Abstract
This study examines the acute and chronic respiratory effects of toner exposure based on markers for interstitial pneumonia, oxidative stress and pulmonary function tests. A total of 112 subjects working in a Japanese toner and photocopier manufacturing company participated in this study in 2004. We annually conducted personal exposure measurements, pulmonary function tests, chest X–ray examinations, biomarkers, and questionnaires on respiratory symptoms to the subjects. We report in this paper the results of the analysis of combined annual survey point data from 2004 to 2008 and data from three annual survey points, 2004, 2008, and 2013. During these survey periods, we observed that none of subjects had a new onset of respiratory disease or died of such a disease. In both the analyses, there were no significant differences in each biomarker and pulmonary function tests within the subjects, nor between a toner–handling group and a non–toner–handling group, except for a few results on pulmonary function tests. The findings of this study suggest that there were no acute and chronic respiratory effects of toner exposure in this cohort group, although the number of subjects was small and the level of toner exposure in this worksite was low.
Keywords: Biomarkers, Occupational toxicology, Respiratory health, Toner dust, Printer
Introduction
A case of siderosilicosis caused by toner exposure was reported in The Lancet in 19941). Subsequently, there have been some additional case reports of granulomatous pneumonitis and other diseases associated with toner exposure2, 3). In 1996, The International Agency for Research on Cancer (IARC) re-classified carbon black, the main component of toner, to Group 2B (possibly carcinogenic to humans), based on experiments on rodents4). Recently, it has been shown in several studies that office machines, such as printers or photocopiers, have the ability to increase indoor air pollution because office machines emit particulate matters (PM), ozone, and volatile organic compounds5, 6, 7, 8). In vivo and in vitro experiments to assess the toxicity of toner particles and emissions from printers suggest that they are, in fact, toxic9, 10, 11, 12, 13). However, most of the previous studies were either case reports of specific diseases, or were based on animal experiments and in vivo and in vitro experiments with extremely high levels of toner exposure, which are unlikely environments to encounter at the workplace. The data from these studies were insufficient to confirm the actual risk of carbon black or toner.
It is therefore necessary to confirm the relationship between toner exposure and human health effects via longitudinal epidemiological studies so the authors started a 10–year cohort study in 2003 using subjects from several Japanese companies. The purpose of this study was to examine the acute and chronic respiratory effects of toner exposure based on markers for interstitial pneumonia, oxidative stress and pulmonary function tests.
Methods
Subjects and data collection
A total of 112 subjects working in a Japanese toner and photocopier manufacturing company participated in this study in 2004. The subjects were divided into a toner–handling group and a non–toner–handling group. The toner–handling group consisted of 53 subjects who were engaged in research and development of toner, machine development, or production and maintenance. The non–toner–handling group consisted of 59 subjects who were office workers. However, we excluded 6 subjects in the non–toner–handling group from this study because they had engaged in toner–handling tasks from 1990 to 2002. This study was conducted with a total of 106 subjects consisting of 53 subjects in each group. From 2004 to 2008, we prospectively followed 77 subjects consisting of 32 toner–handling workers and 45 non–toner–handling workers.
The number of subjects and the quantity of toner–handling work have been drastically declining since 2009 because of decreasing toner and photocopier manufacturing in the company. A total of 61 subjects participated in this study in 2009 while 37 subjects, consisting of 13 toner–handling workers and 24 non–toner–handling workers participated in both 2008 and 2013, the survey’s end point.
Toner exposure measurements
Personal exposure measurements were conducted in subjects who were randomly selected in the total subjects of toner–handling group or non–toner–handling group in each year. We performed exposure assessment using a personal respirable dust sampler, Model PS–47, Roken type (Shibata Scientific Technology, Ltd.) with glass fiber filters, and polytetrafluoroethylene (PTFE) binding, T60A20 type. It had a filter of φ25 mm in diameter with a flow rate of 2.0 m/s. Subjects wore the data recorder of the personal dust sampler on the left side of their waist and the detector around their necks. Respirable dust trapped in the filter was measured with an electric balance, and the measured quantity was divided by the total aspirated air volume to calculate the respirable dust concentration. In order to compare it with the threshold limit value (TLV) as defined by the American Conference of Governmental Industrial Hygienists (ACGIH), which is based on an allowable exposure averaged over a normal 8–hour workday or 40–hour workweek, we calculated an 8–hour time weighted average (TWA8h)14). Actually, personal exposure measurements were conducted only when workers were using toner particles. Personal exposure measurements were calculated to a TWA8h using the following formula: personal exposure concentration (mg/m3)×performing working hours (h)/8=TWA8h (mg/m3). Workers are regularly scheduled to work eight hours but if there has been less than eight hours exposure, time was increased to eight hours, or if more than eight hours exposure time is compressed before being calculated. We calculated the mean TWA8h values in each year and each group.
Questionnaire
We investigated subjects’ work history and current duties with regard to toner–handling work; their medical histories and present respiratory illnesses and symptoms using yearly self–reported questionnaires. Using the Japanese version ATS–DLD–78 Adult questionnaire15), we evaluated five chronic respiratory symptoms: persistent cough, persistent phlegm, persistent cough and phlegm, wheezing without asthmatic response and breathlessness,
Biomarkers for interstitial pneumonia, oxidative stress and other conditions
Several serum or urine biomarkers, along with leukocyte counts, were measured in this study. First, sialylated carbohydrate antigen KL–6 (KL–6) and surfactant protein D (SP–D), being well–known markers of interstitial pneumonia, were measured. Second, C–reactive protein (CRP) and the number of leukocytes as representative inflammatory markers were measured in blood samples. Additionally, 8–hydroxy–2’–deoxyguanosine (8–OHdG) as a marker of oxidative stress was measured in urine. Blood samples were obtained to measure levels of KL–6, SP–D, CRP and leucocyte count, and the tests were carried out at Special Reference Laboratory, Inc. (SRL Inc. Tokyo, Japan), while urine levels of 8–OHdG were measured at OHG Institute Co. Ltd (Fukuoka, Japan). Serum levels of KL–6 and SP–D were measured by Electro–chemiluminescence immunoassay (ECLIA) and enzyme immunoassay (EIA). Leukocyte count was measured using an automated cell counter. Urine level of 8–OHdG was measured using high–performance liquid chromatography (HPLC), and corrected values were adjusted with urine creatinine concentration. The serum level of CRP was measured using turbidimetric immunoassay (TIA) from 2004 to 2006, latex immunoagglutination assay (LA) from 2007 to 2008, and latex nephelometric immunoassay from 2009 onward. Limits of detection (LOD) of SRL Inc. were 0.1 mg/dl for CRP using TIA, 0.01 mg/dl for CRP using LA, 0.004 mg/dl for CRP using latex nephelometric immunoassay, and 17.2 ng/ml for SP–D.
