Abstract
Simulated microgravity (SMG) bioreactors and DNA microarray technology are powerful tools to identify “space genes” that play key roles in cellular response to microgravity. We applied these biotechnology tools to investigate SMG and post-SMG recovery effects on human epidermal keratinocytes by exposing cells to SMG for 3, 4, 9, and 10 d using the high aspect ratio vessel bioreactor followed by recovery culturing for 15, 50, and 60 d in normal gravity. As a result, we identified 162 differentially expressed genes, 32 of which were “center genes” that were most consistently affected in the time course experiments. Eleven of the center genes were from the integrated stress response pathways and were coordinately down-regulated. Another seven of the center genes, which are all metallothionein MT-I and MT-II isoforms, were coordinately up-regulated. In addition, HLA-G, a key gene in cellular immune response suppression, was found to be significantly up-regulated during the recovery phase. Overall, more than 80% of the differentially expressed genes from the shorter exposures (≤4 d) recovered in 15 d; for longer (≥9 d) exposures, more than 50 d were needed to recover to the impact level of shorter exposures. The data indicated that shorter SMG exposure duration would lead to quicker and more complete recovery from the microgravity effect.
Key words: HEK001, HARV, DNA microarray, Northern blotting, expression profiling, microgravity
Introduction
Exposure to microgravity has been recognized as a major environmental factor of spaceflight. Some of the adverse effects resulting from spaceflight are a decline in cellular immune response 1., 2., 3., cardiovascular deconditioning (4), bone deterioration (5), and muscular atrophy (6). Elucidation of the molecular mechanisms underlying microgravity-induced health problems is critical for formulating effective counter-measures for spaceflight side-effects. Due to the cost effectiveness and the ability to separate microgravity effect from other complex factors of spaceflight, ground-based simulated microgravity (SMG) research has become popular and is widely practiced in space life sciences research.
Ground-based SMG conditions for mammalian cell and microorganism cultures are created through the use of high aspect ratio vessel (HARV) bioreactors 7., 8., which simulate microgravity by maintaining the cells in continuous free fall in liquid medium and are most commonly used in the United States 9., 10., 11., 12.. The HARV bioreactor does not allow the cells to receive a gravitational load in any fixed direction. Its constant rotation does not eliminate gravity, but it does allow the g-vector to be time-averaged to near zero (13). Ground-based SMG experiments using bioreactors such as HARV have become increasingly recognized as an effective approach in simulating certain aspects of microgravity, as it readily permits the more detailed experimentation towards the understanding of microgravity effects on genes and cellular activities during microgravity exposure.
Understanding gene and cellular activity changes in microgravity is essential for tackling the problems caused by microgravity exposure and for developing potential countermeasures. Studies at the cellular and molecular levels have been reported from both spaceflight and ground-based microgravity simulators such as HARV 10., 14., 15., 16., 17.. Microgravity has been found to influence major cellular events such as cell proliferation, cell cycle, cell differentiation, and programmed cell death 18., 19., 20.. Many cell types, ranging from bacteria to mammalian cells, are sensitive to the microgravity environment, suggesting that microgravity affects fundamental cellular activities. The study of microgravity effects on cellular activities can benefit enormously from genome-wide gene expression technologies.
Gene expression profiling based on DNA microarrays is a genome-wide gene expression analysis method for assessing cellular and molecular activity changes in response to a change in the growth environment. Environmental change as drastic as sudden gravity change is likely to alter the functions and transcriptional activities of groups of genes. A genome-wide display and comparison of gene expression profiles in cells that are exposed to microgravity and different recovery stages could provide great insight into the array of genes that are directly or indirectly involved in gravity response. This is because any change in the physiological activity of a cell is most likely the result of changes in the expression of certain genes. Thus, the genome-wide expression analysis technology can be a powerful tool to identify the “space genes” that play key roles in cellular response to a microgravity environment. In recent years, microarray technology has become increasingly popular in space biosciences research and most of the microgravity-induced cellular and molecular effects have been reported on cells of the immune system 21., 22., muscle 23., 24., 25., 26., and bone 12., 27., 28., 29., 30.. However, it is not clear if the affected cellular functions would recover once the cells are returned to 1 g conventional growth conditions.
To further understand the cellular and molecular mechanisms by which spaceflight alters cellular activities, the effects of microgravity on various human cell lines should be studied to identify the genes whose functions are most consistently affected by microgravity. Epidermal keratinocytes, the major cell type in the outermost layer of the skin, play an essential role in the first-line defense against invading microorganisms and in innate immunity 31., 32.. To date, the molecular effect of microgravity on keratinocytes is not known. Thus, the aim of this study was to display time course gene expression profiles and identify key gravity sensitive genes for human kerotinocytes in response to microgravity. We studied the microgravity effect on a basal-like type of immortalized human epidermal keratinocytes, HEK001, using the HARV bioreactor SMG system. We applied DNA microarray analysis for genome-wide expression profiling of the cells exposed to HARV bioreactor SMG conditions for 3, 4, 9, and 10 d, followed by recovery of 15, 50, and 60 d at 1 g. Our results indicated that cellular gene activity achieved >80% recovery from the shorter exposure time (≤4 d) in a recovery period of 15 d, while a longer recovery period (≥50 d) was needed for genes to recover to the shorter exposed impact levels after an exposure time as long as 9 and 10 d. In addition, we have identified 32 putative “major space genes” that were most consistently affected by SMG through the interlinked time course analysis. Interestingly, a cluster of eleven genes that are inducible through the integrated stress response pathways were all down-regulated in the presence of SMG. In contrast, a cluster of seven metallothionein genes were up-regulated through all the time points. Moreover, HLA-G, a key gene that mediates cellular immune suppression effect, was up-regulated during the recovery phase of SMG exposure. Our findings contribute significantly to the knowledge of epidermal response to SMG, which may suggest a mechanism in the whole body response to microgravity, particularly in microgravity-associated immune response suppression, integrated stress response, and tumor progression.
Results
Morphology of HEK001 cells cultured in HARV bioreactors and in conventional 2D cell culture flasks
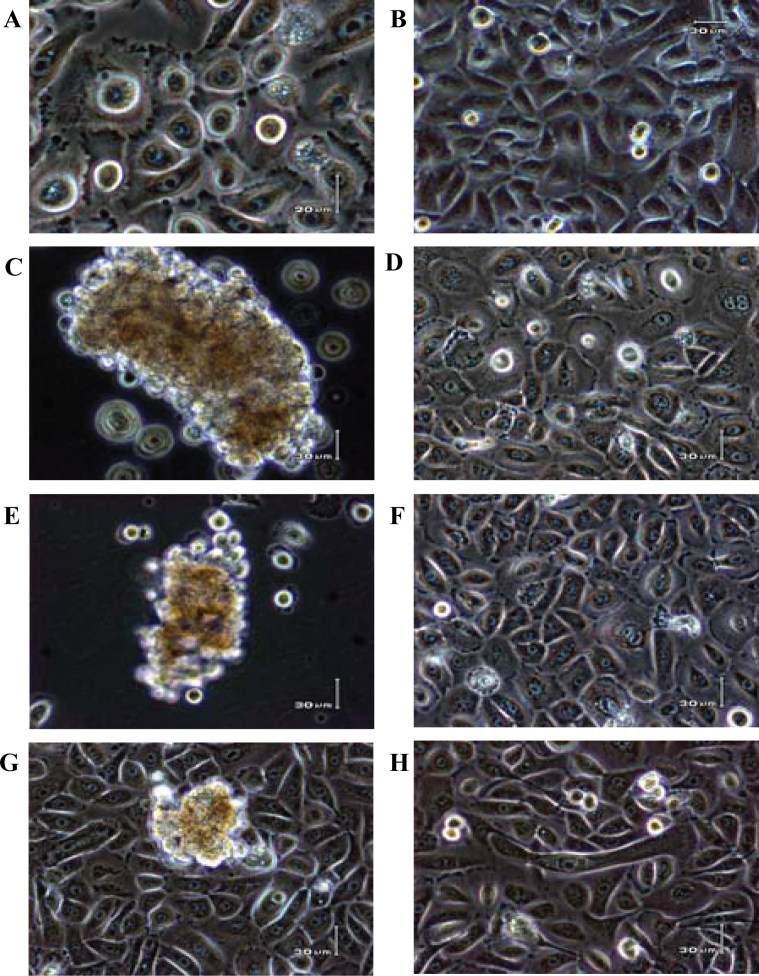
Monolayer keratinocytes, HEK001 cells, formed small aggregates and spherical cellular morphology (Figure 1C and E) typical for cells cultured in the 3D environment of HARV bioreactors. When the HEK001 cells were removed from the HARV bioreactors after the shorter (3 and 4 d) SMG exposures and then cultured in conventional 2D cell culture flasks for 15 d, the keratinocytes spread out to form monolayer growth indistinguishable from the untreated controls (compare Figure 1A and B with D and F). Cells from the longer (9 and 10 d) SMG exposures were not recovered as complete as the shorter ones, and elongated cells were found in the recovery culture after 16 d (Figure 1G and H). Figure 1G shows cells after 7-day recovery from a 9-day SMG treatment; note that cell aggregates were visible at the early recovery stage. Although these cells regained their morphological appearance after extended culturing under normal gravity, the extent to which cellular gene activity was affected by the SMG treatments and whether the altered gene functions would ever recover were of crucial interest. Therefore, we next investigated through DNA microarray analysis for genome-wide gene expression evaluation and through Northern blotting for gene-specific validation.
Fig. 1.
Morphology of HEK001 cells cultured in HARV bioreactors for SMG treatment and in conventional 2D cell culture flasks for normal gravity recovery. A and B. HEK001 cells cultured in normal gravity tissue culture flasks showing different photo fields and magnifications of the control cells. C. Cell aggregates from 3-day SMG culture. D. Cells recovering from 3-day SMG treatment in Panel C by 15-day growth in normal (1 g) gravity (culture flasks). E. Cell aggregates from 4-day SMG treatment. F. Cells recovering from 4-day SMG treatment through 15-day culture in normal gravity. G. Cells after 7-day recovery in normal gravity from 9-day SMG treatment. H. Cells after 16-day recovery in normal gravity from 10-day SMG treatment.
General gene expression profiles and distributions of the time course microarray data
To visualize the general gene expression profiles of the microarray data, we first plotted the extracted microarray data in MA plots (Figure 2). In the MA plots, each point represents one feature from a corresponding microarray. The plots of five different microarray experiments before and after normalization show the general gene expression profiles of the cells exposed to different doses of SMG and during different recovery periods (Arrays 1−5; Figure 2A−E). After normalization and statistical analysis (student t-test) of the microarray data, a total of 314 differentially regulated genes from the five time course microarrays were identified as statistically significant (p ≤0.05). We further filtered the 314 genes to a cut-off point of 1.5-fold up- or down-regulation. Thus, a total of 162 genes were identified from the five SMG experiments to be significantly (≥1.5-fold change, p ≤0.05) differentially expressed, constituting the initial pool of the putative gravity sensitive genes in the SMG-treated keratinocytes (see Table 1 in Appendix). The numbers of differentially regulated genes allocated for each of the five (Arrays 1−5) microarrays were 101, 94, 18, 105, and 86, respectively. These putative gravity sensitive genes of the keratinocytes were subjected to further bioinformatics analysis.
