Abstract
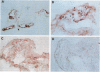
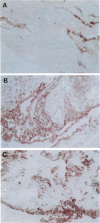
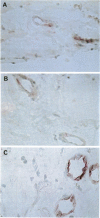
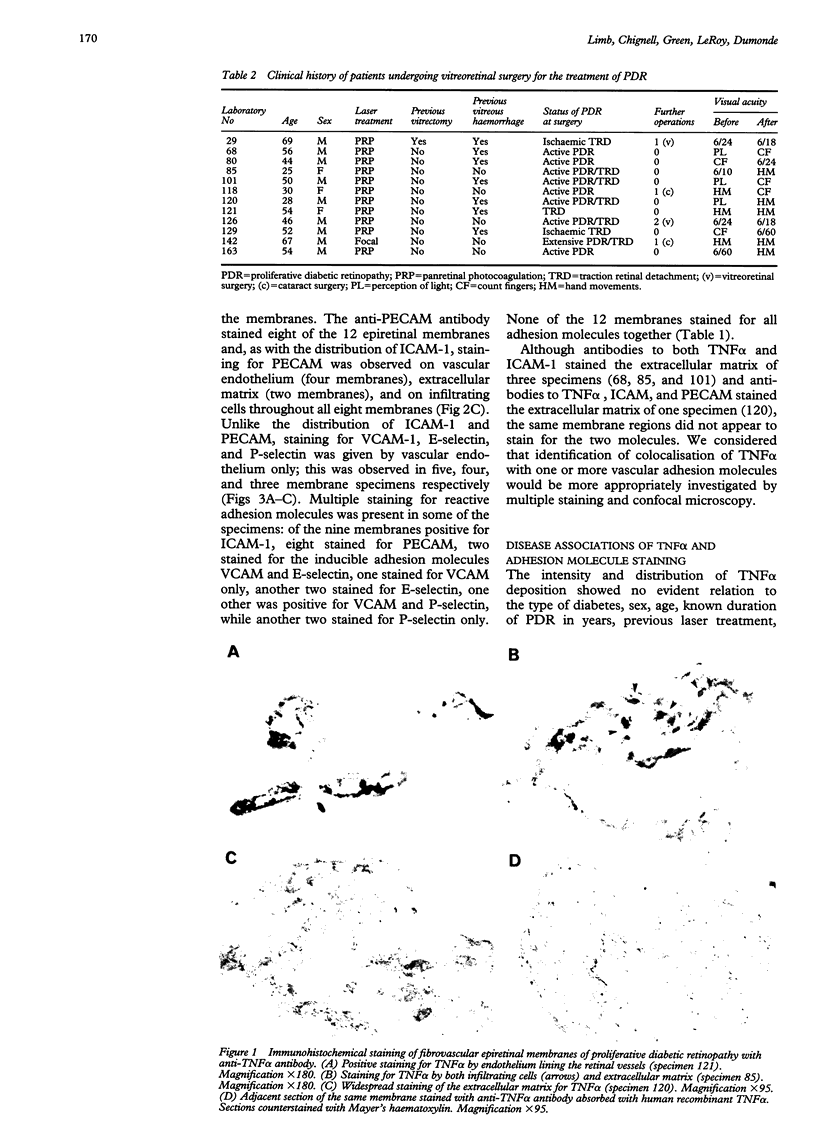
AIMS: This study investigated the presence of the cytokine tumour necrosis factor alpha (TNF alpha) and the vascular adhesion glycoproteins ICAM-1, VCAM-1, E-selectin, P-selectin, and PECAM within fibrovascular membranes of eyes with proliferative diabetic retinopathy (PDR). METHODS: The presence of these molecules was determined by immunohistochemical staining using monoclonal antibodies and the APAAP technique. RESULTS: Staining for TNF alpha was observed on the retinal vascular endothelium of five of 12 specimens, on infiltrating cells within all membranes, and on the extracellular matrix of nine specimens. This staining wa abolished by absorption of the monoclonal antibody with human recombinant TNF alpha. Likewise, ICAM-1 staining was given by infiltrating cells and extracellular matrix of nine membranes and by the endothelium of three of the specimens. VCAM-1, E-selectin, and P-selectin staining was observed on the vascular endothelium of 5/12, 4/12, and 3/12 epiretinal membranes respectively. PECAM was expressed by the endothelium of 4/12 specimens, by infiltrating cells of 8/12 membranes, and also by the extracellular matrix of two of the specimens. CONCLUSION: The widespread distribution of TNF alpha and the nature of the adhesion molecules expressed by vascular endothelial cells in PDR membranes suggest that local activation of TNF alpha and enhanced expression of vascular cell adhesion molecules may play an important role in the development of the proliferative phase of diabetic retinopathy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camussi G., Albano E., Tetta C., Bussolino F. The molecular action of tumor necrosis factor-alpha. Eur J Biochem. 1991 Nov 15;202(1):3–14. doi: 10.1111/j.1432-1033.1991.tb16337.x. [DOI] [PubMed] [Google Scholar]
- Cox A., Gonzalez A. M., Wilson A. G., Wilson R. M., Ward J. D., Artlett C. M., Welsh K., Duff G. W. Comparative analysis of the genetic associations of HLA-DR3 and tumour necrosis factor alpha with human IDDM. Diabetologia. 1994 May;37(5):500–503. doi: 10.1007/s001250050138. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Weissmann G. The adhesion molecules of inflammation. Arthritis Rheum. 1993 Feb;36(2):147–157. doi: 10.1002/art.1780360204. [DOI] [PubMed] [Google Scholar]
- Forrester J. V., Shafiee A., Schröder S., Knott R., McIntosh L. The role of growth factors in proliferative diabetic retinopathy. Eye (Lond) 1993;7(Pt 2):276–287. doi: 10.1038/eye.1993.61. [DOI] [PubMed] [Google Scholar]
- Franks W. A., Limb G. A., Stanford M. R., Ogilvie J., Wolstencroft R. A., Chignell A. H., Dumonde D. C. Cytokines in human intraocular inflammation. Curr Eye Res. 1992;11 (Suppl):187–191. doi: 10.3109/02713689208999531. [DOI] [PubMed] [Google Scholar]
- Garner A. Histopathology of diabetic retinopathy in man. Eye (Lond) 1993;7(Pt 2):250–253. doi: 10.1038/eye.1993.58. [DOI] [PubMed] [Google Scholar]
- Hamilton C. W., Chandler D., Klintworth G. K., Machemer R. A transmission and scanning electron microscopic study of surgically excised preretinal membrane proliferations in diabetes mellitus. Am J Ophthalmol. 1982 Oct;94(4):473–488. doi: 10.1016/0002-9394(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Hiscott P., Waller H. A., Grierson I., Butler M. G., Scott D. L. The extracellular matrix of reparative tissue in the vitreous: fibronectin production in proliferative diabetic retinopathy membranes. Eye (Lond) 1993;7(Pt 2):288–292. doi: 10.1038/eye.1993.62. [DOI] [PubMed] [Google Scholar]