Other examinations
We conducted yearly pulmonary function tests and chest x-ray examination for subjects. The pulmonary function tests were performed with a pneumotach–type spirometry measuring unit, for example, Microspiro HI–701 and Microspiro HI–801 (Chest Corporation, Tokyo, Japan), meeting the standards stipulated by the American Thoracic Society16). We evaluated vital capacity in each subject as percent of predicted (%VC), forced expiratory volume percentile per second (FEV1.0%). Chest X-rays were performed for subjects in accordance with the standard examination method regulated by the Pneumoconiosis Law17, 18). The X–rays were interpreted based on the international classification of pneumoconiosis16), and obtained images were electronically stored using a film digitizer.
Data analysis
We performed analysis to evaluate the changes of within-subjects using the data from 2004 to 2013, and then compare the between-subjects effects using the data in 2004, 2008 and 2013. Biomarkers and pulmonary function tests were analyzed as dependent variables using mixed model and repeated measures analysis of variance (ANOVA). For independent variables, the year of the survey was used as within–subjects factor, and toner–handling or non–toner–handling group, gender, age groups and smoking status were used as between–subjects factors. Smoking status was classified under either never smoker, former smoker 1 (ceased smoking before 2003), former smoker 2 (ceased smoking for the years of the survey) or current smoker. Age groups were classified under either 20–29 years, 30–39 years, 40–49 years, or 50–59 years. Significant statistical differences among other independent variables were assessed with Bonferroni multiple comparison procedure. As mentioned above, the measuring methods and LOD of CRP were different in years, and the CRP levels could not be processed as continuous data within each subjects using repeated ANOVA. In the Kolmogorov–Smirnov test, the values from 2004 to 2008 did not follow the normal distribution, except for the one of 2013. Therefore, The Mann–Whitney U test were used to analyze CRP levels between the toner–handling group and non–toner–handling group in each year of the surveys from 2004 to 2008, and the Student t–test was used in 2013. In addition, the Fisher’s exact test was used to analyze for annual comparisons of prevalence rates of each respiratory symptom between the toner–handling and non–toner–handling groups, because more than 20% of the contingency table cells had expected cell frequencies less than 5. Statistical analyses were conducted using SPSS23.0J (IBM SPSS Inc., former SPSS Inc.). Probability values of 0.05 were considered to indicate statistical significance.
Ethical approval
This study was reviewed and approved by the Human Ethics Committee for Epidemiological Research at the University of Occupational and Environmental Health, in Japan.
Results
The results are described here in the following order toner exposure measurement, annual survey points from 2004 to 2008, and the three survey points of 2004, 2008, and 2013.
Toner exposure measurements
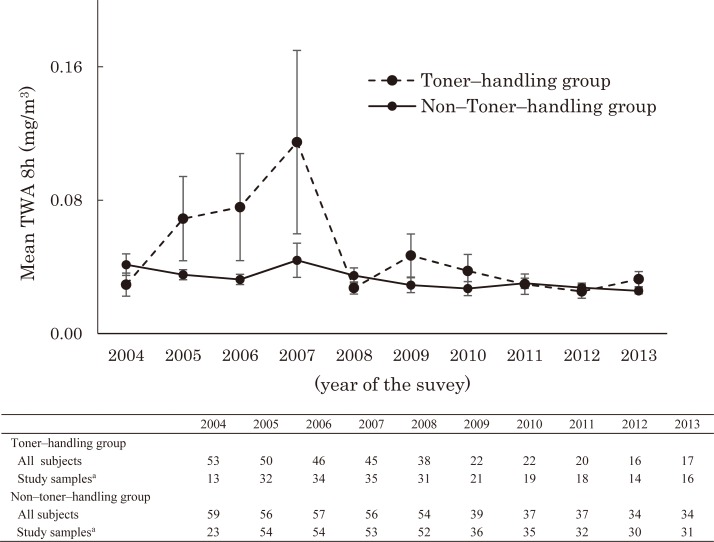
Personal exposure measurements were conducted in subjects who were randomly selected from the entire sample of subjects. The mean TWA8h of personal exposure measurements was analyzed by toner–handling status, for each year (Fig. 1). Personal exposure concentration levels in 2004 and from 2008 to 2013 were almost the same, and there were higher levels of personal exposure concentrations in the toner–handling group than the non–toner–handling group from 2005 to 2007. The highest mean values in 2007 were well below the acceptable value of 3.0 mg/m3, recommended by the ACGIH as the TLV–TWA of particles not otherwise specified.
Fig. 1.
Mean 8–hour time weighted average (TWA8h) of Personal exposure measurement in the toner–handling and non–toner–handling groups from 2004 to 2013.
a Personal exposure measurements were conducted annually in subjects who were randomly selected from among the entire sample population comprising a toner–handling and non–toner–handling group. Error bars represent the standard error of the mean.
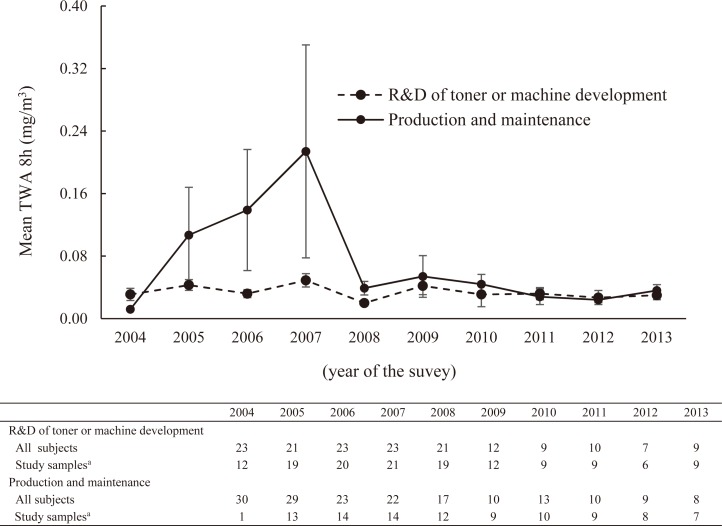
In the toner–handling group, the mean TWA8h of personal exposure measurements was analyzed by work contents, which were “R&D (research and development) of toner or machine development” and “production and maintenance”, for each year (Fig. 2). The mean TWA8h levels in “R&D of toner or machine development” were quite low and were almost the same as the ones of the non–toner–handling group. There were much higher levels of the mean TWA8h in “production and maintenance” than “R&D of toner or machine development” from 2005 to 2007, and the ones from 2008 to 2013 were almost the same. In 2004, the study sample in “production and maintenance” was only one, so it was difficult to compare personal exposure concentrations levels between the two.
Fig. 2.
Mean 8–hour time-weighted average (TWA8h) of personal exposure measurement by work contents from 2004 to 2013.
