Abstract
Stable isotopes of most important biological elements, such as C, H, N and O, affect living organisms. In rapidly growing species, deuterium and to a lesser extent other heavy isotopes reduce the growth rate. At least for deuterium it is known that its depletion also negatively impacts the speed of biological processes. As a rule, living organisms “resist” changes in their isotopic environment, preferring natural isotopic abundances. This preference could be due to evolutionary optimization; an additional effect could be due to the presence of the “isotopic resonance”. The isotopic resonance phenomenon has been linked to the choice of earliest amino acids, and thus affected the evolution of genetic code. To test the isotopic resonance hypothesis, literature data were analyzed against quantitative and qualitative predictions of the hypothesis. Four studies provided five independent datasets, each in very good quantitative agreement with the predictions. Thus, the isotopic resonance hypothesis is no longer simply plausible; it can now be deemed likely. Additional testing is needed, however, before full acceptance of this hypothesis.
Key words: origin of life, stable isotope, isotopic resonance
Introduction
Because of the presence of several stable isotopes of the elements C, H, N and O, molecular masses of biopolymers (proteins, nucleic acids, sugars and lipids) are not just numbers, but rather complex entities called isotopic distributions (1). Therefore, two molecules with seemingly identical chemical structures often differ in terms of their molecular masses. But does this intrinsic heterogeneity of biomolecules influence biology? In other words, do stable isotopes have any impact on life?
Biological impact of heavy isotopes
The answer is very clear concerning the heavy isotope of hydrogen, deuterium 2D, biological effects of which have emerged soon after its discovery in 1932 by Urey et al. (2). Already in the early experiments it was found that deuterium content has profound effect on life. From 1934 to the beginning of the Second World War in 1939, a total of 216 publications appeared dealing with biological effects of deuterium (3). Excess of deuterium in water was found to cause reduction in synthesis of proteins and nucleic acids, disturbance in cell division mechanism, changes in enzymatic kinetic rates, and cellular morphological changes 4, 5. The effects of deuterium were found to be only partly reversible, and lethal for higher organisms in doses above ~20%−30% D2O 6, 7. Even relatively small intakes resulting in enrichment of body fluids by less than 0.5% (and the total body mass of slightly in excess of 0.1%) caused clinically relevant side effects (8).
Unlike deuterium, biological effects of other stable isotopes, such as 13C, 15N and 18O discovered about the same time as deuterium, have not been systematically studied until 1960s. While nothing similar to the biological activity of deuterium was observed, some of them reported notable effects, especially in combination with other factors. The Katz group, who have studied multiple heavy-isotope (13C, 15N and 18O) substitutions in Chlorella vulgaris grown in heavy water, found that all additional isotopic substitutions result into abnormal effects in cell size, appearance, growth rate and division (9). The effects were progressively stronger with the isotopic composition deviated from the normal. The authors postulated that “organisms of different isotopic compositions are actually different organisms, to the degree that their isotopic compositions are removed from naturally occurring compositions” (9).
The biological effects are usually observed shortly after the organism is placed in an isotopically different medium (10). Microorganisms and cells experience at first an “isotopic shock” manifested through the growth arrest and morphology changes. After a period of adaptation, growth resumes, but the rate is usually slower than in normal isotopic environment (11), although a higher growth rate has also been observed (12). The changes in the growth rate can be explained by the impact of isotopic substitutions on the kinetics of enzymes (12), pattern of hydrogen bonds and similar relatively subtle but cumulatively potentially important effects.
Yet it is possible to grow microorganisms (e.g., some variants of Escherichia coli) in a highly substituted medium, and achieve almost complete deuterium substitution in E. coli 13, 14. Even higher organisms, such as mice, have been grown on 80% 13C (15) and in 90% 18O environment (16), in some cases for several generations.
Resistance to isotope incorporation
There is ample evidence that stable isotopes do affect life. One manifestation is the “resistance” of organisms to changes in the isotopic composition of their components. For example, equilibrium concentration of deuterium in urine and serum of mice drinking 50% D2O is ~32%, and it is achieved only after eight days (17). Mice grown on 80% 13C food incorporated only 60% of 13C on average, with even most metabolically active organs, such as liver, showing stark deficit of 13C compared to food, their only source of carbon intake (15). Even for small (few percent) isotopic variations in the growth medium compared to normal abundances, isotopic composition of microorganisms shows deficit of heavy isotopes (17). Because of this “isotopic resistance”, many organisms and cells require several generations of growth in isotopically modified medium to achieve the desired degree of isotope enrichment.
