Abstract
Background and Objectives
We conducted a review of current data on respiratory syncytial virus (RSV) prophylaxis with palivizumab, in Korean children with congenital heart diseases (CHD). In 2009, the Korean guideline for RSV prophylaxis had established up to five shots monthly per RSV season, only for children <1 year of age with hemodynamic significance CHD (HS-CHD).
Subjects and Methods
During the RSV seasons in 2009-2015, we performed a retrospective review of data for 466 infants with CHD, examined at six centers in Korea.
Results
Infants received an average of 3.7±1.9 (range, 1-10) injections during the RSV season. Fifty-seven HS-CHD patients (12.2%) were hospitalized with breakthrough RSV bronchiolitis, with a recurrence in three patients, one year after the initial check-up. Among patients with simple CHD, only five (1.1%) patients received one additional dose postoperatively, as per the limitations set by the Korean guideline. Among the 30 deaths (6.4%), five (1.1%) were attributed to RSV infection; three to simple CHD, one to Tetralogy of Fallot, and one to hypertrophic cardiomyopathy (HCM). Of the three HCM patients that exceeded guidelines for RSV prophylaxis, two (66.6%) were hospitalized, and one died of RSV infection (33.3%).
Conclusion
In accordance to the Korean guideline, minimal injections of palivizumab were administered to patients having HS-CHD <one year of age during the RSV season; the risk of RSV infection remains significant among children with simple CHD, cardiomyopathy, and children above the age of one year with HS-CHD.
Keywords: Respiratory syncytial virus, Congenital heart disease, Pediatrics, Palivizumab, Prophylaxis
Introduction
Respiratory syncytial virus (RSV) infection is one of the most common causes of bronchiolitis and pneumonia in infants who require hospitalization worldwide. The prevalence of hospitalization for RSV infection is highest in infants less than 6 months old, and decreases after two years of age.1)
The risk of severe RSV infection with significant morbidity and mortality is greatest in premature infants, infants with chronic lung disease, immune deficiency, and hemodynamically significant congenital heart disease (HS-CHD).2),3) HS-CHD patients often have several pathophysiological issues such as altered pulmonary blood flow, cyanosis, pulmonary hypertension, and ventilation-perfusion mismatch, which can exacerbate lower respiratory tract infections. Therefore, RSV infection in those infants is associated with longer hospitalization, higher admission rates to the intensive care unit (ICU), and more frequent requirement for mechanical ventilation.4),5),6) Recent studies have suggested that among the infants with congenital heart disease (CHD) who are hospitalized with RSV infection, 33% will require treatment in an ICU, and as many as 7.3% will die as a result of complications from RSV infection. The mortality rates associated with RSV infection in CHD have been reported significantly higher than in infants without underlying diseases.7),8),9)
For the past 10 years, the humanized monoclonal antibody, palivizumab, has been used as prophylaxis for serious RSV infection in high risk infants, and has resulted in a significant reduction in hospitalization rates for children <24 months of age suffering from HS-CHD.10) Reflecting the global trend, Korean guidelines by the by Korean National Health Insurance Review and Assessment Service, have approved the use of palivizumab in children <1 year of age with HS-CHD since 2009 (Table 1). These criteria, as suggested by Feltes et al.,7),11) have been applied in the recommendation of indicated RSV prophylaxis in many countries, but only up to 2 years of age. This is the first study to evaluate the compliance with the Korean guideline on prophylactic treatment for RSV in pediatric CHD patients. We retrospectively analyzed current data on RSV prophylaxis to determine the prevalence of morbidity in children with CHD in Korea, who developed RSV infection despite receiving prophylaxis as per the Korean guidelines.
