Abstract
α-Synuclein (αSyn) is a major constituent of proteinaceous aggregates in neurodegenerative diseases such as Parkinson′s disease (PD) and a potential biomarker candidate for diagnosis and treatment effects. However, studies about αSyn in cerebrospinal fluid (CSF) in diseases are inconsistent and mainly based on immunological assays. Quantitative information about β-synuclein (βSyn) and γ-synuclein (γSyn) in CSF is not available.
Here, we present an alternative method for the simultaneous quantification of αSyn, βSyn and γSyn in CSF by multiple reaction monitoring (MRM) with a high sequence coverage (70%) of αSyn to validate previous, ELISA-based results and characterize synucleins in CSF in more detail.
The MRM has high sensitivity in the low pg/ml range (3–30pg/ml full-length αSyn) using 200 μl CSF. A high portion of CSF αSyn is present in the N-terminally acetylated form and the concentration of unmodified peptides in the nonamyloid component region is about 40% lower than in the N-terminal region. Synuclein concentrations show a high correlation with each other in CSF (r>0.80) and in contrast to αSyn and γSyn, βSyn is not affected by blood contamination. CSF αSyn, βSyn and γSyn concentrations were increased in Alzheimer′s and Creutzfeldt-Jakob disease but not altered in PD, PD dementia (PDD), Lewy body dementia and atypical parkinsonian syndromes. The ratio βSyn/αSyn was increased in PDD (1.49 ± 0.38, p < 0.05) compared with PD (1.11 ± 0.26) and controls (1.15 ± 0.28). βSyn shows a high correlation with CSF tau concentrations (r = 0.86, p < 0.0001, n = 125).
In conclusion, we could not confirm previous observations of reduced αSyn in PD and our results indicate that CSF synuclein concentrations are rather general markers of synaptic degeneration than specific for synucleinopathies. βsyn is an attractive biomarker candidate that might be used as an alternative to or in combination with tau in AD and CJD diagnosis and in combination with αSyn it is a biomarker candidate for PDD.
α-Synuclein (αSyn)1 is a small (14 kDa) presynaptic protein and a key player in the pathogenesis of several neurodegenerative diseases such as Parkinson′s disease (PD), PD dementia (PDD), and Lewy body dementia (LBD). None of these diseases is curable to date and diagnosis is based on clinical symptoms (1). Aggregated αSyn is the main constituent of Lewy bodies which are histopathological hallmarks in the brain of these synucleinopathies. Oligomerization and aggregation of αSyn is neurotoxic and thought to be a causative factor in the neurodegenerative process. Many post-translational modifications (PTMs) have been described for αSyn (e.g. phosphorylation, oxidation) (2) and there is evidence that PTMs might influence its aggregation and toxic potential (in addition to other mechanisms such as αSyn mutations or metal ion binding) (3). Additionally, shorter forms of αSyn with unknown function can be generated by alternative splicing of the αSyn gene (2). Although αSyn is a cytoplasmic protein it is also present in the cerebrospinal fluid (CSF) (4). Because of its importance in the pathogenesis of synucleinopathies, αSyn determination in CSF is a promising biomarker candidate for clinical diagnosis and for the development of αSyn modulating drugs.
Many studies investigated CSF αSyn concentrations in neurodegenerative diseases, especially PD as the most common synucleinopathy (for a review, see ref (5)). Most studies observed slightly reduced αSyn concentrations in PD, although results are inconsistent. Additionally, the reported small alterations seem not to be of diagnostic relevance (5). To date, the method of choice for αSyn determination are immunoassays but antibodies and platforms vary considerably. This led to large concentration differences between studies and hampers the interpretation of inconsistent results. These differences also raise concern about the selectivity of the assays and, thus, an alternative method is needed to confirm previous observation about CSF αSyn concentrations.
The other members of the synuclein protein family, β-synuclein (βSyn) and γ-synuclein (γSyn), are less well studied although they are present in proteinaceous aggregates in some neurodegenerative diseases (6) and there is evidence for a strong interaction of αSyn and βSyn (7). βSyn has been shown to be neuroprotective and inhibits αSyn aggregation (7). γSyn aggregation is also associated with widespread neurodegeneration (8). Both proteins are present in CSF (9). Only a single study investigated γSyn concentrations in CSF of dementia patients by a qualitative Western blot and observed an increase in Alzheimer′s disease (AD) and LBD (10) but this was not validated with further studies and information about other neurodegenerative diseases is missing. CSF βSyn has not been investigated in neurological disorders so far. The determination of βSyn and γSyn in CSF and their relation to αSyn would help to clarify their role in neurodegenerative diseases and the ratio of synuclein protein concentrations in CSF might be more meaningful biomarker candidates than each of the proteins alone.
