Abstract
U7 small nuclear RNA (snRNA) sequences have been described only for a handful of animal species in the past. Here we describe a computational search for functional U7 snRNA genes throughout vertebrates including the upstream sequence elements characteristic for snRNAs transcribed by polymerase II. Based on the results of this search, we discuss the high variability of U7 snRNAs in both sequence and structure, and report on an attempt to find U7 snRNA sequences in basal deuterostomes and non-drosophilids insect genomes based on a combination of sequence, structure, and promoter features. Due to the extremely short sequence and the high variability in both sequence and structure, no unambiguous candidates were found. These results cast doubt on putative U7 homologs in even more distant organisms that are reported in the most recent release of the Rfam database.
Key words: U7 snRNA, non-coding RNA, RNA secondary structure, evolution
Introduction
The U7 small nuclear RNA (snRNA) is the smallest polymerase II transcript known to date, with a length ranging from only 57 nt (sea urchin) to 70 nt (fruit fly). Its expression level of only a few hundred copies per cell in mammals is at least three orders of magnitude smaller than the abundance of other snRNAs. It is part of the U7 small nuclear ribonucleoprotein (snRNP), which plays a crucial role in the 3′ end processing of histone mRNAs (1). Replication-dependent histone mRNAs in metazoa are the only known eukaryotic protein-coding mRNAs that are not polyadenylated ending but contain a conserved stem-loop sequence instead (2). Beyond metazoan animals, non-polyadenylated histone genes have been described in the algae Chlamydomonas reinhardtii and Volvox carteri (3), and Dictyostelium discoideum has a homolog of the histone RNA hairpin-binding protein/stem-loop-binding protein (HBP/SLBP) (DictyBase: DDB0169192). It appears that replication-dependent histone mRNAs are the only mRNAs that are processed in this way (4).
The 5′ region of the U7 snRNA is complementary to the “histone downstream element” (HDE), located just downstream of the conserved hairpin. The interaction of the U7 snRNP with the HDE is crucial for the correct processing of the histone 3′ elements (1). The 3′ part of the U7 snRNA is occupied by a modified binding domain for Sm proteins consisting of a characteristic sequence motif followed by a conserved stem-loop secondary structure motif (5). U7 snRNA binds five of the seven Sm proteins that are present in spliceosomal snRNAs, while the D1 and D2 subunits are replaced by the Sm-like proteins Lsm10 and Lsm11 6., 7., 8.. This difference is likely to be associated with the differences in the Sm-binding sequence. Recently, the U7 snRNP has not only received considerable attention from a structural biology point of view 9., 10., but also has been investigated as a means of modifying splicing dys-regulation. In particular, U7 snRNA-derived constructs that target a mutant dystrophin gene were explored as a gene-therapy approach to Duchenne muscular dystrophy 11., 12..
Given the attention received by histone RNA 3′ end processing and the protein components of the U7 snRNP, it may come as a surprise that the U7 snRNA itself has received little attention in the last decades. In fact, the only two experimentally characterized mammalian U7 snRNAs are those of mouse 13., 14., 15., 16. and human 1., 17., while most of the earliest work on U7 snRNPs concentrated on the sea urchin Psammechinus miliaris 18., 19., 20., 21. and two Xenopus species 22., 23., 24.. More recently, the U7 snRNA sequences have been reported for Drosophila melanogaster (25) and Takifugu rubripes (26).
We are aware of only two studies that considered U7 snRNA from a bioinformatics point of view. In Lück et al. (27), the U7 snRNA was used as an example for the application of the Construct tool to compute consensus secondary structures, and Bompfünewerer et al. (28) briefly reported on a BLAST-based homology search that uncovered candidate sequences for chicken and two teleost fish.
The U7 snRNP-dependent mode of histone end processing is a metazoan innovation 2., 6.. Nevertheless, the most recent release of the Rfam database (29) (Version 8.0; February 2007) lists sequences from eukaryotic protozoa, plants, and even bacteria. This discrepancy prompted us to critically assess the available information on U7 snRNAs.