Table 1. The Korean guideline for the use of palivizumab prophylaxis for infants with congenital heart disease (last updated in 2009).
| Children ≤1 year of age with hemodynamically significant congenital heart disease at the start of RSV season (from October to March) |
|---|
| 1. Infants who are receiving medication for congestive heart failure |
| 2. Infants with moderate to severe pulmonary arterial hypertension |
| 3. Infants with cyanotic heart disease |
RSV: respiratory syncytial virus
Subjects and Methods
We conducted a retrospective, multicenter study at six major tertiary care children's hospitals (Ajou University School of Medicine, Sejong General Hospital, Pucheon, Seoul National University Children's Hospital, Sungkyunkwan University Samsung Medical Center, Seoul, Ulsan University Asan Medical Center and Yonsei University Cardiovascular Center, Korea) during 6-year seasonal period, from 1st October 2009 to 31st March 2015. We examined hospital medical records of all children with CHD who had received palivizumab according to the prophylaxis guideline and clinical decision after approval of the Investigated Research Board (AJIRB-MED-MDB-11-351). A total of 466 children with CHD received 1-5 injections of palivizumab (15 mg/kg) by intramuscular injection every 30 days, over one RSV season (October to February). CHD were identified by code based on the 10th revision of International Classification of Diseases and Related Health Problems (ICD-10). The Korean guidelines for RSV prophylaxis suggest that children less than 1 year of age with HS-CHD are most likely to benefit from prophylaxis. HS-CHD was defined as uncorrected or palliated cyanotic CHD, or acyanotic CHD associated with documented pulmonary hypertension, and/or a requirement for medication to manage congestive heart failure (CHF).
Information collected for each patient included date of birth, age at first dose, follow up period, gender, structural type of CHD, and the number of palivizumab injections received before and after RSV infection. Additional data reviewed included the time between the actual start of treatment and the recommendation in the national guideline (October or the first month of life, if the child was born during the RSV season). Any underlying diagnoses of chromosomal defects, prematurity and low birth weight and status of cardiovascular surgery undergone, that involved cardiopulmonary bypass, were reviewed.
We also investigated the patients hospitalized for RSV infection despite receiving palivizumab; factors included their age and calendar month at RSV infection, and factors related to infection severity. For infection severity, we reviewed their visit to the emergency department and ICU admissions, hospital and ICU length of stay, oxygen requirements, mechanical ventilation use, and delay of elective operation. According to different types of heart disease, the rate of RSV infection, all-cause and RSV related mortality, and doses of palivizumab were analyzed.
RSV infection was confirmed by direct fluorescent antibody staining, culture, or reverse-transcriptase polymerase chain reaction on nasopharyngeal swabs or aspirates according to policy of each center. We noted the number of patients with breakthrough RSV infection and mortality, by comparison to doses of palivizumab prophylaxis according to type of CHD.
Data were entered into the Statistical Package for the Social Sciences (version 18.0, SPSS Inc., Chicago, IL, USA) program and analyzed using descriptive statistics. All continuous variables were analyzed using standard descriptive statistics with mean±standard deviation values. Statistical significance was defined as p<0.05.
Results
From October 2009 to March 2015, 466 patients received RSV prophylaxis with palivizumab at six major pediatric cardiac centers in Korea. The mean age of patients at the start of prophylaxis was 2.9±2.8 months (range, 0-11.2 months), and the mean follow-up period was 24.3±16.4 months. The male to female ratio was 1.03 (male:female=237:229).
The main indication for RSV prophylaxis in this study was moderate to severe pulmonary hypertension (n=213; 45.7%), followed by cyanotic heart disease (n=196; 42.1%) and CHF requiring medication (n=115; 24.7%). A total of 4 patients (0.9%) (3 with HCM and 1 with mitral stenosis) had received RSV prophylaxis according to the clinician's recommendation, regardless of exclusive criteria of the Korean guideline of RSV prophylaxis for CHD patients.
Co-morbidities other than CHD were found in 127 patients (27.3%): chromosomal anomalies were found in 65 (13.9%), low birth weight baby in 33 (7.1%), and prematurity in 35 patients (7.5%).
Most patients (n=442; 94.8%) underwent either palliative or definitive cardiac surgery before (60.3%) and after (39.7%) the start of palivizumab vaccination. After cardiac surgery that involved cardiopulmonary bypass, 5 (1.1%) patients received one additional dose immediately postoperatively (Table 2).