Even in natural environment, isotopic compositions slightly vary (within a few percent or even per mille) depending upon geographical location, source of food and even season. “Isotopic ecology” is an area of research that deduces the details of the life cycle of various biological organisms in their natural environment by studying isotopic composition of their tissues (18). It is a common knowledge in that area that there is a “tissue to diet discrimination”, i.e., the difference between the isotopic composition of food and that of tissues (18). In a stunning control experiment, rats grown on four isotopically different but dietary equivalent diets exhibited little to no variation in isotopic composition of their liver, the most metabolically active organ (19). This and similar results point strongly towards the existence of a preferred isotopic composition of the environment for a given organism, at which the organism achieves maximum growth rate.
Preferred isotopic environment
This preferred composition must be close to the average composition in which the organism has evolved, because evolution pressure acts to optimize the biochemical processes as to maximize the growth rate [more specifically, exergy rate (20)] at prevailing conditions. For instance, the temperature of maximum growth of E. coli is 37°C, very close to the temperature of its natural environment (21). Since it can be expected that the terrestrial organisms will thrive best in the natural isotopic environment, any deviations from natural isotopic compositions (i.e., both enrichment and depletion of heavy isotopes) should have negative impact on the growth rate. Although experimental data are absent for many elements, the example of deuterium supports the above postulate: both enrichment (22) and depletion (23) of deuterium in water have negative impact on growth of cell culture and can induce apoptosis.
Isotopic Resonance Hypothesis
There is also another reason, more hypothetical, to expect that deviation in isotopic compositions will have negative effect on organism growth. Recently, Zubarev et al. discovered the existence of an “isotopic resonance” close to (but somewhat off) the standard natural isotopic abundances of C, H, N and O (24). At the isotopic abundances equal to resonance values, the class of molecules containing C, H, N and O and obeying the rule H=2C−N (to which many amino acids and peptides belong) has a greatly reduced complexity, and their rate reactions are expected to be enhanced (24). The isotopic resonance phenomenon may have had an impact on the choice of earliest amino acids (24), and thus affected the evolution of genetic code.
Thus there are two independent reasons to expect the existence of preferred isotopic composition of environment at which terrestrial organisms thrive best—a general one, related to the evolutionary optimization of the organism to grow in a given isotopic environment, and a specific one, related to the existence of the “isotopic resonance” for a wide class of biomolecules. While the plausibility of the first reason is beyond doubt, the second reason is hypothetical and requires thorough testing. The test for the second mechanism of isotopic sensitivity of living organisms can be based on its prediction that the isotopic compositions of the living cells will strive towards the “resonance” isotopic composition which, as mentioned above, deviates somewhat from the standard natural isotopic abundances. This deviation is element-specific: in order to achieve resonance conditions, the abundance of 2D must be increased by 64%, or that of 18O increased by 1% (24). Alternatively, the isotopic abundance of 13C must be decreased by 22%, or that of 15N decreased by 2% (24).
Testing the hypothesis
Since different elements have different “distances” to the resonance, not only in terms of their values but also in terms of their signs (H and O vs C and N), these distances can provide a testable hypothesis following the resonance mechanism. The line of reasoning can go as follows. Since all reactions are reversible at the molecular level (the principle of microscopic reversibility), the growth rate should affect the isotopic compositions of the growing organism as much as enrichment or depletion of stable isotopes affects the rate of organism growth. The fact that natural isotopic compositions are most distant to the resonance for hydrogen and closest for oxygen means that, in strive to the isotopic resonance, growing cells may need to change the hydrogen isotopic composition significantly more than for other elements, especially oxygen.
In quantitative terms, the degrees of changes in isotopic composition induced by fast growth should be proportional to the values −22%, +64%, −2% and +1% for C, H, N and O, respectively. These numbers represent the specific prediction of the “isotopic resonance” hypothesis. Because evolutionary optimization mechanism makes no specific quantitative prediction, testing these numbers experimentally amounts to testing the validity of the “isotopic resonance” hypothesis.