Results
Bona fide U7 snRNA sequences
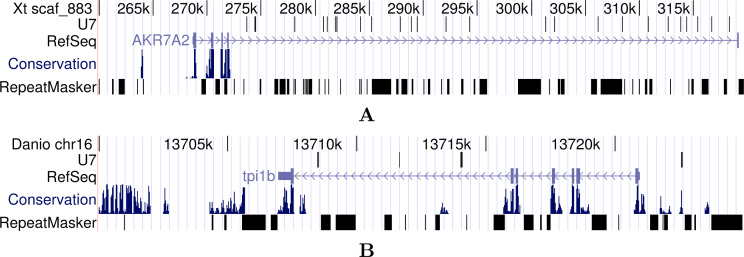
The results of the BLAST-based searches are summarized in Table 1. In most species, only a single gene with clear snRNA-like upstream elements was found. In addition, BLAST identified several pseudogenes. Clusters of U7 snRNAs as previously described for sea urchins and frogs were otherwise only found in zebrafish (Figure 1).
Table 1.
Trusted U7 snRNA sequences*
| Species | Assembly | Sequence | Start | Stop | Ori. | Database ID | ψ |
|---|---|---|---|---|---|---|---|
| Mus musculus | Ensembl 43 | Chr.6 | 124,706,844 | 124,706,905 | − | ENSMUSG00000065217 | 27 |
| Rattus norvegicus | Ensembl 43 | Chr.X | 118,163,804 | 118,163,865 | − | ENSRNOG00000034996 | 31 |
| Rattus norvegicus | Ensembl 43 | Chr.4 | 160,870,934 | 160,870,995 | − | ENSRNOG00000035016 | 31 |
| Homo sapiens | Ensembl 43 | Chr.12 | 6,923,240 | 6,923,302 | + | ENSG00000200368 | 91 |
| Macaca mulatta | Ensembl 43 | Chr.11 | 7,125,496 | 7,125,557 | + | ENSMMUG00000027525 | 95 |
| Otolemur garnettii | PreEnsembl 43 | Scaffold_102959 | 117,572 | 117,633 | − | 0 | |
| Oryctolagus cuniculus | Ensembl 43 | GeneScaffold_1693 | 111,485 | 111,546 | + | 3 | |
| Procavia capensis | NCBI TRACE | 175719230 | 275 | 336 | + | – | |
| Loxodonta africana | Ensembl 43 | Scaffold_60301 | 4,254 | 4,314 | − | 2 | |
| Echinops telfairi | Ensembl 43 | GeneScaffold_2204 | 10,742 | 10,803 | + | ENSETEG00000020899 | 57 |
| Felis catus | Ensembl 43 | GeneScaffold_69 | 192,907 | 192,968 | + | 7 | |
| Canis familiaris | Ensembl 43 | Chr.27 | 41,131,749 | 41,131,810 | − | ENSCAFG00000021852 | 2 |
| Myotis lucifugus | PreEnsembl 43 | Scaffold_168837 | 32,294 | 32,356 | − | 0 | |
| Equus caballus | PreEnsembl 43 | Scaffold_58 | 7,463,562 | 7,463,623 | + | 0 | |
| Bos taurus | Ensembl 43 | Chr.5 | 10,349,126 | 10,349,187 | − | AAFC03061782 | 8 |
| Tursiops truncatus | NCBI TRACE | 194072802 | 598 | 659 | + | – | |
| Dasypus novemcinctus | Ensembl 43 | GeneScaffold_1944 | 24,469 | 24,530 | + | 16 | |
| Spermophilus tridec. | PreEnsembl 43 | Scaffold_139061 | 45,428 | 45,489 | − | 0 | |
| Erinaceus europaeus | Ensembl 43 | GeneScaffold_2232 | 5,133 | 5,194 | + | 30 | |
| Monodelphis domestica | Ensembl 43 | Un | 131,411,333 | 131,411,393 | + | ENSMODG00000022029 | 1 |
| Gallus gallus | Ensembl 43 | Chr.1 | 80,484,148 | 80,484,212 | + | ENSGALG00000017891 | 1 |
| Taeniopygia guttata | NCBI TRACE | TGAB-afg09c06.b1 | 683 | 748 | − | – | |
| Anolis carolinensis | NCBI TRACE | G889P8207RM16.T0 | 106 | 171 | − | – | |
| Xenopus tropicalis | Ensembl 43 | Scaffold_883 | Cluster: ~20 copies from 272,500 to end | ||||
| Xenopus laevis | GenBank | X64404 | Cluster (partial) | ||||
|
Xenopus borealis |
GenBank |
Z54313 |
Cluster (partial) |
||||
| Danio rerio | Ensembl 43 | Chr.16 | Cluster: 4 copies from 13,708,000 to 13,723,000 | ||||
| Takifugu rubripes | Ensembl 43 | Scaffold_205 | 229,679 | 229,736 | + | 0 | |
| Tetraodon nigroviridis | Ensembl 43 | Chr.8 | 9,059,483 | 9,059,541 | + | (1) | |
| Gasterosteus aculeatus | Ensembl 43 | GroupXX | 11,616,333 | 11,616,392 | − | 0 | |
