Abstract
Multiple sclerosis (MS) is a disease in which we can recognize destruction of the myelin that is around nerve cells of brain and spinal cord called as oligodendrocytes. Both genetic and environmental factors play roles in MS. One of these genes is the killer-cell immunoglobulin-like receptor (KIR) which expressed on surface of natural killer cells (NKs). These genes have loci (not locus) in human genome, so they inherit as haplotypes. The results of previous studies show that different genes of KIR may affect both susceptibility and resistance to such autoimmune disorders that their pathogenesis in MS is still unclear. Since NKs play key roles in immune tolerance, we intend to perform a meta-analysis for the correlation of KIR genes and MS. We used the software comprehensive meta-analysis for data of totally 568 MS patients and 280 controls. Among the 14 genes of KIR in the human genome, lack of KIR2DS1 is accompanied by MS. No KIR gene found to be a risk factor for MS. Further studies on other molecules of NKs like CD94 and NKG2a is suggested.
Keywords: Multiple sclerosis, Killer cell immunoglobulin-like receptor, Meta-analysis
1. Introduction
Multiple sclerosis (MS) is a disease in which we can recognize destruction of the myelin that is around nerve cells of brain and spinal cord called as oligodendrocytes. Both genetic and environmental factors play roles in MS [1], [2], [3]. It is better to say that infectious agents as an example for environmental factors can increase the chance of developing MS in the individuals those who are genetically susceptible for that [4].
Among the genes that have been found for the disease, human leucocyte antigen (HLA) system is the most important one [5], [6] and this system has also some associations with other Auto immune diseases like type 1 diabetes [7] and systemic lupus erythematosus (SLE) [8].
As we mentioned, infectious agents also play roles in this disease. Many microbes like different viruses are known for their participating. For instance Herpes virus family specially group four the Epstein-Barr virus (EBV). There is a belief that the persons those who have not been infected by this family of viruses, have lower chance to develop MS than those who had these infections [5].
Different aspects of the immune system of MS patients seem to have functional problems; for example the increase in aggressive activity of Th1, Th17 and T CD8+ or decrease in regulatory T cells activity and also unusual function of B lymphocyte cells, Antibodies, complement system and natural killer cells (NKs) [9].
Morphology wise, NKs are a kind of lymphocyte cells, however their functions are in the innate immune system. These cells are the first part of immune system which defend the body against cancerous or infected cells, but if they cannot be successful in performing their tasks, it can cause a variety of problems like different types of cancers, infections, immunodeficiency syndromes and even auto immune diseases. The majority of NKs have cytotoxic effects of course just against cells with impaired or without HLA class 1 molecules [10], [11], [12], [13]. But on the other hand, there are NKs called CD56bright which kill the activated T lymphocytes which their action is against the myelin surrounding nerve cells [14]. In MS a reduction could be recognized in the amount of this kind of NKs along with reduction of T regulatory cells activity, and it seems to be a cause for recurrence of the clinical symptoms [15].
The destruction caused by MS is usually on the myelin of basal ganglia [16], brainstem [17] and spinal cord [18]. Destroying white matter can lead to low pace of transfer of information to the processing areas. According to the place which the destructions happened we can see different kinds of clinical disorders.
Primarily MS is an inflammatory disorder as a consequence of a local lymphocyte infiltration [19] and this can damage the myelin and even the axons [20]. These damaged parts could be recovered but the recoveries are not stable [21]. In other words oligodendrocytes which are responsible for production and maintenance of myelin, have been destroyed by autoantibodies and the outcome is destruction of the axons [22], because they are much more vulnerable than before due to the absence of the myelin. Axons demolition can be an inducement for the Astrocytosis (also called as Astrogliosis), an abnormal increase in the amount of astrocytes [23].
