016). Synergetic cholesterol-lowering effects of main alkaloids from Rhizoma Coptidis in HepG2 cells and hypercholesterolemia hamsters. Life Sci. 151, 50–60. 10.1016/j.lfs.2016.02.046 [DOI] [PubMed] [Google Scholar]
- Kwak D. H., Lee J. H., Kim D. G., Kim T., Lee K. J., Ma J. Y. (2013). Inhibitory effects of Hwangryunhaedok-tang in 3T3-L1 adipogenesis by regulation of Raf/MEK1/ERK1/2 Pathway and PDK1/Akt Phosphorylation. Evid. Based Complement. Alternat. Med. 2013:413906. 10.1155/2013/413906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie C. J., Milani R. V., Ventura H. O. (2009). Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J. Am. Coll. Cardiol. 53, 1925–1932. 10.1016/j.jacc.2008.12.068 [DOI] [PubMed] [Google Scholar]
- Li G., Li Y., Sun F., Dong X., Zhong F. (2015). Chinese Medicine Composition for Reducing Blood Glucose and Lipid Protecting Blood Vesssel Endothelial, and Preparation Method Thereof. CN. Patent No 104257839 Beijing: State Intellectual Property Office of the P.R.C.
- Li J. C., Shen X. F., Meng X. L. (2013). A traditional Chinese medicine JiuHuangLian (Rhizoma coptidis steamed with rice wine) reduces oxidative stress injury in type 2 diabetic rats. Food Chem. Toxicol. 59, 222–229. 10.1016/j.fct.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Li X., Liu J., Lin L., Guo Y., Lin C., Zhang C., et al. (2014). Traditional Chinese medicine Shuang Shen Ning Xin attenuates myocardial ischemia/reperfusion injury by preserving of mitochondrial function. Evid. Based Complement. Alternat. Med. 2014:180965. 10.1155/2014/180965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou S. F., Ke H. J., Hsu J. H., Liang J. C., Lin H. H., Chen I. J., et al. (2011). San-Huang-Xie-Xin-Tang prevents rat hearts from ischemia/reperfusion-induced apoptosis through eNOS and MAPK Pathways. Evid. Based Complement. Alternat. Med. 2011:915051. 10.1093/ecam/neq061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Lu H. Y. (2014). Nonalcoholic fatty liver disease and cardiovascular disease. World J. Gastroenterol. 20, 8407–8415. 10.3748/wjg.v20.i26.8407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Li J., Wang J., Li J., Janicki J. S., Fan D. (2013). Effects and mechanisms of Chinese herbal medicine in ameliorating myocardial ischemia-reperfusion injury. Evid. Based Complement. Alternat. Med. 2013:925625. 10.1155/2013/925625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chen X., Jin R. (2010). Protective effects of Rhizoma coptidis on acute myocardial ischemia injured cardiomyocytes in vivo and in vitro. Chin. J. Info. Tradit. Chin. Med. 17, 28–30. 10.3969/j.issn.1005-5304.2010.12.012 [DOI] [Google Scholar]
- Lucas A. D., Greaves D. R. (2001). Atherosclerosis: role of chemokines and macrophages. Expert Rev. Mol. Med. 3, 1–18. 10.1017/S1462399401003696 [DOI] [PubMed] [Google Scholar]
- Ma B. L., Yao M. K., Zhong J., Ma Y. M., Gao C. L., Wu J. S., et al. (2012). Increased systemic exposure to rhizoma coptidis alkaloids in lipopolysaccharide-pretreated rats attributable to enhanced intestinal absorption. Drug Metab. Dispos. 40, 381–388. 10.1124/dmd.111.041152 [DOI] [PubMed] [Google Scholar]
- Ma H., Hu Y., Zou Z., Feng M., Ye X., Li X. (2016). Antihyperglycemia and antihyperlipidemia effect of protoberberine alkaloids from Rhizoma Coptidis in HepG2 cell and diabetic KK-Ay mice. Drug Dev. Res. 77, 163–170. 10.1002/ddr.21302 [DOI] [PubMed] [Google Scholar]