| Oryzias latipes | Ensembl 43 | Chr.16 | 17,393,002 | 17,393,059 | + | 0 | |
| Strongylocentrotus p. | BCM_Spur_v2.1 | Cluster: 2 sequences each on scaffolds 83935 and 88560 | |||||
|
Psammechinus miliaris |
GenBank |
Cluster: 5 genes, 1 sequence=M13311.1 |
|||||
| Drosophila melanogaster | UCSC | 3L | 3,577,355 | 3,577,425 | + | CR33504 | 0 |
| Drosophila ananassae | CAF-1 | CH902618.1 | 9,849,345 | 9,849,414 | − | 0 | |
| Drosophila erecta | CAF-1 | CH954178.1 | 6,292,889 | 6,292,959 | + | 1 | |
| Drosophila grimshawi | CAF-1 | CH916366.1 | 10,347,991 | 10,348,062 | + | 1 | |
| Drosophila mojavensis | CAF-1 | CH933809.1 | 2,924,982 | 2,925,053 | − | 1 | |
| Drosophila persimilis | CAF-1 | CH479328.1 | 89,311 | 89,383 | − | 0 | |
| Drosophila pseudoobscura | CAF-1 | CH379070.2 | 5,738,714 | 5,738,786 | + | 1 | |
| Drosophila simulans | CAF-1 | CM000363.1 | 3,136,652 | 3,136,582 | − | 1 | |
| Drosophila virilis | CAF-1 | CH940647.1 | 4,512,836 | 4,512,907 | − | 1 | |
| Drosophila willistoni | CAF-1 | CH964101.1 | 1,418,210 | 1,418,280 | + | 0 | |
| Drosophila yakuba | CAF-1 | CM000159.2 | 4,146,836 | 4,146,905 | + | 0 | |
ψ gives the number of paralog loci, most likely U7 pseudogenes, defined by a BLAST E-value less than 0.001 compared with the functional copy. CAF-1 refers to the genome freezes provided by the Drosophila Comparative Genomics Consortium. These sequences were retrieved from http://rana.lbl.gov/drosophila/caf1.html in December 2006. The D. melanogaster sequence is the one used by the UCSC Genome Browser (http://genome.ucsc.edu/) (Release 4; Apr. 2004, UCSC version dm2). The sea urchin genome BCM_Spur_v2.1 was obtained from ftp://ftp.hgsc.bcm.tmc.edu/pub/data/Spurpuratus/fasta/Spur_v2.1/linearScaff.
Fig. 1.
Clusters of U7 snRNA genes in Xenopus tropicalis (A) and Danio rerio (B) taken from the USCS Genome Browser (http://genome.ucsc.edu/). The “U7” track shows BLAST matches of the U7 snRNA sequences; “Repeat-Masker” refers to annotated repetitive sequence elements; the “RefSeq” track shows the intron/exon structure of protein-coding genes; the “Conservation” panel displays PhastCons score measuring sequence conservation across vertebrates. We refer to the data track description at the USCS Genome Browser for technical details.
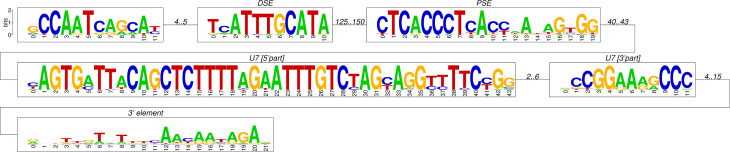
The short length and the substantial divergence of the U7 snRNA sequences make it impossible to distinguish functional U7 snRNAs from pseudogenes based on the U7 sequence alone. To make this distinction, it is necessary to analyze the flanking sequences as well. Bona fide snRNA genes are accompanied by characteristic promoter elements 30., 31.. Figure 2 displays the consensus sequence motifs of the presumably functional amniote U7 snRNAs.
Fig. 2.
Conserved elements in functional U7 snRNA genes. The consensus pattern is the amniote sequences from Table 1. The classical distal sequence element (DSE), proximal sequence element (PSE), and 3′ element of pol-II spliceosomal RNA genes are clearly discernible. The U7 sequence itself is interrupted by a short variable region with substantial length variation.