R&D: research and development; SD: standard deviation
a Personal exposure measurements were conducted annually in subjects who were randomly selected from among the entire sample of subjects. Error bars represent standard error of the mean.
Findings from the annual survey points from 2004 to 2008
Subject demographics
We followed up 77 subjects, all of whom had annual examinations, among 106 subjects from 2004 to 2008. The follow–up rates for the toner–handling group and non–toner– handling group were 60.4% (32/53 subjects) and 84.9% (45/53 subjects). The characteristics of the 77 subjects are shown in Table 1. In terms of presence of respiratory illness, a subject in the toner–handling group and two subjects in the non–toner–handling group had contracted bronchiectasis and other respiratory diseases before 2003, and one subject in the toner handling group had chronic bronchitis. However, no other subjects contracted a respiratory disease between 2004 and 2008. During this survey period, none of the 112 subjects had new onset of, or died of any respiratory disease.
Table 1. Characteristics of 77 subjects in 2004.
| Toner–handling group | Non–toner–handling group | Statistics | p value | ||||
|---|---|---|---|---|---|---|---|
| No. of subjects | 32 | 45 | |||||
| Sex | |||||||
| Male, n / (%) | 28 | (87.5) | 37 | (82.2) | 0.396a | 0.529 | |
| Female, n / (%) | 4 | (12.5) | 8 | (17.8) | |||
| Age, Mean / (SD) | 38.6 | (6.3) | 38.2 | (7.0) | 0.273b | 0.786 | |
| Age group (years), n / (%) | |||||||
| 20–29 | 1 | (3.1) | 4 | (8.9) | |||
| 30–39 | 15 | (46.9) | 21 | (46.7) | |||
| 40–49 | 15 | (46.9) | 20 | (44.4) | |||
| 50–59 | 1 | (3.1) | |||||
| BMI, Mean / (SD) | 23.2 | (4.1) | 22.1 | (2.4) | 1.418b | 0.160 | |
| Smoking | |||||||
| Never smoker, n / (%) | 9 | (28.1) | 16 | (35.6) | 0.677a | 0.879 | |
| Former smoker 1c, n / (%) | 3 | (9.4) | 5 | (11.1) | |||
| Former smoker 2d, n / (%) | 8 | (25.0) | 9 | (20.0) | |||
| Current smoker, n / (%) | 12 | (37.5) | 15 | (33.3) | |||
| PI of respiratory system | |||||||
| bronchiectasis | 1 | ||||||
| chronic bronchitis | 1 | ||||||
| other respiratory diseases | 2 | ||||||
| PMH of respiratory system | |||||||
| pneumonia, bronchitis | 7 | 4 | |||||
| pleuritis | 1 | ||||||
| pulmonary tuberculosis | 1 | ||||||
| other respiratory diseases | 1 | ||||||
BMI: body mass index, SD: standard deviation, PI: present illness, PMH: past medical history
a Statistics were χ2 values. b Statistics were t values. c Former smoker 1 were subjects who had ceased smoking before 2003.
d Former smoker 2 were subjects who had ceased smoking during the years of the survey.
The Japanese version ATS–DLD–78 Adult questionnaire
The number of subjects who had any of the five respiratory symptoms in the toner–handling and non–toner handling groups is shown in Table 2. The rates of subjects who had persistent cough, persistent phlegm and persistent cough and phlegm were the highest in 2004, and the numbers remained the same till 2006, and then declined from 2007. Particularly, we observed statistically significant differences in the number of persistent cough and phlegm in 2006. Subjects who had wheezing without an asthmatic response and breathlessness numbered only a few throughout the survey periods.
Table 2. Respiratory symptoms using self–reported questionnaire in the toner–handling group and the non–toner–handling group from 2004 to 2008.
| Respiratory symptoms | Groups | n | 2004 | 2005 | 2006 | 2007 | 2008 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |||
| Persistent cough | Toner–handling group | 32 | 6 | (18.8) | 5 | (15.6) | 5 | (15.6) | 5 | (15.6) | 3 | (9.4) |
| Non–toner–handling group | 45 | 4 | (8.9) | 2 | (4.4) | 2 | (4.4) | 3 | (6.7) | 0 | (0.0) | |
| p–valuea | 0.304 | 0.120 | 0.120 | 0.265 | 0.068 | |||||||
| Persistent phlegm | Toner–handling group | 32 | 5 | (15.6) | 4 | (12.5) | 5 | (15.6) | 5 | (15.6) | 4 | (12.5) |
| Non–toner–handling group | 45 | 6 | (13.3) | 5 | (11.1) | 2 | (4.4) | 4 | (8.9) | 3 | (6.7) | |
| p–valuea | 1.000 | 1.000 | 0.120 | 0.477 | 0.441 | |||||||
| Persistent cough and phlegm | Toner–handling group | 32 | 4 | (12.5) | 3 | (9.4) | 4 | (12.5) | 1 | (3.1) | 0 | (0.0) |
| Non–toner–handling group | 45 | 5 | (11.1) | 2 | (4.4) | 0 | (0.0) | 0 | (0.0) | 1 | (2.2) | |
| p–valuea | 1.000 | 0.644 | 0.027* | 0.416 | 1.000 | |||||||
| Wheezing without asthmatic response | Toner–handling group | 32 | 0 | (0.0) | 0 | (0.0) | 1 | (3.1) | 1 | (3.1) | 2 | (6.3) |
| Non–toner–handling group | 45 | 2 | (4.4) | 1 | (2.2) | 1 | (2.2) | 0 | (0.0) | 0 | (0.0) | |
| p–valuea | 0.508 | 1.000 | 1.000 | 0.416 | 0.170 | |||||||
| Breathlessness | Toner–handling group | 32 | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) |
| Non–toner–handling group | 45 | 1 | (2.2) | 1 | (2.2) | 1 | (2.2) | 0 | (0.0) | 0 | (0.0) | |
| p–valuea | 1.000 | 1.000 | 1.000 | — | — | |||||||
a Fisher’s exact test was performed for annual comparisons of prevalence rates of each respiratory symptom between the toner–handling and non–toner–handling groups. *p<0.05
Biomarkers for interstitial pneumonia, oxidative stress and others
We evaluated the differences of each biomarker, except CRP, within–subjects and between the toner–handling group and non–toner–handling group in the five–year study period (Table 3). There were no significant differences in the levels of each biomarker between toner–handling group and non–toner–handling group. In addition, interaction effects were not detected among each dependent variable. Statistically, significant differences were not observed in the levels of CRP between the toner–handling group and non–toner–handling group in each year.