- Moghadamtousi S., Goh B., Chan C., Shabab T., Kadir H. (2013). Biological activities and phytochemicals of swietenia macrophylla king. Molecules 18, 10465–10483. 10.3390/molecules180910465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moukdar F., Robidoux J., Lyght O., Pi J., Daniel K. W., Collins S. (2009). Reduced antioxidant capacity and diet-induced atherosclerosis in uncoupling protein-2-deficient mice. J. Lipid Res. 50, 59–70. 10.1194/jlr.M800273-JLR200 [DOI] [PubMed] [Google Scholar]
- Ning N., He K., Wang Y., Zou Z., Wu H., Li X., et al. (2015). Hypolipidemic effect and mechanism of palmatine from Coptis chinensis in hamsters fed high-fat diet. Phytother. Res. 29, 668–673. 10.1002/ptr.5295 [DOI] [PubMed] [Google Scholar]
- Pisto P., Santaniemi M., Bloigu R., Ukkola O., Kesäniemi Y. A. (2014). Fatty liver predicts the risk for cardiovascular events in middle-aged population: a population-based cohort study. BMJ Open 4:e004973. 10.1136/bmjopen-2014-004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remppis A., Bea F., Greten H. J., Buttler A., Wang H., Zhou Q., et al. (2010). Rhizoma coptidis inhibits LPS-induced MCP-1/CCL2 production in murine macrophages via an AP-1 and NFκB-dependent pathway. Mediators Inflamm. 2010:194896. 10.1155/2010/194896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supriady H., Kamarudin M. N. A., Chan C. K., Goh B. H., Kadir H. A. (2015). SMEAF attenuates the production of pro-inflammatory mediators through the inactivation of Akt-dependent NF-κB, p38 and ERK1/2 pathways in LPS-stimulated BV-2 microglial cells. J. Funct. Food. 17, 434–448. 10.1016/j.jff.2015.05.042 [DOI] [Google Scholar]
- Tan H. L., Chan K. G., Pusparajah P., Lee L. H., Goh B. H. (2016). Gynura procumbens: an overview of the biological activities. Front. Pharmacol. 7:52. 10.3389/fphar.2016.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L. T. H., Lee L. H., Yin W. F., Chan C. K., Kadir H. A., Chan K. G., et al. (2015). Traditional uses, phytochemistry, and bioactivities of Cananga odorata (Ylang-Ylang). Evid. Based Complement Alternat Med. 2015:896314. 10.1155/2015/896314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L. Q., Wei W., Chen L. M., Liu S. (2006). Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J. Ethnopharmacol. 108, 109–115. 10.1016/j.jep.2006.04.019 [DOI] [PubMed] [Google Scholar]
- Trapani L., Segatto M., Pallottini V. (2012). Regulation and deregulation of cholesterol homeostasis: the liver as a metabolic “power station.” World J. Hepatol. 4, 184–190. 10.4254/wjh.v4.i6.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan K. (2010). Treatment of dyslipidemia in patients with type 2 diabetes. Lipids Health Dis. 9, 1–12. 10.1186/1476-511X-9-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden R., Tomlinson B. (2011). Cardiovascular disease, in Herbal Medicine: Biomolecular and Clinical Aspects, eds Benzie I. F. F., Wachtel-Galor S. (Boca Raton, FL: LLC; ), 333–361. [PubMed] [Google Scholar]
- Wallace T. C. (2011). Anthocyanins in cardiovascular disease. Adv. Nutr Int. Rev. J. 2, 1–7. 10.3945/an.110.000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Mu W., Shang H., Lin J., Lei X. (2014). The antihyperglycemic effects of Rhizoma Coptidis and mechanism of actions: a review of systematic reviews and pharmacological research. Biomed. Res. Int. 2014:10. 10.1155/2014/798093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhang M., Liang B., Shirwany N., Zhu Y., Zou M. H. (2011). Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: the role of uncoupling protein 2. PLoS ONE 6:e25436. 10.1371/journal.pone.0025436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. (2015). A Chinese Medicine Composition for Treating Obesity Complicated with Hyperlipidemia and Its Application. CN. Patent No 104645055 Beijing: State Intellectual Property Office of the P.R.C.