In human and mouse, several pseudogenes have been described in detail in addition to the functional genes 16., 32.. Notably, several variant U7 snRNA sequences from human HeLa cells were reported in Yu et al. (17). This might indicate that the human genome, in apparent contrast to mouse, also contains more than one functional U7 snRNA gene, or that some of the pseudogenes are transcribed at low levels. Table 1 therefore lists the number of U7-associated loci obtained by BLAST searches that use the presumably functional gene from the same species as query. This number can be fairly large in some mammalian lineages, reaching almost 100 loci in primates. In contrast, in most species there are only a few U7-associated sequences, most of which are readily recognizable as retrogenes by virtue of poly-A tails.
In several genomes, we were not able to find an unambiguous candidate for a functional U7 snRNA, although we found sequences that are clearly derived from U7 but are not accompanied by a recognizable proximal sequence element (PSE). Examples include Sorex araneus and platypus. Most likely, these BLAST hits are pseudogenes, although many of them are annotated with Ensembl gene IDs. This annotation derives from sequence homology with the examples stored in the Rfam database. In Figure 3 and Table 1, we compile the results of our BLAST-based homology search, which contain only sequences that are either experimentally known to be expressed or are predicted to be functional genes based on the presence of conserved upstream elements.
Fig. 3.
Manually curated alignment of functional U7 snRNA sequences. A. The 3′ stem-loop, the Sm-binding site, and the histone-binding regions are highlighted. The 5′ most part of the histone-binding region is not aligned between vertebrate and drosophilid sequences. B. Sequence logos for the partial alignment comprising only tetrapods, teleosts, sea urchins, and flies, respectively, as well as the consensus pattern arising from combining all the data.
Separate multiple sequence alignments of amniotes, teleosts, frogs, sea urchins, and flies reveal strong conservation of the Sm-binding motif, consisting of the deviant Sm-binding site RUUUNUCYNG and the 3′ hairpin structure. Furthermore, the histone-binding region contains a universally conserved box UCUUU (33). Using these features as anchors, we obtained the alignment in Figure 3, which highlights the differences between major clades. Notable variations within the vertebrates are in particular the A-rich 5′ and the reduced stem in teleosts, and their A-rich sequences in the hairpin loop. The hairpin region is very poorly conserved at the sequence level between vertebrates, sea urchins, and flies, although its structural variation is limited in essence to the length of the stem and a few short interior loops or single-nucleotide bulges.
More distant homologs?
The U7 snRNA sequences evolve rather fast. Together with the short sequence length, this limits the power of sequence-based approaches to distant homology search. The consensus pattern in Figure 3 indicates quite clearly that such methods are bound to fail outside the four groups with experimentally known sequences (tetrapods, teleosts, sea urchins, and flies). Indeed, both BLAST and Fragrep (34) did not provide additional candidates that could be unambiguously classified as U7 snRNAs based on sequence information alone.
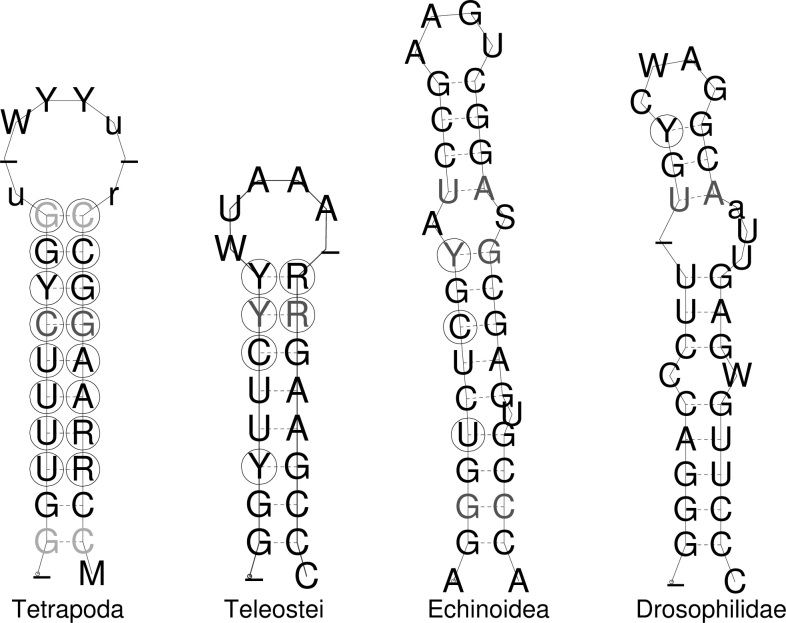
The comparison of the U7 hairpins in the different clades (Figure 4) reveals significant differences in the secondary structures between invertebrates and vertebrates: vertebrates have smaller stem-loop structures with smaller or no interior loops or bulges. The stem in teleosts, furthermore, is systematically shorter than that in tetrapods. These structural differences between clades have to be taken into account for homology search. In fact, as a consensus rule, we can only deduce that the stem-loop structure has a total of 8–15 bp, which is nearly symmetric, and it is enclosed by an uninterrupted stem at least 5 bp in length with 2 GC pairs at its base.