Fig. 2.
MA scatter plots showing the average trend of the log ratio as a function of intensity of features in Arrays 1−5 using Lowess normalization. Each point represents one feature from the corresponding microarray. F633 represents the intensity of the red (R) laser wavelength (633 nm) that excites the cyanine 5 dye. F545 represents the intensity of the green (G) laser wavelength (545 nm) that excites the cyanine 3 dye. The MA plot shows the log intensity ratio M=log2(R/G) versus the average intensity A= ½(log2R+log2G). A. MA plot for 3-day SMG (Array 1). B. MA plot for 4-day SMG (Array 2). C. MA plot for 4-day SMG followed by 15-day recovery at 1 g (Array 3). D and E. MA plots for 9-day and 10-day SMG followed by 50-day and 60-day recovery at 1 g, respectively (Arrays 4 and 5).
Functional grouping and time course expression profiles of the 162 gravity sensitive genes
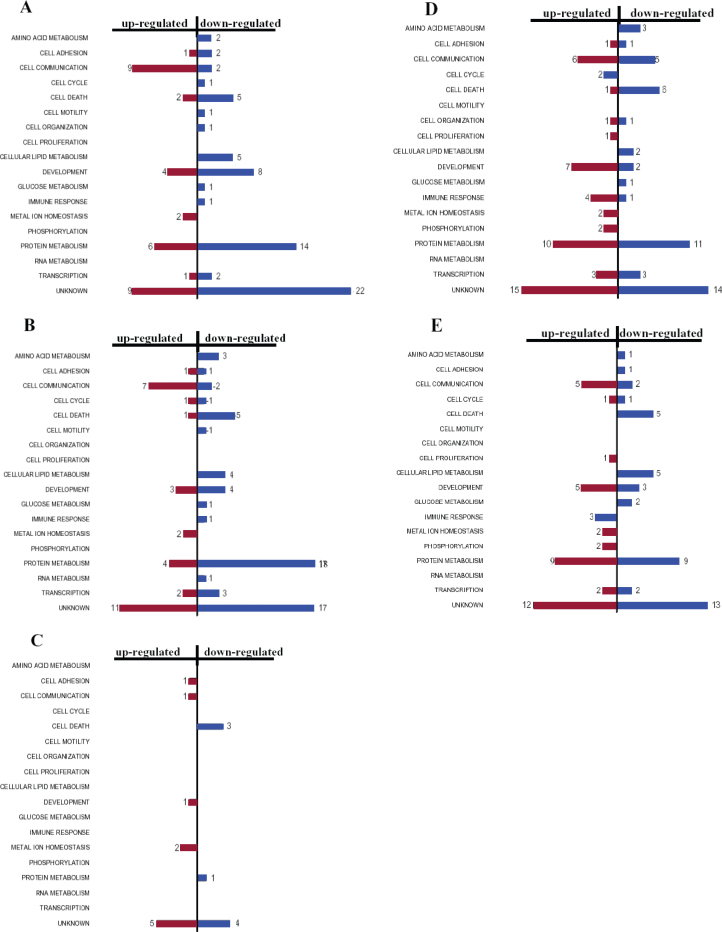
The significantly differentially expressed genes in each of the five experiments were further categorized into 18 functional groups using histograms as shown in Figure 3. For each experiment, the number of genes induced (red) and suppressed (blue) was graphed for each of the 18 functional categories, generating an individual histogram profile for the corresponding microarray. Figure 3A shows the functional distribution of 101 SMG-affected genes exposed to 3-day microgravity (Array 1) with 34 induced and 67 suppressed. Those exposed to 4-day SMG (Array 2) exhibited a similar expression pattern, with 32 genes up-regulated and 62 down-regulated (Figure 3B). In contrast, the cells that were cultured for 4-day SMG and then re-cultured and grown at 1 g for 15 d recovered from the microgravity effect more efficiently, with only 18 genes exhibiting significant differential regulation (10 up and 8 down) (Figure 3C). The cells that were grown in SMG for 9 and 10 d followed by recovery culturing at 1 g for 50 and 60 d, respectively, exhibited a similar expression pattern to each other and to the first two time points (compare Figure 3D and E with A and B). The effect of the longer exposures followed by the near two-month recovery time seemed to be equivalent to the shorter exposures without recovery. Most notably across the four similar profiles (Figure 3A, B, D, E), cellular functions affected by SMG mainly were metabolism, cell communication, cell development, and cell death. The data suggest a critical role of the recovery growth in mitigating the alteration of cellular gene functions from prolonged SMG exposure. The recovery effect was even more apparent in Array 3 (Figure 3C), which showed that only 18 genes were differentially expressed compared with the non-treated control, while >80% of the genes had returned to homeostasis after 15-day recovery in normal gravity.
Fig. 3.
Functional grouping and time course expression profiles of gravity sensitive genes. The gene expression profiles of the 162 putative gravity sensitive genes that were statistically significant (p≤0.05) and showed at least 1.5-fold up- or down-regulation from the five experiments were categorized into 18 functional groups and displayed as histograms. The red histograms represent the up-regulated genes and the blue histograms represent down-regulated genes. A. Category of 101 differentially regulated genes exposed to 3-day SMG. B. Category of 93 differentially regulated genes exposed to 4-day SMG. Note that the patterns of gene regulation exhibited in Panels A and B are very similar. C. Category of 18 differentially genes exposed to 4-day SMG followed by 15-day recovery at 1 g. D. Category of 105 differentially regulated genes exposed to 9-day SMG followed by 50-day recovery. E. Category of 95 differentially regulated genes exposed to 10-day SMG followed by 60-day recovery.
Cluster analysis of genes differentially expressed under microgravity and recovery
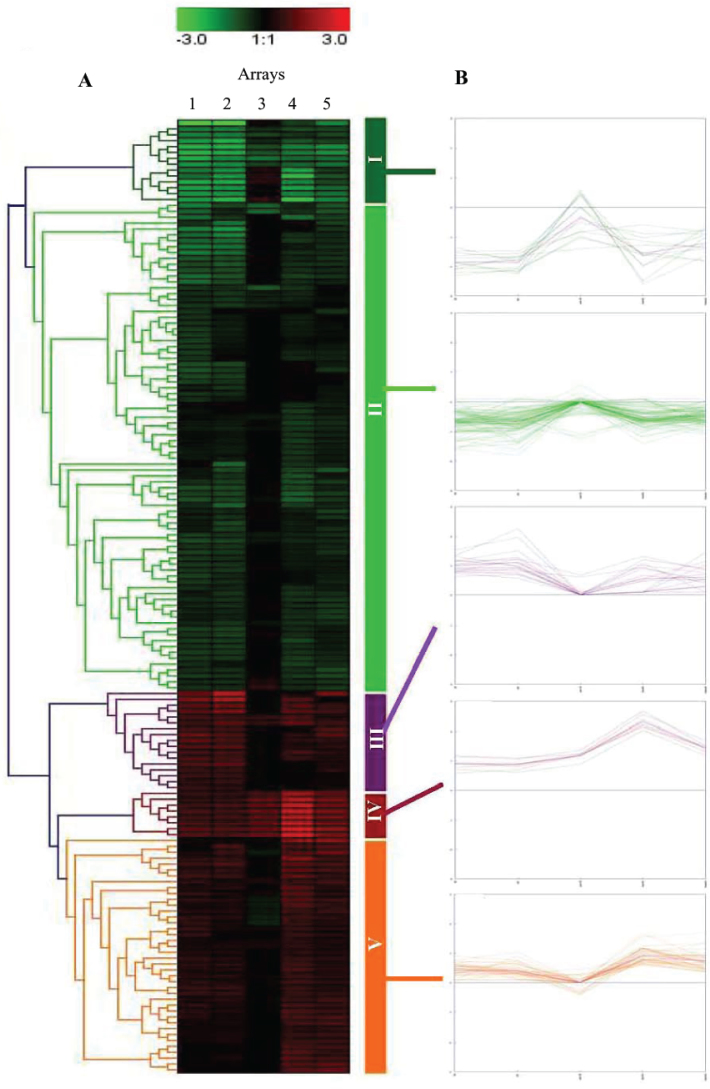
The 162 differentially regulated genes were clustered hierarchically into five clusters (Figure 4; Table 1) using average-linkage clustering (33). Cluster I was coordinately down-regulated, consisting of 14 genes, 11 of which were from the integrated stress response pathway. The genes in Cluster I from the cells cultured for 3 d (Array 1) and 4 d (Array 2) in SMG were on average down-regulated ~3.8 fold. When allowed to recover at 1 g for 15 d (Array 3), >80% of these genes recovered to non-exposed level. The genes that were exposed to SMG for 9 and 10 d and were allowed to recover at 1 g for 50 d (Array 4) and 60 d (Array 5), respectively, showed a level of down-regulation similar to those exposed to SMG for 3 and 4 d. Cluster II has 83 genes. The expression pattern was very similar between 3-day and 4-day SMG, with the average down-regulation of ~1.7 fold, and similar between 9-day and 10-day SMG followed by 50-day and 60-day recovery at 1 g, respectively, with the average down-regulation of ~1.5 fold. This cluster did not show significant differential expression after exposure to 4-day SMG followed by 15-day recovery at 1 g. There are 17 genes represented in Cluster III. The average expression level for 3-day and 4-day SMG exposure was ~2 fold. Again, this cluster did not show significant differential expression after exposure to 4-day SMG followed by 15-day recovery. Cluster IV is comprised of eight metallothione genes that were all up-regulated across the five time points. The level of up-regulation was ~2 fold across the first three time points. After 9-day SMG followed by 50-day recovery, the Cluster IV gene expression increased to ~4-fold up-regulation. After 10-day SMG followed by 60-day recovery, however, the Cluster IV gene expression returned to the level similar to the levels of the shorter exposure time points (~2-fold up-regulation). It may be that an additional 10 more days’ recovery time was needed to allow the longest exposed cells to recover to the impact level of shorter exposed cells. In Cluster V (40 genes), there was an average up-regulation of ~1.5 fold after 3-day and 4-day SMG and a drop to little (no differential regulation or complete recovery) after 15-day recovery. However, for 9-day and 10-day SMG followed with recovery at 1 g for 50 and 60 d, it showed a similar expression pattern (~1.6-fold up-regulation) to those cultured under SMG for 3 and 4 d. Thus, the close to two-month recovery time again reduced the SMG effect to the level of shorter exposures for the 40 genes in this cluster.
Fig. 4.
Cluster analysis of 162 differentially regulated genes from five microarray experiments showing the trend in time course. A. The average-linkage hierarchal clustering of the expression data (log2) of all the 162 significant genes. The rows represent individual genes categorized into five hierarchical clusters according to differential expression levels. The columns represent the five microarray experiments showing the gene expression pattern for each time point according to the five clusters. B. The time course trend of expression of all of the genes represented in each cluster.