Slow vs fast growth
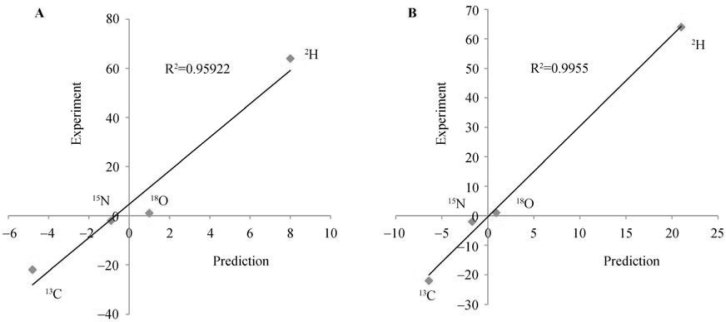
A recent study by Harvard researchers provides a dataset for such suitable testing (25). They have analyzed isotopic abundances of C, H, N and O in pigs grown in the same conditions and on the same diet but for some reasons exhibiting vastly different growth rates. The study has found significant differences for all four elements. The deviations in the isotopic compositions for C, H, N and O for fast-growing pigs compared to their initial state correlate with the predictions surprisingly well both in terms of signs of the deviations (positive for H and O and negative for C and N) and of absolute values (R2>0.955, P<0.01) (Figure 1A). For slow-growing pigs, where the isotopic deviations from the initial state are smaller and thus have larger experimental errors, the correlation with the predictions is poorer but still decent (R2>0.925). The largest (and thus most reliable) deviations are the differences between the fast- and the slow-growing pigs. For these differences, the correlation with the predictions is the best (R2>0.995, P<0.001) (Figure 1B).
Figure 1.
Correlation between the changes (in ‰) in the isotopic compositions of collagen in growing pigs (25) and the predictions of the isotopic resonance hypothesis (in arbitrary units) (24). A. Fast-growing pigs. B. Fast- and slow-growing pigs.
Hair and nail studies
The above dataset provides an important piece of evidence in favor of the isotopic resonance hypotheses; however, much more testing is required to fully support its validity. Unfortunately, there is a lack of relevant studies where the only varied parameter was the growth rate, and where isotopic compositions of all four elements were measured. There are however several studies where isotopic compositions in human hair and nails were measured. To utilize the published datasets, the predictions of the isotopic resonance hypothesis must be modified. The new prediction is that the variations in the isotopic compositions within large population groups should scale as the absolute values of the deficit, i.e., 22%, 64%, 2% and 1% for C, H, N and O, respectively.
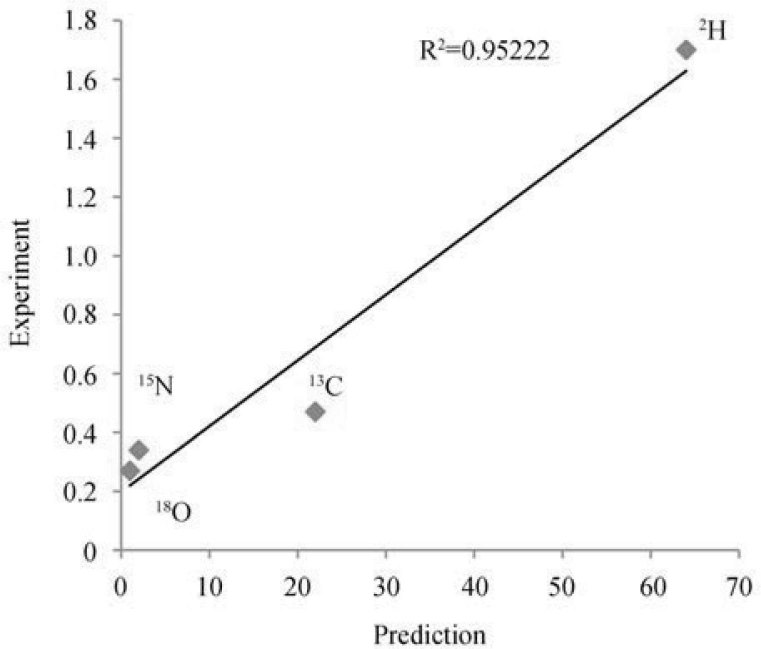
In the work of Fraser et al., standard deviations in isotopic compositions of hair and nail of 70 individuals have been reported (26). These deviations correlated well with the above prediction (R2>0.95), with the order of the elements (O, N, C and H) reproduced correctly (Figure 2).