The inflammatory response is a reason for releasing different kinds of cytokines and autoantibodies and this can cause more destruction in blood brain barrier (BBB), releasing and activating more macrophages, cytokines and proteins. As we know one role of BBB is not to let entrance of T cells to central nervous system (CNS), but sometimes because of some previous infections some T cells can find a way to CNS and remain there even after getting cleaned of infection agents and be a reason for future problems [24], [25], [26]. Such immune responses and cytokines also induce apoptosis (programmed cell death) in CNS [27], [28], [29], [30]; this apoptosis can be intensify complications of MS [14], [31]. Since neurodegeneration occurs after demyelination, apoptosis of the nerve cells is recently approved with better evidences than before [32].
Killer-cells immunoglobulin-like receptors (KIRs) are one of the markers on surface of NKs, and like HLAs, they are inherited as the form of haplotypes. KIR has 14 genes and 2 pseudo-genes which 8 of them are of the inhibitors and 6 of them are activators. KIRs which are expressed on the surface of NKs interact with HLA class I on surface of the other nuclear cells of human. The result of this interaction is the immune tolerance if their targeted cells are healthy, and cytotoxic activity of NKs against the cancerous targeted cells of theirs. According to the fact that HLA is the most polymorphic loci in human genome and also KIR has different types of genes and alleles for each gene, different interactions of KIR-HLA can be along with susceptibility to different diseases like cancers among different ethnicities and populations; this is called as “disease association” in medical anthropology. Some of these 14 genes are seems to be associated as risk factors with some cancers while the other ones are known for their protective effects [13], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42].
Since NKs play key roles in immune tolerance and on the other hand KIRs are of their functional surface molecules, we intend to perform a meta-analysis for the correlation of KIR genes and MS.
2. Methods
As our search strategy in the current meta-analysis, we used the meta-search engines Pubmed, Google scholar and Researchgate. Our key word was KIR AND “multiple sclerosis” in titles. Finally we found five articles. Among them 3 articles of those found in google scholar seemed to be fake! Because their bibliography was for the journals tissue antigens and European journal of neurology, but we couldn't trace them in web site of these journals. Therefor only two studies were used for our meta-analysis. Both of them had been published in Journal of neuroimmunology.
We used the software comprehensive meta-analysis version 2 for final analyses. The test Chi-squared 2 multiplied by 2 with Yate's correction was staffed for each gene. Totally there were the informations of 568 MS patients and 280 controls.
3. Results