- Wang Y., Liu J., Ma A., Chen Y. (2015). Cardioprotective effect of berberine against myocardial ischemia/reperfusion injury via attenuating mitochondrial dysfunction and apoptosis. Int. J. Clin. Exp. Med. 8:14513. [PMC free article] [PubMed] [Google Scholar]
- Wu H., He K., Wang Y., Xue D., Ning N., Zou Z., et al. (2014a). The antihypercholesterolemic effect of jatrorrhizine isolated from Rhizoma Coptidis. Phytomedicine 21, 1373–1381. 10.1016/j.phymed.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Wu H., Tang Q., Yang J., Ding J., Ye M., Dong W. (2014b). Atorvastatin ameliorates myocardial ischemia/reperfusion injury through attenuation of endoplasmic reticulum stress-induced apoptosis. Int. J. Clin. Exp. Med. 7, 4915–4923. [PMC free article] [PubMed] [Google Scholar]
- Wu H., Wang Y. Z., Wang D. Z., Pang J., Ye X. L., Li X. G. (2014c). Effects of alkaloids from coptidis rhizoma on blood lipid metabolism and low-denstity lipoprotein receptor mRNA in golden hamsters. Zhongguo Zhong Yao Za Zhi 39, 2102–2105. 10.4268/cjcmm20141131 [DOI] [PubMed] [Google Scholar]
- Wu J., Zhang H., Hu B., Yang L., Wang P., Wang F., et al. (2016). Coptisine from Coptis chinensis inhibits production of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. Eur. J. Pharmacol. 780, 106–114. 10.1016/j.ejphar.2016.03.037 [DOI] [PubMed] [Google Scholar]
- Xiao J., Fai So K., Liong E. C., Tipoe G. L. (2013). Recent advances in the herbal treatment of non-alcoholic Fatty liver disease. J. Tradit. Complement. Med. 3, 88–94. 10.4103/2225-4110.110411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Gu D., Li J., Cui K., Zhang Y. (2011). Effects and action mechanisms of berberine and Rhizoma coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PLoS ONE 6:e24520. 10.1371/journal.pone.0024520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X., Li X., Yuan L. (2009). Hypolipidemia Composite Preparation Comprising Rhizoma Coptidis Alkaloid and Crataegus Pinnatifida Flavanoid. CN. Patent No 10485752 Beijing: State Intellectual Property Office of the P.R.C.
- Yi J., Ye X., Wang D., He K., Yang Y., Liu X., et al. (2013). Safety evaluation of main alkaloids from Rhizoma Coptidis. J. Ethnopharmacol. 145, 303–310. 10.1016/j.jep.2012.10.062 [DOI] [PubMed] [Google Scholar]
- Yokozawa T., Ishida A., Cho E. J., Nakagawa T. (2003). The effects of Coptidis Rhizoma extract on a hypercholesterolemic animal model. Phytomedicine 10, 17–22. 10.1078/094471103321648610 [DOI] [PubMed] [Google Scholar]
- Yu L., Li F., Zhao G., Yang Y., Jin Z., Zhai M., et al. (2015). Protective effect of berberine against myocardial ischemia reperfusion injury: role of Notch1/Hes1-PTEN/Akt signaling. Apoptosis 20, 796–810. 10.1007/s10495-015-1122-4 [DOI] [PubMed] [Google Scholar]
- Yuan X., Wang J., Tang X., Li Y., Xia P., Gao X. (2015). Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J. Transl. Med. 13:24. 10.1186/s12967-015-0383-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Li B., Meng X., Yao S., Jin L., Yang J., et al. (2016). Berberine prevents progression from hepatic steatosis to steatohepatitis and fibrosis by reducing endoplasmic reticulum stress. Sci. Rep. 6:20848. 10.1038/srep20848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G. L., Yu L. M., Gao W. L., Duan W. X., Jiang B., Liu X. D., et al. (2016b). Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol. Sin. 37, 354–367. 10.1038/aps.2015.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Xu L., Qiao Z., Gao L., Ding S., Ying X., et al. (2016a). YiXin-Shu, a ShengMai-San-based traditional Chinese medicine formula, attenuates myocardial ischemia/reperfusion injury by suppressing mitochondrial mediated apoptosis and upregulating liver-X-receptor α. Sci. Rep. 6:23025. 10.1038/srep23025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Cao S., Wang Y., Xu P., Yan J., Bin W., et al. (2014). Berberine metabolites could induce low density lipoprotein receptor up-regulation to exert lipid-lowering effects in human hepatoma cells. Fitoterapia 92, 230–237. 10.1016/j.fitote.2013.11.010 [DOI] [PubMed] [Google Scholar]
- Zhu Q. W., Li Y. G. (2016). Berberine attenuates myocardial ischemia reperfusion injury by suppressing the activation of PI3K/AKT signaling. Exp. Ther. Med. 11, 978–984. 10.3892/etm.2016.3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z. Y., Hu Y. R., Ma H., Feng M., Li X. G., Ye X. L. (2016). Epiberberine reduces serum cholesterol in diet-induced dyslipidemia Syrian golden hamsters via network pathways involving cholesterol metabolism. Eur. J. Pharmacol. 774, 1–9. 10.1016/j.ejphar.2015.11.017 [DOI] [PubMed] [Google Scholar]