Abstract
MicroRNAs (miRNAs) usually contain 19-24 nucleotides and have been identified as important eukaryotic gene regulators. Applications of various computational approaches have simplified the task by predicting miRNAs from available sequence data sources. In this study, we identified a conserved miR414 from a computational analysis of EST sequence data available from Stevia rebaudiana. In addition, we also identified six conserved miRNAs namely miR169, miR319, miR414, miR164, miR167 and miR398 using stem-loop RT-PCR analysis. Hence, miR414 was commonly identified using both methods. The expression analysis of these miRNAs documented their roles in growth and development of Stevia. Furthermore, the detected miRNAs were found to target genes involved in plant growth, development, metabolism and signal transduction. This is the first study reporting these conserved miRNAs and their expression in Stevia.
Key words: Stevia, miRNA, computational prediction, expression analysis, target prediction, stem-loop reverse transcription
Introduction
MicroRNAs (miRNAs) are 19-24 nucleotides (nt)-long riboregulators in plants, animals and viruses. They act on mRNAs and regulate the gene expression post-transcriptionally through mRNA degradation or translational repression (1). miRNAs are processed from intergenic regions of the host genome 2, 3. Pri-miRNA is transcribed from miRNA-coding genes by RNA polymerase II and processed by Dicer-like 1 protein (DCL1) to produce pre-miRNA and eventually the mature miRNA duplex. The processed mature miRNA binds to Argonaute protein that is present on the RNA-induced silencing complex (RISC) 1, 2, 3. This binding directs miRNA towards its complementary target mRNA sequence. This particular mode of action of miRNAs has become the basis of various plant processes such as developmental changes, physiological responses, metabolism or any defensive response against abiotic or biotic factors 2, 3, 4. Presently, a large number of miRNAs have been characterized from various monocots and dicots (1). The sequence information about these is publicly available on the miRBase website (http://www.mirbase.org/).
Stevia rebaudiana, a dicotyledonary plant of the Asteraceae family, produces clinically and commercially important secondary metabolites, steviol glycosides (5). Biosynthesis pathway of these metabolites consists of multiple steps that are catalyzed by different enzymes. The yield of steviol glycosides and expression of genes encoding the biosynthetic enzymes are known to be developmentally regulated 6, 7. Therefore, better understanding of the genetic regulation of this pathway could be very useful for manipulating the yield of steviol glycosides. Modern genomics approaches have documented the importance of miRNAs in such metabolic pathway regulation 4, 8. For example, in Arabidopsis, TEOSINTE BRANCHED/CYCLOIDEA/PC (TCP) transcription factors that control the biosynthesis of jasmonic acid are regulated by miR319 (8).
Despite the clinical and commercial value of S. rebaudiana, no conserved or novel miRNA has been identified from this species. In view of this, a computational approach was employed to identify potential miRNAs using EST data of Stevia available at NCBI and one conserved miR414 was identified. In addition, we used stem-loop reverse transcription PCR (SL RT-PCR) analysis to identify more miRNAs experimentally. In doing so, six miRNAs were identified including the computationally identified miR414. Furthermore, expression of these 6 miRNAs was analyzed. Target protein analysis of these identified miRNAs suggested their roles in growth and development of Stevia. For the first time, a total of six conserved miRNAs were identified and experimentally validated in Stevia in the present study.
Results and Discussion
Identification of miRNAs has remained a tedious job. Cloning of miRNAs was possible only for organisms with completely sequenced genomes (9). With the advent of novel next-generation technology, computational approaches were introduced and currently software tools can identify miRNAs from EST/GSS sources and predict their secondary structures, targets and homologues (10). However, computationally predicted data need to be validated experimentally. Thus, combined computational and experimental approaches would provide more reliable data. In this paper, we attempted to identify conserved miRNAs from S. rebaudiana using computational and experimental methods.