Table 3. Comparison of biomarkers and pulmonary function tests between the toner–handling group and the non–toner handling group from 2004 to 2008.
| Parameters (Unit) | Groups | n | 2004 | 2005 | 2006 | 2007 | 2008 | F valuea | p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | |||||
| WBC (/μl) |
Toner–handling group | 32 | 7056.3 | (1447.1) | 7337.5 | (2116.1) | 6696.9 | (1711.3) | 6159.4 | (1267.2) | 6987.5 | (1838.4) | 0.270 | 0.847 |
| Non–toner–handling group | 45 | 6571.1 | (1931.6) | 6500.0 | (2167.2) | 6293.3 | (1829.4) | 6171.1 | (1655.8) | 6386.7 | (1758.0) | |||
| Total | 77 | 6772.7 | (1752.8) | 6848.1 | (2172.2) | 6461.0 | (1781.1) | 6166.2 | (1497.4) | 6636.4 | (1804.7) | |||
| KL–6 (U/ml) |
Toner–handling group | 32 | 235.2 | (84.4) | 247.0 | (79.5) | 244.7 | (82.1) | 226.5 | (83.5) | 222.8 | (82.2) | 0.411 | 0.757 |
| Non–toner–handling group | 45 | 204.6 | (76.6) | 209.4 | (76.0) | 208.6 | (66.7) | 199.4 | (75.2) | 202.9 | (85.1) | |||
| Total | 77 | 217.4 | (80.8) | 225.0 | (79.2) | 223.6 | (75.1) | 210.7 | (79.4) | 211.1 | (84.0) | |||
| SP–D (ng/ml) |
Toner–handling group | 32 | 51.2 | (24.4) | 44.7 | (22.2) | 39.1 | (18.5) | 41.1 | (22.2) | 46.9 | (22.7) | 1.231 | 0.289 |
| Non–toner–handling group | 45 | 58.1 | (30.1) | 53.6 | (27.3) | 49.6 | (28.4) | 49.6 | (26.1) | 52.6 | (28.0) | |||
| Total | 77 | 55.2 | (27.9) | 49.9 | (25.5) | 45.2 | (25.2) | 46.1 | (24.7) | 50.2 | (25.9) | |||
| 8–OHdG (mg/dl) |
Toner–handling group | 27 | 4.3 | (1.7) | 4.8 | (2.3) | 4.3 | (1.7) | 4.2 | (1.7) | 3.9 | (1.4) | 0.145 | 0.941 |
| Non–toner–handling group | 42 | 5.0 | (1.9) | 5.0 | (1.6) | 4.9 | (1.7) | 4.5 | (1.6) | 4.5 | (1.3) | |||
| Total | 69 | 4.7 | (1.9) | 4.9 | (1.9) | 4.7 | (1.7) | 4.4 | (1.6) | 4.2 | (1.4) | |||
| CRPb (mg/dl) |
Toner–handling group | 32 | 0.22 | (0.26) | 0.53 | (1.08) | 0.17 | (0.13) | 0.12 | (0.20) | 0.17 | (0.32) | ||
| Non–toner–handling group | 45 | 0.21 | (0.38) | 0.18 | (0.28) | 0.22 | (0.68) | 0.07 | (0.08) | 0.17 | (0.52) | |||
| Total | 77 | 0.21 | (0.34) | 0.33 | (0.74) | 0.20 | (0.53) | 0.09 | (0.14) | 0.17 | (0.44) | |||
| %VC (%) |
U values / p valuec | 634.5 / 0.274 | 601.0 / 0.110 | 582.5 / 0.064 | 577.5 / 0.139 | 584.5 / 0.160 | ||||||||
| Toner–handling group | 31 | 111.0 | (14.1) | 107.9 | (15.2) | 109.0 | (13.3) | 108.4 | (14.6) | 113.2 | (13.9) | 3.534 | 0.020* | |
| Non–toner–handling group | 39 | 112.7 | (11.3) | 111.6 | (14.8) | 113.4 | (12.4) | 113.8 | (12.2) | 118.3 | (15.4) | |||
| Total | 70 | 112.0 | (12.6) | 109.9 | (15.0) | 111.4 | (12.9) | 111.4 | (13.5) | 116.1 | (14.9) | |||
| FEV1.0% (%) |
Toner–handling group | 31 | 82.1 | (5.8) | 81.1 | (9.1) | 82.8 | (5.5) | 83.0 | (6.0) | 82.0 | (5.2) | 1.588 | 0.201 |
| Non–toner–handling group | 38 | 84.5 | (6.0) | 85.4 | (6.7) | 83.9 | (5.7) | 82.4 | (6.8) | 81.1 | (7.3) | |||
| Total | 69 | 83.4 | (6.0) | 83.5 | (8.1) | 83.4 | (5.6) | 82.7 | (6.4) | 81.5 | (6.4) | |||
SD: standard deviation, WBC: white ball cell, KL–6: Sialylated carbohydrate antigen KL–6, SP–D: surfactant protein D, 8–OHdG: 8–hydroxy–2’–deoxyguanosine, CRP: C–reactive protein, %VC: vital capacity as percent of predicted, FEV1.0%: forced expiratory volume percentile in one second.
a In mixed model repeated ANOVA, the sphericity assumption was violated; to account for this violation, degrees of freedom were Greenhouse–Geisser adjusted.
b The serum level of CRP was measured using turbidimetric immunoassay (TIA) from 2004 to 2006 and latex immunoagglutination assay (LA) from 2007 to 2008. Limits of detection (LOD) for CRP were 0.1 mg/dl in using TIA and 0.01 mg/dl in using LA.
c Mann–Whitney U test was performed on the serum levels of CRP between the toner–handling group and non–toner–handling groups in each year of the survey.
* p<0.05. The levels of %VC in 2008 were significantly more than the level of 2006 to 2007 in the toner–handling group, and the levels in 2008 were significantly more than the levels from 2004 to 2007 in the non–toner–handling group with Bonferroni multiple comparison procedure.
Other examinations
We evaluated within–subject effects on pulmonary function as %VC and FEV1.0%, between the toner–handling group and non–toner–handling group in the five–year study period (Table 3). There were no significant differences in FEV1.0% between the toner–handling group and non–toner–handling group. Significant differences were observed in %VC, so we consequently analyzed multiple comparisons according to toner–handling group or non–toner–handling group. The levels of %VC in 2008 were significantly more than the ones of 2006 to 2007 in the toner–handling group, and the ones in 2008 were significantly more than the ones from 2004 to 2007 in the non–toner–handling group. We could not observed significant differences in the main effects of gender, age groups and smoking status. In addition, interaction effects were detected among the toner–handling and non–toner–handling groups and age groups. There were significant differences in the toner–handling and 40–49 years group, and the non–toner–handling and 30–39 years group in the analysis of multiple comparisons. The levels of %VC of the non–toner–handling group and 30–39 years group were significantly more in 2008 than between 2004 to 2006.
The chest X–rays showed bilateral or unilateral pleural thickening of the pulmonary apex in two and six subjects in the toner–handling group and non–toner–handling group, respectively, and emphysema in two subjects in the non–toner–handling group.
