Abstract
In order to obtain a high-resolution electrophorogram of rice young panicle proteome, we evaluated various protocols commonly used in two-dimensional (2D) polyacrylamide gel electrophoresis (PAGE) of proteins, including gel staining protocol, pH range of immobilized pH gradient (IPG) strips and sample loading quantity. Results showed that a silver staining protocol using sensitized solution containing glacial acetic acid, sodium acetate and sodium thiosulfate (reported by Heukeshoven and Dernick in 1988) and a Coomassie Brilliant Blue staining method using solution containing G-250, ammonium sulfate and phosphoric acid (reported by Pink et al. in 2010) demonstrated the superior staining effect. In addition, we also showed that higher resolution was achieved when IPG gel strip with pH range of 5-8 was used, compared to that with pH range of 4-7. Finally, the optimal loading quantity was determined as 130 µg using the 17 cm-long nonlinear IPG strip with pH 5-8 in combination with the silver nitrate staining protocol. The evaluated results would be helpful in proteome analysis of young rice caryopsis.
Key words: protocol, 2-DE, proteome, rice caryopsis
Introduction
Separation and visualization of proteins extracted from tissues or cells by two-dimensional electrophoresis (2-DE) gel followed by identification and characterization using mass spectrometry (MS) is commonly used in proteomic analysis 1, 2. High resolution of 2-DE gel is not only important for identification of a characteristic MS spectrum but also crucial for quantitative analysis of differentially expressed proteins. High resolution of protein profile is usually achieved by improving the 2-DE procedure that involves separating by charge and mass under denaturing conditions via isoelectric focusing (IEF) and polyacryl amide gel electrophoresis (PAGE) and optimizing the staining protocol 3, 4.
With the continuous improvement and application of methods in proteomics, 2-DE technology and the employed instruments and equipments have been developed accordingly (5). In particular, the invention and use of immobilized pH gradient (IPG) gel strips has greatly improved the resolution and reproducibility of 2-DE (6). Currently, Coomassie Brilliant Blue (CBB) staining and silver nitrate staining is most frequently used in developing protein spots following 2-DE separation. Many studies have been reported for improving the detection efficiency of these two staining methods 7, 8, 9. As a result, thousands of proteins can now be separated and visualized on a 2-DE gel for protein samples from various tissues or cell types. However, optimization of the basic separation procedure is still mandatory for improving sensitivity and obtaining reproducible results, due to technical noise and the variable properties and components of protein samples extracted from different tissues or cells 10, 11.
Rice is a critically important food plant on our planet and serves as an excellent model plant for genetic and genomic research of crops because of the smallest genome among the cereal crops examined, easy genetic operation and high collinearity with other cereal crops (12). Rice caryopsis, especially at early milky stage, is very sensitive to environmental stress including high temperature, which may result in yield loss (13).
In the present study, we evaluated various procedures and protocols commonly used in resolving high resolution of 2-DE, including staining protocols, pH range of IPG strip and sample loading quantity. We believe that this evaluation would help to obtain a high-resolution proteomic profile of young rice caryopsis, which will facilitate the proteomic research at the stage important for yield.
Results and Discussion
Comparison of silver staining protocols
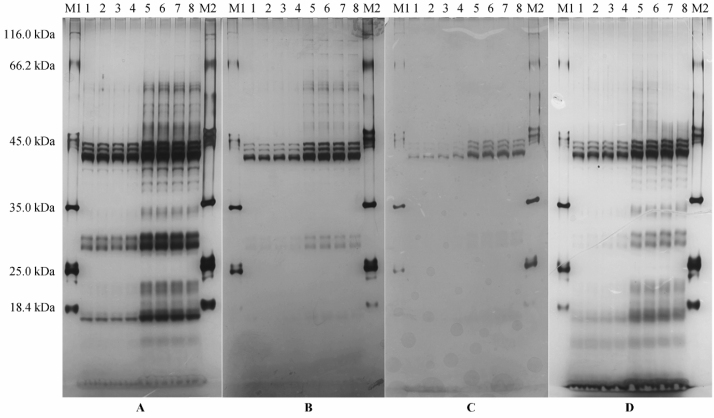
Silver staining is a useful, sensitive, non-radioactive method for permanently staining proteins in polyacrylamide gels (14). In order to obtain a highly sensitive silver staining protocol for protein imaging of young rice caryopsis, four previously reported silver staining protocols were examined, which were referred as silver protocol A (15), B (16), C (17) and D (18), respectively, in this study. Protein staining effects using protocol A, B, C or D were evaluated after separation by sodium dodecyl sulfate (SDS)-PAGE. Among these 4 silver staining protocols (Figure 1), silver protocol A provided the highest protein resolution, which is superior to other protocols, especially for proteins with either high molecular weight or low molecular weight. Moreover, when the loading quantity of the protein sample was only 2 µg in SDS-PAGE gel, some protein bands with molecular weights lower than 14.4 kDa were also detected using the silver protocol A. In addition, a good staining was also obtained for proteins with molecular weights ranging from 35.0 to 116.0 kDa using silver protocol B, which were easily distinguished with 4 µg of sample loading quantity (lane 5-8). However, silver protocol B provided a poor resolution for proteins with low molecular weight. Silver protocol C showed a relatively poor protein staining and only a few highly abundant proteins could be distinguished for proteins with either low or high molecular weight when the sample loading quantity was 4 µg (lane 5-8). In contrast to the silver protocol B, silver protocol D showed a good staining for proteins with low molecular weight, but poor for proteins with high molecular weight.