In silico identification of conserved miR414 from Stevia ESTs
Micro PC (µPC) is a computational tool that allows the identification of conserved miRNAs from plant EST data, predicts their secondary structure and identifies homologues from other plant species 9, 11, 12, 13. This software compares the submitted query sequence against the known miRNAs in miRBase Release 18 (www.mirbase.org), which contains 18,226 entries of miRNAs from animal as well as plant species.
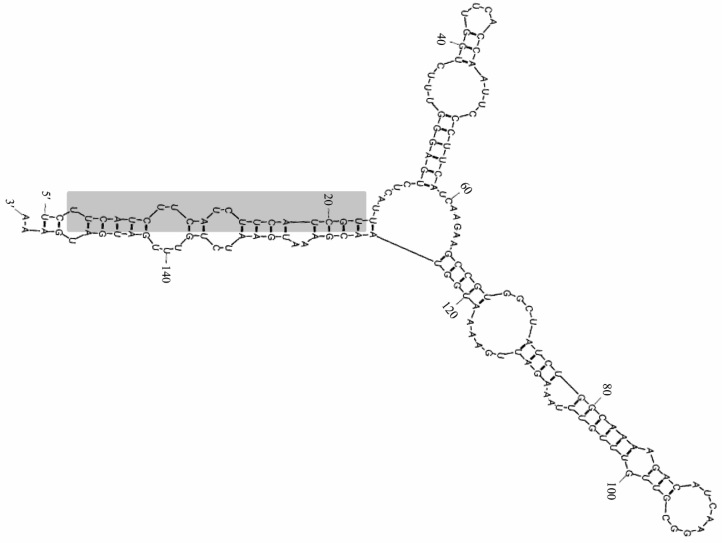
Out of the 5,548 ESTs available at NCBI for Stevia, only 33 ESTs showed complementarities with miRNAs from other plant species. The data were further analyzed based on the number of mismatches and secondary structures. It is known that potential miRNAs are allowed to have maximum alignment and less than four mismatches with the query sequence. In addition, the secondary structure should possess higher negative minimum free energy (MFE), 30%-70% A+U content and the absence of a break or loop in the miRNA sequence 9, 14. According to these criteria, only one miRNA designated as miR414 was found on EST gi|16948646 of Stevia. In this case, only 2-bp mismatch was present between the query and miR414, A+U content was 61.59%, MFE was −33.4 and MFEI was calculated as −0.57. These observations satisfied the defined criteria (Figure 1), suggesting that EST gi|16948646 is a precursor sequence for miR414. Homologues for miR414 were also predicted and this miR414 showed homology with those from Oryza sativa and Raphanus raphanis (Table S1).
Figure 1.
Predicted secondary structure of identified precursor miR414 in Stevia. Structure was generated using UNAFold program. The mature miR414 sequence is shown with shade. The actual size of the precursor may be slightly shorter or longer than presented here.
Targets for miR414 were also predicted using the target scanner utility program of µPC. No target was found related to the synthesis of steviol glycosides. A total of 117 target proteins were predicted from Arabidopsis. Annotations of target proteins with GO terms suggested that these proteins are involved in transcriptional and translational regulation, protein and chromatin modifications, and DNA repair (Table S2). GO enrichment analysis showed that 40 out of 117 predicted target proteins were associated with transcriptional regulation, followed by 16 targets that regulate protein modifications and 10 targets involved in DNA repair. Moreover, 9 out of 117 play roles in chromatin modification and only one was found to regulate the translation process (Table S3). The observation thus suggests that among the various predicted targets, miR414 primarily targets transcriptional regulators. The target transcription factors include the bZIP family transcription factors, WRKY, MYB, B3, scarecrow, heat shock proteins and TCP, all of which play roles in plant growth, development, physiological and morphological changes, metabolism and defense responses 15, 16, 17, 18, 19, 20, 21. Hence, miR414 seems to play a very important role in the regulation of plant growth and development. Other targets identified for miR414 were found to play roles in post-transcriptional modifications, including high mobility group proteins, SNF2 transcriptional regulator, C2H2 zinc finger proteins, pentatricopeptide repeat-containing proteins, F-Box family proteins, DNA store keepers and RNA recognition motifs 19, 20, 22, 23.