Finding from the three survey points of 2004, 2008, and 2013
Subject demographics
We followed up 37 subjects in three survey years, 2004, 2008, and 2013. The follow up rates of the toner–handling group and non–toner–handling group were 24.5% (13/53 subjects) and 45.2% (24/53 subjects). The characteristics of the 37 subjects are shown in Table 4. In terms of the presence of respiratory illness, a subject in the non–toner–handling group had contracted bronchiectasis before 2003, but no other subjects contracted a respiratory disease from 2004 to 2013. During this survey periods, none of the 106 subjects had a new onset or died of a respiratory disease.
Table 4. Characteristics of the 37 subjects in 2004.
| Parameters | Toner–handling group | Non–toner–handling group | t value | p value | |||
|---|---|---|---|---|---|---|---|
| No. of subjects | 13 | 24 | |||||
| Sex | |||||||
| Male, n / (%) | 12 | (92.3) | 23 | (95.8) | |||
| Female, n / (%) | 1 | (8.3) | 1 | (4.2) | |||
| Age, Mean / (SD) | 40.5 | (7.4) | 39.1 | (6.5) | 0.588 | 0.565 | |
| Age group (years), n / (%) | |||||||
| 20–29 | 1 | (7.7) | 2 | (8.3) | |||
| 30–39 | 4 | (30.8) | 10 | (41.7) | |||
| 40–49 | 7 | (53.8) | 11 | (45.8) | |||
| 50–59 | 1 | (7.7) | 1 | (4.2) | |||
| BMI, Mean / (SD) | 23.9 | (4.6) | 23.0 | (2.4) | 0.784 | 0.438 | |
| Smoking | |||||||
| Never smoker, n / (%) | 4 | (30.8) | 8 | (33.3) | |||
| Former smoker 1a, n / (%) | 1 | (7.7) | 4 | (16.7) | |||
| Former smoker 2b, n / (%) | 4 | (30.8) | 6 | (25.0.) | |||
| Current smoker, n / (%) | 4 | (30.8) | 6 | (25.0) | |||
| PI of respiratory system | |||||||
| bronchiectasis | 1 | ||||||
| PMH of respiratory system | |||||||
| pneumonia, bronchitis | 1 | 1 | |||||
| pleuritis | 1 | ||||||
BMI: body mass index, SD: standard deviation, PI: present illness, PMH: past medical history,
a Former smoker 1 were group of subjects who had ceased smoking before 2003. b Former smoker 2 were subjects who had ceased smoking during the years of the survey.
The participation rates at the three survey points of 2004, 2008, and 2013 that also came under the annual survey points between 2004 to 2008 were 51.1% (11/32 subjects) in the toner–handling group and 34.4% (23/45 subjects) in the non–toner–handling group.
The Japanese version ATS–DLD–78 Adult questionnaire
The number of subjects who had any of the five respiratory symptoms in the toner–handling group and non–toner–handling group are shown in Table 5. The rate of subjects who had persistent cough, persistent phlegm and persistent cough and phlegm was the most in 2004 out of the three survey points, after which point the number declined. The subjects who had wheezing without an asthmatic response and breathlessness numbered a few over the three survey points.
Table 5. Respiratory symptoms using self–reported questionnaire in the toner–handling group and the non–toner–handling group in 2004, 2008, and 2013.
| Respiratory symptoms | Groups | n | 2004 | 2008 | 2013 | |||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |||
| Persistent cough | Toner–handling group | 13 | 3 | (23.1) | 1 | (7.7) | 0 | (0.0) |
| Non–toner–handling group | 24 | 2 | (8.3) | 0 | (0.0) | 2 | (8.3) | |
| p valuea | 0.321 | 0.351 | 0.532 | |||||
| Persistent phlegm | Toner–handling group | 13 | 3 | (23.1) | 2 | (15.4) | 1 | (7.7) |
| Non–toner–handling group | 24 | 3 | (12.5) | 0 | (0.0) | 0 | (0.0) | |
| p valuea | 0.643 | 0.117 | 0.351 | |||||
| Persistent cough and phlegm | Toner–handling group | 13 | 2 | (15.4) | 0 | (0.0) | 0 | (0.0) |
| Non–toner–handling group | 24 | 2 | (8.3) | 0 | (0.0) | 0 | (0.0) | |
| p valuea | 0.602 | — | — | |||||
| Wheezing without asthmatic response | Toner–handling group | 13 | 1 | (7.7) | 2 | (15.4) | 0 | (0.0) |
| Non–toner–handling group | 24 | 1 | (4.2) | 0 | (0.0) | 1 | (4.2) | |
| p valuea | 1.000 | 0.117 | 1.000 | |||||
| Breathlessness | Toner–handling group | 13 | 1 | (7.7) | 0 | (0.0) | 0 | (0.0) |
| Non–toner–handling group | 24 | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | |
| p valuea | 0.351 | — | — | |||||
a Fisher’s exact test was performed for annual comparisons of prevalence rates of each respiratory symptom between the toner–handling and non–toner–handling groups.
Biomarkers for interstitial pneumonia, oxidative stress and others
Except for CRP, we evaluated within–subjects effects on each biomarker between the toner–handling group and the non–toner–handling group, at the three survey points of 2004, 2008 and 2013. There were no significant differences in the levels of each biomarker between the toner–handling group and the non–toner–handling group (Table 6). In addition, interaction effects were not detected for each dependent variables. Statistically significant differences were not observed in the levels of CRP between the toner–handling group and the non–toner–handling group in each year.
Table 6. Comparison of biomarkers and pulmonary function tests between the toner–handling group and the non–toner handling group in 2004, 2008, and 2013.