Fig. 4.
Comparison of U7 hairpin structures. Consensus secondary structures are computed using RNAalifold program (39) on the manually improved alignments of tetrapods, teleosts, sea urchins, and flies, respectively. Circles indicate consistent and compensatory mutations that leave the structure intact. Gray letters indicate that one or two of the aligned sequences cannot form the base pair.
Even combined with the conserved sequence motifs in the 5′ part of the molecule, it yields only a rather loose definition of the U7 snRNA. Release 8.0 of the Rfam database (29) lists several sequences in its U7 RNA section that are surprising. Neither contained in the literature nor contained in the manually curated U7 “seed-set”, these candidate sequences were found using a homology search based on Infernal (35) and the seed alignment. While the Danio rerio sequences are identical with the sequences we identified in work starting from the much closer homolog in T. rubripes, the candidates reported for Caenorhabditis elegans and Girardia tigrina raise serious doubts. The C. elegans sequence, although ostensibly well conserved in comparison with the deuterostome sequences, has no recognizable homologs in any one of the other three sequenced Caenorhabditis species, C. briggsae, C. remanei, and C. reinhardii. The G. tigrina sequence is located in the 3′ UTR of the DthoxE-Hox gene (X95413). Both sequences furthermore do not share even the core UUUNUC of the consensus Sm-binding motif.
Several additional candidates were reported in the Rfam database for higher plants and even bacteria. Higher plants apparently do not have the replication-dependent metazoan-style histone 3′ end processing machinery 2., 6., and bacteria do not even have proper histones. It is very unlikely that these sequences are real U7 snRNAs. No conclusive argument can be given at this point for the few isolated U7 snRNA candidates listed in the Rfam database. These examples show once again that at least for very short non-coding RNAs, the results from homology searches have to be taken with caution, in particular when they are not corroborated by additional supporting evidence.
The poor sequence conservation between major groups highlighted in Figure 3 suggests that purely sequence-based homology searches have little chance of success in insect or basal deuterostome genomes. Indeed, neither BLAST nor Fragrep found convincing candidates. We therefore resorted to structure-based approaches and explicitly included the PSE in the search procedure (see Materials and Methods for details). We used RNABOB software with a non-restrictive pattern to find plausible initial candiates, which were then manually compared with the alignment in Figure 3. The most plausible candidates are shown in Figure 5, albeit none of them is unambiguous. No convincing candidates were found in the mosquito Anopheles gambiae and in the honeybee Apis mellifera.
Fig. 5.
Best candidates from searches using RNABOB in Petromyzon marinus, Branchiostoma floridae, and Bombyx mori. In addition to the putative U7 snRNA sequences shown here, these candidate sequences also have a putative PSE associated with them.
Discussion
Since U7 snRNA has its primary function in histone 3′ maturation, it is virtually certain that this class of non-coding RNAs is restricted to metazoan animals—after all, the process in which they play a crucial role is unknown outside multicellular animals. With its length of 70 nt or less, U7 snRNA is the smallest known polymerase II transcript. Each of its three major domains, the histone-binding region, the Sm-binding sequence, and the 3′ stem-loop structure exhibits substantial variation in both sequence and structural details, as can be seen from the detailed sequence alignments (Figure 3) and the structural models of the terminal stem-loop structure (Figure 4). As a consequence, our computational survey not only compiles a large number of previously undescribed U7 homologs from vertebrates and drosophilids, but also stresses the limits of current approaches to RNA homology search.
While BLAST already fails to unambiguously recognize teleost fish homology from mammalian queries and vice versa, even more sophisticated (and computationally expensive) methods have limited success when applied to basal deuterostome or insect genomes. On the other hand, not only the limited sensitivity of current approaches poses a problem; conversely, the most sensitive methods are fooled by false positives, as exemplified by the plant and bacterial sequences in Rfam.