Figure 1.
Comparison of staining using four silver staining protocols. Proteins extracted from young rice caryopsis were loaded as sample and SDS-PAGE was carried out at 10 mA/gel for 15 min followed by 40 mA/gel for 3 h until the dye front just ran off the edge of the gel. The gel was cut into four pieces equally, which were stained using four sliver staining protocols (referred as A, B, C, D) respectively and shown in panel A, B, C, D, respectively. Lane M1 and M2 were loaded with the standard protein marker; lanes 1-4 were loaded with 2 µg of sample; lanes 5-8 were loaded with 4 µg of sample. These experiments were repeated four times.
Comparison of CBB staining protocols
One of the current challenges in the field of proteomics is the development of highly sensitive protein staining methods compatible with sophisticated identification techniques such as matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization (ESI). Studies for improving the detection efficiency of protein’s staining have been reported 7, 8, 9. Silver staining methods have raised the detection limit to the nanogram range, but protein identification of excised spots is often an obstacle that cannot be overcome easily. On the other hand, protocols using CBB, for instance, are highly compatible with MS but are not at all sensitive. To take advantage of both approaches, we implemented the following strategy. In the first step, analytical 2-DE is carried out using a highly sensitive silver staining protocol for image analysis of the gels and afterwards, a second experimental run comprises semipreparative 2-DE utilizing a CBB staining protocol for spot excision and subsequent protein identification. Therefore, in order to obtain a highly sensitive CBB staining protocol for protein spot excision, we compared the staining effect of four CBB staining protocols after SDS-PAGE that were reported previously, which were referred as CBB protocol A (19), B (20), C (21) and D (22), respectively.
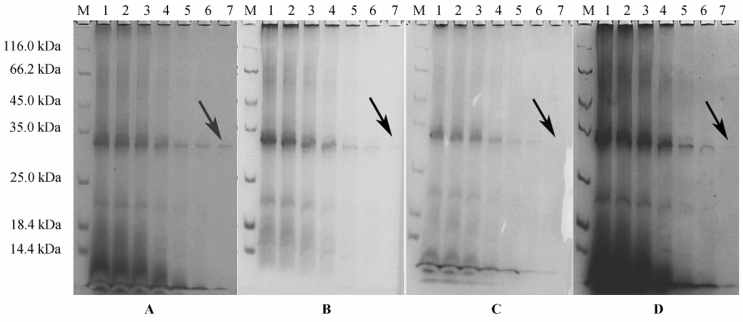
Of the four CBB staining methods (Figure 2), CBB protocol A provided the highest staining resolution with low background, and the abundant 30-kDa protein was readily detected with 5 µg of sample loading quantity (Figure 2A, the band indicated by the arrow in lane 7). As for CBB protocols B and C, the backgrounds were low, but the resolutions were also compromised, and the bands were hardly detected with 5 µg of sample loaded (Figure 2B and C, lane 7). In addition, CBB protocol D presented similar staining efficiency to that using CBB protocol A. However, the higher background using CBB protocol D discourages its usage for following software analysis and automatic detection of protein spots, compared to CBB protocol A.
Figure 2.
