Abstract
Background: The main aim of this study was to quantify and compare 6-year mortality risk attributable to smoking, hypertension and diabetes among English and Brazilian older adults. This study represents a rare opportunity to approach the subject in two different social and economic contexts. Methods: Data from the data from the English Longitudinal Study of Ageing (ELSA) and the Bambuí Cohort Study of Ageing (Brazil) were used. Deaths in both cohorts were identified through mortality registers. Risk factors considered in this study were baseline smoking, hypertension and diabetes mellitus. Both age–sex adjusted hazard ratios and population attributable risks (PAR) of all-cause mortality and their 95% confidence intervals for the association between risk factors and mortality were estimated using Cox proportional hazards models. Results: Participants were 3205 English and 1382 Brazilians aged 60 years and over. First, Brazilians showed much higher absolute risk of mortality than English and this finding was consistent in all age, independently of sex. Second, as a rule, hazard ratios for mortality to smoking, hypertension and diabetes showed more similarities than differences between these two populations. Third, there was strong difference among English and Brazilians on attributable deaths to hypertension. Conclusions: The findings indicate that, despite of being in more recent transitions, the attributable deaths to one or more risk factors was twofold among Brazilians relative to the English. These findings call attention for the challenge imposed to health systems to prevent and treat non-communicable diseases, particularly in populations with low socioeconomic level.
Introduction
Non-communicable diseases (NCDs) are a rising epidemic in high, as well as low and middle income countries. Of the 57 million global deaths in 2008, 63% were due to NCDs, particularly cardiovascular diseases, diabetes, cancer and chronic respiratory diseases.1 As populations age, annual NCD deaths are projected to rise substantially, to 52 million in 2030.2
Nearly 80% of NCD deaths occur in low- and middle-income countries and are the most frequent causes of death in most countries, except in sub-Sahara Africa.2 Vulnerable and socially disadvantaged people get ill and die sooner as a result of NCDs than people from higher socioeconomic groups. There is a strong inverse correlation between a host of social determinants, particularly education, and the prevalence of NCDs and risk factors.2
In terms of attributable deaths, the leading NCD risk factor globally is raised blood pressure (to which 13% of global deaths are attributed), followed by tobacco use (9%), raised blood glucose (6%), physical inactivity (6%) and overweight and obesity (5%).3 In looking at these estimates it is important to consider that most knowledge about chronic disease epidemiology comes from cohort studies conducted in the United States and Western Europe.3–6 There is a need for further research comparing attributable deaths to major cardiovascular risk factors and diseases between high and low or middle income countries. Such comparison will provide an opportunity to broaden our understanding of the consequences of cardiovascular diseases and risk factors in populations where the epidemiologic transition is more recent.
NCDs are estimated to account to 74% of all deaths in Brazil (88% in England), and death rates due to cardiovascular diseases and diabetes are twice as higher in Brazilians (304 in men and 226 per 100 000 thousand in women) relative to English (166 and 102 per 100 000, respectively).7 Further, Brazilians report worse health than did English. Country-specific differences are higher among the poorest, but also exist across the income spectrum.8
We used data from the English Longitudinal Study of Ageing (ELSA)9 and the Bambuí Cohort Study of Ageing (Brazil)10 to quantify and compare 6-year mortality risk attributable to smoking, hypertension and diabetes among older adults. Given that the Bambui study was designed to investigate predictors of adverse health outcomes in individuals with low income and schooling levels, this study represents a rare opportunity to approach the subject in two very different social and economic contexts.
Methods
Data
For England, data came from the wave 2 (2004–2005) of the English Longitudinal Study of Ageing (ELSA), a cross-disciplinary panel study of ageing. The ELSA sample at wave 2 comprised 9432 respondents and was designed to be representative of the English community-dwelling population aged 50 years and older. The aim of ELSA is to explore the unfolding dynamic relationships between health, functioning, social networks and economic position in English older adults. ELSA members have a face-to-face interview every two years and there is an additional health examination every other interview (i.e. every four years). ELSA’s methodology is described elsewhere.9 All participants from wave 2 aged 60 and over were eligible for the present analysis.