Figure 2.
Correlation between the standard deviations (in ‰) in isotopic compositions of C, H, N and O in human hair and nail of 70 individuals (26) and the predictions of the isotopic resonance hypothesis (in arbitrary units) (24).
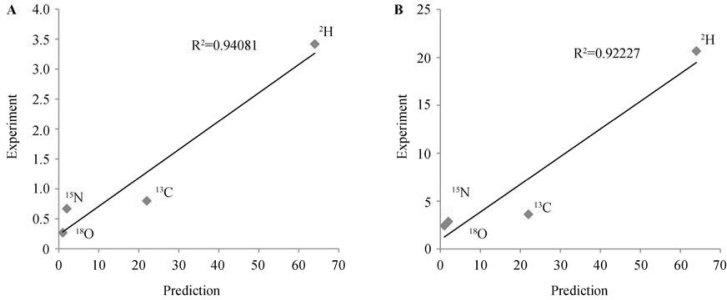
In another study, Bowen et al. have studied compositions in human hair of hundreds of individuals from 17 groups of indigenous populations of all populated continents (27). The published dataset provides two independent tests: one for group-to-group variations, and one for inter-individual variations within the group. Both comparisons are shown in Figure 3, and both correctly reproduce the order of the elements and provide good quantitative correlations (R2>0.94 and >0.92, respectively).
Figure 3.
Correlation between the standard deviations (in ‰) in isotopic compositions of C, H, N and O in human hair of hundreds of individuals from 17 groups (nations) of indigenous populations of all populated continents (27) and the predictions of the isotopic resonance hypothesis (in arbitrary units) (24). A. Nation-to-nation variations. B. Person-to-person variations within the nation.
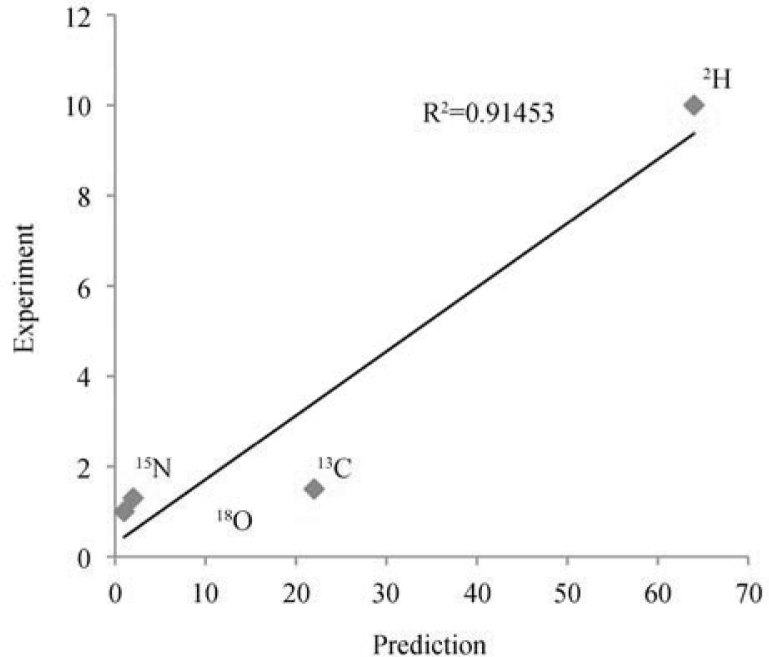
Thompson et al. have studied human hair in Asian population (28). The largest dataset was from China (112 individual measurements for H and O, and 74 measurements for C and N). In line with previous observations, this dataset gives the correct order of elements and good quantitative correlation (R2>0.91) with the hypothesis predictions (Figure 4).
Figure 4.
Correlation between the standard deviations (in ‰) in isotopic compositions of C, H, N and O in Chinese population (112 individual measurements for H and O, and 74 measurements for C and N) (28) and the predictions of the isotopic resonance hypothesis (in arbitrary units) (24).