Overlapping patterns of differentially expressed genes among the five SMG experiments and the identification of “center genes”
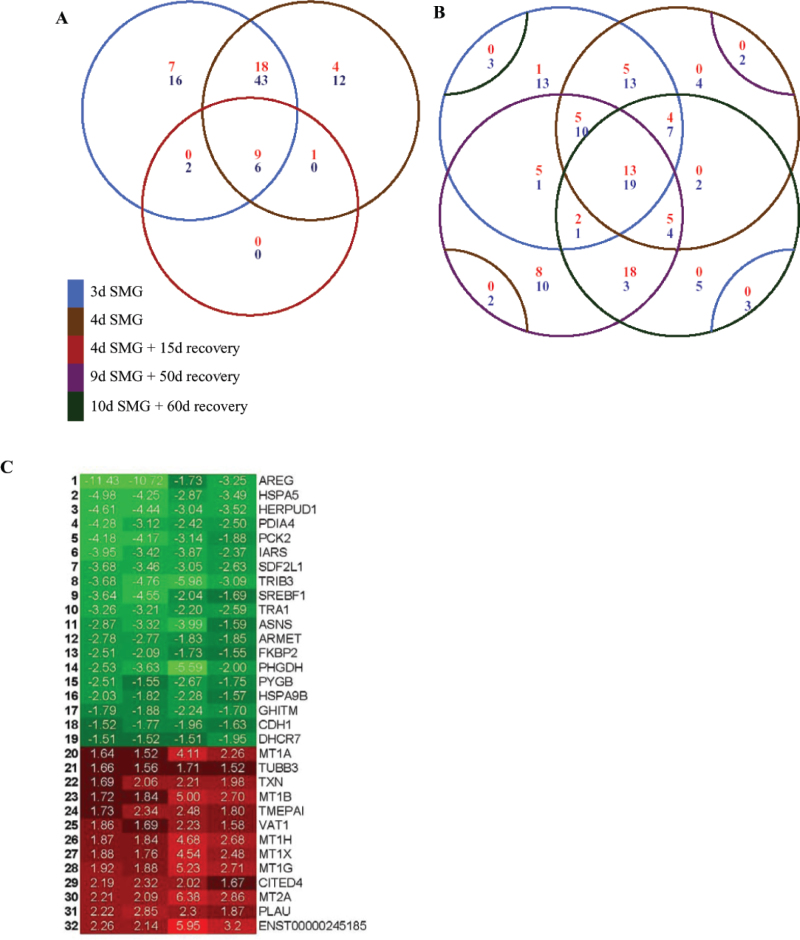
When comparing Arrays 1 and 2 for the 3-day and 4-day SMG (Figure 5A) using the Venn diagram, we found that a total of 76 differentially regulated genes (27 up and 49 down) were common to both microarrays, showing a consistency of ~75% between the two different time points. A total of merely 18 differentially expressed genes in Array 3 indicated that >80% of the genes had recovered from the 4-day microgravity effect after 15 d in normal gravity culture. Out of these 18 significant genes, there were 17 and 16 genes shared with the 3-day and 4-day microgravity treatment alone, respectively; this constituted about 90% of all the significant genes identified from Array 3. The data suggest a causal link for the remnant SMG affected genes, which resulted from having not yet completely recovered from the SMG treatment. Figure 5B allowed us to further compare the expression patterns between the shorter exposures alone and the longer exposures followed with up to a two-month recovery period (Arrays 4 and 5). We found a significant overlap of the differentially regulated genes among the four different time points. Similar to the two shorter exposures (3-day and 4-day SMG) shown in Figure 5A, the two longer exposures (plus longer recovery) shared ~69% common differentially expressed genes. An important function of the Venn diagram is that it allowed us to readily identify the genes affected by all the SMG treatments through the center of the diagrams: all four time point microarrays shared 32 genes (13 up and 19 down) that are differentially regulated by at least 1.5 fold (Figure 5B and C). These “center genes” were the best candidates for the “major space genes” because they were most consistently affected by SMG.
Fig. 5.
Venn diagrams of overlapping gene expression patterns of the 162 significant genes across the five time points. The red numbers represent up-regulated genes and the blue numbers represent down-regulated genes. A. Comparison of gene expression data of 3-day and 4-day SMG exposure with 4-day SMG exposure followed by 15-day recovery at 1 g. B. Comparison of gene expression data of 3-day and 4-day SMG exposure with 9-day SMG followed by 50-day recovery and 10-day SMG followed by 60-day recovery. The center of the Venn diagrams shows that there are 32 differentially regulated genes between all four array experiments. C. Heat map of the expression pattern for the 32 differentially regulated genes identified from the center of the Venn diagrams in Panel B.
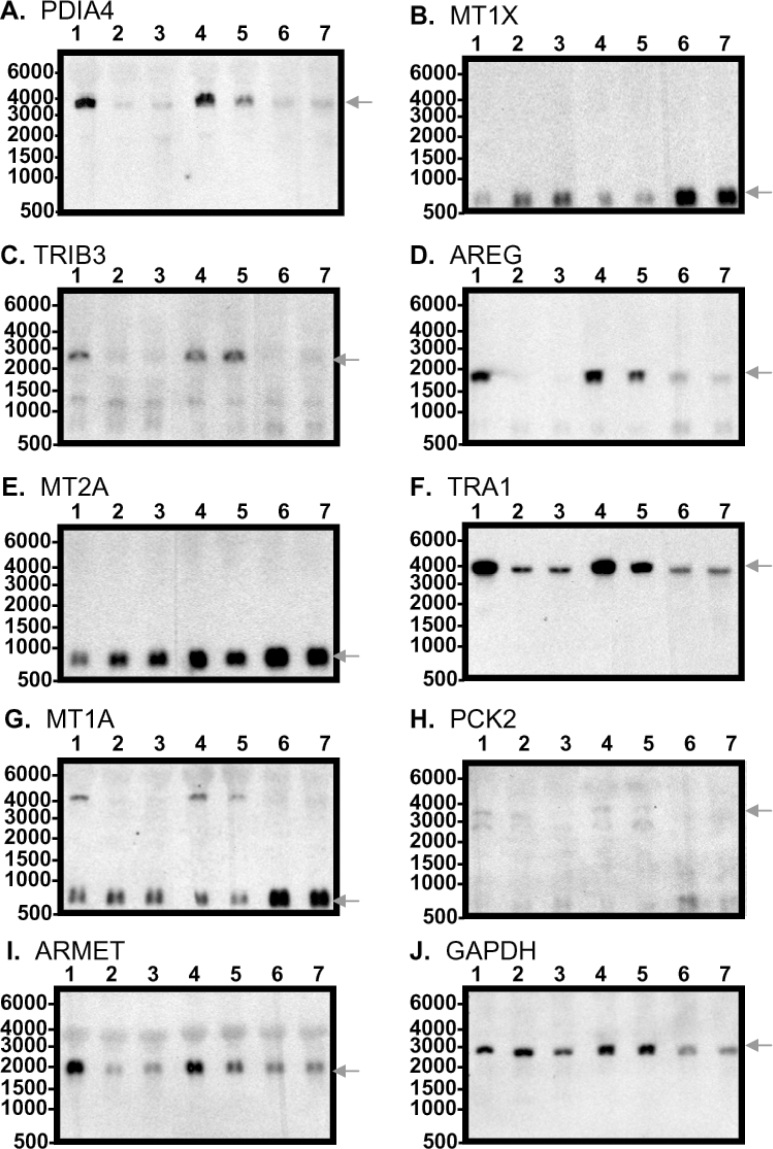
Validation of microarray results through Northern blotting analysis of ten cellular genes
We also performed Northern hybridization to verify the results from the microarray analysis of the SMG time course experiments. The uniqueness of Northern blotting analysis is that it measures the abundance as well as the size of the RNA of interest (34). Figure 6 shows the size identity and expression levels for ten genes, nine of which were significant genes identified through microarray experiments, and GAPDH was used as an internal control. The Northern hybridization reactions were performed sequentially with the ten cDNA probes as shown in the figure. All the hybridizations shown in the figure resulted in one predominant band of the expected sizes (marked with arrows) for the particular mRNAs. However, some minor bands were visible in a few panels. For the TRIB3 probe, the level of the larger band (~2,500 to 2,800 bp) was down-regulated in SMG; the expression level for another isoform (alternatively spliced) of TRIB3 mRNA (Figure 6C) did not seem to be affected by SMG. There was a higher band found in lanes 1, 4, and 5 of Figure 6G (MT1A probe), which most likely resulted from the incomplete stripping of the TRA1 probe hybridized immediately prior to the hybridization of MT1A.
Fig. 6.
Validation of microarray results using Northern blotting analysis of genes differently expressed under modeledmicrogravity conditions. An amount of 10 μg total RNA from each sample was loaded onto a 1% formaldehyde agarosegel. Lanes 1−7 were loaded with samples as the following: Lane 1, conventional stationary control; Lane 2, three-day modeled microgravity; Lane 3, four-day modeled microgravity; Lane 4, three-day microgravity recovery; Lane 5, four-day microgravity recovery; Lane 6, nine-day microgravity recovery; Lane 7, ten-day microgravity recovery. The Northern blot was sequentially hybridized with ten different probes as indicated in Panels A−J: A. PDIA4; B. MT1X; C. TRIB3; D. AREG; E. MT2A: F. TRA1; G. MT1A: H. PCK2; I. ARMET; J. GAPDH. The expected mRNA bandfor each probe was indicated with an arrow to the right side of each panel.