Table 2. Patients' characteristics (N=466).
| Characteristics | Numbers (%) |
|---|---|
| Male:female | 237 (50.9%):229 (49.1%) |
| Age at first injection of palivizumab (month) | 2.9±2.8 (range, 0–11.2) |
| Mean doses of palivizumab underwent cardiovascular surgery (n=442) | 3.7±1.9 (range, 1–10) (94.8%) |
| Co-Morbidities | |
| Prematurity | 35 (7.5%) |
| Low birth weight | 33 (7.1%) |
| Chromosomal anomalies or syndromes | 65 (13.9%) |
| RSV bronchiolitis | 57 (12.2%) |
| Respiratory infection associated with other viruses requiring in-hospitalization treatment | 40 (8.6%) |
| Total deaths | 30 (6.4%) |
RSV: respiratory syncytial virus
The mean number of received injections was of 3.7±1.9 per patient (range, 1-10). Only 146 patients (31%) received more than five injections following the guidelines, whereas the majority (n=320; 69%) received less than four injections, including 166 patients (36%) who received only one injection (Fig. 1). Among the patients, 7 who had been born prematurely received palivizumab injections until they reached 2 years of age, according to the Korean guideline for prematurity. There were 182 patients (39%) who did not start the first injection of palivizumab at the recommended time – in the first October or the first month of life if the child was born during the RSV season – according to the guideline, with an average delay of 2.7±2.6 months (range, 1-4 months) (Figs. 1, 2, and 3).
Fig. 1. The number of patients according to palivizumab injections (N=466). The mean number who received injections was 3.7±1.9 per patient (range, 1-10). Only 146 patients (31%) received more than five injections because of prematurity, whereas the majority (n=320; 69%) received less than four injections, including 166 patients (36%) who received only one injection.
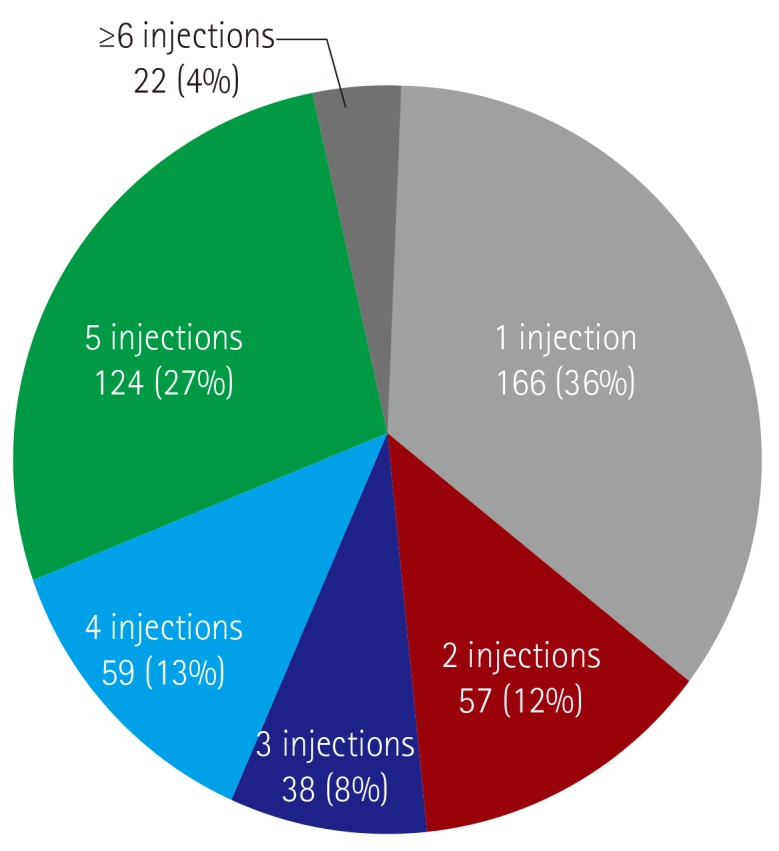
Fig. 2. The number of patients starting treatment during the recommended time of the year, in accordance with the national guideline. There were 182 patients (39%) who did not start the first injection of palivizumab at the right time according to the guideline, with an average delay of 2.7±2.6 months (range, 1-4 months).
Fig. 3. The serial infection rate of RSV according to each calendar month. Fifty-seven patients (12.2%) were hospitalized with breakthrough RSV bronchiolitis after prophylaxis. The mean age of these patients was 7.9±5.7 months (range, 1.6-23.8), and most (86%) experienced frequent RSV infections between October and the following January. RSV: respiratory syncytial virus.