Comparison of staining using four CBB staining protocols. Protein samples were loaded and separated similarly as indicated in Figure 1. SDS-PAGE gel was cut into 4 equal pieces, which were stained using four CBB staining protocols (referred as A, B, C, D) respectively and shown in panel A, B, C, D, respectively. Lane M indicated the standard protein marker; lane 1-7 was loaded with 35, 30, 25, 20, 15, 10 and 5 µg of sample, respectively. These experiments were repeated four times.
Resolution comparison between pH 4-7 and 5-8 IPG strips to IEF
For 2-DE with protein sample of unknown features, the optimal pH range of IPG strip needs to be determined empirically. Generally, better separation can be achieved with strips of a narrow pH range. In addition, sample loading amount can also be increased so that more low-abundance proteins can be detected. To improve the resolution and enhance the detection of low-abundance proteins, separation using 2-DE gel with pH 4-7 and 5-8 IPG strips to IEF was compared.
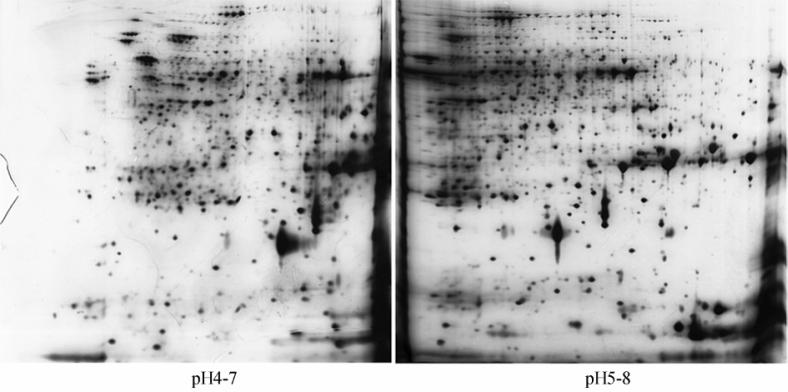
2-DE profiles (Figure 3) showed that proteins from young rice caryopsis were almost distributed throughout the gel using the pH 5-8 IPG strip, which was beneficial to automatic protein identification with software and protein collection. In the profile using pH 4-7 strip to IEF, barely no protein spots were present at the acidic end of the gel while too many protein spots were found to be stacked at the alkaline end, which were difficult to be identified by Imagemaster 2D Platinum 5.0 software automatically. As a result, 1,051 protein spots were detected in the gel with the pH 5-8 IPG strip, while only 851 protein spots were detected in the gel with pH 4-7 IPG strip to IEF.
Figure 3.
Comparison of separation of 2-DE using IPG strips with pH 4-7 and pH 5-8 to IEF. Total proteins were extracted from young rice caryopsis on the 10th day after heading and 120 µg of protein was loaded as sample. 2-DE was carried out using 17 cm-long nonlinear IPG strips with pH 4-7 (left panel) and pH 5-8 (right panel) to IEF, respectively. These experiments were repeated four times.
Separation comparison with different sample loading quantities in 2-DE gel
Sample loading quantity of 2-DE is also an important factor determining the resolution of the protein profile. If the gel was overloaded with excessive amount of sample, protein spots would present a stacking state in the profile, which makes them difficult to be differentiated by software. On the other hand, low-abundance proteins will not be visualized and are difficult to be detected if the protein sample loading quantity is too small. Thus, in order to obtain an optimal sample loading quantity for 2-DE gel, evaluation of resolution was performed on 100 µg, 115 µg, 130 µg and 145 µg of sample loading quantity using 17 cm-long nonlinear IPG strip with pH 5-8 for IEF.