Note the striking similarity of the relative positions of the datapoints in Figure 2, Figure 3, Figure 4, despite the fact that the vertical scales differ by more than one order of magnitude. The typical experimental errors, ±0.1%– 0.2%, are smaller than typical differences between the datapoints.
Antagonistic effects
Another prediction that could be made is that the biological effects of 2H and 13C substitution should be antagonistic, as the signs of the deficits of H and C (+64% and −24%) are opposite. Flaumenthaft et al. studied the growth of C. vulgaris in media substituted with 2H and/or 13C (29). The most pronounced difference seen between the cultures was in the thickness of the cell walls. Walls of 13C-grown cells were much thicker than those of the other cells, while the 2H−13C cell walls were even thicker than those of 13C cells. But overall, the authors note that “the most remarkable feature of these results is the fact that introduction of 13C into a cell tends to diminish some of the consequences arising from the introduction of 2H” (29). In particular, cell size distribution for the 2H, 13C-medium was much closer to that of the normal medium than to 2H-medium. The authors were unable to explain the observed antagonism between heavy isotope effects. They found it surprising, because both 2H and 13C substitutions should result in slower reactions. The authors conclude that “the antagonism between 2H and 13C may be fortuitous, or may reflect a general principle. Further studies will be required to clarify this point” (29).
Conclusion
Taken together, the above observations from independent studies provide the first test of the isotopic resonance hypothesis. Since all so far generated predictions were confirmed, the status of the hypothesis can be elevated from “plausible” to “likely”. Given the fundamental nature of the isotopic resonance hypothesis and its potential implications, e.g., for the theory of the terrestrial life’s origin, additional experimental testing is required before its full acceptance.
Acknowledgements
This work was supported by the Swedish Research Council (Grant 2007–4410).
References
- 1.Yergey J. Isotopic distributions in mass spectra of large molecules. Anal. Chem. 1983;55:353–356. [Google Scholar]
- 2.Urey H.C. A hydrogen isotope of mass 2. Phys. Rev. 1932;39:164–165. [Google Scholar]
- 3.Koletzko B. Safety of stable isotope use. Eur. J. Pediatr. 1997;156:S12–S17. doi: 10.1007/pl00014267. [DOI] [PubMed] [Google Scholar]
- 4.Katz J.J. Some observations on biological effects of deuterium, with special reference to effects on neoplastic processes. J. Natl. Cancer Inst. 1957;18:641–659. [PubMed] [Google Scholar]
- 5.Katz J.J., Crespi H.L. Isotope effects in biological systems. In: Collins C.J., Bowman N.S., editors. Isotope Effects in Chemical Reactions. Van Nostrand Reinhold; New York, USA: 1971. pp. 286–363. [Google Scholar]
- 6.Czajka D.M. Physiological effects of deuterium on dogs. Am. J. Physiol. 1961;201:357–362. doi: 10.1152/ajplegacy.1961.201.2.357. [DOI] [PubMed] [Google Scholar]
- 7.Katz J.J. Course of deuteration and some physiological effects of deuterium in mice. Am. J. Physiol. 1962;203:907–913. doi: 10.1152/ajplegacy.1962.203.5.907. [DOI] [PubMed] [Google Scholar]
- 8.Jones P.J. Plasma cholesterol synthesis using deuterated water in humans: effects of short-term food restriction. J. Lab. Clin. Med. 1988;111:627–633. [PubMed] [Google Scholar]
- 9.Uphaus R.A. A living organism of unusual isotopic composition. Sequential and cumulative replacement of stable isotopes in Chlorella vulgaris. Biochim. Biophys. Acta. 1967;141:625–632. doi: 10.1016/0304-4165(67)90191-2. [DOI] [PubMed] [Google Scholar]
- 10.Katz J.J., Crespi H.L. Deuterated organisms: cultivation and uses. Science. 1966;151:1187–1194. doi: 10.1126/science.151.3715.1187. [DOI] [PubMed] [Google Scholar]