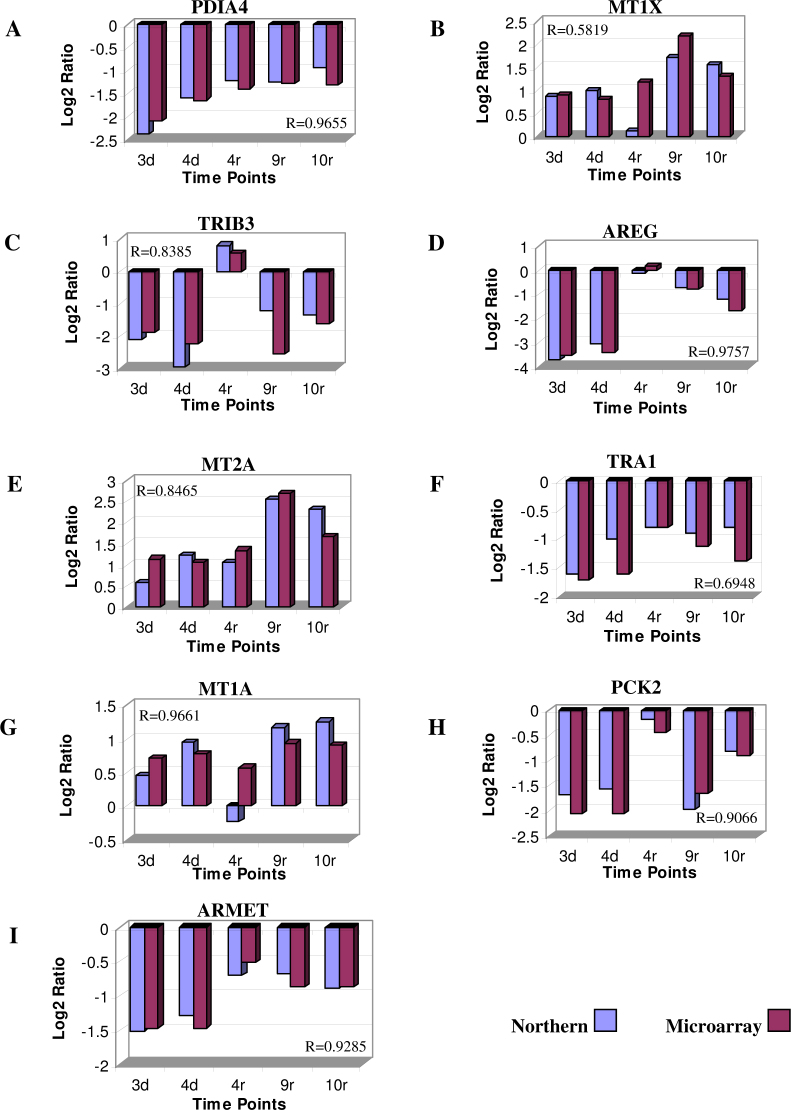
The bands of the expected sizes for the probed mRNA (marked with arrows) were subjected to quantitative analysis. To quantitatively analyze the mRNA levels from the Northern blots, we first normalized all the mRNA bands in Figure 6A−J against the GAPDH level in each corresponding lane. The normalized levels of the mRNA in each panel were then plotted in histograms as shown in Figure 7. The overall Northern blotting results demonstrated a remarkable resemblance to those obtained from microarray analysis, with an overall high level of correlation between the results from the two methods (R=0.86; Figure 7). The mRNA levels for genes PDIA4, TRIB3, AREG, TRA1, PCK2, and ARMET were shown to be decreased significantly by both microarray analysis and Northern blotting, with correlation coefficient values of 0.97, 0.84, 0.98, 0.69, 0.91, and 0.93, respectively. The MT1X, MT2A, and MT1A mRNA levels were found to be significantly increased by both microarray and Northern blotting analysis, with correlation coefficient values of 0.58, 0.85, and 0.97, respectively. The overall high level of correlation (R=0.86) between the analytical results from microarray and Northern blotting further validated our genome-wide analysis using the microarray technology.
Fig. 7.
Quantitative comparison of gene expression data obtained from Northern blotting analysis and microarray analysis. The mRNA bands in Figure 6A−J were first normalized against the GAPDH level in each corresponding lane. The normalized levels of the mRNA in each panel were then plotted in histograms as shown in Figure 7A−I. In the histograms, the Northern blotting data (blue) were plotted side by side with the microarray data (red) to directly compare the expression levels of the same nine significant genes. The values of the correlation coefficient (R values) for each comparison were indicated in each panel.
Discussion
Microgravity effects have been closely associated with bone loss, muscle atrophy, and decreased immune function of astronauts. Consequently, extensive studies on cells of the bone, muscle, and blood origin have been documented in literature 18., 19., 20.. Since astronauts experience whole body exposure to microgravity, all the organ systems in the body are potentially affected by the gravity change and may function differently to counterbalance the microgravity stress. The different organ systems may react differently to gravity change. It is also possible that a common set of genes in different cell lineages are preferentially altered in microgravity conditions. To fully understand the microgravity effect on human health, it is important to study the microgravity response of cells of various organ origins. The identification of the common set of gravity sensitive genes may lead to the identification of “major space genes” that together play a major check-and-balance role ultimately determining the outcome of a cell, or an organism such as an individual person, in the response to microgravity conditions.
The gene expression profiles for the five time points were achieved mainly from the 18 functional groups of genes in histograms and hierarchical cluster analysis of all the 162 significantly differentially regulated genes. The nearly identical expression profiles between the 3-day and 4-day SMG treatment indicated a high level of consistency in the number and type of gravity sensitive genes between the closely spaced time points (Figure 3A and B). Similar expression profiles were also found between longer time points, that is, cells recovering from 9-day and 10-day SMG treatment in normal gravity for 50 and 60 d, respectively (Figure 3D and E). The validity of the microarray data was further confirmed with Northern blotting analysis of ten genes (Fig. 6, Fig. 7). For SMG exposure time as short as 4 d, a 15-day recovery period resulted in >80% of the SMG affected gene expression returning to homestasis (compare Figure 3C with B). A two-month recovery period mitigated the affected gene expression profile to that equivalent to the 4-day SMG exposure level. Thus, our expression profiling results suggest that the time duration of microgravity exposure as well as post-SMG recovery are critical determinants of the extent to which cellular gene expression is affected by SMG. The shorter the SMG exposure and the longer the recovery time, the better the recovery from the SMG stress. Based on our results, it is advisable that shorter mission duration time and longer recovery interval would be beneficial to astronauts’ health.
We further identified 32 “key” or “center” gravity sensitive genes from the 162 significantly differentially regulated genes detected from the time course experiments. This was achieved by the use of Venn diagrams of the shorter SMG exposures (3-day and 4-day SMG) and the longer exposures (9-day and 10-day SMG followed by 50-day and 60-day recovery) locating the genes that were in the very center of the four interlinked time points (Figure 5B and C). These genes were considered to be most consistently impacted by the microgravity treatment because they were affected by all the interlinked time points of the SMG exposures. These key gravity sensitive genes may be candidates for the “major space genes” whose activities were most significantly affected by the SMG environmental factor and most likely play a determinant role coordinately on the outcome of the SMG exposure effects. Many of these genes were found to be coordinately regulated in specific clusters belonging to particular pathways and are known to be coordinately regulated in non-space environment. One of the most notable groups of coordinately regulated genes was the eleven “center genes” (AREG, HSPA5, HERPUD1, PDIA4, PCK2, SDF2L1, TRIB3, SREBF1, TRA1, ASNS, and ARMET) that all came from Cluster I and were all down-regulated in SMG (Figure 5C; Table 1). These genes are known to be coordinately regulated in integrated stress response pathways such as the endoplasmic reticulum (ER) stress response pathway and the amino acid starvation response pathway 35., 36.. They are typically induced coordinately in the presence of ER stress or in response to amino acid starvation 35., 36.. It is not clear why these genes were all down-regulated under reduced gravity conditions. It maybe that microgravity is favorable for protein folding and transport, making the machinery for the ER protein unfold stress response pathway unnecessary. To our knowledge, this is the first report to connect this set of integrated stress response genes to microgravity response. Nonetheless, in a study comparing the gene expression from cells grown in microgravity on the space shuttle with those grown in SMG, TRA1 was found to be down-regulated for 5.5 fold and 1.2 fold, respectively (37). In agreement with the reported space shuttle and SMG results, TRA1 in our experiment was also down-regulated across all the time points (from ~3.2 fold to ~1.75 fold) in SMG. TRA1 has also been found to be up-regulated in a variety of tumor cells in response to glucose depletion, hypoxia, and decreased pH. Therefore, TRA1 seems to be implicated in cell immunity against cancer cells (38).
Another group of coordinately regulated genes that were also found in the center of the four interlinked time points includes seven metallothionein genes (MT1A, MT1B, MT1G, MT1H, MT1X, MT2A, and a MT2A clone ENST00000245185) in Cluster IV. These genes were all major isoforms of metallothioneins and were up-regulated in all the SMG time points, even more significant during recovery periods (Fig. 4, Fig. 5; Table 1). Consistent with our result, MT1A and MT2A expressions have been found to be up-regulated in space-flown (16 d) rat skeletal muscles (20). MT-I and MT-II isoforms have very similar structures and functions and are coordinately regulated in most cells 39., 40.. They have cellular functions primarily in metal ion homeostasis, scavenging of ROS, redox status, immune defense responses, cell proliferation, and cell death 40., 41.. MT-I and MT-II are induced by most inflammatory or pathological stimulus such as proinflammatory cytokines and oxidative stress (42). In addition, our microarray data showed several genes involved in immune response such as HLA-G and IL-1β to be affected by SMG (Table 1, Cluster V). Although IL-1β induction has been reported in a macrophage cell line in space (43), it is not known whether HLA-G gene expression would be altered by microgravity. In normal gravity environment, HLA-G has been shown to have direct inhibitory effect on T, APC, and NK cell functions in the immune system and induces suppressor T-cells. HLA-G also plays a role in tumordriven immune escape mechanism of cancer cells during the later phase in host and tumor cell interactions (44). Our finding that HLA-G mRNA levels were up-regulated in microgravity-exposed keratinocytes may suggest an underlining mechanism for microgravity-associated immune response suppression.
Conclusion
In summary, our results indicated that cellular gene activity achieved >80% recovery from the shorter exposure time (≤4 d) in a recovery period of 15 d, while a longer recovery period (≥50 d) was needed for genes to recover to the shorter exposed impact levels after an exposure time as long as 9 and 10 d. Interestingly, a cluster of eleven genes in the integrated stress response pathway that are inducible by ER stress and amino acid starvation were all down-regulated in the presence of SMG. In contrast, a number of genes involved in proliferation, development, and cancer were up-regulated. A cluster of seven metallothionein genes were all up-regulated through all the time points. In addition, HLA-G, a key gene that mediates cellular immune suppression effect, was up-regulated during recovery phases of SMG exposure. Our findings contribute significantly to the knowledge of epidermal response to SMG, which may suggest a mechanism in the whole body response to microgravity, particularly in microgravity-associated immune response suppression, integrated stress response, and tumor progression. Overall, the data suggests, for health’s sake, shorter mission duration in space may lead to quicker and more complete recovery from the microgravity effects.
Materials and Methods
Cell culture
HEK001, an immortalized human skin keratinocyte cell line of basal-like type (45), was purchased from American Type Culture Collection (Manassas, VA, USA) and cultured at 37°C with 95% humidity and 5% CO2 in Ketatinocyte-Serum Free medium with 5 ng/mL human recombinant EGF, 50 ug/mL bovine pituitary extract, and 2 mM L-glutamine.