In summary, thus, this study calls both for more experimental data on U7 snRNAs—Which, if any, of our U7 candidate sequences in lamprey or silkworm are really U7 snRNAs in these species?—and for improved bioinformatics approaches for homology search that can deal with such small and rapidly evolving genes.
Materials and Methods
The experimentally known U7 snRNA sequences were retrieved from GenBank database (http://www.ncbi.nlm.nih.gov/entrez). Starting from the known functional mouse gene (GenBank X54748.4), we used the built-in BLAST search function of Ensembl (http://www.ensembl.org; Release 43) to retrieve homologous regions in other mammalian genomes and the chicken genome. Parameters were set to “distance homologies” and repeat-masking was disabled. The resulting sequences were downloaded and aligned using both DIALIGN2 (36) and ClustalW (37) to determine whether the characteristic up- and downstream elements were present. In order to check for consistency, we compared these alignments with the Ensembl genomic alignments of the homologous human locus. In all cases, Ensembl data and our own search gave consistent results. The T. rubripes U7 snRNA sequence described in Myslinksi et al. (26) was used as starting point for searching the teleost fish genomes.
Drosophilid sequences, with the exception of D. melanogaster (which was retrieved from Ensembl), were obtained from the website of the Drosophila Comparative Genomics Consortium (http://rana.lbl.gov/drosophila/caf1.html). The D. melanogaster U7 snRNA region (25) was used as BLAST query, resulting in a unique hit in each of the other drosophilid genomes that exhibits the characteristic upstream elements. In addition, at most one putative pseudogene was found in some species.
Sequence alignments of U7 sequences were generated separately for mammals, sauropsids, teleosts, frogs, sea urchins, and flies using ClustalW. These alignments were combined manually using the RALEE mode for Emacs (38). Consensus secondary structure for a given sequence alignment was computed using RNAalifold (39).
We expanded the tool aln2pattern, the component of the Fragrep distribution (34) that generates a collection of position weight matrixs as search patterns with a “Sequence-Logo” style output derived from the WebLogo PostScript code (40). This provides a convenient way of generating graphical representations of sequence patterns that consist of collections of local motifs from a single multiple sequence alignment.
In addition to purely sequence-based methods, we also searched for more distant homologies based on combined sequence/structure patterns using Sean Eddy’s RNABOB software (downloaded from ftp://ftp.genetics.wustl.edu/pub/eddy/software/rnabob-2.1.tar.Z). We constructed search patterns comprising the most conserved motifs of the histone-binding site, the Sm-binding motif, and the stem-loop structure at the 3′ end that is enclosed by two GC pairs. In order to increase specifity, we additionally included a species-specific model of the PSE, which was derived from the upstream regions of the spliceosomal snRNAs U1, U2, U4, U5, U4atac, U11, and U12. These snRNAs are larger and better conserved than the U7 snRNAs. Hence they were straightforward to find in most of the metazoan genomes where they were not annotated previously. The RNABOB descriptors are listed in Supporting Online Material (http://www.bioinf.uni-leipzig.de/Publications/SUPPLEMENTS/07-010/).
Authors’ contributions
All authors collaborated in data analysis and homology search as well as in the interpretation of the data. AM and PFS conceived the study and wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors have declared that no competing interests exist.
Note added in proof
While this manuscript was in production, two relevant papers have appeared: Higuchi et al. (U7 snRNA acts as a transcriptional regulator interacting with an inverted CCAAT sequence-binding transcription factor NF-Y. Biochim. Biophys. Acta. Epub ahead of print 2007 Nov 22, doi:10.1016/j.bbagen.2007.11.005) demonstrated that U7 snRNA also acts as a transcriptional regulator, and Dávila López and Samuelsson (Early evolution of histone mRNA 3′ end processing. 2008. RNA 14: 1–10. Epub 2007 Nov 12) reported evidence for an origin of the metazoan-like histone 3′ end processing machinery early in eukaryotic evolution. These authors also reported several computationally predicted U7 snRNA sequences, most of which agree with our results.
Acknowledgements
BMRS and PFS thank the PICB in Shanghai for its hospitality, where much of this work was performed in spring 2007. This work was supported by the DFG-funded Graduiertenkolleg Wissensrepräsentation to MM and the DFG Bioinformatics Initiative to PFS. We thank an anonymous referee for bringing evidence for the special histone 3′ end processing mechanism outside metazoa to our attention.