Among the 466 patients with CHD during the follow up period, 39% had no respiratory symptoms, 61% had respiratory symptoms. Of the patients having respiratory symptoms, 49% had non-RSV respiratory infections and 12% had RSV infection. Fifty-seven patients (12.2%) were hospitalized with breakthrough RSV bronchiolitis after prophylaxis. Other respiratory virus infections with rhinovirus, parainfluenza virus, influenza virus, adenovirus, metapneumovirus, bocavirus, coronavirus, and Epstein-Barr virus affected 24 patients (42.1%). Prophylactically treated CHD patients with RSV infection who required hospitalization had received an average of 3.6±1.9 injections (range, 1-8). The mean age of these patients was 7.9±5.7 months (range, 1.6-23.8), with a maximum (86%) experiencing frequent RSV infections between October and the following January (RSV season; Fig. 3). These CHD patients were diagnosed as systemic-pulmonary shunt defects (42.1%), univentricular heart (10.5%), pulmonary hypertension (3.5%), left side outflow obstruction (12.2%), right side outflow obstruction (1.7%), Tetralogy of Fallot (22.8%), other complex cyanotic heart disease (5.2%) and cardiomyopathy (5.2%). Patient information is displayed in Tables 3 and 4. About 60% of the patients visited the emergency room and were hospitalized for an average of 15.7±5.2 days (range, 3-60 days). One-fourth of the patients (24.6%) required admission to the ICU; 8 patients (14%) needed supplemental oxygen, and 5 patients (8.8%) needed mechanical ventilation; however, extracorporeal membrane oxygenation was not required for any patient (Table 3). Three patients were hospitalized twice, once before and once after the 1-year-old check-up. Total of 5 patients were diagnosed as RSV infection after the first year follow-up, including these 3 patients.
Table 3. Characteristics of prophylactically treated CHD patents requiring hospitalization for RSV infection (N=57).
| Characteristics | Numbers |
|---|---|
| Total number of injections (shots, mean±SD ) | 3.6±1.9 (range, 1–8) |
| Number of injections prior to RSV infection | 1.4±1.6 (range, 0–6) |
| Number of injections post to RSV infection | 2.1±1.7 (range, 0–5) |
| Age at RSV infection (months, mean±SD) | 7.9±5.7 (range, 1.6–23.8) |
| Co-Morbidities | 13 (22.8%) |
| Prematurity | 6 (10.5%) |
| Low birth weight | 2 (3.5%) |
| Chromosomal anomalies or syndromes | 5 (8.8%) |
| Other respiratory viral co-infections | 24 (42.1%) |
| Visits to emergency room | 33 (57.9%) |
| Days of hospitalization (days, mean±SD) | 15.7±5.2 (range, 3–60) |
| Required oxygen | 8 (14.0%) |
| Delay of elective surgery | 4 (7.0%) |
| ICU admission | 14 (24.6%) |
| Ventilator requirement | 5 (8.8%) |
| Deaths related to RSV infection | 5 (8.8%) |
| Simple left-to-right shunt lesions | 3 |
| Tetralogy of Fallot | 1 |
| Hypertrophic cardiomyopathy | 1 |
CHD: congenital heart disease, RSV: respiratory syncytial virus, SD: standard deviation, ICU: intensive care unit
Table 4. Comparison data of death, RSV infection, and the number of palivizumab injections according to underlying heart diseases (N=466).