Simulated microgravity and cell exposure
Ground-based modeled microgravity was achieved using the 50 mL HARV units of the rotating wall vessel (RWV) culture apparatus (a NASA JSC invention) from Synthecon, Inc (Houston, TX, USA). Cell viability and cell concentration were determined by Vi-Cell 1.01 cell counter of Beckman Coulter (Fullerton, CA, USA). For the 3-day and 4-day modeled microgravity exposures, a density of 2.0×105 cell/mL with viability of 93.2% of the HEK001 cells were cultured in RWV bioreactors at 17.2 rpm to achieve the constant free-fall experience for cell aggregates. At the end of the 3-day and 4-day microgravity exposure, the contents of the bioreactor vessels were poured out into a 50 mL sterile centrifuge tube to collect cell pellets, and 5 mL of the cell suspension from the bioreactor vessels was transferred to T75 flasks for morphological observation and for further stationary culture. Hence, the recovery stage for the exposed cells as well as post 3-day and post 4-day microgravity recovery cell samples were achieved. After the removal of the cells from the vessels, fresh medium was used to refill the vessels for extended microgravity exposure to 9 and 10 d, respectively. The resulted cells were all transferred to stationary culture for recovery of 50 and 60 d, with medium change twice per week. Non-exposed stationary controls and the recovery stage of microgravity-exposed HEK001 cells were cultured in tissue culture flasks with vented caps (TPP Techno Plastic Products, Trasadingen, Switzerland) in the same incubator at 37°C with 5% CO2.
Total RNA isolation and DNA microarray hybridization
HEK001 cells cultured in modeled microgravity HARV bioreactors and control flasks were removed at their respective time points, washed with PBS for three times and lysed in guanidinium isothiocyanate buffer. The cell lysates were stored at —80°C prior to ultracentrifugation for total RNA isolation 46., 47.. Total cellular RNA was labeled using the Agilent Low RNA Input fluorescent Linear Amplification Kit (Agilent Technologies, Santa Clara, CA, USA) following the manufacturer’s protocols (48). The fluorescently labeled cRNA probes were further purified and hybridized to Agilent 22K Human Microarray V2 according to the specified procedures within the kit.
Microarray scanning, feature extraction, and functional grouping
The microarrays were scanned using a ScanArray microarray scanner (Perkin-Elmer, Waltham, MA, USA). The images generated from the scanning were imported into GenePix 6.0 (Molecular Devices, Sunnyvale, CA, USA) for alignment and initial quantitation. Lowess normalization and some statistical analyses were performed in Acuity 4.0 (Molecular Devices). The normalized genes were then subjected to a student t-test to determine statistical significance. An alteration in gene expression level was considered significant if it passed a statistically significant level of p ≤0.05 and met the minimum cut-off of 1.5-fold differential regulation. The significant genes thus identified were further processed using EASE (49) to identify 18 functional categories and relevant pathways. The functional categories were then used to organize the gene table (Table 1) and to generate the comparative histograms (Figure 3).
Hierarchical clustering and Venn diagrams
We also used the average-linkage hierarchical clustering to better organize and visualize the expression patterns in the data 33., 50.. The hierarchical clustering was performed using Genesis 1.0 (51). To more easily identify the genes affected by all the SMG treatments, we generated Venn diagrams using AFM 4.0 (52).
Northern blotting, normalization, and quantitative analysis
Some of the significantly regulated microgravity sensitive genes identified from the DNA microarray analysis were further verified using Northern blotting. Briefly, 10 μg total RNA was loaded per lane on a 1% formaldehyde agarose gel for electrophoresis separation of RNA species. The gel-separated RNAs were capillarilly transferred onto a nylon membrane, which was subjected to a hybridization procedure using chemilluminascently (Pierce Biotechnology, Rockford, IL, USA) labeled cDNA probe fragments. The blot was sequentially hybridized with ten different probe fragments. The cDNA probe fragments were generated from RT-PCR reactions using total cellular RNA as the templates. RT-PCR reactions were carried out to generate the cDNA probes required for the Northern blotting analysis, using the Reverse Transcription System of Promega (Madison, WI, USA) and the Bio-Line Red DNA Polymerase PCR kit (Taunton, MA, USA). For Northern blot quantitation, the autoradiographs of the Northern blots were scanned and saved as TIF images for further analysis. The images were then opened in Unscan-It Gel (Silk Scientific, Orem, UT, USA) for quantitation. To quantitatively analyze the mRNA levels from the Northern blots, we first normalized the mRNA bands in Figure 6A−J against the GAPDH level in each corresponding lane. The normalized levels of the mRNA in each panel were then plotted in histograms as shown in Figure 7.
Authors’ contributions
JQC conceived and supervised the project, collected the data, conducted the data analysis, and prepared the manuscript. SML assisted with data collection and analysis. BLW supervised all the URC projects. All authors read and approved the final manuscript.
Competing interests
The authors have declared that no competing interests exist.
Acknowledgements
We thank the NASA University Research Center for Biotechnology and Environmental Health at Texas Southern University for discussion and support. We are especially grateful for the encouragement and support provided by the Technical Review Group of NASA Johnson Space Center. This work was funded by NASA JSC Grant No. NCC9-165 and NIH Grant No. RR03045-12A1.
Appendix
Table 1.
Identification and clustering of 162 putative gravity sensitive genes*
| Accession No. | Gene symbol | 1# | 2# | 3# | 4# | 5# | Gene description | Biological process |
|---|---|---|---|---|---|---|---|---|
| CLUSTER I | ||||||||
| NM_001657 | AREG | −11.43 | −10.72 | 1.13 | −1.73 | −3.25 | amphiregulin (schwannoma-derived growth factor) | Cell communication |
| NM_006010 | ARMET | −2.78 | −2.77 | −1.43 | −1.83 | −1.85 | arginine-rich, mutated in early stage tumors | Unknown |
| NM_001673 | ASNS | −2.87 | −3.32 | 1.31 | −3.99 | −1.59 | asparagine synthetase (ASNS), transcript variant 2 | Amino acid metabolism |
| NM_004343 | CALR | −3.08 | −2.92 | −1.77 | −1.49 | −1.75 | calreticulin | Cell cycle |
| NM_014685 | HERPUD1 | −4.61 | −4.44 | −1.94 | −3.04 | −3.52 | homocysteine-inducible, endoplasmic reticulum stress-inducible, ubiquitin-like domain member 1, transcript variant 1 | Protein metabolism |
| NM_004134 | HSPA5 | −4.98 | −4.25 | −2.03 | −2.87 | −3.49 | heat shock 70 kDa protein 5 (glucose-regulated protein, 78 kDa) | Cell cycle |
| NM_013417 | IARS | −3.95 | −3.42 | −3.87 | −2.37 | isoleucine-tRNA synthetase (IARS), transcript variant long | Protein metabolism | |
| NM_004563 | PCK2 | −4.18 | −4.17 | 1.37 | −3.14 | −1.88 | phosphoenolpyruvate carboxykinase 2 (mitochondrial), nuclear gene encoding mitochondrial protein, transcript variant 1 | Glucose metabolism |
| NM_004911 | PDIA4 | −4.28 | −3.12 | −2.63 | −2.42 | −2.50 | protein disulfide isomerase family A, member 4 | Unknown |
| NM_006623 | PHGDH | −2.53 | −3.63 | 1.39 | −5.59 | −2.00 | phosphoglycerate dehydrogenase | Development |
| NM_022044 | SDF2L1 | −3.68 | −3.46 | −3.05 | −2.63 | stromal cell-derived factor 2-like 1 | Unknown | |
| NM_001005291 | SREBF1 | −3.64 | −4.55 | −1.30 | −2.04 | −1.69 | sterol regulatory element binding transcription factor 1, transcript variant 1 | Cellular lipid metabolism |
| NM_003299 | TRA1 | −3.26 | −3.21 | −1.75 | −2.20 | −2.59 | tumor rejection antigen (gp96) 1 | Cell cycle |
| NM_021158 | TRIB3 | −3.68 | −4.76 | 1.48 | −5.98 | −3.09 | tribbles homolog 3 (Drosophila) | Unknown |
| CLUSTER II | ||||||||
| NM_001605 | AARS | −1.73 | −1.83 | −1.01 | −1.43 | −1.22 | alanyl-tRNA synthetase | Protein metabolism |
| NM_001001937 | ATP5A1 | −1.64 | −1.12 | 1.04 | −1.20 | −1.25 | ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit, isoform 1 | Development |
| NM_001697 | ATP5O | −2.06 | −1.93 | 1.09 | −1.39 | −1.60 | ATP synthase, H+ transporting, mitochondrial F1 complex, O subunit | Development |
| BC000228 | BC000228 | −3.02 | −1.83 | −2.23 | −1.41 | −2.03 | Similar to transducin-like enhancer of split 1, homolog of Drosophila E (sp1) | Unknown |