For Brazil, data came from the baseline interview (1997) of the Bambui Cohort Study of Aging, which comprised 1606 (92% of the total population) aged 60 and over in Bambui city (∼15 000 inhabitants), Minas Gerais State, Southern Brazil. This cohort study was designed and developed to investigate the incidence and predictors of adverse health outcomes in an elderly Brazilian population with low schooling and income levels and in epidemiological transition (i.e. with high prevalence of NCDs but also widespread Trypanosoma cruzi infection, a protozoan that causes Chagas disease, whose main feature is heart involvement). The Bambui cohort members have a face-to-face interview every year and there was an additional health examination at baseline and in selected years of follow-up. The Bambui cohort methodology is described elsewhere.10 All participants of the baseline survey aged 60 and over were eligible for this analysis.
ELSA ethical approval was obtained from the National Research Ethics Service, UK. The Bambuí cohort study was approved by the Ethics Board of the Fundação Oswaldo Cruz, Rio de Janeiro, Brazil. Participants gave full informed consent to participate in both cohorts and authorized death certificate verification.
Mortality data source
For England, deaths occurring from the wave 2 (2004) until 31 December 2009 were included in this analysis. The individual participant data was linked with death records from National Health Service mortality registry; deaths certificates were obtained for 96.5% of individuals. For Brazil, deaths occurring from the study enrollment in 1997 to 31 December 2002 were included in this analysis. Deaths were reported by next of kin during the annual follow-up interview and ascertained through the Brazilian System of Information on Mortality; deaths certificates were obtained for 98.9% of individuals. For both sites, deaths assigned to any cause were considered in this analysis.
Risk factors
Risk factors considered in this study were baseline smoking, hypertension and diabetes mellitus. For both studies, current smokers were those who had smoked during their lifetime and were currently smoking. The measurement of hypertension and diabetes mellitus was based on self-reported doctor-diagnosis i.e. ‘Did a doctor ever tell you that you had…?’ Systolic blood pressure (SBP) and diastolic blood pressure (DBP) was defined as the mean of two out of three measures by using standard protocols. Hypertension was defined as a previous medical diagnosis for the disease and/or systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg (10). Given that blood glucose was based on different laboratory measures, diabetes mellitus in this study was defined as a previous medical diagnosis for the disease and/or glycated hemoglobin level ≥ 6.5% (for English)11 or fasting blood glucose ≥ 126 mg/dL (for Brazilians). Fasting blood glucose was determined by using standard enzymatic methods and glycated hemoglobin by using the principles of ion exchange high performance liquid chromatography (HPLC). Furthermore, we assessed T. cruzi infection status among the Bambui cohort population by means of three different assays performed concurrently as previously described.10 Further details on how these measures were performed can be found elsewhere.9,10
Statistical analyses
The univariate analysis were based on the chi square test, Student’s t-test or Wilcoxon rank test for differences between frequencies, means and medians, respectively. To examine the sex adjusted association between age group and all-cause mortality, we computed sex-adjusted cumulative survival curves using Kaplan–Meier estimates, by age group. We estimated age–sex adjusted hazard ratios and 95% confidence intervals for the association between risk factors and mortality using Cox proportional hazards models, after confirming that the assumption of proportionality among the hazards was met on the basis of Schoenfeld residuals. Further, we estimated age–sex adjusted population attributable risk (PAR) of all-cause mortality separately for each risk factor. In a subsequent analysis, we additionally adjusted the PARs for smoking, hypertension and diabetes, besides age and sex. This method provides an estimate of the reduction in mortality that would be observed if the individuals were unexposed to the risk factors. PARs were derived from Cox proportional hazard models (Poisson regression estimates)12 and were based on the method for estimation recommended by Greenland and Drescher.13 Because individuals may experience multiple exposures simultaneously, we additionally estimated age–sex adjusted PAR for the presence of at least one of the risk factors, and a combined PAR, assuming that all the exposures were eliminated. Due to lack of statistical power (mainly in Bambui) for separate analyses for sex, all the analyses were for both men and women with sex as a covariate. Statistical analyses were conducted using version 13.0 of STATA statistical software (Stata Corp, College Station, Texas). All P values were 2-tailed (P < 0.05).
