Introduction
In 2003, the Takaue group observed clinical features such as fever, skin rash and fluid retention that mimicked engraftment syndrome (ES) in cord blood transplantation (CBT) patients who received reduced-intensity conditioning regimens, and referred to this event as an "early inflammatory syndrome (EIS)" (personal communication). Around the same time, we also observed these manifestations in patients following CBT and reported the condition as "EIS" [1]. Kishi et al. [2] labeled similar symptoms that appeared after reduced-intensity CBT as "early immune reaction". Based on these observations, we retrospectively analyzed the early toxicities in the pre-engraftment period of hematopoietic stem cell transplantation (HSCT) and designated the clinical features mimicking ES (Table 1) as "pre-engraftment syndrome (pES)" [3]. The rationale of using the name pES was that the signs appeared earlier than those of ES, even though the clinical features of pES in the pre-engraftment period were highly similar to those of ES. Since that report on pES, many investigators have tried to establish the incidence, risk factors, and clinical outcomes of pES after CBT.
Table 1. Clinical features of pre-engraftment syndrome.
Clinical significance
Many complications attributable to regimen-related toxicities arise during HSCT. The cytoreductive regimens used in conventional HSCT destroy rapidly dividing cell populations, particularly bone marrow (BM) progenitor cells and mucosal epithelial cells. Depletion of these cells can lead to infections as well as defective immune responses during the neutropenic pre-engraftment period. Besides the conditioning regimens, the graft itself can contribute to transplant-related toxicities. During the pre-engraftment period, transplant physicians sometimes encounter post-transplant events with clinical features similar to those of acute graft versus host disease (aGVHD); these include hyperacute GVHD, ES, and the recently documented pES. The defining features and pathogenesis of GVHD are well understood, but those of hyperacute GVHD, ES, and pES are not. Moreover, the relationships between these post-transplant events are also unclear.
The reported incidence of pES ranges from 20% to 77%. Although the risk factors and clinical outcomes of pES are poorly understood, most investigators agree that it is most common after myeloablative conditioning, and is associated with increased risk of aGVHD but not with transplant-related mortality, relapse, or decreased overall survival [4,5]. Among the clinical features of pES, pulmonary manifestations tachypnea, hypoxemia, and pulmonary edema can occur along with other radiographic findings of diffuse ground glass opacities and/or pleural effusion. Brownback et al found that over 50% of patients with pES developed hypoxemia and their chest CT scan and bronchoalveolar lavage findings were consistent with noncardiogenic pulmonary edema. Non-significant trends toward increased mortality have been observed in patients with pES who developed hypoxemia and in those who were treated with corticosteroids [6]. However, we would like to emphasize that clinicians should carefully check for the clinical features of pES, particularly for the pulmonary manifestations. Although pES does not affect transplant outcomes, failure to recognize this syndrome in transplant recipients could result in the recipient being subjected to unnecessary diagnostic procedures or treatments for various suspected causes of the pulmonary complications. Early recognition of pES and treatment with a short course of corticosteroids can also avoid unnecessarily long, empirical treatments that could promote opportunistic infections.