Identification of conserved miRNAs experimentally
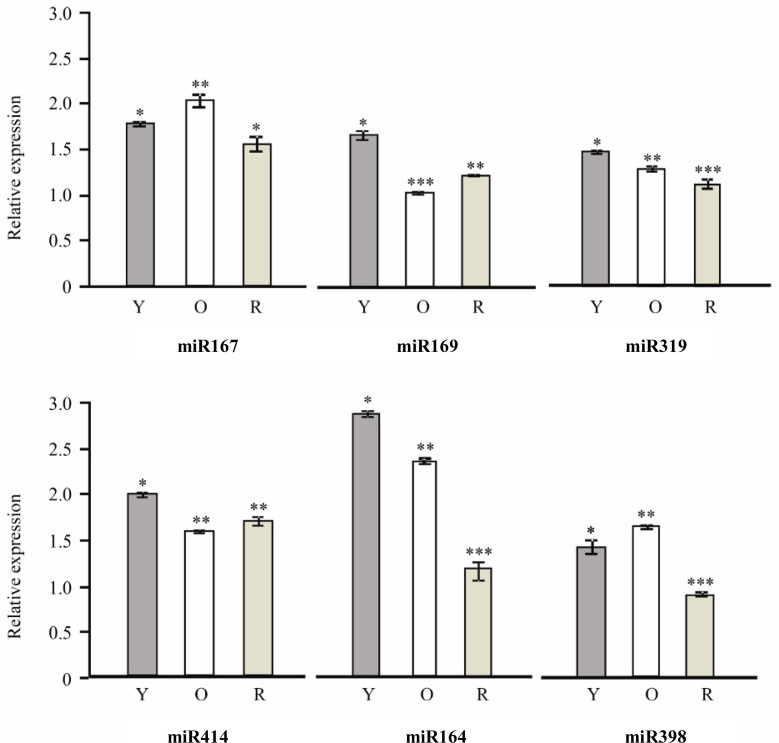
miRNAs are known to be evolutionarily conserved among plant species (24). Ten miRNAs were used in this study for experimental validation, since they are conserved among various dicots and monocots such as Arabidopsis, poplar, grape, rice, maize and sorghum (24). It has been reported that cloning, hybridization and microarray-like techniques are not sensitive enough to detect less abundant miRNAs (25). However, SL RT-PCR has been found to be a more reliable method for the detection of miRNAs from various plant tissues in Arabidopsis, poppy and tobacco 25, 26, 27. Therefore, in this work, the presence of 10 conserved miRNAs (miR167, miR169, miR319, miR414, miR164, miR398, miR408, miR395, miR397 and miR399) was detected using SL RT-PCR. RNA from three tissues, including young leaves, old leaves and roots, was extracted and miRNA specific cDNA was synthesized and utilized for the following amplification reactions with SL RT-PCR. Among the 10 miRNAs examined, the expression of miR167, miR169, miR319, miR414, miR164 and miR398 was detected. Identity of these miRNA PCR products was confirmed by sequencing. Interestingly, miR414 was also detected using SL RT-PCR, which validates our result of the previous in silico prediction.
All of the six detected miRNAs were found to be expressed in both leaves and roots. However, they showed differential expression in the tested tissues. All these miRNAs were expressed significantly higher in young leaves than in roots. To evaluate the developmental effect on the miRNA expression, we conducted expression profiling of these miRNAs in young and old leaf samples. Of the six miRNAs detected, four miRNAs including miR169, miR319, miR414 and miR164 were expressed significantly higher in young leaves than in old leaves, while miR167 and miR398 were expressed significantly higher in older leaves than young ones. Interestingly, steviol glycosides are reported to be accumulated at higher levels in young leaves than old leaves and least found in roots 6, 28. Therefore, it is possible that miR169, miR319, miR414 and miR164 might be involved in regulation of genes of the steviol glycoside biosynthesis pathway in young leaves, which might explain why the detected miRNAs showed tissue and developmental age-dependent expression in Stevia. Importantly, miR164 showed highest expression in leaf tissues, while miR414 showed highest expression in root tissues. Differential expression of various identified miRNAs implies their involvement in various physiological and developmental processes including steviol glycoside biosynthesis, which needs to be unraveled in future.