| Heart diseases | Number of patients (%) | Number of RSV positive (%) | Total deaths | Deaths related to RSV | Average number of palivizumab injections |
|---|---|---|---|---|---|
| Systemic-pulmonary shunt defects (AVSD, ASD, VSD) | 176 (37.7) | 24 (13.6) | 7 | 3 | 2.51 |
| Tetralogy of Fallot | 114 (24.4) | 13 (11.4) | 6 | 1 | 3.37 |
| Univentricular heart | 59 (12.7) | 6 (10.2) | 9 | - | 3.63 |
| Left side outflow obstructions (AS, CoA, IAA) | 43 (9.2) | 7 (16.3) | - | - | 2.77 |
| Right side outflow obstructions (PA, PS) | 23 (4.9) | 1 (4.3) | - | - | 3.55 |
| PH (Prematurity, Down syndrome with small L-R shunt defect) | 13 (2.8) | 2 (15.4) | 3 | - | 3.85 |
| Other complex cyanotic heart disease | |||||
| Complete TGA with/without VSD | 15 (3.2) | 1 (6.7) | - | - | 2.98 |
| TAPVR | 14 (3.0) | 1 (7.1) | 2 | - | 3.07 |
| Anomalous LCA from pulmonary artery | 3 (0.6) | 1 (33.3) | - | - | 3.67 |
| Cardiomyopathy | |||||
| Hypertrophic cardiomyopathy | 3 (0.6) | 2 (66.6) | 2 | 1 | 3.67 |
| Dilated cardiomyopathy | 5 (1.1) | 1 (20.0) | - | - | 2.59 |
| Total | 466 | 57 (12.2) | 30 (6.4) | 5 (1.1) | 3.71 |
RSV: respiratory syncytial virus, AVSD: atrioventricular septal defect, ASD: atrial septal defect, VSD: ventricular septal, AS: aortic stenosis, CoA: coarctation of aorta, IAA: interrupted aortic arch, PA: pulmonary atresia, PS: pulmonary stenosis, PH: pulmonary hypertension, TGA: transposition of great arteries, TAPVR: total anomalous pulmonary venous return, LCA: left coronary artery
Comparison of mortality data between RSV infection and doses of palivizumab according to the structural type of CHD, revealed that one-third of the children (n=176; 37.7%) were classified as having shunt lesions with pulmonary hypertension. Among these, 24 patients (13.6%) were diagnosed with RSV infection. There were 7 in-hospital deaths of patients with shunt lesions, 3 of which were related to RSV infection. In the group of 59 patients (12.7%) with univentricular heart defects, there were 9 deaths, but none was related to RSV infection. The group with HCM had larger breakthrough rate of RSV infection: out of 3 patients, 2 (66.6%) were treated for RSV infection. In addition, of the 2 deaths reported in this group, 1 was related to RSV infection.
During follow-up, all-cause mortality was reported as 30 for this group (6.4%). In 19 patients who died from cardiac issues, 3 deaths were from early postoperative complications; 11 patients died from other morbidities, including 5 patients (1.1%) due to RSV infection (Table 4).
Discussion
The effectiveness of prophylactic palivizumab for prevention of RSV infection in CHD patients was shown by a randomized controlled study in 1998.7) Palivizumab, a humanized monoclonal antibody against RSV, has been the gold standard for RSV prophylaxis in high risk groups, and recently was shown by a Cochrane meta-analysis to have significantly reduced the hospitalization rate of infected patients.10) The study showed a 45% relative reduction in RSV hospitalization among high risk children receiving palivizumab (9.7% vs. 5.3%; p=0.003). before the palivizumab era, about more than 10% of all CHD infants required hospital admission for RSV infection and some needed intensive care; however, palivizumab vaccination at the start of RSV season has been proven to reduce the rate of hospitalizations by half (~5%).7),12),13)
In Korea, according to the biweekly reports from the Korea Centers for Disease Control and Prevention, the seasonal outbreaks of RSV infection occur during the winter months, namely September through March. Almost all children are susceptible to RSV infection until they reach the age of 2 years, and on an average, nearly half will experience two episodes of infection.2),9) Prior to the palivizumab era (from 2003 to 2006), in a study of 213 patients diagnosed with lower respiratory infection, 45 CHD patients (21.1%) were hospitalized due to RSV infection, 22 (48.9%) were treated in the ICU, 12 (26.7%) required mechanical ventilation, and 2 (4.4%) died; however, no deaths occurred among non-CHD patients.14)
In our study, the rate of mortality related to RSV infection (1.1%) was reduced significantly but was still a threat to infants with HS-CHD even after palivizumab injection, because the monoclonal antibody tends to attenuate but not eradicate RSV disease.5),9),15),16),17) Moreover, the rate of RSV-associated hospitalization of children <12 months old with HS-CHD who received more than one prophylactic injection was 12.5%, which was relatively higher than in previous reports from other countries (0.46% to 5.3%).18) One-fourth of the patients were admitted to the ICU with RSV infection, and 3 patients were hospitalized for longer than 4 weeks. This could relate to incomplete vaccination, which would point to a lack of compliance with the guideline, as well as increased testing for RSV.5),7),11),19),20),21),22),23)