| Parameters (Unit) | Group | n | 2004 | 2008 | 2013 | F valuea | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |||||
| WBC (/μl) |
Toner–handling group | 13 | 6707.7 | (1489.7) | 6376.9 | (1874.9) | 6800.0 | (1383.8) | 0.493 | 0.615 |
| Non–toner–handling group | 24 | 6791.7 | (1941.7) | 6112.5 | (1953.4) | 6712.5 | (2015.2) | |||
| Total | 37 | 6762.2 | (1774.9) | 6205.4 | (1904.2) | 6743.2 | (1798.5) | |||
| KL–6 (U/ml) |
Toner–handling group | 13 | 236.2 | (90.4) | 222.6 | (85.7) | 263.2 | (104.6) | 0.040 | 0.961 |
| Non–toner–handling group | 24 | 201.7 | (70.3) | 198.5 | (94.0) | 226.7 | (73.1) | |||
| Total | 37 | 213.8 | (78.5) | 207.0 | (90.7) | 239.5 | (85.9) | |||
| SP–D (ng/ml) |
Toner–handling group | 13 | 63.1 | (29.6) | 58.5 | (22.5) | 44.8 | (24.4) | 0.496 | 0.614 |
| Non–toner–handling group | 24 | 60.8 | (32.2) | 53.3 | (26.0) | 51.3 | (30.3) | |||
| Total | 37 | 61.6 | (30.9) | 55.1 | (24.7) | 49.0 | (28.2) | |||
| 8–OHdG (mg/dl) |
Toner–handling group | 13 | 4.0 | (1.7) | 3.5 | (1.3) | 3.5 | (1.0) | 2.159 | 0.131 |
| Non–toner–handling group | 24 | 4.8 | (2.1) | 4.3 | (1.3) | 4.0 | (1.6) | |||
| Total | 37 | 4.5 | (2.0) | 4.0 | (1.3) | 3.8 | (1.4) | |||
| CRP b (mg/dl) |
Toner–handling group | 13 | 0.22 | (0.21) | 0.16 | (0.17) | 0.17 | (0.20) | ||
| Non–toner–handling group | 24 | 0.22 | (0.42) | 0.22 | (0.63) | 0.07 | (0.14) | |||
| Total | 37 | 0.22 | (0.38) | 0.20 | (0.56) | 0.10 | (0.17) | |||
| Statistics / P value | 120.0 / 0.128c | 119.0 / 0.235c | 2.037 / 0.060d | |||||||
| %VC (%) |
Toner–handling group | 12 | 109.5 | (19.5) | 107.1 | (19.0) | 107.6 | (15.9) | 3.701 | 0.064 |
| Non–toner–handling group | 23 | 112.6 | (9.3) | 117.3 | (11.8) | 109.3 | (17.7) | |||
| Total | 35 | 111.5 | (13.5) | 113.8 | (15.2) | 108.7 | (16.9) | |||
| FEV1.0% (%) |
Toner–handling group | 12 | 81.6 | (6.6) | 82.1 | (5.4) | 80.6 | (6.2) | 4.056 | 0.027* |
| Non–toner–handling group | 23 | 82.8 | (6.2) | 79.0 | (9.6) | 81.0 | (7.7) | |||
| Total | 35 | 82.4 | (6.3) | 80.0 | (8.5) | 80.9 | (7.1) | |||
SD: standard deviation, WBC: white ball cell, KL–6: Sialylated carbohydrate antigen KL–6, SP–D: surfactant protein D, 8–OHdG: 8–hydroxy–2’–deoxyguanosine, CRP: C–reactive protein, %VC: vital capacity as percent of predicted, FEV1.0%: forced expiratory volume percentile in one second.
a In mixed model repeated ANOVA, the sphericity assumption was violated; to account for this violation, degrees of freedom were Greenhouse–Geisser adjusted.
b The serum level of CRP was measured using turbidimetric immunoassay (TIA) in 2004, latex immunoagglutination assay (LA) in 2008, and latex nephelometric immunoassay in 2013. Limits of detection (LOD) for CRP were 0.1 mg/dl in using TIA, 0.01 mg/dl in using LA, 0.004 mg/dl in using latex nephelometric immunoassay.
c Mann–Whitney U test and d Student t test were conducted between toner–handling group and non–toner–handling group in each year of the survey.
*p<0.05, FEV1.0% was not significant with Bonferroni multiple comparison procedure.
Other examinations
We evaluated within–subjects effects on pulmonary function as %VC and FEV1.0% between the toner–handling group and the non–toner–handling group in the three survey points of 2004, 2008, and 2013 (Table 6). There were no significant differences in %VC between the toner–handling group and the non–toner–handling group. A significant difference was observed in FEV1.0%, so we consequently analyzed multiple comparisons by toner–handling group and non–toner–handling group. In the non–toner–handling group, the levels of FEV1.0% in 2004 were less than the ones in 2008 or 2013, but there was no statistically significant difference. In addition, interaction effects were detected between the toner–handling group and the non–toner–handling group for gender. We analyzed multiple comparisons by toner–handling group and the non–toner–handling group in males, but observed no significant differences. We observed statistically significant differences of FEV1.0% in the main effects of smoking status, so we consequently analyzed multiple comparisons according by smoking status. We found statistically significant differences among the category “former smoker 2”. The levels of FEV1.0% in the former smoker 2 category declined in 2008 and then increased again in 2013, but there were no statistically significant differences in the multiple comparisons. In addition, interaction effects were detected among the toner–handling and non–toner–handling groups and gender, but there were not significant differences to analyze multiple comparisons by the toner–handling and non–toner–handling groups and gender. We excluded female subjects because there were only three in both groups. In the findings of chest X–ray, we observed bilateral or unilateral pleural thickening of the pulmonary apex in one and five subjects in the toner–handling group and non–toner–handling group, respectively, and emphysema in two subjects of the non–toner–handling group.
Discussion
We evaluated the levels of markers for interstitial pneumonia and oxidative stress, and pulmonary function tests, and additionally investigated the onset of respiratory diseases or respiratory symptoms using questionnaires or chest X–ray examinations among a cohort of workers in a toner and photocopier manufacturing company, to clarify the effects of toner exposure on the human respiratory system.
In respiratory symptoms, the prevalence of subjects who had persistent cough, persistent phlegm and persistent cough and phlegm from 2004 to 2007 were higher in the toner–handling group than the non–toner–handling group. Mean TWA8h levels of the toner–handling group tended to be high from 2004 to 2007. We considered that some subjects might be sensitive to dust exposure and be prone to symptoms such as coughing and sputum production. We consider that these symptoms were not morbid findings because other examinations in subjects who had the respiratory symptoms were within normal ranges.
KL–6 and SP–D is increased in patients with interstitial lung diseases and is a sensitive indicator for the active phase of interstitial lung diseases19, 20, 21). Hamaguchi et al. reported that indium–exposed workers showed significantly higher values of KL–6 and SP–D than non–exposed workers, and the exposure–effect and exposure response relations between indium in serum and KL–6, and between indium in serum and SP–D were very pronounced22). For these reasons, it is suggested that KL–6 and SP–D are useful indicators of exposure to material that includes respiratory diseases. Many studies have reported that various diseases, as well as dangers in lifestyle factors such as habitual smoking and drinking, are associated with the urinary 8–OHdG levels23, 24, 25). In terms of respiratory disorders, Takahashi et al. reported that leukocytic 8–OHdG levels correlated with the cumulative asbestos–exposure indices26). Additionally, Kim et al. reported the association between exposure to particulate matter less than 2.5 microns in diameter (PM2.5) and urinary 8–OHdG concentrations27). The level of 8–OHdG is thus also useful as indicator of exposure to materials that include respiratory diseases. The pulmonary function test generally show abnormal levels when human beings are suffer sever lung diseases. Some studies supposed that the amount of change in %VC and FEV1.0% reflects the degree of dust exposure28, 29).