| NM_032621 | BEX2 | −2.86 | −2.69 | −1.22 | −1.57 | brain expressed X-linked 2 | Unknown | |
| NM_001005266 | C15orf21 | −2.40 | −3.07 | −1.58 | −1.43 | chromosome 15 open reading frame 21, transcript variant 1 | Unknown | |
| NM_001009924 | C20orf30 | −1.71 | −1.11 | −1.41 | −1.35 | chromosome 20 open reading frame 30, transcript variant 2 | Unknown | |
| NM_000071 | CBS | −1.98 | −1.93 | −1.91 | −1.38 | cystathionine-beta-synthase | Amino acid metabolism | |
| NM_014550 | CARD10 | −1.47 | −1.38 | −1.52 | −1.29 | caspase recruitment domain family, member 10 | Cell cycle | |
| NM_004360 | CDH1 | −1.52 | −1.77 | −1.96 | −1.63 | cadherin 1, type 1, E-cadherin (epithelial) | Cell adhesion | |
| NM_025233 | COASY | −1.51 | −1.32 | Coenzyme A synthase | Unknown | |||
| NM_001316 | CSE1L | −1.26 | −1.62 | −1.57 | −1.52 | CSE1 chromosome segregation 1-like (yeast), transcript variant 1 Cell cycle | ||
| D00022 | D00022 | −1.57 | −1.32 | −1.01 | −1.33 | −1.13 | mRNA for F1 beta subunit, complete cds | Unknown |
| NM_032552 | DAB2IP | −1.24 | −1.85 | −1.33 | −1.19 | DAB2 interacting protein, transcript variant 1 | Unknown | |
| NM_004394 | DAP | −1.66 | −1.29 | 1.05 | −1.27 | −1.13 | death-associated protein | Cell cycle |
| NM_033657 | DAP3 | −1.23 | −1.28 | −1.01 | −1.61 | −1.24 | death-associated protein 3 | Cell cycle |
| NM_001919 | DCI | −3.00 | −3.51 | −1.55 | dodecenoyl-Coenzyme A delta isomerase (3,2-trans-enoyl-Coenzyme A isomerase), nuclear gene encoding mitochondrial protein | Cellular lipid metabolism | ||
| NM_001360 | DHCR7 | −1.51 | −1.52 | −1.51 | −1.95 | 7-dehydrocholesterol reductase | Cellular lipid metabolism | |
| NM_006824 | EBNA1BP2 | −1.56 | −1.22 | −1.31 | −1.09 | EBNA1 binding protein 2 | Cell organization and biogenesis | |
| NM_006579 | EBP | −1.54 | −1.58 | −1.25 | −1.43 | emopamil binding protein (sterol isomerase) | Development | |
| NM_001398 | ECH1 | −1.73 | −1.63 | −1.27 | −1.60 | enoyl Coenzyme A hydratase 1, peroxisomal | Cellular lipid metabolism | |
| NM_001404 | EEF1G | −1.65 | −1.51 | −1.29 | −1.30 | −1.44 | eukaryotic translation elongation factor 1 gamma | Protein metabolism |
| NM_013234 | EIF3S12 | −1.66 | −1.28 | −1.27 | −1.12 | eukaryotic translation initiation factor 3, subunit 12 | Unknown | |
| NM_003756 | EIF3S8 | −1.45 | −1.51 | −1.55 | −1.62 | eukaryotic translation initiation factor 3, subunit 3 gamma, 40 kDa | Protein metabolism | |
| BC047866 | ENST 00000265423 | −1.78 | −1.26 | −1.12 | −1.06 | chromosome 6 open reading frame 62, mRNA (cDNA clone MGC:57512 IMAGE:6499979) | Unknown | |
| NM_004446 | EPRS | −1.96 | −1.91 | −1.24 | glutamyl-prolyl-tRNA synthetase | Protein metabolism | ||
| NM_144503 | F11R | −1.10 | −1.14 | −1.56 | −1.36 | F11 receptor | Immune response | |
| NM_004470 | FKBP2 | −2.51 | −2.09 | −1.09 | −1.73 | −1.55 | FK506 binding protein 2, 13 kDa, transcript variant 1 | Protein metabolism |
| NM_003910 | G10 | −1.59 | −1.78 | 1.16 | −1.48 | −1.28 | maternal G10 transcript | Transcrition |
| NM_002047 | GARS | −1.70 | −1.61 | 1.02 | −1.56 | −1.36 | glycyl-tRNA synthetase | Protein metabolism |
| NM_014394 | GHITM | −1.79 | −1.88 | −2.24 | −1.70 | growth hormone inducible transmembrane protein | Unknown | |
| NM_006098 | GNB2L1 | −1.02 | −1.13 | −1.26 | −1.55 | −1.36 | guanine nucleotide binding protein (G protein), beta polypeptide 2-like 1 | Cell communication |
| NM_000183 | HADHB | −1.57 | −1.61 | hydroxyacyl-Coenzyme A dehydrogenase/3-ketoacyl-Coenzyme A thiolase/enoyl-Coenzyme A hydratase (trifunctional protein), beta subunit | Cellular lipid metabolism | |||
| NM_080820 | HARS2 | −2.56 | −1.60 | −1.31 | histidyl-tRNA synthetase 2 | Amino acid metabolism | ||
| NM_178580 | HM13 | −1.49 | −1.14 | −1.82 | −1.51 | histocompatibility (minor) 13 | Unknown | |
| NM_001540 | HSPA9B | −2.03 | −1.82 | −1.12 | −2.28 | −1.57 | heat shock 70 kDa protein 9B (mortalin-2), nuclear gene encoding mitochondrial protein | Cell cycle |
| NM_006764 | IFRD2 | −1.51 | −1.42 | −1.20 | interferon-related developmental regulator 2 | Development | ||
| NM_000210 | ITGA6 | −1.82 | −1.42 | −1.10 | −1.29 | integrin, alpha 6 | Cell adhesion | |
| NM_002229 | JUNB | −1.19 | −1.83 | −1.79 | −2.13 | jun B protooncogene | Transcrition | |
| NM_015005 | KIAA0284 | −1.12 | −1.26 | −1.63 | −1.29 | KIAA0284 | Unknown | |
| NM_000421 | KRT10 | −1.24 | −1.29 | −1.05 | −1.76 | −1.50 | keratin 10 (epidermolytic hyperkeratosis; keratosis palmaris et plantaris) | Development |
| NM_000422 | KRT17 | 1.54 | 1.25 | 1.37 | 1.73 | 1.85 | keratin 17 | Development |
| NM_004138 | KRTHA3A | −1.47 | −1.67 | −1.30 | −1.43 | −1.02 | keratin, hair, acidic, 3A | Unknown |
| NM_005561 | LAMP1 | −1.94 | −1.83 | −1.23 | −1.47 | −1.41 | lysosomal-associated membrane protein 1 | Unknown |
| NM_018407 | LAPTM4B | −1.54 | −1.42 | −1.34 | lysosomal-associated protein transmembrane 4 beta | Unknown | ||
| M_002300 | LDHB | −1.33 | −1.49 | 1.08 | −1.44 | −1.62 | lactate dehydrogenase BN | Glucose metabolism |
| NM_031484 | MARVELD1 | −1.89 | −2.13 | −1.53 | −1.49 | MARVEL domain containing 1 | Unknown | |
| NM_014874 | MFN2 | −1.53 | −1.48 | −1.26 | −1.22 | mitofusin 2, nuclear gene encoding mitochondrial protein | Cell cycle | |
| NM_014046 | MRPS18B | −1.90 | −1.74 | mitochondrial ribosomal protein S18B, nuclear gene encoding mitochondrial protein | Protein metabolism | |||
| NM_002467 | MYC | −1.48 | −2.64 | −1.74 | v-myc myelocytomatosis viral oncogene homolog (avian) | Cell cycle | ||
| NM_004559 | NSEP1 | −1.38 | −1.45 | 1.24 | −1.44 | −1.55 | nuclease sensitive element binding protein 1 | Transcrition |
| NM_000918 | P4HB | −1.65 | −1.63 | −1.81 | −1.27 | procollagen-proline, 2-oxoglutarate 4-dioxygenase, beta polypeptide (protein disulfide isomerase-associated 1) | Protein metabolism | |
| NM_005742 | PDIA6 | −2.35 | −2.52 | −1.65 | −1.46 | protein disulfide isomerase family A, member 6 | Unknown | |
| NM_024895 | PDZK7 | −1.53 | −1.47 | −2.11 | −1.53 | 1.02 | PDZ domain containing 7 | Unknown |
| NM_203473 | PORCN | −2.87 | −2.43 | −1.07 | −1.49 | −1.47 | porcupine homolog (Drosophila) (PORCN), transcript variant B | Unknown |
| NM_138689 | PPP1R14B | −1.35 | −1.40 | 1.02 | −1.49 | −1.68 | protein phosphatase 1, regulatory (inhibitor) subunit 14B | Unknown |
| NM_000310 | PPT1 | −1.97 | −1.97 | −1.53 | −1.43 | palmitoyl-protein thioesterase 1 (ceroid-lipofuscinosis, neuronal 1, infantile) | Protein metabolism | |
| NM_012094 | PRDX5 | −1.76 | −1.58 | −1.07 | −1.07 | peroxiredoxin 5, nuclear gene encoding mitochondrial protein, transcript variant 1 | Immune response | |
| NM_212472 | PRKAR1A | −1.28 | −1.34 | −1.52 | −1.66 | protein kinase, cAMP-dependent, regulatory, type I, alpha (tissue specific extinguisher 1) | Cell communication | |
| NM_002862 | PYGB | −2.51 | −1.55 | −2.67 | −1.75 | phosphorylase, glycogen; brain | Protein metabolism | |
| NM_000967 | RPL3 | −1.56 | −1.71 | −1.02 | −1.51 | −1.33 | ribosomal protein L3 | Protein metabolism |
| NM_000968 | RPL4 | −1.45 | −1.57 | −1.32 | −1.36 | −1.16 | ribosomal protein L4 | Protein metabolism |
| NM_033251 | RPL13 | −1.25 | −1.34 | −1.30 | −1.74 | ribosomal protein L13 | Protein metabolism | |
| NM_002948 | RPL15 | −1.35 | −1.57 | 1.19 | −1.53 | −1.52 | ribosomal protein L15 | Protein metabolism |
| NM_033251 | RPL30 | 1.37 | 1.35 | 1.06 | 1.68 | 1.42 | ribosomal protein L30 | Protein metabolism |
| NM_033625 | RPL34 | 1.47 | 1.32 | −1.30 | 1.75 | 1.51 | ribosomal protein L34 | Protein metabolism |
| NM_053275 | RPLP0 | −1.29 | −1.49 | 1.10 | −1.17 | −1.55 | ribosomal protein, large, P0 | Protein metabolism |
| NM_002950 | RPN1 | −2.03 | −1.65 | −1.59 | Homo sapiens ribophorin I | Protein metabolism | ||
| NM_001005 | RPS3 | −1.57 | −1.66 | −1.34 | −1.18 | −1.02 | ribosomal protein S3 | Protein metabolism |
| NM_183352 | SEC13L1 | −1.51 | −1.26 | −1.02 | −1.20 | SEC13-like 1 (S. cerevisiae), transcript variant 2 | Development | |
| NM_006640 | SEPT9 | −1.27 | −1.78 | −1.29 | −1.53 | septin 9 | Unknown | |
| NM_152264 | SLC39A14 | −1.62 | −1.55 | −1.29 | −1.21 | solute carrier family 39 (zinc transporter), member 13 | Unknown | |
| NM_014426 | SNX5 | −1.16 | −1.26 | −1.53 | −1.35 | Sorting Nexin 5 | Cell communication | |
| NM_021102 | SPINT2 | −1.57 | −1.67 | 1.07 | −1.14 | −1.23 | serine protease inhibitor, Kunitz type, 2 | Cell motility |
| NM_003145 | SSR2 | −1.22 | −1.36 | −1.14 | −1.58 | −1.38 | signal sequence receptor, beta (translocon-associated protein beta) | Cell organization and biogenesis |
| NM_003146 | SSRP1 | −1.20 | −1.69 | −1.22 | structure specific recognition protein 1 | Transcrition | ||