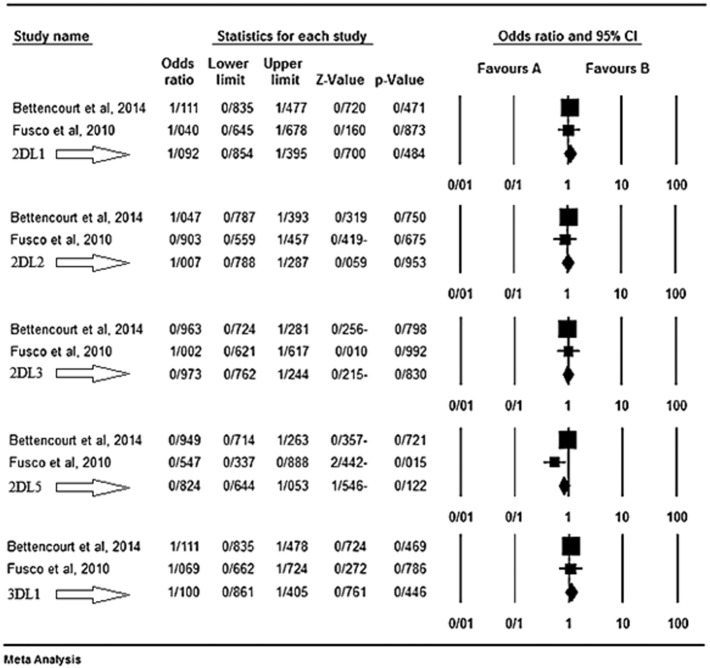
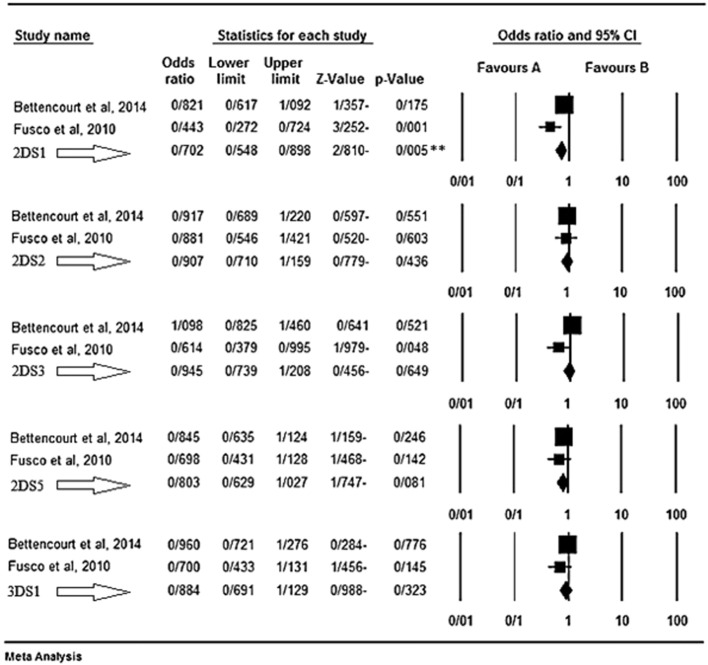
Based on the results of our meta-analysis KIR2DS1 seems as a protective factor for MS disease. Fusco et al. [43] believed that different KIR genes may affect both susceptibility and resistance to such autoimmune disorders that their pathogenesis in MS is still unclear. They found a possible protective role of the activating gene KIR2DS1, enhanced in the presence of its ligand group HLA-C2. In their belief, the presence of such KIRs accompanied by their HLA ligands results in the immunomodulatory function of NKs [43]. Bettencourt et al. [44] believed that KIR genes may influence both susceptibility and resistance to MS. They observed a negative association between the activating gene KIR2DS1 and MS independently from the presence of HLA-DRB1*15 allele. They believed that this activating KIR seems to play a protection role against MS via modulation of autoreactive T cells by NKs [44]. The results of the meta-analyses for the inhibitory and activating genes are shown respectively in Fig. 1, Fig. 2.
Fig. 1.
KIR inhibitory genes. The favours A shows protecting effect and the B shows the genes as risk factors.
Fig. 2.
KIR activating genes. The favours A shows protecting effect and the B shows the genes as risk factors.
4. Discussion
NKs are a part of innate lymphoid cells (ILCs) and they have CD3-phenotype generally and also have 2 subsets, CD56bright and CD56dim which have differences in their amount and activities. NKs can be found in blood, peripheral organs and secondary lymphoid organs. Most NKs are CD56dim and only about 10% of them are the CD56bright which have regulatory roles in immune system whereas CD56dims are mostly act in cytotoxicity [10], [11], [12], [13]. NKs have a variety of roles in MS; their maturity which is in CNS and their engagement with different kinds of receptors in neural cells, seem to result a correlation with MS. For instance they have some receptors like the CX3CR1 which is more expressed in relapse phase of MS than the stable phase. As another example, a decrease in NK population can cause more attacks in relapse phase of the disease. NK activity regulates with different kinds of receptors that help them to recognize their targets; for example the KIR system works with lectin-like NKG2 receptors to modulate immune response. KIRs are divided into two groups, one the inhibitory which their ligands are usually HLA class 1 molecules and the other group is the stimulatory with unknown ligands. According to some researches, there is a significant correlation between KIRs and their HLA ligands activity with MS disease. In some patients a significant deficit of HLA BW4 were found, this reduction in the amount of HLABW4 can influence NK activity and cause a diminished response to infectious disease and increased susceptibility to MS. Some in vitro studies also show that NKs can cause tissue injury in MS because they can directly lyse neural tissues [45], [46], [47].