Proposed pathophysiology
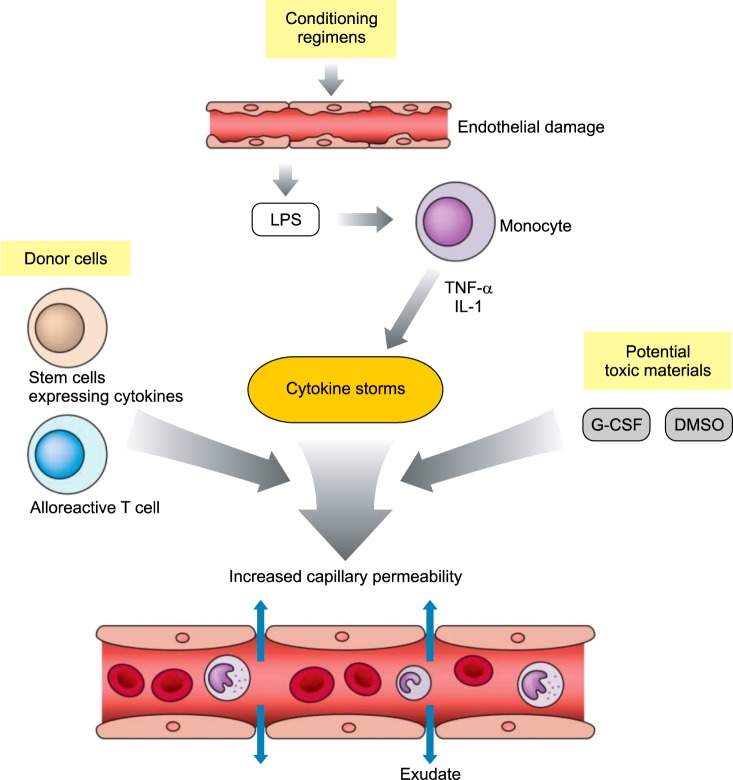
ES results from endothelial cell injury, cytokine production, and recruitment of effector cells in response to pre-transplant conditioning therapy. The inflammatory nature of ES is supported by the elevated concentrations of C-reactive protein and several cytokines, including interleukin (IL)-1, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ [7]. The pathogenesis of pES is not clear, but capillary leak syndrome (CLS) initiated by a cytokine storm could be a cause (Fig. 1). CLS is the escape of protein and fluid from the vascular system into the extravascular spaces, possibly due to cellular damage resulting from the preparative regimen and the use of toxic materials, and it leads to an associated cytokine storm. The granulocyte colony-stimulating factor (G-CSF) administered during CBT, dimethylsulfoxide (DMSO) used for the cryopreservation of cord blood (CB), and proinflammatory cytokine secretion elicited by CB could all contribute to the cytokine storm and thereby to CLS. Moreover, the percentage of natural killer (NK) cells in CB is much higher than that in adult peripheral blood [8]. CD56hi cells, a subset of NK cells, make major contributions to their proinflammatory effect by secreting cytokines such as IFN-γ, TNF-α, granulocyte monocyte (GM)-CSF, IL-5, IL-10 and IL-13. Moreover, CB cells have significantly higher necrosis-mediated cytotoxic activity than BM-derived cells [9] and this could also contribute to the high incidence of CLS or pES after CBT.
Fig. 1. Pathophysiology of capillary leak syndrome, the probable cause of pre-engraftment syndrome. Conditioning chemoradiotherapy can damage the endothelial lining of the gastrointestinal tract, allowing immunostimulatory microbial products such as LPS to enter the circulation. These molecules can stimulate the secretion of inflammatory cytokines (TNF-α, IL-1), leading to secondary release of inflammatory cytokines (cytokine storms). In addition, potentially toxic materials such as G-CSF and the cryoprotectant DMSO, donor stem cells expressing proinflammatory cytokines, and alloreactive donor T cells can also enhance the cytokine storms, thereby contributing to increased vascular permeability to fluids and low-molecular-weight substances.
Abbreviations: LPS, lipopolysaccharide; TNF, tumor necrosis factor; IL, interleukin; G-CSF, granulocyte colony-stimulating factor; DMSO, dimethylsulfoxide.
Questions to be answered
Some investigators have labeled the clinical features of pES as hyperacute GVHD [10], ES, or peri-engraftment syndrome [11], with those of many of the patients being compatible with pES. These early toxicities have been classified based on the time to neutrophil engraftment or aGVHD. However, pES may be a distinct clinical syndrome related to the cytokine storms associated with regimen-related toxicities, graft source, and toxic materials such as DMSO or G-CSF. It could also be related to aGVHD against a major or minor mismatched antigen by donor T cells. Thus, a thorough understanding of their pathogenesis is needed in order to elucidate the relationships between these early post-transplant events. In addition, transplant physicians have mainly reported pES in patients who received CBT. Therefore, pES is thought be specific to CBT recipients; however, the clinical features of patients receiving BM or mobilized peripheral blood stem cells (mPBSCs) should also be compared with those of pES.