Results
From the 5264 (ELSA) and 1606 (Bambui) participants eligible, 3205 and 1382 for whom complete data was available for all study variables were included in current analyses, respectively. Those included in these analyses were slight younger than those excluded for the English (70.1 [SD = 7.2] vs. 71.2 [SD = 7.8]; P < 0.001) and the Brazilian surveys (68.8 [SD = 6.9] vs. 72.7 [SD = 9.1]; P < 0.001). For the ELSA sample, men and women were similarly represented (62.1% and 60.4%, respectively; P = 0.195). Among Brazilians, there was a higher proportion of women participants (82.9% of men and 88.2% of women; P = 0.003).
Selected baseline characteristics of the study participants are reported in table 1. Relative to English, Brazilian participants were slightly younger, showed a higher proportion of women, a much lower schooling level, and a 5 times lower household income. The prevalence of smoking, medical diagnosis for hypertension and diabetes, as well as mean DBP were all higher among Brazilians compared with English. Mean SBP was similar in both populations. Overall, only 30.7% of English and 20.4% of Brazilians had none of the 3 risk factors considered in the present analysis.
Table 1.
Selected baseline characteristics of participants, by cohort (The ELSA and Bambui cohort studies)
| Characteristics | ELSA (n = 3205) | Bambui (n = 1382) |
|---|---|---|
| Mean age in years (SD) | 70.1 (7.2) | 68.8 (6.9)# |
| Women (%) | 54.4 | 61.5# |
| ≥11 years of schooling | 83.2 | 5.5# |
| Median monthly household income in GBP (interquartile range) | 1088 (711–1688) | 205 (103–342)# |
| Current smoking (%) | 9.8 | 17.3# |
| Previous medical diagnosis for hypertension (%) | 45.6 | 57.1# |
| Mean systolic blood pressure in mmHg (SD) | 137.1 (18.8) | 137.4 (22.5) |
| Mean diastolic blood pressure in mmHg (SD) | 74.2 (10.9) | 83.4 (12.6)# |
| Hypertensiona (%) | 65.2 | 70.4# |
| Previous medical diagnosis for diabetes (%) | 8.6 | 12.3# |
| Glycated hemoglobin ≥6.5% (ELSA) or fasting blood glucose ≥125 mg/Dl (Bambui) (%) | 7.0 | 13.1# |
| Diabetesb (%) | 10.3 | 18.5# |
| Number of risk factorsc (%) | ||
| None | 30.7 | 20.4# |
| One | 54.8 | 54.6 |
| Two or more | 14.5 | 25.0 |
Medical diagnosis for hypertension and/or systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg.
Medical diagnosis for diabetes and/or glycated hemoglobin ≥ 6.5% (ELSA) or fasting blood glucose ≥ 125 mg/Dl (Bambui).
Smoking, hypertension or diabetes.
#P < 0.05 for differences between ELSA and Bambui.
For English, during an average period of 5.80 years, 265 participants died, yielding 18 594 person-years (pyrs) of observation. For Brazilians, during an average period of 5.45 years, 241 participants died and 37 (2.7%) were lost (i.e. their vital status could not be assessed), yielding 7575 pyrs of observation. Overall, mortality rate was about twice higher among Brazilians (31.8 per 1000 pyrs) than among English (14.3 per 1000 pyrs).