It is necessary to carefully consider the effects on pulmonary function or biomarkers by smoking status and ageing as existing research has already shown that they both affect, pulmonary function30, 31, 32, 33). We observed there were no significant differences between the toner–handling and the non–toner–handling group by subjects’ smoking status or average age, and that interaction effects were not detected between toner exposure and smoking status by the use of the repeated ANOVA. This study suggests that there were no respiratory effects of toner exposure regardless of smoking status, because smoking was adjusted for in the statistical models. In the non–toner–handling group, the levels of FEV1.0% from 2004 to 2006 were higher than the levels at other survey points. We speculate that the results might be influenced by the motivation of the subjects in the non–toner–handling group or by the skill of examiners, but not due to the effects of smoking or ageing.
The authors of this study consider that the analysis of annual surveys from 2004 to 2008 identify acute effects of toner exposure. The analysis of the three survey points in 2004, 2008, and 2013, on the other hand, identify chronic effects, because subjects in the toner–handling group had not been exposed to toner particles since 2008, based on the analyses of personal exposure concentrations. For example, there might be acute effects due to toner exposure if we observed significant changes of some indicators from 2004 to 2008, when subjects exposed to toner particles with the production increase, yet no significant worsening in the three survey points in 2004, 2008, and 2013. There might be chronic effects due to toner exposures if we observed significant changing for the worse of some indicators in the three survey points in 2004, 2008, and 2013. We did not observe either of these n this study. In addition, we conducted multifaceted examinations of the subjects’ respiratory systems and performed analyses to adjust for smoking status, gender and aging, and did not find any respiratory effects in the toner–handling group. The findings of this study suggest that there were no acute and chronic respiratory effects of toner exposure.
Some limitations exist in this study. First, the number of subjects at the first survey point was small, and therefore type II statistical errors could have possibly occurred. The follow–up rate over 10 years was also small due to decreasing toner and photocopier manufacturing in the company since 2009. However, we were able to make substantial estimates of within–subject effects of toner exposure, because most of the remaining subjects in the toner–handling group had been continuously engaged in toner handling work without changing the type of job. Second, we could not evaluate the degree of subjects’ past toner exposure because we did not conduct personal exposure measurements prior to 2004. This is an important limitation because Nakadate et al. suggested that cumulative toner exposure affects pulmonary functions34). We need to investigate methods of estimating past toner exposure levels using records of work environment conditions, workers’ types of jobs, and their length of service. Third, the toner exposure concentration in this worksite may be quite low. One of the reasons is that the work environment was properly controlled for prevention of dust scattering. The other reason is that the subjects who were particularly engaged in R&D of toner or machine development, had not been exposed to significant concentrations of toner particles when dealing with toner in draft chambers or charging equipment with toner. However, subjects in the toner–handling–group must have been exposed to toner particles albeit in potentially low concentrations. Fourth, survey periods of this study might be still short. For example, incubation periods of asbestosis and asbestos–related lung cancer are generally 10 to 20 years and 15 to 40 years or more. We might need to observe subjects in the toner–handling group in future. Fifth, the investigations instituted must be effective for the accurately and early detection of small airway disease by providing measurement of closing volume (CV) or high resolution CT. It is therefore necessary to consider the inclusion of these examinations in future studies.
Recently, it has been speculated that nanoparticles emitted from laser printers and photocopiers affect health, in addition to the effects of toner35, 36). As for photocopiers and laser printers, emissions such as VOC or ozone are produced by the parts where heat is generated. These parts include heat fixing units and scanning units. Subjects in this study were engaged in tasks such as toner production and maintenance, R&D of toner, and machine development, and did not be engage in tasks on operation tests or quality inspections of printers or photocopiers. Therefore, VOC or ozone were not likely to have affected the subjects in this study. However, further studies on harmful emissions are necessary to prove human effects.
Conclusion
We could not detect any differences in various indicators concerning the respiratory system between the toner–handling group and the non–toner–handling group. This study suggests that there were no respiratory effects of toner exposure. This report is significant because it is a 10–year longitudinal epidemiological survey of the health effects of toner exposure. However, evidence might be insufficient because the number of subjects was small. Further studies are needed to assess large–scale longitudinal epidemiological effects of toner exposure on human health.
Acknowledgments
We would like to thank Ms. Ying Jiang of the University of Occupational and Environmental Health in Japan for her help with suggestions on statistical analysis, and the Public Health Research Foundation, the Science Center of Industrial Hygiene, SRL Inc., OHG Institute Co., Ltd., Bio communication Inc., and Soft Wave Pro Co., Ltd. for their help and the cooperation with laboratory or data analysis works.
Conflicts of Interest
This study was funded by a grant from Panasonic System Networks Co., Ltd. However, the research fund had no control over the design, interpretation of data, writing, or publication of this study.
References
- 1.Gallardo M, Romero P, Sánchez-Quevedo MC, López-Caballero JJ (1994) Siderosilicosis due to photocopier toner dust. Lancet 344, 412–3. [DOI] [PubMed] [Google Scholar]
- 2.Armbruster C, Dekan G, Hovorka A (1996) Granulomatous pneumonitis and mediastinal lymphadenopathy due to photocopier toner dust. Lancet 348, 690. [DOI] [PubMed] [Google Scholar]
- 3.Wieriks J, Armbruster C, Dekan G, Hovorka A (1996) Photocopier toner dust and lung disease. Lancet 348, 1518–9. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer IARC Monographs on the Evaluation of Carcinogenic Risks to Humans volume 93: Carbon Black, Titanium Dioxide and Non–Asbestiform. http://monographs.iarc.fr/ENG/Monographs/vol93/mono93.pdf. Accessed August 13, 2015.