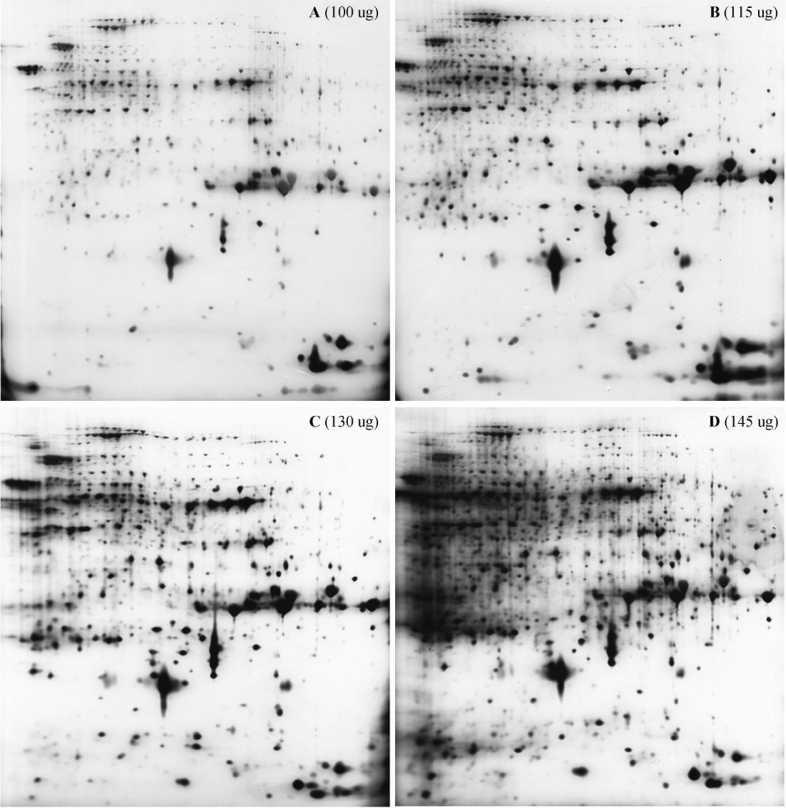
The protein profiles with different loading quantities were shown in Figure 4. Among the examined loading quantities, loading with 130 µg of protein sample appeared to provide superior resolution with clear protein spots (Figure 4C). In total, 1,235 protein spots were detected with 130 µg of loading quantity. In addition, acceptable resolution was also achieved for profiles of protein with 100 µg and 115 µg of loading quantity (Figure 4, A and B). 1,073 and 876 protein spots were detected for 115 µg and 100 µg loading, respectively. The lower number of protein spots detected by the Imagemaster 2D Platinum 5.0 software indicated that some low-abundance proteins could not be detected. On the other hand, although more spots were present in the protein profile with 145 µg of loading quantity, many proteins with high molecular weight and high abundance were stacked together at the acidic end of gel (Figure 4D), which were not automatically detected using the Imagemaster 2D Platinum 5.0 software. As a result, only 821 protein spots were detected when 145 µg of protein sample was loaded.
Figure 4.
Evaluation of sample loading quantity for 2-DE. Proteins extracted from young rice caryopsis were loaded with various quantities including 100 µg (A), 115 µg (B), 130 µg (C) and 145 µg (D) as indicated, and 2-DE was carried out using 17 cm-long nonlinear IPG strips with pH 5-8 to IEF. These experiments were repeated four times.
Test of the optimized 2-DE protocols
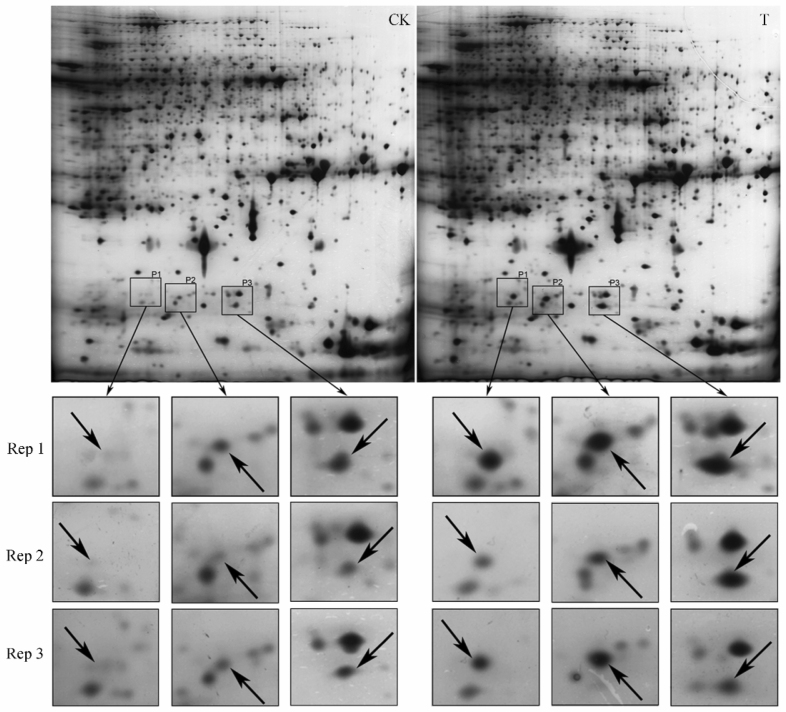
The improved protocols obtained above was validated by a comparative proteome procedure to detect differentially expressed proteins between young rice caryopsis exposed to normal condition and high temperature stress (38.0/25.0±0.5°C, treatment/control), on the 10th day after heading. Three significantly up-regulated proteins, renamed as P1, P2 and P3, were detected (Figure 5). MS analysis of the three proteins was further conducted and the result showed that P1, P2 and P3 were all heat shock proteins (HSPs). They are 18 kDa HSP, 17.9 kDa HSP and class 1 HSP, respectively, which is consistent with the previous report (23).