Future perspective
Most cases of pES have been reported in patients who have received CBT; however, we have previously reported that its incidence does not depend on the source of the graft [5]. In addition, the occurrence of hyperacute GVHD before engraftment has been reported extensively in patients receiving BM or mPBSCs, particularly prior to the CBT era. Therefore, further research should include prospective clinical studies on the effect of the source of stem cells (BM, mPBSCs, CB) on the incidence of pES; differences between the causes of pES and hyperacute GVHD, ES, and aGVHD; and cytokine studies, including assessment of NK cell/T cell activities.
Acknowledgments
This study was supported by grants from the Korea Healthcare Technology R&D Project of the Ministry for Health & Welfare Affairs of the Republic of Korea (HI16C0972).
Footnotes
Authors' Disclosure of Potential Conflict of Interest: The authors have no potential conflict of interest relevant to this article.
References
- 1.Lee YH, Lee SW, Noh KT, Lee YS, Lee YA. Early inflammatory syndrome following cord blood stem cell transplantation. Korean J Hematol. 2004;39:66–70. [Google Scholar]
- 2.Kishi Y, Kami M, Miyakoshi S, et al. Early immune reaction after reduced-intensity cord-blood transplantation for adult patients. Transplantation. 2005;80:34–40. doi: 10.1097/01.tp.0000163289.20406.86. [DOI] [PubMed] [Google Scholar]
- 3.Lee YH, Lim YJ, Kim JY, Kim YD, Lee SW. Pre-engraftment syndrome in hematopoietic stem cell transplantation. J Korean Med Sci. 2008;23:98–103. doi: 10.3346/jkms.2008.23.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Liu H, Li L, et al. Pre-engraftment syndrome after unrelated donor umbilical cord blood transplantation in patients with hematologic malignancies. Eur J Haematol. 2012;88:39–45. doi: 10.1111/j.1600-0609.2011.01709.x. [DOI] [PubMed] [Google Scholar]
- 5.Park M, Lee SH, Lee YH, et al. Pre-engraftment syndrome after unrelated cord blood transplantation: a predictor of engraftment and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:640–646. doi: 10.1016/j.bbmt.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Brownback KR, Simpson SQ, McGuirk JP, et al. Pulmonary manifestations of the pre-engraftment syndrome after umbilical cord blood transplantation. Ann Hematol. 2014;93:847–854. doi: 10.1007/s00277-013-1981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornell RF, Hari P, Drobyski WR. Engraftment syndrome after autologous stem cell transplantation: An update unifying the definition and management approach. Biol Blood Marrow Transplant. 2015;21:2061–2068. doi: 10.1016/j.bbmt.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardiner CM, Meara AO, Reen DJ. Differential cytotoxicity of cord blood and bone marrow-derived natural killer cells. Blood. 1998;91:207–213. [PubMed] [Google Scholar]
- 9.Kotylo PK, Baenzinger JC, Yoder MC, Engle WA, Bolinger CD. Rapid analysis of lymphocyte subsets in cord blood. Am J Clin Pathol. 1990;93:263–266. doi: 10.1093/ajcp/93.2.263. [DOI] [PubMed] [Google Scholar]
- 10.Kim DH, Sohn SK, Kim JG, Suh JS, Lee KS, Lee KB. Clinical impact of hyperacute graft-versus-host disease on results of allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;33:1025–1030. doi: 10.1038/sj.bmt.1704479. [DOI] [PubMed] [Google Scholar]
- 11.Hong KT, Kang HJ, Kim NH, et al. Peri-engraftment syndrome in allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013;48:523–528. doi: 10.1038/bmt.2012.171. [DOI] [PubMed] [Google Scholar]