- Kundu S. K., Merigan T. C. Relationship of HIV-1 provirus load, CD8+ CD11+ T cells and HIV-1 envelope-specific cytotoxic T lymphocytes in HIV-infected asymptomatic offients. Immunology. 1994 Sep;83(1):81–85. [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Polverini P. J., Shepard H. M., Wiseman D. M., Shively V., Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987 Oct 15;329(6140):630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- Limb G. A., Alam A., Earley O., Green W., Chignell A. H., Dumonde D. C. Distribution of cytokine proteins within epiretinal membranes in proliferative vitreoretinopathy. Curr Eye Res. 1994 Nov;13(11):791–798. doi: 10.3109/02713689409025133. [DOI] [PubMed] [Google Scholar]
- Limb G. A., Franks W. A., Munasinghe K. R., Chignell A. H., Dumonde D. C. Proliferative vitreoretinopathy: an examination of the involvement of lymphocytes, adhesion molecules and HLA-DR antigens. Graefes Arch Clin Exp Ophthalmol. 1993 Jun;231(6):331–336. doi: 10.1007/BF00919029. [DOI] [PubMed] [Google Scholar]
- Limb G. A., Meager A., Woolley J., Wadhwa M., Biggerstaff J., Brown K. A., Wolstencroft R. A. Release of cytokines during generation of lymphokine-activated killer (LAK) cells by IL-2. Immunology. 1989 Dec;68(4):514–519. [PMC free article] [PubMed] [Google Scholar]
- Luscinskas F. W., Ding H., Lichtman A. H. P-selectin and vascular cell adhesion molecule 1 mediate rolling and arrest, respectively, of CD4+ T lymphocytes on tumor necrosis factor alpha-activated vascular endothelium under flow. J Exp Med. 1995 Mar 1;181(3):1179–1186. doi: 10.1084/jem.181.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pociot F., Briant L., Jongeneel C. V., Mölvig J., Worsaae H., Abbal M., Thomsen M., Nerup J., Cambon-Thomsen A. Association of tumor necrosis factor (TNF) and class II major histocompatibility complex alleles with the secretion of TNF-alpha and TNF-beta by human mononuclear cells: a possible link to insulin-dependent diabetes mellitus. Eur J Immunol. 1993 Jan;23(1):224–231. doi: 10.1002/eji.1830230135. [DOI] [PubMed] [Google Scholar]
- Romer L. H., McLean N. V., Yan H. C., Daise M., Sun J., DeLisser H. M. IFN-gamma and TNF-alpha induce redistribution of PECAM-1 (CD31) on human endothelial cells. J Immunol. 1995 Jun 15;154(12):6582–6592. [PubMed] [Google Scholar]
- Scheiffarth O. F., Kampik A., Günther H., von der Mark K. Proteins of the extracellular matrix in vitreoretinal membranes. Graefes Arch Clin Exp Ophthalmol. 1988;226(4):357–361. doi: 10.1007/BF02172967. [DOI] [PubMed] [Google Scholar]
- Stolpen A. H., Guinan E. C., Fiers W., Pober J. S. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol. 1986 Apr;123(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Tang S., Le-Ruppert K. C., Gabel V. P. Expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on proliferating vascular endothelial cells in diabetic epiretinal membranes. Br J Ophthalmol. 1994 May;78(5):370–376. doi: 10.1136/bjo.78.5.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellicome S. M., Thornhill M. H., Pitzalis C., Thomas D. S., Lanchbury J. S., Panayi G. S., Haskard D. O. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol. 1990 Apr 1;144(7):2558–2565. [PubMed] [Google Scholar]