Figure 5.
Detection of differentially expressed proteins from rice young caryopsis exposed to high temperature stress. Rice was treated at 38.0/25.0±0.5°C (high temperature stress / control) for 9 h on the 10th day after heading. Total protein was extracted from young rice caryopsis under high temperature stress and control condition, respectively. The improved method of 2-DE was carried out to detect differentially expressed proteins between young caryopsis from control rice (CK) and that subjected to high temperature stress (T). These experiments were repeated three times. Rep indicates replication.
Conclusion
In our study, the silver staining protocol reported by Heukeshoven and Dernick in 1988 and the CBB staining protocol reported by Pink et al. in 2010, were determined to provide the highest resolution for protein staining. In addition, more protein spots were detected using pH 5-8 gel strip, compared to pH4-7 gel strip for IEF. Finally, the optimal loading quantity of protein was 130 µg when a 17 cm-long nonlinear IPG strip with pH 5-8 was used in combination with the silver nitrate staining protocol. The results obtained with the improved protocols of 2-DE agree with the previous report. We believe that the current study would be helpful for the proteome research related to young rice caryopsis.
Materials and Methods
Protein exaction
Total protein of young rice caryopsis on the 10th day after heading was extracted using ice-cold acetone buffer containing 10% (w/v) trichloroacetic acid (TCA), 1% (w/v) polyvinylpolypyrrolidone (PVPP) and 2% (v/v) β-mercaptoethanol, following the protocol described previously (24). The obtained protein precipitate (dry protein powder) was sealed and stored at −80°C until use after vacuum drying at 25°C for 30 min. The dry protein powder, 100 mg, was dissolved in 1 mL of lysis buffer containing (8 M urea, 50 mM dithiothreitol (DTT), 2% 3-[(3-cholamidopro-pyl) dimethylaminol]-1-propanesulfonate (CHAPS) and 0.2% Bio-Lyte ampholytes by ultrasound at 20°C -28°C for 1 h. After centrifugation with 13,000 rpm for 45 min at 20°C, the supernatant was transferred to a 1.5 mL centrifuge tube and protein concentration was determined by a Bradford assay using the bovine albumin standard curve (25).
IEF
The IPG strips were rehydrated with 300 µL rice caryopsis protein in buffer containing 7 M urea, 2 M thiourea, 1.2% (w/v) CHAPS, 0.005% (w/v) bromophenol blue, 20 mM DTT and 0.25% (v/v) ampholytes of the same pH range as the IPG strips. After rehydration for 14 h, the IPG strips were removed to a focusing tray and any proteins that had not been absorbed into the gel strip were removed. IEF was conducted using a Protean IEF Cell (Bio-Rad) according to the following IEF parameters. Rapid ramping to 100 V, 300 V, 500 V, 800 V and 1,000 V was performed, desalting for 1 h, 1.5 h, 2.5 h, 2.5 h and 2.5 h, respectively, linear ramping to 8,000 V for 3 h, rapid ramping to 10,000 V and a constant of 10,000 V until approximately 65 kVh was reached. The gel strips were removed to run on the second dimension or stored at −80°C until use.