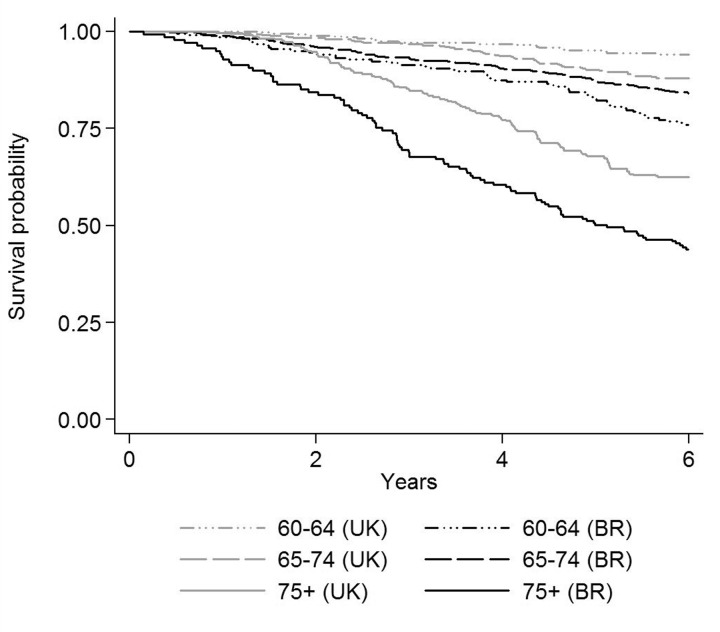
Figure 1 shows the predicted probability of 6-year survival among English and Brazilians, by age group. For each age group, Brazilians show clear low probabilities of survival relative to their English counterparts. Among Brazilians aged 60–64 years, the probability of survival is much lower than that of English of the same age and lower to that of English aged 65–74 years. The gap between the two populations is even more pronounced among the oldest old (75+ years).
Figure 1.
Six-year age adjusted survival probability among English (UK) and Brazilians (BR), by age group (The ELSA and Bambui cohort studies)
Mortality rates and hazard ratios (HR) for 6-year mortality by baseline risk factors, stratified by cohort, are showed in table 2. An excess mortality rate was found among Brazilians relative to English, in all risk groups. Among English, in the full adjusted Cox proportional model, statistically significant hazard ratios for mortality were found for smoking (HR= 2.06; P < 0.001) and diabetes (HR= 1.53; P = 0.012), but not for hypertension (HR= 1.06; P = 0.682). Among Brazilians, significant hazard ratios were found for hypertension (HR= 1.43; P = 0.028) and diabetes (HR= 1.57; P = 0.012); the corresponding value for smoking was at the borderline of the statistical significance (HR= 1.31; P = 0.054). In both populations, there was a graded association between number of risk factors and subsequent mortality, with significant hazard ratios for 2 or more risk factors (HR = 1.83 in English and HR = 2.18 in Brazilians). Importantly, the hazard ratios for each risk factor considered in this analysis, despite some heterogeneity, showed no statistically significant difference between English and Brazilians (P > 0.05 in the Wald’s test).
Table 2.
Mortality rates and hazard ratios for 6-year mortality among English and Brazilians, by selected baseline risk factors (the ELSA and Bambui cohort studies)
| Risk factor | No. deaths (mortality rate per 1.000 pyrs) |
Age–sex adjusted HR (95% CI) |
Full adjusted HR (95% CI) |
|||
|---|---|---|---|---|---|---|
| ELSA | Bambui | ELSA | Bambui | ELSA | Bambui | |
| Smoking | ||||||
| No | 227 (13.5) | 192 (30.5) | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 38 (21.3) | 49 (38.4) | 2.06 (1.46–2.91) | 1.31 (0.94–1.81) | 2.06 (1.45–2.91) | 1.38 (0.99–1.92) |
| P < 0.001 | P = 0.054 | |||||
| Hypertensiona | ||||||
| No | 77 (11.6) | 56 (24.7) | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 188 (15.7) | 185 (34.9) | 1.11 (0.85–1.45) | 1.49 (1.11–2.02) | 1.06 (0.81–1.38) | 1.43 (1.06–1.94) |
| P = 0.682 | P = 0.028 | |||||
| Diabetesb | ||||||
| No | 222 (13.2) | 180 (29.9) | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 43 (22.9) | 61 (44.3) | 1.54 (1.11–2.14) | 1.61 (1.20–2.16) | 1.53 (1.10–2.13) | 1.57 (1.17–2.11) |
| P = 0.003 | P = 0.012 | |||||
| No. risk factors | ||||||
| None | 61 (10.6) | 36 (22.9) | 1.0 | 1.0 | – | – |
| One | 146 (12.2) | 122 (29.1) | 1.12 (0.83–1.51) | 1.33 (0.92–1.93) | – | – |
| Two or more | 58 (16.9) | 83 (45.8) | 1.83 (1.28–2.63) | 2.18 (1.47–3.22) | – | – |
Medical diagnosis for hypertension and/or systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg.