- 5.Barthel M, Pedan V, Hahn O, Rothhardt M, Bresch H, Jann O, Seeger S (2011) XRF-analysis of fine and ultrafine particles emitted from laser printing devices. Environ Sci Technol 45, 7819–25. [DOI] [PubMed] [Google Scholar]
- 6.Lee CW, Hsu DJ (2007) Measurements of fine and ultrafine particles formation in photocopy centers in Taiwan. Atmos Environ 41, 6598–609. [Google Scholar]
- 7.McGarry P, Morawska L, He C, Jayaratne R, Falk M, Tran Q, Wang H (2011) Exposure to particles from laser printers operating within office workplaces. Environ Sci Technol 45, 6444–52. [DOI] [PubMed] [Google Scholar]
- 8.Morawska L, He C, Johnson G, Jayaratne R, Salthammer T, Wang H, Uhde E, Bostrom T, Modini R, Ayoko G, McGarry P, Wensing M (2009) An investigation into the characteristics and formation mechanisms of particles originating from the operation of laser printers. Environ Sci Technol 43, 1015–22. [DOI] [PubMed] [Google Scholar]
- 9.Tang T, Gminski R, Könczöl M, Modest C, Armbruster B, Mersch-Sundermann V (2012) Investigations on cytotoxic and genotoxic effects of laser printer emissions in human epithelial A549 lung cells using an air/liquid exposure system. Environ Mol Mutagen 53, 125–35. [DOI] [PubMed] [Google Scholar]
- 10.Bai R, Zhang L, Liu Y, Meng L, Wang L, Wu Y, Li W, Ge C, Le Guyader L, Chen C (2010) Pulmonary responses to printer toner particles in mice after intratracheal instillation. Toxicol Lett 199, 288–300. [DOI] [PubMed] [Google Scholar]
- 11.Umezawa M, Kudo S, Yanagita S, Shinkai Y, Niki R, Oyabu T, Takeda K, Ihara T, Sugamata M (2011) Maternal exposure to carbon black nanoparticle increases collagen type VIII expression in the kidney of offspring. J Toxicol Sci 36, 461–8. [DOI] [PubMed] [Google Scholar]
- 12.Alessandrini F, Schulz H, Takenaka S, Lentner B, Karg E, Behrendt H, Jakob T (2006) Effects of ultrafine carbon particle inhalation on allergic inflammation of the lung. J Allergy Clin Immunol 117, 824–30. [DOI] [PubMed] [Google Scholar]
- 13.Morimoto Y, Kim H, Oyabu T, Hirohashi M, Nagatomo H, Ogami A, Yamato H, Obata Y, Kasai H, Higashi T, Tanaka I (2005) Negative effect of long-term inhalation of toner on formation of 8-hydroxydeoxyguanosine in DNA in the lungs of rats in vivo. Inhal Toxicol 17, 749–53. [DOI] [PubMed] [Google Scholar]
- 14.The American Conference of Governmental Industrial Hygienists (2001) Documentation of the Threshold Limit Values and Biological Exposure Indices, 7th edition. Ohio: The American Conference of Governmental Industrial Hygienists.
- 15.Ferris BG. (1978) Epidemiology standardization project (American thoracic society). Am Rev Respir Dis 118, 1–120. [PubMed] [Google Scholar]
- 16.American Thoracic Society (1995) Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med 152, 1107–36. [DOI] [PubMed] [Google Scholar]
- 17.Japan Ministry of Labour (1982) Jinpai Hyojun x–ray films (Standard x–ray films for diagnosis of pneumoconiosis). Tokyo: Japan Industrial Safety and Health Association (in Japanese). [Google Scholar]
- 18.Japan Ministry of Labour (1978) Jinpai Shinsa Handbook (Pneumoconiosis examination handbook. Tokyo: Japan Industrial Safety and Health Association (in Japanese). [Google Scholar]
- 19.Hermans C, Bernard A (1999) Lung epithelium-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med 159, 646–78. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama A, Kohno N, Hamada H, Sakatani M, Ueda E, Kondo K, Hirasawa Y, Hiwada K (1998) Circulating KL-6 predicts the outcome of rapidly progressive idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 158, 1680–4. [DOI] [PubMed] [Google Scholar]
- 21.Kohno N. (1999) Serum marker KL-6/MUC1 for the diagnosis and management of interstitial pneumonitis. J Med Invest 46, 151–8. [PubMed] [Google Scholar]
- 22.Hamaguchi T, Omae K, Takebayashi T, Kikuchi Y, Yoshioka N, Nishiwaki Y, Tanaka A, Hirata M, Taguchi O, Chonan T (2008) Exposure to hardly soluble indium compounds in ITO production and recycling plants is a new risk for interstitial lung damage. Occup Environ Med 65, 51–5. [DOI] [PubMed] [Google Scholar]
- 23.Kasai H, Iwamoto-Tanaka N, Miyamoto T, Kawanami K, Kawanami S, Kido R, Ikeda M (2001) Life style and urinary 8-hydroxydeoxyguanosine, a marker of oxidative DNA damage: effects of exercise, working conditions, meat intake, body mass index, and smoking. Jpn J Cancer Res 92, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu LL, Chiou CC, Chang PY, Wu JT (2004) Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta 339, 1–9. [DOI] [PubMed] [Google Scholar]
- 25.Irie M, Tamae K, Iwamoto-Tanaka N, Kasai H (2005) Occupational and lifestyle factors and urinary 8-hydroxydeoxyguanosine. Cancer Sci 96, 600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi K, Pan G, Kasai H, Hanaoka T, Feng Y, Liu N, Zhang S, Xu Z, Tsuda T, Yamato H, Higashi T, Okubo T (1997) Relationship between asbestos exposures and 8–hydrox–ydeoxyguanosine levels in leukocytic DNA of workers at a Chinese asbestos–material plant. Int J Occup Environ Health 3, 111–9. [DOI] [PubMed] [Google Scholar]
- 27.Kim JY, Mukherjee S, Ngo LC, Christiani DC (2004) Urinary 8-hydroxy-2′-deoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to fine particulates. Environ Health Perspect 112, 666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cowie HA, Miller BG, Rawbone RG, Soutar CA (2006) Dust related risks of clinically relevant lung functional deficits. Occup Environ Med 63, 320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harber P, Muranko H, Solis S, Torossian A, Merz B (2003) Effect of carbon black exposure on respiratory function and symptoms. J Occup Environ Med 45, 144–55. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz J, Weiss ST (1994) Cigarette smoking and peripheral blood leukocyte differentials. Ann Epidemiol 4, 236–42. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa N, Mazur W, Toljamo T, Vuopala K, Rönty M, Horimasu Y, Kohno N, Kinnula VL (2011) Ageing and long-term smoking affects KL-6 levels in the lung, induced sputum and plasma. BMC Pulm Med 11, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA Jr, Enright PL, Kanner RE, O’Hara P, Owens GR, Scanlon PD, Tashkin DP, Wise RA (1994) Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 272, 1497–505. [PubMed] [Google Scholar]
- 33.Loft S, Vistisen K, Ewertz M, Tjønneland A, Overvad K, Poulsen HE (1992) Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis 13, 2241–7. [DOI] [PubMed] [Google Scholar]
- 34.Nakadate T, Yamano Y, Adachi C, Kikuchi Y, Nishiwaki Y, Nohara M, Satoh T, Omae K (2006) A cross sectional study of the respiratory health of workers handling printing toner dust. Occup Environ Med 63, 244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khatri M, Bello D, Pal AK, Cohen JM, Woskie S, Gassert T, Lan J, Gu AZ, Demokritou P, Gaines P (2013) Evaluation of cytotoxic, genotoxic and inflammatory responses of nanoparticles from photocopiers in three human cell lines. Part Fibre Toxicol 10, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pirela S, Molina R, Watson C, Cohen JM, Bello D, Demokritou P, Brain J (2013) Effects of copy center particles on the lungs: a toxicological characterization using a Balb/c mouse model. Inhal Toxicol 25, 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]