KIR2DS1 based on the results of our meta-analysis seems as a protective factor for MS disease, contrary to what had been expected; because we anticipated that these receptors were considered as a risk factor for this type of inflammation-related disease.
The followings can be considered as reasons to justify this contradiction: First, MS is a disease caused by hypersensitivity type IV or cell-mediated immune responses, and not by inflammation. Inflammation is just a cause for progression and recurrence of the disease. Second, KIRs have different effects in combination with different ligands. For example, it may be something entirely different in result if KIR combined with different types of HLA. Lack of former studies about this issue and also lack of studies on other molecules like CD94 and NKG2a are of the limitations of ours in this paper. It is worth noting that these receptors are more abundant in healthy groups compared with patients but this does not mean that the lesser amount of these receptors is a risk factor for the disease. Third, the reasoning of Fusco et al. and Bettencourt et al. mentioned above, could be good responses to this contradiction in turn. Forth, as we mentioned in introduction, NKs fight with autoreaction of T cells [14]; hence the activation of NKs through activating KIRs like 2DS1 can impede the autoreaction [44].
5. Conclusions
Among the 14 genes of KIR in the human genome, lack of the activating gene KIR2DS1 is accompanied by MS. No KIR gene found to be a risk factor for MS. Further studies on other molecules of NKs like CD94 and NKG2a is suggested.
Conflict of interest
We declare that there is no conflict of interest.
References
- 1.Tolou-Ghamari Z., Mazdak H. Fingolimod for multiple sclerosis; mechanism of action, safety and toxicity. Arch. Neurosci. 2016;3 [Google Scholar]
- 2.Tolou-Ghamari Z. Efficacy and toxicity of rituximab in multiple sclerosis. Arch. Neurosci. 2016;3 [Google Scholar]
- 3.Tolou-Ghamari Z. A review of geoepidemiological differences of multiple sclerosis in Iran and other Middle East countries. Arch. Neurosci. 2015;2 [Google Scholar]
- 4.Kallaur A.P., Reiche E.M.V., Oliveira S.R., Pereira W.L.d.C.J., Alfieri D.F., Flauzino T., de Meleck Proença C., Lozovoy M.A.B., Kaimen-Maciel D.R., Maes M. Genetic, immune-inflammatory, and oxidative stress biomarkers as predictors for disability and disease progression in multiple sclerosis. Mol. Neurobiol. 2016:1–14. doi: 10.1007/s12035-015-9648-6. [DOI] [PubMed] [Google Scholar]
- 5.Wergeland S., Myhr K.M., Løken-Amsrud K., Beiske A., Bjerve K., Hovdal H., Midgard R., Kvistad S., Holmøy T., Riise T. Vitamin D, HLA-DRB1 and Epstein–Barr virus antibody levels in a prospective cohort of multiple sclerosis patients. Eur. J. Neurol. 2016;23:1064–1070. doi: 10.1111/ene.12986. [DOI] [PubMed] [Google Scholar]
- 6.Elmongui A.E., Khalil M.H., Salama H.H., Askar H., Eltoukhy K.M. Association and linkage of definite multiple sclerosis with HLA and the potential protective role of helicobacter pylori infection among Egyptians. Int. Arch. Med. 2016;9 [Google Scholar]