Medical diagnosis for diabetes and/or glycated hemoglobin ≥ 6.5% (ELSA) or fasting blood glucose ≥ 125 mg/Dl (Bambui).
HR (95%CI): Hazard ratios and 95% CI estimated by Cox proportional regression.
Table 3 shows population attributable risk (PARs) for mortality by risk factor in each population. Among Brazilians, attributable deaths to hypertension largely predominated, (Full adjusted PAR = 25.3%; 95% CI 8.2, 39.3), followed by diabetes (PAR = 9.2%; 95% CI 4.4, 13.8) and smoking (PAR = 5.6%; 95% CI 0.6, 10.3). Among English, attributable death to smoking was highest (Full adjusted PAR = 7.4%; 95% CI 4.9, 9.7) followed by diabetes (PAR = 5.6; 95% CI 2.1, 9.1); given that hazard ratios for mortality associated with hypertension was at the borderline of the statistical significance (see above), attributable deaths to hypertension among English lack of precision (PAR = 3.9; 95% CI − 15.9, 20.3). Overall, attributable deaths to one or more risk factors was twofold higher in Brazilians (Age–sex adjusted PAR= 31.2%; 95% CI 9.3, 47.9) relative to English (PAR = 16.0; 95% CI − 3.4, 31.8). The combined PAR for hypertension, diabetes and smoking was similar to those observed above. Among Brazilians, the estimated age–sex adjusted PAR was 34.1% (95% CI 18.2, 47.0) and among English, 15.8% (95% CI 1.1, 30.0).
Table 3.
Population attributable risk for 6-year mortality among English and Brazilians, by selected baseline risk factors (the ELSA and Bambui cohort studies)
| Risk factor | Age–sex adjusted PAR (95%CI) |
Full adjusted PAR (95%CI)c |
||
|---|---|---|---|---|
| ELSA | Bambui | ELSA | Bambui | |
| Smoking | 7.4 (4.9, 9.8) | 4.8 (−0.4, 9.7) | 7.4 (4.9, 9.7) | 5.6 (0.6, 10.2) |
| Hypertensiona | 7.3 (−11.4,22.8) | 25.4 (8.2, 39.3) | 3.9 (−15.9, 20.3) | 23.1 (5.2, 37.8) |
| Diabetesb | 5.7 (2.2, 9.1) | 9.6 (4.9, 14.1) | 5.6 (2.1, 9.1) | 9.2 (4.4, 13.8) |
| At least one of the above | 16.0 (−3.4, 31.8) | 31.2 (9.3, 47.9) | – | – |
| Combinedd | 15.8 (−1.1, 30.0) | 34.1 (18.2, 47.0) | – | – |
Medical diagnosis for hypertension and/or systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg.
Medical diagnosis for diabetes and/or glycated hemoglobin ≥ 6.5% (ELSA) or fasting blood glucose ≥ 125 mg/Dl (Bambui).
PAR (95%CI): Population attributable risk derivate from the Cox proportional hazard models.
Adjusted for age, sex, plus the two remaining risk factors.
Combined estimate for smoking, hypertension and diabetes.