Many cases started prophylaxis either earlier (n=70, 15%) or later (n=182, 39%) than the recommendation, and it can be argued that delays in identifying patients at risk could have affected the outcome.23) Monthly injections of up to five injections are recommended once patients start treatment; however, the majority (69%) of patients in this study received less than four, including 36% who received only one injection. Therefore, factors involved in effective prophylactic treatment include ensuring that treatment is initiated at the right time, and that children with CHD are identified and followed up. Missing or delayed injections may lead to increased rates of re-hospitalization, making it essential to adhere to prophylactic guidelines.24),25)
According to many studies that have reported advantages in socio-economic aspects of prophylaxis of RSV infection in CHD patients under the age of 2 years, recommendations from the guidelines of Canada, Japan, Germany, and Austria for the use of palivizumab have included children younger than 24 months of age.8) In contrast, the Korean guideline has recommend 12 months as the upper age limit. Our study noted that 3 patients were hospitalized with RSV infection after 1 year of age, who were not treated after the recommended age of 12 months.
The majority of CHD patients in this study (35.6%) had shunt lesions with pulmonary hypertension, and 94.8% had undergone corrective surgery. Because the Korean guideline for the use of palivizumab in infants with CHD limits the vaccination to the infants who are receiving medication for congestive heart failure, patients who showed improvement of heart failure after cardiovascular surgery usually had terminated medication, and therefore, were not eligible for additional vaccinations. Moreover, RSV pneumonia is associated with a high risk of postoperative complications.4),26),27) In this study, there were 5 deaths related to RSV infection; of these, underlying heart diseases for 3 among the 5 deaths were associated with simple left-right shunt lesion. The American Association of Pediatricians has recommended administration of another injection of palivizumab (15 mg/kg) as soon as possible in postoperative patients because serum palivizumab levels decrease by 58% after cardiopulmonary bypass.15),16),28) Therefore, it is essential to supplement this guideline, as well as adhere to the existing guideline, ensuring that treatment is started at the right time and that an additional postoperative injection is given immediately. The emphasis should be the identification of children with CHD.
Cardiomyopathies as well as other structural heart diseases are major risk factors for RSV hospitalization in infants with CHD.20),29),30) Furthermore, in our study, HCM patients were placed into the high risk group with respect to RSV infection because 66.6% (n=2) had contracted RSV infection and 20% (n=1) of all RSV-related mortality occurred in the HCM group. Stagnant pulmonary flow due to cardiomyopathy results in significantly abnormal hemodynamics with perivascular infiltrates, leading to altered vascular tone and permeability changes in the alveolar-capillary interface, which is further exaggerated by the inflammation caused by RSV.29) Therefore, the USA, UK, Japan, France, and Austria have adopted the recommendations for infants with cardiomyopathy as co-morbidity.8) These findings should be taken into consideration when deciding which children should receive prophylaxis.
There were a few of limitations to this study. A recent systematic review of the cost-effectiveness of palivizumab prophylaxis compared with no prophylaxis in infants and young children with CHD showed a favorable trend, but were not addressed in this study.30) We are also still collecting data for further study of the treatment effect of palivizumab against RSV in a large population, which is warranted.
For CHD patients in Korea, small doses and omitting or delaying palivizumab prophylaxis were found in major pediatric centers during the 2009-2015 RSV seasons. Even though the current data on RSV prophylaxis has been collected for infants with CHD in Korea, the data might actually be helpful for CHD patients if the Korean guideline would be extended to children up to 24 months of age with HS-CHD, including cardiomyopathy, and postoperative CHD patients. We recommend that the guideline should be updated based on the key findings: (i) the risk of hospitalization due to RSV infection remains high among children with CHD, (ii) children with CHD exhibit a higher rate of morbidity despite prophylactic treatment than that reported in recent studies,11),18),19),20),21),22) and (iii) since the RSV season of Korea is not different with that of other countries, the national guideline might be regarded as outdated by international guidelines.23) In Korean guidelines, the patient group with HCM was not supported financially. Thus, only a few patients with HCM were included because patients who had received palivizumab injection were rare.