Targets for these conserved miRNAs have been reported in Arabidopsis. Due to lack of genomic data in Stevia, targets have not been identified from Stevia mRNA. The target genes for these miRNAs mainly encode proteins involved in plant growth, hormonal signaling, stress responses, secondary metabolism and signal transduction 12, 29, 30. Out of the six miRNAs, miR398, miR164 and miR169 have been shown to be mechanistically conserved in the regulation of a variety of nutrient, abiotic, mechanical and physiological stresses in Arabidopsis and poplar (31). Identification of these conserved miRNAs in Stevia and targets in Arabidopsis suggested their possible role in growth and development of Stevia.
In conclusion, our work documents the identification of conserved miR414 from computational EST analysis of Stevia for the first time. In addition, six miRNAs were also identified using SL RT-PCR, including miR414, which was predicted computationally. The expression analysis of miRNAs in various tissues suggests their possible regulatory role in growth, development and metabolism of Stevia. Also, Stevia can be added to the list of plant species containing miRNAs and supporting the concept of evolutionary conservation of miRNAs.
Materials and Methods
EST collection and in silico miRNA prediction
A total of 5,548 Stevia EST sequences were obtained from the NCBI dbEST Database (http://www.ncbi.nlm.nih.gov/dbEST/). µPC (http://www.biotec.or.th/isl/micropc) was used to predict conserved miRNAs. Stevia ESTs were submitted as input query sequences against miRBase Release 18 with condition <3000 bp with default parameters to obtain possible complementary miRNAs.
RNA isolation
Stevia seeds were surface sterilized using 1% sodium hypochlorite and germinated on medium (pH 5.8) containing Murashige and Skoog (MS) salts supplemented with vitamins solution (1000X), 0.8% agar, and 3% sucrose. The germinated seedlings were allowed to grow in tissue culture conditions for two months. Afterwards, the plants were transferred to a green house in pots. RNA was isolated using fourth leaf from the top (designated as young leaf, Y), eighth leaf from the top (designated as old leaf, O) and roots (designated as R). Total RNA was isolated from individual samples using Qiagen miRNeasy Plant Minikit as instructed by the manufacturer. The quality and quantity of isolated RNA samples were measured using Nanodrop ND-1000 (Nanodrop Technologies, USA).
Stem-loop reverse transcription
Stem-loop reverse transcription (SL RT) primers were designed manually for miR408, miR399, miR398, miR397, miR395, miR319, miR169, miR167, miR164 and miR414 (Table S4) following the criteria as described earlier (32). The forward primer was designed to be specific to the miRNA sequence excluding the last six nucleotides at the 3’ end of the miRNA. At the 5’ end of the forward primer, 5-7 GC-rich nucleotides were added to raise the melting temperature of primers. The reverse primer was universal for all sets of stem-loop primers (32). miRNA SL RT was carried out using 50 ng of leaf and root small RNA fractions. 0.5 µL of 10 mM dNTP mix was added to RNA, incubated for 5 min at 65°C and then kept on ice for 2 min. To the above mix, 2 µL of 5× First Strand Buffer, 1 µL of 0.1 M DTT and 0.5 µL of Superscript III reverse transcriptase (100 units) were added. A master mix of the above recipe was prepared for ten tubes and distributed equally. Thereafter, 1 µL of miRNA-specific SL RT primer was added. The reaction was performed as 16°C, 30 min for 1 cycle; 30°C, 30 s; 42° C, 30 s; 50°C, 1 s for 60 cycles followed by incubation at 85°C for 5 min.and finally hold at 4°C.