Because the prevalence of T. cruzi infection in the Bambuí population was high (37.5%), we implemented a sensitivity analysis to assess the influence of this infection on our results. The results of these analysis showed that all-cause mortality rate in non-infected elderly (27.6 per pyrs) remained largely higher than that among English, previously described. Also, further adjustment for T. cruzi infection had little impact on the attributable deaths to hypertension (Full plus infection adjusted PAR = 22%; 95% CI 3.6, 36.9, as well on the attributable deaths attributable to one or more risk factors (Age–sex plus infection adjusted PAR = 32.1%; 95% CI 10.4, 48.5). Interactions between the risk factors were tested and were not statistically significant (P > 0.05).
Discussion
The key findings from this analysis comparing 6-year mortality risk attributable to smoking, hypertension and diabetes in well-defined populations of English and Brazilian older adults are as follows. First, Brazilians showed much higher absolute risk of mortality than English and this finding was consistent in all age, independently of sex. Second, as a rule, hazard ratios for mortality to smoking, hypertension and diabetes showed more similarities than differences between these two populations. Third, there was strong difference among English and Brazilians on deaths attributable to hypertension.
The excess mortality rates among elderly Brazilians in relation to English might be explained by several factors. These include differences in health behaviours along the life course, factors related with health care systems, and differences in social policy context that indirectly affect health determinants and risk factors.8,14 The health care systems of Brazil and England have similarities and differences. Both countries have universal health systems, with medical care accessible to all and a strong primary care orientation not found in many countries.15,16 However, the per capita health expenditure in Brazil (US $875.00) is only a quarter of that in England (US $3222.00), and the ratio of physicians is much lower (17.0 vs. 27.4/10 000).17 Further, current and past social policy contexts, as well as life course exposures to risk are likely to play an important role. Cohort participants in both countries were born before 1960, and these age cohorts (particularly in Brazil) have experienced dramatic political and social changes during their lifetime. Brazil has rapidly transitioned from a low-income, primarily rural country in the mid-1950s to the seventh largest economy in the world, with 84% of the population living in urban areas.18 Living conditions in Brazil have also changed substantially.
Cerebrovascular diseases rank the second leading cause of death after ischemic heart disease globally.19 Mortality rates related to stroke in elderly Brazilians have decreased in the last two decades, but it has remained the primary cause of death higher than ischaemic heart disease.20 At a population level, blood pressure and tobacco use are the two most important modifiable risk factors to address the levels of strokes due to their strong associations with strokes, high prevalence and the possibility for intervention.21 Epidemiological research has shown that raised blood pressure is the single most important risk factor for ischemic stroke.22 Anti-hypertensive treatment has been shown to reduce stroke risk by about 38%.23 In the United States, efforts in hypertension control appear to have had the most substantial influence on the accelerated decline in stroke mortality.24 Tobacco use increases the risk of ischemic stroke about twofold and is furthermore also associated with a higher risk of haemorrhagic stroke.25 Our results show much higher mortality risk attributable to hypertension in Brazilians than in English, even after further adjustments for smoking and diabetes. This is in agreement with the fact that stroke is the first leading cause of death in elderly Brazilians,20 and with previous evidence indicating a poor control of blood pressure in the elderly using anti-hypertensive drugs in the Brazilian studied cohort population.26 The size of the effect of smoking was very different in the two populations too. This is a very important finding which might be due to non-studied additional confounders or to different distribution of complementary causes, effect modifiers of the mortality in the two populations.
Brazil has undergone rapid demographic and nutritional transitions.27 Dietary shifts towards low consumption of fibre and heavy consumption of saturated fatty acids and sugar and sedentary lifestyles are key contributors to the incidence of type 2 diabetes.28 Mortality rates due to diabetes in elderly Brazilians have increased about 18% in the last two decades19 Brazilian estimates indicate that people with diabetes have a 57% greater risk of death relative to the general population.27 Similar increased risks were found in our study for both English (53%) and Brazilians (57%). However, given the baseline higher prevalence of diabetes in the latter, attributable death risk to diabetes was about twofold in this population relative to English.