- 11.Borek E., Rittenberg D. Anomalous growth of microorganisms produced by changes in isotopes in their environment. Proc. Natl. Acad. Sci. USA. 1960;46:777–782. doi: 10.1073/pnas.46.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler E.B. Kinetic studies of C. pyrenoidosa using 94% 13C CO2. Biotechnol. Bioengineer. 1972;14:819–829. [Google Scholar]
- 13.Rokop S. Purification and characterization of fully deuterated enzymes. Biochim. Biophys. Acta. 1969;191:707–715. doi: 10.1016/0005-2744(69)90365-9. [DOI] [PubMed] [Google Scholar]
- 14.Paliy O. Improved methods of cultivation and production of deuteriated proteins from E. coli strains grown on fully deuteriated minimal medium. J. Appl. Microbiol. 2003;94:580–586. doi: 10.1046/j.1365-2672.2003.01866.x. [DOI] [PubMed] [Google Scholar]
- 15.Gregg C.T. Substantial replacement of mammalian body carbon with carbon-13. Life Sci. 1973;13:775–782. doi: 10.1016/0024-3205(73)90068-4. [DOI] [PubMed] [Google Scholar]
- 16.Klein P.D., Klein E.R. Stable isotopes: origins and safety. J. Clin. Pharmacol. 1986;26:378–382. doi: 10.1002/j.1552-4604.1986.tb03544.x. [DOI] [PubMed] [Google Scholar]
- 17.Kreuzer-Martin H.W., Jarman K.H. Stable isotope ratios and forensic analysis of microorganisms. Appl. Envir. Microbiol. 2007;73:3896–3908. doi: 10.1128/AEM.02906-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.del Rio C.M. Isotopic ecology: ten years after a call for more laboratory experiments. Biol. Rev. Camb. Philos. Soc. 2009;84:91–111. doi: 10.1111/j.1469-185X.2008.00064.x. [DOI] [PubMed] [Google Scholar]
- 19.Caut S. Caution on isotopic model use for analyses of consumer diet. Canadian J. Zool. 2008;86:438–445. [Google Scholar]
- 20.Jørgensen S.E., Fath B.D. Application of thermodynamic principles in ecology. Ecol. Complex. 2004;1:267–280. [Google Scholar]
- 21.Kovarova K. Temperature-dependent growth kinetics of Escherichia coli ML 30 in glucose-limited continuous culture. J. Bacteriol. 1996;178:4530–4539. doi: 10.1128/jb.178.15.4530-4539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartmann J. Effects of heavy water (D2O) on human pancreatic tumor cells. Anticancer Res. 2005;25:3407–3411. [PubMed] [Google Scholar]
- 23.Somlyai G. Naturally occurring deuterium is essential for the normal growth rate of cells. FEBS Lett. 1993;317:1–4. doi: 10.1016/0014-5793(93)81479-j. [DOI] [PubMed] [Google Scholar]
- 24.Zubarev R.A. Early life relict feature in peptide mass distribution. Cent. Eur. J. Biol. 2010;5:190–196. [Google Scholar]
- 25.Warinner C., Tuross N. Tissue isotopic enrichment associated with growth depression in a pig: implications for archaeology and ecology. Am. J. Phys. Antropology. 2010;141:486–493. doi: 10.1002/ajpa.21222. [DOI] [PubMed] [Google Scholar]
- 26.Fraser I. The role of stable isotopes in human identification: a longitudinal study into the variability of isotopic signals in human hair and nails. Rapid Commun. Mass Spectrom. 2006;20:1109–1116. doi: 10.1002/rcm.2424. [DOI] [PubMed] [Google Scholar]
- 27.Bowen G.J. Dietary and physiological controls on the hydrogen and oxygen isotope ratios of hair from mid-20th century indigenous populations. Am. J. Phys. Antropol. 2009;139:494–504. doi: 10.1002/ajpa.21008. [DOI] [PubMed] [Google Scholar]
- 28.Thompson A.H. Stable isotope analysis of modern human hair collected from Asia (China, India, Mongolia, and Pakistan) Am. J. Phys. Antropol. 2010;141:440–451. doi: 10.1002/ajpa.21162. [DOI] [PubMed] [Google Scholar]
- 29.Flaumenhaft E. Isotope biology of 13C extensive incorporation of highly enriched 13C in the alga Chlorella vulgaris. Biochim. Biophys. Acta. 1970;215:421–429. doi: 10.1016/0304-4165(70)90092-9. [DOI] [PubMed] [Google Scholar]