- 7.Pugliese A., Boulware D., Yu L., Babu S., Steck A.K., Becker D., Rodriguez H., DiMeglio L., Evans-Molina C., Harrison L.C. The HLA-DRB1* 15: 01-DQA1* 01: 02-DQB1* 06: 02 haplotype protects autoantibody-positive relatives from type 1 diabetes throughout the stages of disease progression. Diabetes. 2016 doi: 10.2337/db15-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jucaud V., Ravindranath M., Terasaki P., Morales-Buenrostro L., Hiepe F., Rose T., Biesen R. Serum antibodies to human leucocyte antigen (HLA)-E, HLA-F and HLA-G in patients with systemic lupus erythematosus (SLE) during disease flares: clinical relevance of HLA-F autoantibodies. Clin. Exp. Immunol. 2016;183:326–340. doi: 10.1111/cei.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato W., Yamamura T. Neuroimmunological Diseases. Springer; 2016. Cellular immunity and multiple sclerosis: current understanding; pp. 3–20. [Google Scholar]
- 10.Beigi Boroujeni N., Beigi Boroujeni M., Rafiei Alavi E., Shafiei A. The effect of ethanolic extract of Salvia officinalis on the uterine natural killer cells population at day 7 of pregnancy. J. Med. Plants. 2015;14:15–24. [Google Scholar]
- 11.Ghafourian M., Band N.A., Pour A.F., Kooti W., Rad M.F., Badiee M. The role of CD16 +, CD56 +, NK (CD16 +/CD56 +) and B CD20 + cells in the outcome of pregnancy in women with recurrent spontaneous abortion. Int. J. Wom. Health Reprod. Sci. 2015;3:61–66. [Google Scholar]
- 12.Mousavi T., Poormoghim H., Moradi M., Tajik N., Shahsavar F., Soofi M. Phenotypic study of natural killer cell subsets in ankylosing spondylitis patients. Iran. J. Allergy Asthma Immunol. 2009;8:193–198. [PubMed] [Google Scholar]
- 13.Ahmadi S.A.Y., Shahsavar F., Akbari S. A review on controversies about the role of immune and inflammatory systems in implantation process and durability of pregnancy. Int. J. Wom. Health Reprod. Sci. 2016;4:96–102. [Google Scholar]
- 14.Macchi B., Mastino A. Programmed cell death and natural killer cells in multiple sclerosis: new potential therapeutic targets? Neural Regen. Res. 2016;11:733. doi: 10.4103/1673-5374.182695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandenbark A.A., Huan J., Agotsch M., La Tocha D., Goelz S., Offner H., Lanker S., Bourdette D. Interferon-beta-1a treatment increases CD56 bright natural killer cells and CD4 + CD25 + Foxp3 expression in subjects with multiple sclerosis. J. Neuroimmunol. 2009;215:125–128. doi: 10.1016/j.jneuroim.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Schmalbrock P., Prakash R., Schirda B., Janssen A., Yang G., Russell M., Knopp M., Boster A., Nicholas J., Racke M. Basal ganglia iron in patients with multiple sclerosis measured with 7T quantitative susceptibility mapping correlates with inhibitory control. Am. J. Neuroradiol. 2016;37:439–446. doi: 10.3174/ajnr.A4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnano I., Pes G., Ginatempo F., Cabboi M., Pilurzi G., Conti M., Rothwell J., Deriu F. Assessment of brainstem reflexes improves the diagnostic sensitivity of multimodal evoked potentials, MRI and clinical testing in the investigation of brainstem function in multiple sclerosis. Clin. Neurophysiol. 2016;127 [Google Scholar]
- 18.Casserly C., Sankar S., Baral S., Oh J. MRI quantification of spinal cord atrophy in multiple sclerosis: a systematic review and meta-analysis (P3. 016) Neurology. 2016;86 doi: 10.1111/jon.12553. (P3. 016) [DOI] [PubMed] [Google Scholar]