SDS-PAGE
Prior to SDS-PAGE, the IPG strip was equilibrated twice for 15 min with gentle vertical shaking. The first equilibration solution contained 50 mM of Tris-HCl, pH 8.8, 6 M urea, 20% (v/v) glycerol, 2% (m/v) SDS and 1% (m/v) DTT. In the second equilibration solution, DTT was replaced with 2.5% (m/v) iodoacetamide. To remove the excess equilibration buffer on the surface of strip after equilibration, the strip was immersed for 1 s in 1× Tris/glycine electrophoresis buffer containing 0.3% (m/v) Tris-HCl, 1.44% (m/v) glycine and 0.1% (m/v) SDS. Then, the strip was applied to a precast 12.5% SDS-PAGE using a PROTEAN II XL system (Bio-Rad) at 10 mA/gel for 30 min followed by 40 mA/gel for 6.5 h until the dye front just ran off the edge of the SDS-PAGE gel.
Gel staining, imaging and data analysis
Proteins were developed using the silver staining protocol and differentially expressed proteins were analyzed using the Imagemaster 5.0 software (GE Healthcare).
Identification of differentially expressed proteins
After differentially expressed proteins were detected, CBB staining protocol was carried out to visualize a new 2-DE gel and the differentially expressed proteins were collected and sent to Shanghai Boyuan Biological Technology (Shanghai, China) for MS analysis (ABI, MALDI-TOF/TOF 4800 mass spectrograph).
Protein identification using the peptide mass fingerprints (PMF) was performed with a MAS-COT Distiller (http://www.matrixscience.com/, Matrix Science, UK) against the Swiss-Prot protein database. The search parameters were as follows: the taxonomy (Oryza sativa), the enzyme (trypsin), the number of missed cleavage sites (up to 1), the fixed modification (carbamidomethylation), the variable modification (oxidation), the peptide tolerance (50 ppm) and the mass value (MH+). Searching range was pI value ±0.5 pH unit and the experimental mass range (Mr) was ±20%. The criteria for positive identification of proteins were set as follows: (1) the MS match consists of a minimum of five peptides; (2) the matched peptides cover at least 20% of the whole protein sequence; (3) the MASCOT score of a protein match is higher than 50 (P<0.05).
Comparative proteome analysis for total protein of young rice caryopsis exposed to normal condition and high temperature stress
In order to test the feasibility of the improved method of 2-DE protocol, a heat-tolerant rice line inbred in our lab previously, XN0437T, was treated on the 10th day after heading at 38.0/25.0±0.5°C (treatment/control) for 9 h (26). After treatment, protein was extracted from rice caryopsis using the TCA/acetone precipitation method (24). IEF was carried out on 17 cm-long nonlinear IPG strip with pH 5-8 loaded with 130 µg of total protein. Protein spots were visualized by the silver nitrate staining protocol after SDS-PAGE. Differentially expressed proteins were identified by the Imagemaster 2D Platinum 5.0 software and visualized using CBB staining protocol followed by mass spectroscopy analysis.
Authors’ contributions
JL conceived the method, designed and carried out the validation of the study and drafted the manuscript. YH designed the study and revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors have declared that no competing interests exist.
Acknowledgements
We thank Professor Wen-Xiong Lin from Fujian Agriculture and Forestry University for valuable advice on this project, and thank Dr Steven Tudor from Genetic Transformation Lab, The Samuel Roberts Noble Foundation for critical reading of the manuscript. This study was funded by the National Natural Science Foundation of China (Grant No. 30860141).