| NM_152295 | TARS | −1.24 | −1.57 | −2.05 | −1.19 | threonyl-tRNA synthetase | Protein metabolism | |
| NM_012458 | TIMM13 | −1.63 | −1.45 | −1.02 | −1.07 | −1.35 | translocase of inner mitochondrial membrane 13 homolog (yeast), nuclear gene encoding mitochondrial protein | Development |
| NM_003722 | TP73L | −1.51 | −2.42 | 1.08 | −1.82 | −1.40 | tumor protein p73-like | Cell communication |
| NM_003329 | TXN | 1.69 | 2.06 | 2.21 | 1.98 | thioredoxin | Cell communication | |
| NM_005080 | XBP1 | −1.61 | −1.68 | −1.64 | −1.41 | X-box binding protein 1 | Transcrition | |
| NM_003404 | YWHAB | −1.58 | −1.46 | 1.49 | −1.45 | −1.61 | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, beta polypeptide, transcript variant 1 | Unknown |
| CLUSTER III | ||||||||
| NM_006317 | BASP1 | 1.85 | 1.88 | 1.88 | 1.42 | brain abundant, membrane attached signal protein 1 | Unknown | |
| NM_000610 | CD44 | 1.83 | 1.78 | 1.52 | 1.92 | 1.31 | CD44 antigen | Cell adhesion |
| NM_133467 | CITED4 | 2.19 | 2.32 | 2.02 | 1.67 | Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 4 | Transcrition | |
| NM_024045 | DDX50 | 1.71 | 1.63 | 1.47 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 50 | Unknown | ||
| NM_001967 | EIF4A2 | 2.28 | 1.98 | 1.41 | 1.63 | eukaryotic translation initiation factor 4A, isoform 2 | Protein metabolism | |
| NM_004004 | GJB2 | 2.49 | 1.95 | 1.47 | 2.21 | gap junction protein, beta 2, 26 kDa (connexin 26) | Cell communication | |
| NM_004832 | GSTO1 | 2.52 | 4.80 | 2.73 | glutathione S-transferase omega 1 | Unknown | ||
| NM_005345 | HSPA1A | 2.06 | 2.14 | 1.38 | 1.46 | heat shock 70 kDa protein 1A | Cell cycle | |
| NM_006644 | HSPH1 | 1.55 | 1.61 | 1.21 | heat shock 105 kDa/110 kDa protein 1 | Protein metabolism | ||
| NM_002658 | PLAU | 2.22 | 2.85 | 2.30 | 1.87 | plasminogen activator, urokinase | Cell communication | |
| NM_002835 | PTPN12 | 2.05 | 2.43 | 1.24 | protein tyrosine phosphatase, non-receptor type 12 | Protein metabolism | ||
| NM_002872 | RAC2 | 2.89 | 3.88 | 2.10 | ras-related C3 botulinum toxin substrate 2 | Cell communication | ||
| NM_000539 | RHO | 1.74 | 1.69 | rhodopsin (opsin 2, rod pigment) | Cell communication | |||
| NM_020150 | SARA1 | 1.75 | 1.84 | 2.04 | SAR1a gene homolog 1 (S. cerevisiae) | Cell communication | ||
| NM_021199 | SQRDL | 1.90 | 1.92 | 1.59 | 1.38 | sulfide quinone reductase-like (yeast) | Unknown | |
| NM_020182 | TMEPAI | 1.73 | 2.34 | 1.59 | 2.48 | 1.80 | transmembrane, prostate androgen induced RNA, transcript variant 1 | Cell communication |
| NM_005499 | UBA2 | 1.67 | 1.48 | 1.10 | 1.21 | SUMO-1 activating enzyme subunit 2 | Protein metabolism | |
| CLUSTER IV | ||||||||
| NM_005946 | MT1A | 1.64 | 1.52 | 1.85 | 4.11 | 2.26 | metallothionein 1A (functional) | Unknown |
| NM_005947 | MT1B | 1.72 | 1.84 | 2.37 | 5.00 | 2.70 | metallothionein 1B (functional) | Unknown |
| NM_175617 | MT1E | 1.49 | 1.63 | 2.33 | 4.40 | 2.81 | metallothionein 1E (functional) | Unknown |
| NM_005950 | MT1G | 1.92 | 1.88 | 2.25 | 5.23 | 2.71 | metallothionein 1G | Unknown |
| NM_005951 | MT1H | 1.87 | 1.84 | 2.28 | 4.68 | 2.68 | metallothionein 1H | Unknown |
| NM_005952 | MT1X | 1.88 | 1.76 | 2.28 | 4.54 | 2.48 | metallothionein 1X | Development |
| NM_005953 | MT2A | 2.21 | 2.09 | 2.57 | 6.38 | 2.86 | metallothionein 2A | Metal ion homeostasis |
| BC007034 | ENST 00000245185 | 2.26 | 2.14 | 2.35 | 5.95 | 3.20 | metallothionein 2A, mRNA (cDNA clone MGC:12397 IMAGE:4051220) | Metal ion homeostasis |
| CLUSTER V | ||||||||
| NM_001101 | ACTB | 1.19 | 1.14 | 1.21 | 1.51 | 1.29 | actin, beta | Cell cycle |
| NM_001102 | ACTN1 | 2.08 | 2.23 | actinin, alpha 1 | Unknown | |||
| NM_004039 | ANXA2 | 1.26 | 1.32 | 1.20 | 1.55 | 1.29 | Annexin A2 | Development |
| NM_006476 | ATP5L | 1.41 | 1.23 | 1.83 | 1.64 | ATP synthase, H+ transporting, mitochondrial F0 complex, subunit g, nuclear gene encoding mitochondrial protein | Phophorylation | |
| NM_178508 | C6orf1 | 1.39 | 1.34 | 1.98 | 1.67 | chromosome 6 open reading frame 1 | Unknown | |
| NM_016289 | CAB39 | 2.01 | 1.77 | calcium binding protein 39 | Unknown | |||
| NM_006367 | CAP1 | 1.47 | 1.44 | 1.84 | 1.30 | adenylate cyclase-associated protein 1 (yeast) | Unknown | |
| NM_152221 | CSNK1E | 1.60 | 1.22 | 1.16 | 1.60 | 1.33 | casein kinase 1, epsilon, transcript variant 1 | Cell communication |
| AK074742 | ENST 00000361624 | −2.28 | −1.38 | −2.38 | −1.49 | −2.09 | cDNA FLJ90261 fis, clone NT2RM4000761 | Unknown |
| NM_001997 | FAU | 1.15 | 1.10 | 1.03 | 1.53 | 1.60 | Finkel-Biskis-Reilly murine sarcoma virus (FBR-MuSV) ubiquitously expressed (fox derived); ribosomal protein S30 (FAU) | Protein metabolism |
| NM_001436 | FBL | −1.24 | −1.57 | −1.03 | −1.14 | fibrillarin | RNA metabolism | |
| M90686 | HLA-G | 2.95 | 3.24 | lymphocyte antigen (HLA-G3) | Immune response | |||
| NM_001540 | HSBP1 | 1.86 | 1.87 | 1.92 | heat shock 27 kDa protein 1 | Transcrition | ||
| NM_000576 | IL1B | 1.25 | 1.41 | 2.26 | 1.39 | interleukin 1, beta | Immune response | |
| NM_002192 | INHBA | 1.40 | 1.55 | 3.20 | 1.77 | inhibin, beta A (activin A, activin AB alpha polypeptide) | Cell cycle | |
| NM_002305 | LGALS1 | 1.55 | 1.18 | 1.22 | 2.25 | 1.36 | lectin, galactoside-binding, soluble, 1 (galectin 1) | Cell communication |
| NM_004528 | MGST3 | 2.23 | 1.59 | microsomal glutathione S-transferase 3 | Immune response | |||
| NM_079423 | MYL6 | 1.49 | 1.40 | −1.24 | 2.12 | 1.67 | myosin, light polypeptide 6, alkali, smooth muscle and non-muscle | Development |
| NM_004552 | NDUFS5 | 1.19 | 1.25 | −1.07 | 1.58 | 1.63 | NADH dehydrogenase (ubiquinone) Fe-S protein 5, 15 kDa (NADH-coenzyme Q reductase) (NDUFS5) | Phophorylation |
| NM_002624 | PFDN5 | 1.55 | 1.09 | 1.68 | 1.37 | prefoldin 5, transcript variant 1 | Protein metabolism | |
| NM_007273 | PHB2 | −1.66 | −1.50 | 1.11 | −1.68 | −1.76 | prohibitin 2 | Unknown |
| NM_021128 | POLR2L | 1.34 | 1.33 | 1.91 | 1.45 | polymerase (RNA) II (DNA directed) polypeptide L, 7.6 kDa | Transcrition | |
| NM_002791 | PSMA6 | 1.41 | 1.18 | 1.91 | 1.92 | proteasome (prosome, macropain) subunit, alpha type, 6 | Protein metabolism | |
| NM_006263 | PSME1 | 1.04 | 1.29 | 2.09 | 1.85 | proteasome (prosome, macropain) activator subunit 1 (PA28 alpha) | Immune response | |
| NM_006907 | PYCR1 | −2.57 | −1.89 | −1.00 | −1.47 | −1.36 | pyrroline-5-carboxylate reductase 1, transcript variant 1 | Development |
| NM_175744 | RHOC | 1.30 | 1.28 | 1.57 | 1.28 | ras homolog gene family, member C | Unknown | |
| NM_001026 | RPS24 | 1.33 | 1.31 | −1.19 | 1.71 | 1.68 | ribosomal protein S24 | Protein metabolism |
| NM_005979 | S100A13 | 1.62 | 1.63 | 1.64 | 1.38 | S100 calcium binding protein A13, transcript variant 2 | Development | |
| NM_005978 | S100A2 | 1.26 | 1.24 | −1.27 | 2.29 | 1.59 | S100 calcium binding protein A2 | Development |
| NM_001018108 | SERF2 | 1.27 | 1.49 | 1.09 | 1.71 | 1.78 | small EDRK-rich factor 2 | Unknown |
| NM_004607 | TBCA | 1.53 | 1.32 | 1.63 | 1.50 | tubulin-specific chaperone a | Protein metabolism | |
| NM_207013 | TCEB2 | 1.22 | 1.26 | 1.85 | 1.89 | transcription elongation factor B (SIII), polypeptide 2 (18 kDa, elongin | Protein metabolism | |
| THC2276562 | THC2276562 | 1.24 | 1.69 | −1.31 | 1.96 | 2.56 | limbic system-associated membrane protein | Unknown |
| NM_021103 | TMSB10 | 1.40 | 1.27 | 1.09 | 1.76 | 1.38 | thymosin, beta 10 | Cell organization and biogenesis |
| NM_000365 | TPT1 | 1.64 | 1.26 | −1.34 | 1.73 | 1.43 | triosephosphate isomerase 1 | Cell cycle |
| NM_006086 | TUBB3 | 1.66 | 1.56 | 1.71 | 1.52 | tubulin, beta 3 | Unknown | |
| NM_006701 | TXNL4A | −1.21 | −1.18 | −1.59 | −1.23 | thioredoxin-like 4A | Unknown | |
| NM_024292 | UBL5 | 1.46 | 1.57 | 2.21 | 1.87 | ubiquitin-like 5 | Protein metabolism | |
| NM_003761 | VAMP8 | 1.19 | 1.23 | 1.66 | 1.72 | vesicle-associated membrane protein 8 | Protein metabolism | |
| NM_006373 | VAT1 | 1.86 | 1.69 | 2.23 | 1.58 | vesicle amine transport protein 1 homolog (T californica) | Development | |
| NM_006887 | ZFP36L2 | 2.37 | 2.54 | zinc finger protein 36, C3H type-like 2 | Cell proliferation | |||
A total of 162 genes were identified from the five SMG experiments to be significantly (≥1.5-fold change, p ≤0.05) differentially expressed. They were further categorized into 5 clusters and 18 functional groups. #1. Regulation value of 3-day SMG. #2. Regulation value of 4-day SMG. #3. Regulation value of 4-day SMG plus 15-day recovery. #4. Regulation value of 9-day SMG plus 50-day recovery. #5. Regulation value of 10-day SMG plus 60-day recovery.