PCR amplification of miRNAs
Experimental identification of conserved miRNAs was performed by polymerase chain reaction (PCR) amplification with the above synthesized cDNAs. The 20 µL reaction recipe consisted of 1 µL miRNA-specific cDNA, 0.4 µL forward primer (10 µМ), 0.4 µL reverse primer (10 µМ), 0.4 µL of 10 mM dNTP mix, 2 µL of 10X PCR Buffer and 0.4 µL of Taq polymerase. The reaction was incubated in a thermal cycler at 94°C, 3 min for 1 cycle; 94°C, 15 s and 60°C, 1 min for 25 cycles followed by hold at 4°C. The reaction products were analyzed on a 3.5% agarose gel in 1×TAE buffer and scanned using an Alpha DigiDoc gel documentation system (Alpha Innotech, USA). The experiment for each miRNA was repeated three times for statistical analysis. 18sRNA was used as the internal control and relative expression was calculated by determining the Integrated Density Values (IDV) of gel bands using AD-1000 software.
Statistical analysis
The data are presented as mean±standard deviation (n=3). A least significant difference test (LSD-test) was employed to test the relative expression levels of miRNAs in young leaf (Y), old leaf (O) and root (R) tissues of Stevia at the 5% level.
Authors’ contributions
SKY planned the experiments and provided guidance to PG. PG performed all the experimental work. PG and SKY wrote the manuscript.
Competing Interests
The authors have no competing interests to declare.
Figure 2.
Expression profile of miRNAs in leaf and root. Expression of miR167, miR169, miR319, miR414, miR164 and miR398 in young leaf (Y), old leaf (O) and root (R) was detected using SL RT-PCR. 18sRNA was used as the internal control to equalize cDNA quantity. Each bar in the diagram corresponds to the IDV of the intensity of the individually amplified band in the gel. A least significant difference test was employed to test the relative expression levels of miRNAs. The data are presented as mean±standard deviation (n=3). Same number of asterisks above error bars represents non-significant difference and different number of asterisks represents significant difference, P<0.05.
Acknowledgements
Authors are thankful to the Director, IHBT for his continuous support and encouragement to pursue the research in this direction. Authors would like to thank Council of Scientific and Industrial Research (CSIR), Govt. of India for financial support. PG would like to thank CSIR for providing fellowship in the form of Senior Research Fellowship (SRF).
Supplementary Material
Tables S1-S4
References
- 1.Sunkar R., Zhu J.K. Micro RNAs and Short-interfering RNAs in Plants. J. Integr. Plant Biol. 2007;49:817–826. [Google Scholar]
- 2.Guleria P. Plant small RNAs: biogenesis, mode of action and their role during abiotic stresses. Genomics Proteomics Bioinformatics. 2011;9:183–199. doi: 10.1016/S1672-0229(11)60022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckardt N.A. Small RNA on the move. Plant Cell. 2004;16:1951–1954. [Google Scholar]
- 4.Guleria P. MicroRNAs and their role in plants during abiotic stresses. In: Parvaiz A., Prasad M.N.V., editors. Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change. Springer-Verlag; New York, USA: 2011. [Google Scholar]
- 5.Brandle J.E., Telmer P.G. Steviol glycoside biosynthesis. Phytochem. 2007;68:1855–1863. doi: 10.1016/j.phytochem.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Bondarev N.I. Steviol glycoside content in different organs of Stevia rebaudiana and its dynamics during ontogeny. Biol. Plantarum. 2003/4;47:261–264. [Google Scholar]
- 7.Yadav S.K., Guleria P. Steviol glycosides from Stevia: biosynthesis pathway review and their application in foods and medicine. Crit. Rev. Food. Sci. Nutr. 2011 doi: 10.1080/10408398.2010.519447. [DOI] [PubMed] [Google Scholar]