Supporting Online Material
Alignments of U7 sequences and other data: http://www.bioinf.uni-leipzig.de/Publications/SUPPLEMENTS/07-010/
References
- 1.Mowry K.L., Steitz J.A. Identification of the human U7 snRNP as one of several factors involved in the 3′ end maturation of histone premessenger RNAs. Science. 1987;238:1682–1687. doi: 10.1126/science.2825355. [DOI] [PubMed] [Google Scholar]
- 2.Marzluff W.F. Metazoan replication-dependent histone mRNAs: a distinct set of RNA polymerase II transcripts. Curr. Opin. Cell Biol. 2005;17:274–280. doi: 10.1016/j.ceb.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Fabry S. The organization structure and regulatory elements of Chlamydomonas histone genes reveal features linking plant and animal genes. Curr. Genet. 1995;28:333–345. doi: 10.1007/BF00326431. [DOI] [PubMed] [Google Scholar]
- 4.Townley-Tilson W.H. Genome-wide analysis of mRNAs bound to the histone stem-loop binding protein. RNA. 2006;12:1853–1867. doi: 10.1261/rna.76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golembe T.J. Specific sequence features, recognized by the SMN complex, identify snRNAs and determine their fate as snRNPs. Mol. Cell. Biol. 2005;25:10989–11004. doi: 10.1128/MCB.25.24.10989-11004.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azzouz T.N., Schümperli D. Evolutionary conservation of the U7 small nuclear ribonucleoprotein in Drosophila melanogaster. RNA. 2003;9:1532–1541. doi: 10.1261/rna.5143303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pillai R.S. Unique Sm core structure of U7 snRNPs: assembly by a specialized SMN complex and the role of a new component, Lsm11, in histone RNA processing. Genes. Dev. 2003;17:2321–2333. doi: 10.1101/gad.274403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schümperli D., Pillai R.S. The special Sm core structure of the U7 snRNP: far-reaching significance of a small nuclear ribonucleoprotein. Cell. Mol. Life Sci. 2004;61:2560–2570. doi: 10.1007/s00018-004-4190-0. [DOI] [PubMed] [Google Scholar]
- 9.Kolev N.G., Steitz J.A. In vivo assembly of functional U7 snRNP requires RNA backbone flexibility within the Sm-binding site. Nat. Struct. Mol. Biol. 2006;13:347–353. doi: 10.1038/nsmb1075. [DOI] [PubMed] [Google Scholar]
- 10.Jaeger S. Binding of human SLBP on the 3′-UTR of histone precursor H4-12 mRNA induces structural rearrangements that enable U7 snRNA anchoring. Nucleic Acids Res. 2006;34:4987–4995. doi: 10.1093/nar/gkl666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brun C. U7 snRNAs induce correction of mutated dystrophin pre-mRNA by exon skipping. Cell. Mol. Life Sci. 2003;60:557–566. doi: 10.1007/s000180300047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goyenvalle A. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science. 2004;306:1796–1799. doi: 10.1126/science.1104297. [DOI] [PubMed] [Google Scholar]
- 13.Soldati D., Schümperli D. Structural and functional characterization of mouse U7 small nuclear RNA active in 3′ processing of histone pre-mRNA. Mol. Cell. Biol. 1988;8:1518–1524. doi: 10.1128/mcb.8.4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruber A. Isolation of an active gene and of two pseudogenes for mouse U7 small nuclear RNA. Biochim. Biophys. Acta. 1991;1088:151–154. doi: 10.1016/0167-4781(91)90167-k. [DOI] [PubMed] [Google Scholar]
- 15.Phillips S.C., Turner P.C. A transcriptional analysis of the gene encoding mouse U7 small nuclear RNA. Gene. 1992;116:181–186. doi: 10.1016/0378-1119(92)90514-p. [DOI] [PubMed] [Google Scholar]
- 16.Phillips S.C., Turner P.C. Sequence and expression of a mouse U7 snRNA type II pseudogene. DNA Seq. 1991;1:401–404. doi: 10.3109/10425179109020796. [DOI] [PubMed] [Google Scholar]
- 17.Yu Y.T. More Sm snRNAs from vertebrate cells. Exp. Cell Res. 1996;229:276–281. doi: 10.1006/excr.1996.0372. [DOI] [PubMed] [Google Scholar]