This study has strengths and limitations. Strengths of our study are: its cross-country comparative character, the analysis of large population based cohorts followed for a long time, the standardized and systematic measurement of parameters at baseline, comparable continuous surveillance for mortality according to standardized criteria, and minimal losses to follow-up. These strengths allowed meaningful estimates of population attributable risk for 6-year mortality due to conventional risk factors in two very different populations of older adults, adding to previous cross-country research on the subject. Although the Brazilian cohort members had a high prevalence of a chronic parasitic disease (Chagas disease), sensitivity analyses showed that infection status did not change our mains conclusions. Further, risk factors were assessed at the baseline and thus we were not able to capture changes afterwards. Thus, the association between these baseline measures and subsequent mortality are prone to a regression dilution effect that tends to attenuate the associations found.4 It is important to note that PAR depends on the size of the effect of a risk factor and also on its prevalence in the population. Therefore, the increased PAR of one or more risk factors in Brazil compared to England is because both the HRs and the prevalence of one or more risk factors were higher in Brazil.
Monitoring morbidity and mortality of NCDs and their risk factors are key components for resource allocation and policy implementation and advocacy, especially in light of the global ageing trend and increasing health inequalities. Our results indicate that, despite of being in more recent transitions, the attributable deaths to one or more risk factors was twofold among Brazilians relative to the English. These findings are indicative of the challenge health systems face to prevent and treat NCDs, particularly in populations with low socioeconomic level.
Acknowledgements
The English Longitudinal Study of Ageing (ELSA) data were made available through the UK Data Archive. The ELSA was developed by a team of researchers based at University College London, the Institute for Fiscal Studies and the National Centre for Social Research. The Bambui cohort study of ageing was developed by a team of researchers based at the Oswaldo Cruz Foundation in Minas Gerais State, Brazil.
Funding
The funding is provided by the National Institute on Aging (NIA-NIH) in the United States (grant number 5 R01 AG017644-16) and a consortium of UK government departments coordinated by the Office for National Statistics. The funding is provided by Financiadora de Estudos e Projetos (FINEP), Conselho Nacional de Desenvolvimento Científico e Tecnológicos (CNPq) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
Conflicts of interest: None declared.
Key points
What is already known on this subject?
Most knowledge about chronic disease epidemiology comes from cohort studies conducted in the United States and Western Europe, with few such studies having been conducted in other regions of the world, particularly in low or middle income countries.
There is a need for further research comparing attributable deaths to major cardiovascular risk factors and diseases between high and low or middle income countries.
What this study adds?
This study represents a rare opportunity to approach the subject in two very different social and economic contexts.
Such comparison provides an opportunity to broaden our understanding of the consequences of cardiovascular diseases and risk factors in populations where the epidemiologic transition is more recent.
The growth of chronic diseases could overwhelm public and private healthcare systems in emerging countries like Brazil. These countries need to improve their capacity to care for older people by, among others, promoting healthy lifestyles to eliminate the causes of premature death. It is worth to mention that evidence shows that lifestyle such as smoking and blood pressure largely determines health outcomes.
References
- 1.United Nations. General Assembly. Prevention and control of non-communicable diseases. Report of the Secretary-General. 2011.
- 2.WHO. Global Status Report on Non-communicable Diseases. Geneva: World Health Organization, 2010. (Chapter 1). [Google Scholar]
- 3.WHO Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: World Health Organization, 2009. [Google Scholar]
- 4.Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–13. [DOI] [PubMed] [Google Scholar]
- 5.Lawes CM, Parag V, Bennett DA, et al. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care 2004;27:2836–42. [DOI] [PubMed] [Google Scholar]
- 6.Danaei G, Ding E, Taylor B, et al. Mor tality effects of lifestyle, dietary, and metabolic risk factors in the United States: comparative risk assessment. PLoS Medicine 2009;6:e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Health statistics. http://www.who.int/nmh/countries/gbr_en.pdf (26 February 2013, date last accessed).