This is the first and largest study to evaluate compliance according to the Korean guideline for prophylactic treatment, and to determine that morbidity and mortality due to RSV infection is still significant in children with CHD. According to the Korean guidelines, the risk of RSV infection remains significant among children with hemodynamically insignificant CHD, cardiomyopathy, and children above 1 year-old with HS-CHD.
Acknowledgments
This study was supported by Korean Pediatric Cardiology Society in 2011.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Schanzer DL, Langley JM, Tam TW. Hospitalization attributable to influenza and other viral respiratory illnesses in Canadian children. Pediatr Infect Dis J. 2006;25:795–800. doi: 10.1097/01.inf.0000232632.86800.8c. [DOI] [PubMed] [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. 2003;143(5 Suppl):S112–S117. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 4.Altman CA, Englund JA, Demmler G, et al. Respiratory syncytial virus in patients with congenital heart disease: a contemporary look at epidemiology and success of preoperative screening. Pediatr Cardiol. 2000;21:433–438. doi: 10.1007/s002460010103. [DOI] [PubMed] [Google Scholar]
- 5.Chang RK, Chen AY. Impact of palivizumab on RSV hospitalizations for children with hemodynamically significant congenital heart disease. Pediatr Cardiol. 2010;31:90–95. doi: 10.1007/s00246-009-9577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabalka AK. Physiologic risk factors for respiratory viral infections and immunoprophylaxis for respiratory syncytial virus in young children with congenital heart disease. Pediatr Infect Dis J. 2004;23(1 Suppl):S41–S45. doi: 10.1097/01.inf.0000108220.94201.1a. [DOI] [PubMed] [Google Scholar]
- 7.Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–540. doi: 10.1067/s0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 8.Resch B, Michel-Behnke I. Respiratory syncytial virus infections in infants and children with congenital heart disease: update on the evidence of prevention with palivizumab. Curr Opin Cardiol. 2013;28:85–91. doi: 10.1097/HCO.0b013e32835dce2f. [DOI] [PubMed] [Google Scholar]
- 9.Jung JW. Respiratory syncytial virus infection in children with congenital heart disease: global data and interim results of Korean RSV-CHD survey. Korean J Pediatr. 2011;54:192–196. doi: 10.3345/kjp.2011.54.5.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev. 2013;4:CD006602. doi: 10.1002/14651858.CD006602.pub4. [DOI] [PubMed] [Google Scholar]
- 11.Feltes TF, Sondheimer HM, Tulloh RM, et al. A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatr Res. 2011;70:186–191. doi: 10.1203/PDR.0b013e318220a553. [DOI] [PubMed] [Google Scholar]
- 12.Yount LE, Mahle WT. Economic analysis of palivizumab in infants with congenital heart disease. Pediatrics. 2004;114:1606–1611. doi: 10.1542/peds.2004-0224. [DOI] [PubMed] [Google Scholar]
- 13.Meissner HC, Long SS American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics. 2003;112(6 Pt 1):1447–1452. doi: 10.1542/peds.112.6.1447. [DOI] [PubMed] [Google Scholar]
- 14.Shim WS, Lee JY, Song JY, et al. Respiratory syncytial virus infection cases in congenital heart disease patients. Korean J Pediatr. 2010;53:380–391. [Google Scholar]
- 15.Saji T, Nakazawa M, Harada K. Nationwide survey of palivizumab for respiratory syncytial virus prevention in Japanese children with congenital heart disease. Pediatr Infect Dis J. 2008;27:1108–1109. doi: 10.1097/INF.0b013e3181801d76. [DOI] [PubMed] [Google Scholar]
- 16.Rackham OJ, Thorburn K, Kerr SJ. The potential impact of prophylaxis against bronchiolitis due to the respiratory syncytial virus in children with congenital cardiac malformations. Cardiol Young. 2005;15:251–255. doi: 10.1017/S1047951105000533. [DOI] [PubMed] [Google Scholar]