- 19.Gentile A., Musella A., Bullitta S., Fresegna D., De Vito F., Piras E., Gargano F., Borsellino G., Battistini L., Mandolesi G. Effects of siponimod (BAF312) on inflammation-driven synaptopathy in experimental multiple sclerosis (P5. 311) Neurology. 2016;86 doi: 10.1186/s12974-016-0686-4. (P5. 311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonio-Santos A., Eggenberger E.R., Fiona Costello M., Balcer L. Optical coherence tomography retinal nerve fiber analysis: a measure of axon loss in multiple sclerosis. Immunol. Infect. Dis. 2016;4:13–19. [Google Scholar]
- 21.Tur C., Goodkin O., Altmann D.R., Jenkins T.M., Miszkiel K., Mirigliani A., Fini C., Wheeler-Kingshott C.A.G., Thompson A.J., Ciccarelli O. Longitudinal evidence for anterograde trans-synaptic degeneration after optic neuritis. Brain. 2016 doi: 10.1093/brain/awv396. (awv396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spadaro M., Meinl E. Detection of autoantibodies against myelin oligodendrocyte glycoprotein in multiple sclerosis and related diseases. Mult. Scler. Methods Protoc. 2016:99–104. doi: 10.1007/7651_2015_223. [DOI] [PubMed] [Google Scholar]
- 23.Ludwin S.K., Rao V.T., Moore C.S., Antel J.P. Astrocytes in multiple sclerosis. Mult. Scler. J. 2016 doi: 10.1177/1352458516643396. (1352458516643396) [DOI] [PubMed] [Google Scholar]
- 24.Minagar A., Alexander J.S. Blood-brain barrier disruption in multiple sclerosis. Mult. Scler. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 25.A. Varatharaj, I. Galea, The blood-brain barrier in systemic inflammation, Brain Behav. Immun., http://dx.doi.org/10.1016/j.bbi.2016.03.010 (in press). [DOI] [PubMed]
- 26.Fraussen J., de Bock L., Somers V. B cells and antibodies in progressive multiple sclerosis: contribution to neurodegeneration and progression. Autoimmun. Rev. 2016;15:896–899. doi: 10.1016/j.autrev.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Gholami M., Khayat Z.K., Anbari K., Obidavi Z., Varzi A., Boroujeni M.B., Alipour M., Niapoor A., Gharravi A.M. Quercetin ameliorates peripheral nerve ischemia–reperfusion injury through the NF-kappa B pathway. Anat. Sci. Int. 2016:1–8. doi: 10.1007/s12565-016-0336-z. [DOI] [PubMed] [Google Scholar]
- 28.Khalatbary A.R., Ahmadvand H. Neuroprotective effect of oleuropein following spinal cord injury in rats. Neurol. Res. 2012;34:44–51. doi: 10.1179/1743132811Y.0000000058. [DOI] [PubMed] [Google Scholar]
- 29.Khalatbary A.R., Ahmadvand H. Anti-inflammatory effect of the epigallocatechin gallate following spinal cord trauma in rat. Iran. Biomed. J. 2011;15:31. [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmadvand H., Ahmadi S.A.Y., Sayahi A., Rezaian J. Role of apoptosis in CNS emphasizing spinal cord injuries: a commentary. Iran. J. Neurosurg. 2016;1:30–31. [Google Scholar]
- 31.Zipp F. Apoptosis in multiple sclerosis. Cell Tissue Res. 2000;301:163–171. doi: 10.1007/s004410000179. [DOI] [PubMed] [Google Scholar]
- 32.Haider L., Zrzavy T., Hametner S., Höftberger R., Bagnato F., Grabner G., Trattnig S., Pfeifenbring S., Brück W., Lassmann H. The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain. 2016;139:807–815. doi: 10.1093/brain/awv398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varzi A.M., Shahsavar F., Tarrahi M.J. Distribution of HLA-DRB1 and HLA-DQB1 alleles in Lak population of Iran. Hum. Immunol. July 2016;77(7):580–583. doi: 10.1016/j.humimm.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Tajik N., Shahsavar F., Mousavi T., Radjabzadeh M.F. Distribution of KIR genes in the Iranian population. Tissue Antigens. 2009;74:22–31. doi: 10.1111/j.1399-0039.2009.01263.x. [DOI] [PubMed] [Google Scholar]