- 8.Schommer C. Control of jasmonate biosynthesis and senescence by miR319 targets. Plos Biol. 2008;6 doi: 10.1371/journal.pbio.0060230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B.H. Identification and characterization of new plant microRNAs using EST analysis. Cell Res. 2005;15:336–360. doi: 10.1038/sj.cr.7290302. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B.H. Computational identification of microRNAs and their targets. Comput. Biol. Chem. 2006;30:395–407. doi: 10.1016/j.compbiolchem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Mhuantong W., Wichadakul D. MicroPC (µPC): A comprehensive resource for predicting and comparing plant microRNAs. BMC Genomics. 2009;10:366–373. doi: 10.1186/1471-2164-10-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhoades M.W. Prediction of plant microRNA targets. Cell. 2002;110:513–520. doi: 10.1016/s0092-8674(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 13.Jones-Rhoades M.W., Bartel D.P. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol. Cell. 2004;14:787–799. doi: 10.1016/j.molcel.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eulgem T. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/s1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- 16.Gurley W.B. HSP101: a key component for the acquisition of thermotolerance in plants. Plant Cell. 2000;12:457–460. doi: 10.1105/tpc.12.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakoby M. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002;7:106–111. doi: 10.1016/s1360-1385(01)02223-3. [DOI] [PubMed] [Google Scholar]
- 18.Palatnik J.F. Control of leaf morphogenesis by microRNAs. Nature. 2003;425:257–263. doi: 10.1038/nature01958. [DOI] [PubMed] [Google Scholar]
- 19.Suo J. Identification of GhMYB109 encoding a R2R3MYB transcription factor that expressed specifically in fiber initials and elongating fibers of cotton (Gossypium hirsutum L.) Biochim. Biophys. Acta. 2003;1630:25–34. doi: 10.1016/j.bbaexp.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Guo Q. Bioinformatic identification of microRNAs and their target genes from Solanum tuberosum expressed sequence tags. Chin. Sci. Bull. 2007;52:2380–2389. [Google Scholar]
- 21.Romanel E.A. Evolution of the B3 DNA binding superfamily: new insights into REM family gene diversification. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W.S. Interaction of wheat high-mobility-group proteins with four-way-junction DNA and characterization of the structure and expression of HMGA gene. Arch. Biochem. Biophys. 2003;409:357–366. doi: 10.1016/s0003-9861(02)00630-6. [DOI] [PubMed] [Google Scholar]
- 23.Flaus A. Identification of multiple distinct SNF2 subfamilies with conserved structural motifs. Nucleic Acids Res. 2006;43:2887–2905. doi: 10.1093/nar/gkl295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin Z.J., Shen F.F. Identification and characterization of conserved microRNAs and their target genes in wheat (Triticum aestivum) Genet. Mol. Res. 2010;9:1186–1196. doi: 10.4238/vol9-2gmr805. [DOI] [PubMed] [Google Scholar]
- 25.Chen C. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33 doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unver T. Identification of conserved microRNAs and their target transcripts in opium poppy (Papaver somniferum L.) Plant Cell Rep. 2010;29:757–769. doi: 10.1007/s00299-010-0862-4. [DOI] [PubMed] [Google Scholar]
- 27.Frazier T.P. Salt and drought stresses induce the aberrant expression of microRNA genes in tobacco. Mol. Biotechnol. 2011;49:159–165. doi: 10.1007/s12033-011-9387-5. [DOI] [PubMed] [Google Scholar]
- 28.Kumar H. A comprehensive analysis of fifteen genes of steviol glycosides biosynthesis pathway in Stevia rebaudiana (Bertoni) Gene. 2012;492:276–284. doi: 10.1016/j.gene.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Sunkar R. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell. 2006;18:2051–2065. doi: 10.1105/tpc.106.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia X. Differential and dynamic regulation of miR398 in response to ABA and salt stress in Populus tremula and Arabidopsis thaliana. Plant Mol. Biol. 2009;71:51–59. doi: 10.1007/s11103-009-9508-8. [DOI] [PubMed] [Google Scholar]
- 31.Liu H.H. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA. 2008;14:836–843. doi: 10.1261/rna.895308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varkonyi-Gasic E. Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods. 2007;3:12. doi: 10.1186/1746-4811-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-S4