- 18.Strub K. The cDNA sequences of the sea urchin U7 small nuclear RNA suggest specific contacts between histone mRNA precursor and U7 RNA during RNA processing. EMBO J. 1984;3:2801–2807. doi: 10.1002/j.1460-2075.1984.tb02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Lorenzi M. Analysis of a sea urchin gene cluster coding for the small nuclear U7 RNA, a rare RNA species implicated in the 3′ editing of histone precursor mRNAs. Proc. Natl. Acad. Sci. USA. 1986;83:3243–3247. doi: 10.1073/pnas.83.10.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilmartin G.M. Functional analysis of the sea urchin U7 small nuclear RNA. Mol. Cell. Biol. 1988;8:1076–1084. doi: 10.1128/mcb.8.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Southgate C., Busslinger M. In vivo and in vitro expression of U7 snRNA genes: cis- and trans-acting elements required for RNA polymerase II-directed transcription. EMBO J. 1989;8:539–549. doi: 10.1002/j.1460-2075.1989.tb03408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips S.C., Birnstiel M.L. Analysis of a gene cluster coding for the Xenopus laevis U7 snRNA. Biochim. Biophys. Acta. 1992;1131:95–98. doi: 10.1016/0167-4781(92)90104-8. [DOI] [PubMed] [Google Scholar]
- 23.Watkins N.J. The U7 small nuclear RNA genes of Xenopus borealis. Biochem. Soc. Trans. 1992;20:301S. doi: 10.1042/bst020301s. [DOI] [PubMed] [Google Scholar]
- 24.Wu C.H., Gall J.G. U7 small nuclear RNA in C snurposomes of the Xenopus germinal vesicle. Proc. Natl. Acad. Sci. USA. 1993;90:6257–6259. doi: 10.1073/pnas.90.13.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dominski Z. Cloning and characterization of the Drosophila U7 small nuclear RNA. Proc. Natl. Acad. Sci. USA. 2003;100:9422–9427. doi: 10.1073/pnas.1533509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myslinksi E. Characterization of snRNA and snRNA-type genes in the pufferfish Fugu rubripes. Gene. 2004;330:149–158. doi: 10.1016/j.gene.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 27.Lück R. Construct: a tool for thermodynamic controlled prediction of conserved secondary structure. Nucleic Acids Res. 1999;27:4208–4217. doi: 10.1093/nar/27.21.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bompfünewerer A.F. Evolutionary patterns of non-coding RNAs. Theory Biosci. 2005;123:301–369. doi: 10.1016/j.thbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths-Jones S. Rfam: annotating non-coding RNAs in complete genomes. Nucleic Acids Res. 2005;33:D121–D124. doi: 10.1093/nar/gki081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez N. Small nuclear RNA genes: a model system to study fundamental mechanisms of transcription. J. Biol. Chem. 2001;276:26733–26736. doi: 10.1074/jbc.R100032200. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez G., Jr. Insect small nuclear RNA gene promoters evolve rapidly yet retain conserved features involved in determining promoter activity and RNA polymerase specificity. Nucleic Acids Res. 2007;35:21–34. doi: 10.1093/nar/gkl982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soldati D., Schümperli D. Structures of four human pseudogenes for U7 small nuclear RNA. Gene. 1990;95:305–306. doi: 10.1016/0378-1119(90)90378-5. [DOI] [PubMed] [Google Scholar]
- 33.Dominski Z. Differences and similarities between Drosophila and mammalian 3′ end processing of histone pre-mRNAs. RNA. 2005;11:1835–1847. doi: 10.1261/rna.2179305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosig A. Fragrep: an efficient search tool for fragmented patterns in genomic sequences. Genomics Proteomics Bioinformatics. 2006;4:56–60. doi: 10.1016/S1672-0229(06)60017-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nawrocki E.P., Eddy S.R. Query-dependent banding (QDB) for faster RNA similarity searches. PLoS Comput. Biol. 2007;3 doi: 10.1371/journal.pcbi.0030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morgenstern B. DIALIGN2: improvement of the segment-to-segment approach to multiple sequence alignment. Bioinformatics. 1999;15:211–218. doi: 10.1093/bioinformatics/15.3.211. [DOI] [PubMed] [Google Scholar]
- 37.Thompson J.D. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffiths-Jones S. RALEE—RNA alignment editor in Emacs. Bioinformatics. 2005;21:257–259. doi: 10.1093/bioinformatics/bth489. [DOI] [PubMed] [Google Scholar]
- 39.Hofacker I.L. Secondary structure prediction for aligned RNA sequences. J. Mol. Biol. 2002;319:1059–1066. doi: 10.1016/S0022-2836(02)00308-X. [DOI] [PubMed] [Google Scholar]
- 40.Crooks G.E. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]