- 17.Feltes TF, Sondheimer HM. Palivizumab and the prevention of respiratory syncytial virus illness in pediatric patients with congenital heart disease. Expert Opin Biol Ther. 2007;7:1471–1480. doi: 10.1517/14712598.7.9.1471. [DOI] [PubMed] [Google Scholar]
- 18.Butt M, Symington A, Janes M, et al. Respiratory syncytial virus prophylaxis in children with cardiac disease: a retrospective single-centre study. Cardiol Young. 2014;24:337–343. doi: 10.1017/S1047951113000401. [DOI] [PubMed] [Google Scholar]
- 19.Medrano López C, García-Guereta L CIVIC Study Group. Community-acquired respiratory infections in young children with congenital heart diseases in the palivizumab era: the Spanish 4-season civic epidemiologic study. Pediatr Infect Dis J. 2010;29:1077–1082. doi: 10.1097/INF.0b013e3181efdac5. [DOI] [PubMed] [Google Scholar]
- 20.Alexander PM, Eastaugh L, Royle J, Daley AJ, Shekerdemian LS, Penny DJ. Respiratory syncytial virus immunoprophylaxis in high-risk infants with heart disease. J Paediatr Child Health. 2012;48:395–401. doi: 10.1111/j.1440-1754.2011.02219.x. [DOI] [PubMed] [Google Scholar]
- 21.Cohen SA, Zanni R, Cohen A, et al. Palivizumab use in subjects with congenital heart disease: results from the 2000-2004 Palivizumab Outcomes Registry. Pediatr Cardiol. 2008;29:382–387. doi: 10.1007/s00246-007-9039-5. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell I, Paes BA, Li A, Lanctôt KL CARESS investigators. CARESS: the Canadian registry of palivizumab. Pediatr Infect Dis J. 2011;30:651–655. doi: 10.1097/INF.0b013e31821146f7. [DOI] [PubMed] [Google Scholar]
- 23.Granbom E, Fernlund E, Sunnegårdh J, Lundell B, Naumburg E. Evaluating national guidelines for the prophylactic treatment of respiratory syncytial virus in children with congenital heart disease. Acta Paediatr. 2014;103:840–845. doi: 10.1111/apa.12658. [DOI] [PubMed] [Google Scholar]
- 24.Parnes C, Guillermin J, Habersang R, et al. Palivizumab prophylaxis of respiratory syncytial virus disease in 2000-2001: results from The Palivizumab Outcomes Registry. Pediatr Pulmonol. 2003;35:484–489. doi: 10.1002/ppul.10288. [DOI] [PubMed] [Google Scholar]
- 25.Golombek SG, Berning F, Lagamma EF. Compliance with prophylaxis for respiratory syncytial virus infection in a home setting. Pediatr Infect Dis J. 2004;23:318–322. doi: 10.1097/00006454-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Khongphatthanayothin A, Wong PC, Samara Y, et al. Impact of respiratory syncytial virus infection on surgery for congenital heart disease: postoperative course and outcome. Crit Care Med. 1999;27:1974–1981. doi: 10.1097/00003246-199909000-00042. [DOI] [PubMed] [Google Scholar]
- 27.Moler FW, Khan AS, Meliones JN, Custer JR, Palmisano J, Shope TC. Respiratory syncytial virus morbidity and mortality estimates in congenital heart disease patients: a recent experience. Crit Care Med. 1992;20:1406–1413. doi: 10.1097/00003246-199210000-00008. [DOI] [PubMed] [Google Scholar]
- 28.American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:415–420. doi: 10.1542/peds.2014-1665. [DOI] [PubMed] [Google Scholar]
- 29.Chantepie A bureau de la Filiale de Cardiologie Pédiatrique de la Société Française de Cardiologie. Use of palivizumab for the prevention of respiratory syncytial virus infections in children with congenital heart disease. Recommendations from the French Paediatric Cardiac Society. Arch Pediatr. 2004;11:1402–1405. doi: 10.1016/j.arcped.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Kristensen K, Stensballe LG, Bjerre J, et al. Risk factors for respiratory syncytial virus hospitalisation in children with heart disease. Arch Dis Child. 2009;94:785–789. doi: 10.1136/adc.2008.143057. [DOI] [PubMed] [Google Scholar]