References
- 1.Herbert B. Advances in protein solubilisation for two-dimensional electrophoresis. Electrophoresis. 1999;20:660–663. doi: 10.1002/(SICI)1522-2683(19990101)20:4/5<660::AID-ELPS660>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 2.Sarma A.D. Plant protein isolation and stabilization for enhanced resolution of two-dimensional polyacrylamide gel electrophoresis. Anal. Biochem. 2008;379:192–195. doi: 10.1016/j.ab.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 3.Anderson L., Anderson N.G. High resolution two-dimensional electrophoresis of human plasma proteins. Proc. Natl. Acad. Sci. USA. 1977;74:5421–5425. doi: 10.1073/pnas.74.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Farrell P.H. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 5.Walton S.P., Jayaraman A. Proteomics: technology development and applications. Expert Rev. Proteomics. 2009;6:23–25. doi: 10.1586/14789450.6.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez J.C., Hochstrasser D.F. High-resolution, IPG-based, mini two-dimensional gel electrophoresis. Methods Mol. Biol. 1999;112:227–233. doi: 10.1385/1-59259-584-7:227. [DOI] [PubMed] [Google Scholar]
- 7.Keating D.H. Optimized two-dimensional thin layer chromatography to monitor the intracellular concentration of acetyl phosphate and other small phosphorylated molecules. Biol. Proced. Online. 2008;10:36–46. doi: 10.1251/bpo141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keidel E.M. Evaluation of protein loading techniques and improved separation in OFFGEL isoelectric focusing. Electrophoresis. 2011;32:1659–1666. doi: 10.1002/elps.201000544. [DOI] [PubMed] [Google Scholar]
- 9.Poon H.F. Improving image analysis in 2DGE-based redox proteomics by labeling protein carbonyl with fluorescent hydroxylamine. Biol. Proced. Online. 2007;9:65–72. doi: 10.1251/bpo134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn P. From genome to proteome: looking at a cell’s proteins. Science. 1995;270:369–370. doi: 10.1126/science.270.5235.369. [DOI] [PubMed] [Google Scholar]
- 11.Wilkins M.R. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol. Genet. Eng. Rev. 1996;13:19–50. doi: 10.1080/02648725.1996.10647923. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy D. The importance of rice. Science. 2002;296:13. doi: 10.1126/science.296.5565.13. [DOI] [PubMed] [Google Scholar]
- 13.Jagadish S.V. High temperature stress and spikelet fertility in rice (Oryza sativa L.) J. Exp. Bot. 2007;58:1627–1635. doi: 10.1093/jxb/erm003. [DOI] [PubMed] [Google Scholar]
- 14.Rabilloud T. Silver staining of 2-D electrophoresis gels. Methods Mol. Biol. 1999;112:297–305. doi: 10.1385/1-59259-584-7:297. [DOI] [PubMed] [Google Scholar]
- 15.Heukeshoven J., Dernick R. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis. 1988;9:28–32. doi: 10.1002/elps.1150090106. [DOI] [PubMed] [Google Scholar]
- 16.Sinha P. A new silver staining apparatus and procedure for matrix-assisted laser desorption/ionization-time of flight analysis of proteins after two-dimensional electrophoresis. Proteomics. 2001;1:835–840. doi: 10.1002/1615-9861(200107)1:7<835::AID-PROT835>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Hochstrasser D.F. Development of polyacrylamide gels that improve the separation of proteins and their detection by silver staining. Anal. Biochem. 1988;173:412–423. doi: 10.1016/0003-2697(88)90208-4. [DOI] [PubMed] [Google Scholar]
- 18.Yan J.X. A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization-mass spectrometry. Electrophoresis. 2000;21:3666–3672. doi: 10.1002/1522-2683(200011)21:17<3666::AID-ELPS3666>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Pink M. CBB staining protocol with higher sensitivity and mass spectrometric compatibility. Electrophoresis. 2010;31:593–598. doi: 10.1002/elps.200900481. [DOI] [PubMed] [Google Scholar]
- 20.Wang X. A modified Coomassie Brilliant Blue staining method at nanogram sensitivity compatible with proteomic analysis. Biotechnol. Lett. 2007;29:1599–1603. doi: 10.1007/s10529-007-9425-3. [DOI] [PubMed] [Google Scholar]
- 21.Choi J.K., Yoo G.S. Fast protein staining in sodium dodecyl sulfate polyacrylamide gel using counter ion-dyes, Coomassie Brilliant Blue R-250 and neutral red. Arch Pharm Res. 2002;25:704–708. doi: 10.1007/BF02976948. [DOI] [PubMed] [Google Scholar]
- 22.Chen H. One-step Coomassie Brilliant Blue R-250 staining of proteins in polyacrylamide gel. Anal. Biochem. 1993;212:295–296. doi: 10.1006/abio.1993.1330. [DOI] [PubMed] [Google Scholar]
- 23.Lin S.K. Proteomic analysis of the expression of proteins related to rice quality during caryopsis development and the effect of high temperature on expression. Proteomics. 2005;5:2140–2156. doi: 10.1002/pmic.200401105. [DOI] [PubMed] [Google Scholar]
- 24.Tsugita A. Separation and characterization of rice proteins. Electrophoresis. 1994;15:708–720. doi: 10.1002/elps.1150150198. [DOI] [PubMed] [Google Scholar]
- 25.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Liao J.L. Identification on heat tolerance of backcross recombinant rice lines and screening of backcross introgression lines with heat tolerance at milky stage. Rice Science. 2011;18:279–286. [Google Scholar]