References
- 1.Leach C.S. Medical considerations for extending human presence in space. Acta Astronaut. 1990;21:659–666. doi: 10.1016/0094-5765(90)90077-x. [DOI] [PubMed] [Google Scholar]
- 2.Cogoli A. The effect of hypogravity and hypergravity on cells of the immune system. J. Leukoc. Biol. 1993;54:259–268. doi: 10.1002/jlb.54.3.259. [DOI] [PubMed] [Google Scholar]
- 3.Cogoli A. Mitogenic signal transduction in T lymphocytes in microgravity. J. Leukoc. Biol. 1993;53:569–575. doi: 10.1002/jlb.53.5.569. [DOI] [PubMed] [Google Scholar]
- 4.Fritsch-Yelle J.M. Microgravity decreases heart rate and arterial pressure in humans. J. Appl. Physiol. 1996;80:910–914. doi: 10.1152/jappl.1996.80.3.910. [DOI] [PubMed] [Google Scholar]
- 5.Atkov O.Yu. Some medical aspects of an 8-month’s space flight. Adv. Space Res. 1992;12:343–345. doi: 10.1016/0273-1177(92)90303-f. [DOI] [PubMed] [Google Scholar]
- 6.Aubert A.E. Cardiovascular function and basics of physiology in microgravity. Acta Cardiol. 2005;60:129–151. doi: 10.2143/AC.60.2.2005024. [DOI] [PubMed] [Google Scholar]
- 7.Hammond T.G., Hammond J.M. Optimized suspension culture: the rotating-wall vessel. Am. J. Physiol. Renal Physiol. 2001;281:F12–F25. doi: 10.1152/ajprenal.2001.281.1.F12. [DOI] [PubMed] [Google Scholar]
- 8.Nickerson C.A. Low-shear modeled microgravity: a global environmental regulatory signal affecting bacterial gene expression, Physiology, and Pathogenesis. J. Microbiol. Methods. 2003;54:1–11. doi: 10.1016/s0167-7012(03)00018-6. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz R.P. Cell culture for three-dimensional modeling in rotating-wall vessels: an application of simulated microgravity. J. Tissue Cult. Methods. 1992;14:51–58. doi: 10.1007/BF01404744. [DOI] [PubMed] [Google Scholar]
- 10.Rucci N. Characterization of the osteoblast-like cell phenotype under microgravity conditions in the NASA-approved Rotating Wall Vessel bioreactor (RWV) J. Cell. Biochem. 2002;85:167–179. [PubMed] [Google Scholar]
- 11.Ontiveros C. Hypoxia suppresses runx2 independent of modeled microgravity. J. Cell. Physiol. 2004;200:169–176. doi: 10.1002/jcp.20054. [DOI] [PubMed] [Google Scholar]
- 12.Patel M.J. Identification of mechanosensitive genes in osteoblasts by comparative microarray studies using the rotating wall vessel and the random positioning machine. J. Cell. Biochem. 2007;101:587–599. doi: 10.1002/jcb.21218. [DOI] [PubMed] [Google Scholar]
- 13.Klaus D.M. Clinostats and bioreactors. Gravit. Space Biol. Bull. 2001;14:55–64. [PubMed] [Google Scholar]
- 14.Cooper D. Suppression of antigen-specific lymphocyte activation in modeled microgravity. In Vitro Cell. Dev. Biol. Anim. 2001;37:63–65. doi: 10.1290/1071-2690(2001)037<0063:SOASLA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Risin D., Pellis N.R. Modeled microgravity inhibits apoptosis in peripheral blood lymphocytes. In Vitro Cell. Dev. Biol. Anim. 2001;37:66–72. doi: 10.1290/1071-2690(2001)037<0066:MMIAIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Plett P.A. Proliferation of human hematopoietic bone marrow cells in simulated microgravity. In Vitro Cell. Dev. Biol. Anim. 2001;37:73–78. doi: 10.1290/1071-2690(2001)037<0073:POHHBM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Sytkowski A.J., Davis K.L. Erythroid cell growth and differentiation in vitro in the simulated microgravity environment of the NASA rotating wall vessel bioreactor. In Vitro Cell. Dev. Biol. Anim. 2001;37:79–83. doi: 10.1290/1071-2690(2001)037<0079:ECGADI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Graebe A. Physiological, pharmacokinetic, and pharmacodynamic changes in space. J. Clin. Pharmacol. 2004;44:837–853. doi: 10.1177/0091270004267193. [DOI] [PubMed] [Google Scholar]
- 19.Crawford-Young S.J. Effects of microgravity on cell cytoskeleton and embryogenesis. Int. J. Dev. Biol. 2006;50:183–191. doi: 10.1387/ijdb.052077sc. [DOI] [PubMed] [Google Scholar]
- 20.Nichols H.L. Proteomics and genomics of microgravity. Physiol. Genomics. 2006;26:163–171. doi: 10.1152/physiolgenomics.00323.2005. [DOI] [PubMed] [Google Scholar]
- 21.Hatton J.P. Microgravity modifies protein kinase C isoform translocation in the human monocytic cell line U937 and human peripheral blood T-cells. J. Cell. Biochem. 2002;87:39–50. doi: 10.1002/jcb.10273. [DOI] [PubMed] [Google Scholar]
- 22.Walther I. Simulated microgravity inhibits the genetic expression of interleukin-2 and its receptor in mitogen-activated T lymphocytes. FEBS Lett. 1998;436:115–118. doi: 10.1016/s0014-5793(98)01107-7. [DOI] [PubMed] [Google Scholar]
- 23.Fitts R.H. Effect of spaceflight on the isotonic contractile properties of single skeletal muscle fibers in the rhesus monkey. J. Gravit. Physiol. 2000;7:S53–S54. [PubMed] [Google Scholar]
- 24.Lalani R. Myostatin and insulin-like growth factor-I and -II expression in the muscle of rats exposed to the microgravity environment of the NeuroLab space shuttle flight. J. Endocrinol. 2000;167:417–428. doi: 10.1677/joe.0.1670417. [DOI] [PubMed] [Google Scholar]
- 25.Yamakuchi M. Type I muscle atrophy caused by micreogravity-induced decrease of myocyte enhancer factor 2C (MEF2C) protein expression. FEBS Lett. 2000;477:135–140. doi: 10.1016/s0014-5793(00)01715-4. [DOI] [PubMed] [Google Scholar]
- 26.Yu Z.B. A proteolytic NH2-terminal truncation of cardiac troponin I that is up-regulated in simulated microgravity. J. Biol. Chem. 2001;276:15753–15760. doi: 10.1074/jbc.M011048200. [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet G. Gene expression related to the differentiation of osteoblastic cells is altered by microgravity. Bone. 1998;22:139S–143S. doi: 10.1016/s8756-3282(98)00007-6. [DOI] [PubMed] [Google Scholar]
- 28.Hughes-Fulford M. Physiological effects of microgravity on osteoblast morphology and cell biology. Adv. Space Biol. Med. 2002;8:129–157. doi: 10.1016/s1569-2574(02)08017-6. [DOI] [PubMed] [Google Scholar]
- 29.Klein-Nulend J. Microgravity and bone cell mechanosensitivity. Adv. Space Res. 2003;32:1551–1559. doi: 10.1016/S0273-1177(03)90395-4. [DOI] [PubMed] [Google Scholar]
- 30.Meyers V.E. Modeled microgravity disrupts collagen I/integrin signaling during osteoblastic differentiation of human mesenchymal stem cells. J. Cell. Biochem. 2004;93:697–707. doi: 10.1002/jcb.20229. [DOI] [PubMed] [Google Scholar]
- 31.Williams I.R., Kupper T.S. Immunity at the surface: homeostatic mechanisms of the skin immune system. Life Sci. 1996;58:1485–1507. doi: 10.1016/0024-3205(96)00042-2. [DOI] [PubMed] [Google Scholar]
- 32.Abbas A.K. second edition. W.B. Saunders Company; Philadelphia, USA: 1994. Cellular and Molecular Immunology; pp. 223–235. [Google Scholar]
- 33.Quackenbush J. Computational analysis of microarray data. Nat. Rev. Genet. 2001;2:418–427. doi: 10.1038/35076576. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J., Russell D.W. third edition. Vol. 1. Cold Spring Harbor Press; New York, USA: 2001. Molecular Cloning: A Laboratory Manual. 7.21. [Google Scholar]
- 35.Ni M., Lee A.S. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein resposnse. Nat. Rev. Mol. Cell. Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 37.Hammond T.G. Mechanical culture conditions effect gene expression: gravity-induced changes on the space shuttle. Physiol. Genomics. 2000;3:163–173. doi: 10.1152/physiolgenomics.2000.3.3.163. [DOI] [PubMed] [Google Scholar]
- 38.Esseghir S. Identification of NTN4, TRA1, and STC2 as prognostic markers in breast cancer in a screen for signal sequence encoding proteins. Clin. Cancer Res. 2007;13:3164–3173. doi: 10.1158/1078-0432.CCR-07-0224. [DOI] [PubMed] [Google Scholar]
- 39.Searle P.F. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol. Cell. Biol. 1984;4:1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penkowa M. Metallothionein I+II expression and roles during neuropathology in the CNS. Dan. Med. Bull. 2006;53:105–121. [PubMed] [Google Scholar]
- 41.Nath R. Metallothioneins, oxidative stress and the cardiovascular system. Toxicology. 2000;155:17–26. doi: 10.1016/s0300-483x(00)00273-0. [DOI] [PubMed] [Google Scholar]
- 42.Vasto S. Inflammation, genes and zinc in ageing and age-related diseases. Biogerontology. 2006;7:315–327. doi: 10.1007/s10522-006-9046-6. [DOI] [PubMed] [Google Scholar]
- 43.Chapes S.K. Cytokine secretion by immune cells in space. J. Leukoc. Biol. 1992;52:104–110. doi: 10.1002/jlb.52.1.104. [DOI] [PubMed] [Google Scholar]
- 44.Rouas-Freiss N. Expression of tolerogenic HLA-G molecules in cancer prevents antitumor responses. Semin. Cancer Biol. 2007;17:413–421. doi: 10.1016/j.semcancer.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Sugerman P.B., Bigby M. Preliminary functional analysis of human epidermal T cells. Arch. Dermatol. Res. 2000;292:9–15. doi: 10.1007/pl00007461. [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson M.F. Purification of RNA. In: Brown T.A., editor. Vol.1. Oxford University Press; New York, USA: 1991. pp. 69–86. (Essential Molecular Biology: A Practical Approach). [Google Scholar]
- 47.Clement J.Q. The stability and fate of a spliced intron from vertebrate cells. RNA. 1999;5:206–220. doi: 10.1017/s1355838299981190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolber P.K. The Agilent in situ-synthesized microarray platform. Methods Enzymol. 2001;410:28–57. doi: 10.1016/S0076-6879(06)10002-6. [DOI] [PubMed] [Google Scholar]
- 49.Hosack D.A. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eisen M.B. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sturn A. Genesis: cluster analysis of microarray data. Bioinformatics. 2002;18:207–208. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 52.Brietkruetz B.J. AFM 4.0: a toolbox for DNA microarray analysis. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-8-software0001. [DOI] [PMC free article] [PubMed] [Google Scholar]