- 35.Mousavi T., Poormoghim H., Moradi M., Tajik N., Shahsavar F., Asadifar B. Inhibitory killer cell immunoglobulin-like receptor KIR3DL1 in combination with HLA-B Bw4iso protect against ankylosing spondylitis. Iran. J. Immunol. 2010;7:88–95. [PubMed] [Google Scholar]
- 36.Shahsavar F., Tajik N., Entezami K.Z., Radjabzadeh M.F., Asadifar B., Alimoghaddam K., Dahaghi M.O., Jalali A., Ghashghaie A., Ghavamzadeh A. KIR2DS3 is associated with protection against acute myeloid leukemia. Iran. J. Immunol. 2010;7:8–17. [PubMed] [Google Scholar]
- 37.Tajik N., Shahsavar F., Nasiri M., Radjabzadeh M.F. Compound KIR-HLA genotype analyses in the Iranian population by a ovel PCR-SSP assay. Int. J. Immunogenet. 2010;37:159–168. doi: 10.1111/j.1744-313X.2010.00906.x. [DOI] [PubMed] [Google Scholar]
- 38.Mousavi T., Shahsavar F., Farnia P., Tajik N., Soofi M. Study of KIR expression and HLA ligands in CD56 + lymphocytes of drug resistant tuberculosis patients. Iran. J. Allergy Asthma Immunol. 2011;10:189–194. [PubMed] [Google Scholar]
- 39.Tajik N., Shahsavar F., Poormoghim H., Radjabzadeh M.F., Mousavi T., Jalali A. KIR3DL1 + HLA-B Bw4 Ile80 and KIR2DS1 + HLA-C2 combinations are both associated with ankylosing spondylitis in the Iranian population. Int. J. Immunogenet. 2011;38:403–409. doi: 10.1111/j.1744-313X.2011.01024.x. [DOI] [PubMed] [Google Scholar]
- 40.Shahsavar F., Mousavi T., Azargoon A., Entezami K. Association of KIR3DS1 + HLA-B BW4lle8 combination with susceptibility to tuberculosis in Lur population of Iran. Iran. J. Immunol. 2012;9:39–47. [PubMed] [Google Scholar]
- 41.Shahsavar F., Asadifar B., Jafarzadeh M., Forutani S. Distribution of KIR genes in the Lur population of Iran. Life Sci. J. 2013;10:11–16. [Google Scholar]
- 42.Shahsavar F., Tajik N., Entezami K., Asadifar B., Alimoghaddam K., Ghavamzadeh A. Donor KIR genotype with KIR2DS3 and/or KIR3DS1 increases survival after non-T-cell depleted HLA-identical sibling hematopoietic stem cell transplantation for acute myeloid leukemia. Life Sci. J. 2013;10:17–25. [Google Scholar]
- 43.Fusco C., Guerini F.R., Nocera G., Ventrella G., Caputo D., Valentino M.A., Agliardi C., Gallotti J., Morra V.B., Florio C. KIRs and their HLA ligands in remitting–relapsing multiple sclerosis. J. Neuroimmunol. 2010;229:232–237. doi: 10.1016/j.jneuroim.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Bettencourt A., Silva A.M., Carvalho C., Leal B., Santos E., Costa P.P., Silva B.M. The role of KIR2DS1 in multiple sclerosis-KIR in Portuguese MS patients. J. Neuroimmunol. 2014;269:52–55. doi: 10.1016/j.jneuroim.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Trachtenberg E.A. Understanding the role of natural killer cell receptors and their human leukocyte antigen ligands in multiple sclerosis. Ann. Neurol. 2009;65:626–628. doi: 10.1002/ana.21747. [DOI] [PubMed] [Google Scholar]
- 46.Fogel L.A., Yokoyama W.M., French A.R. Natural killer cells in human autoimmune disorders. Arthritis Res. Ther. 2013;15:1. doi: 10.1186/ar4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khaksari M., Zarkesh-Esfahani S.H., Etemadifar M., Masjedi M., Aliyari R., Rahmati M. Differential expression of CD16 and CD56 on natural killer (NK) cell subsets in multiple sclerosis and neuromyelitis optica. Int. J. Health Stud. 2015;1:1–7. [Google Scholar]