- 8.Lima-Costa MF, De Oliveira C, Macinko J, Marmot M. Socioeconomic inequalities in health in older adults in Brazil and England. Am J Public Health 2012. Aug; 102:1535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steptoe A, Breeze E, Banks J, Nazroo J. Cohort profile: the English Longitudinal Study of Ageing. Int J Epidemiol 2013;42:1640–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lima-Costa MF, Firmo JO, Uchoa E. Cohort profile: the Bambui (Brazil) Cohort Study of Ageing. Int J Epidemiol 2011; 40:862–7. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus. 2011. http://www.who.int/diabetes/publications/report-hba1c_2011.pdf (10 August 2011, date last accessed)
- 12.Newson RB. Attributable and unattributable risks and fractions and other scenario comparisons. Stata J 2013;13:672–98. [Google Scholar]
- 13.Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics 1993; 49:865–72. [PubMed] [Google Scholar]
- 14.Avendano M, Glymour MM, Banks J, Mackenbach JP. Health disadvantage in US adults aged 50 to 74 years: a comparison of the health of rich and poor Americans with that of Europeans. Am J Public Health 2009;99:540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paim J, Travassos C, Almeida C, et al. The Brazilian health system: history, advances and challenges. Lancet 2011;377:1778–97. [DOI] [PubMed] [Google Scholar]
- 16.Macinko J, Starfield B, Shi L. The contribution of primary care systems to health outcomes within Organization for Economic Cooperation and Development (OECD) countries, 1970–1998. Health Serv Res 2003;38:831–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Statistical Information System. Geneva, Switzerland: World Health Organization, 2011b. [Google Scholar]
- 18.IBGE Censo de 2010 [2010 census]. Rio de Janeiro, Brazil: Instituto Brasileiro de Geografia e Estatística, 2011. [Google Scholar]
- 19.Murray CJL, Lopez AD. The Global Burden of Disease. Boston, MA, USA: Harvard School of Public Health; 1996. [Google Scholar]
- 20.Lima-Costa MF, Stevens A, Duncan B. Mortalidade entre idosos no Brasil: tendências em 20 anos (1991 a 2010). [Mortality in elderly Brazilians: 20 year trends; 1991 to 2010] In: Brasil. Ministério da Saúde. Saúde Brasil 2011 [Health in Brazil 2011]. Brasília: Editora do Ministério da Saúde; 2012: 209–26. [Google Scholar]
- 21.Truelsen T, Begg S, Mathers C. The Global Burden of Cerebrovascular Disease. Geneva: World Health Organization; 2006. www.who.int/healthinfo/statistics/bod_cerebrovasculardiseasestroke.pdf (27 February 2014, date last accessed). [Google Scholar]
- 22.Dunbabin DW, Sandercock P. Preventing stroke by the modification of risk factors. Stroke 1990;21:36–9. [PubMed] [Google Scholar]
- 23.Singh RF, Suh IF, Singh VF, et al. Hypertension and stroke in Asia: prevalence, control and strategies in developing countries for prevention. J Hum Hypertens 2000;14:749–63, 749–63. [DOI] [PubMed] [Google Scholar]
- 24.Lackland DT, Rocella EJ, Deutsch AF, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke 2014;45:315–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. BMJ 1989;298:789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Firmo JO, Peixoto SV, Loyola Filho AI, et al. Birth cohort differences in hypertension control in a Brazilian population of older elderly: the Bambuí Cohort Study of Aging (1997 and 2008). Cad Saude Publica 2011;27:S427–34. [DOI] [PubMed] [Google Scholar]
- 27.Bertold AD, Kanavos P, França GVA, et al. Epidemiology, management, complications and costs associated with typ2 diabetes in Brazil: a comprehensive literature review. Global Health 2013;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sartorelli DS, Franco LJ. Trends in diabetes mellitus in Brazil: the role of the nutritional transition. Cad Saúde Pública 2003;19:S29–36. [DOI] [PubMed] [Google Scholar]