Multiple reaction monitoring mass spectrometry (MRM) can be used for accurate, absolute quantification of proteins using stable-labeled protein standards (protein standard absolute quantification, PSAQ) (11) and is an excellent alternative to immunoassays for quantification of αSyn with a high selectivity and the ability for multiplexing, i.e. simultaneous quantification of βSyn and γSyn. MRM has already successfully been applied for the determination of biomarker candidates in CSF (12). In addition, it allows a more detailed characterization of the whole protein regarding truncations or PTMs by analyzing several peptides across the protein sequence after proteolytic digestion and αSyn PTMs are in discussion as promising biomarker candidates in synucleinopathies (13). Detailed protein characterization by MRM has recently successfully been shown for the tau protein in CSF, a biomarker used in the diagnostics of AD, which seems to be predominantly N- and C-terminally truncated in CSF (14). However, it was not possible so far to quantitatively measure αSyn in CSF by MRM in a useful sample volume because of the low concentration in the pg/ml range.
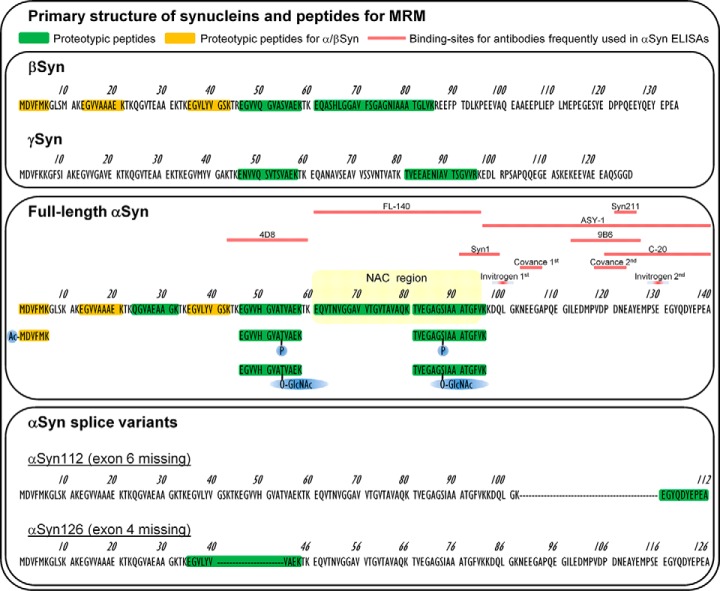
We present here an MRM method for the simultaneous and absolute quantification of unmodified αSyn, βSyn, γSyn and hemoglobin in the low pg/ml range in 200 μl CSF using a stable-labeled protein standard (αSyn) and stable-labeled peptides (βSyn, γSyn, PTMs) as internal standards. Seven of eight possible, unmodified tryptic peptides of αSyn are included covering 70% of the αSyn sequence for a more detailed characterization of αSyn. In addition, several PTMs are included for αSyn (Ser87P, Ser87O-GlcNAc, Thr54P, Thr54O-GlcNAc, N-terminal acetylation) as well as two proteotypic peptides for the αSyn splice variants αSyn126 and αSyn112 (Fig. 1). We used the method to characterize synucleins in CSF of patients without neurodegenerative diseases and compared the results with ELISA data. We then measured synucleins in a panel of neurodegenerative diseases including PD, PDD, LBD, progressive supranuclear palsy (PSP), corticobasal syndrome (CBS), AD and Creutzfeldt-Jakob disease (CJD) to validate previous results of CSF αSyn with immunoassays and to gain new information about βSyn, γSyn and the αSyn peptide pattern in CSF.
Fig. 1.
Primary structure of synucleins and selected peptides for MRM. Protein sequences were obtained from the UniProt database (αSyn: P37840, βSyn: Q16143, γSyn: O76070). Peptides used for MRM are indicated in yellow and green. Proteotypicity of the peptides was tested using the human proteome fasta from UniProt (23 Jan 2014) and Skyline software 3.1. Peptides with posttranslational modifications were also included in the MRM (Ac: N-terminal acetylation, P: phosphorylation, O-GlcNAc: O-linked β-N-acetylglucosamine). Alternative splicing of αSyn generates two new proteotypic peptides. The NAC (non-Abeta component) region which is important for aggregation is highlighted in the αSyn sequence. Frequently used combinations of antibodies in ELISAs for αSyn measurement in CSF are: Syn211 (1st) and FL-140 (2nd) (66, 67), ASY-1 (1st) and polyclonal anti-αSyn (2nd) (17), Syn1 (1st) and C-20 (2nd) (68), 9B6 (1st) and 4D8 (2nd) (39), MJF-1 (1st) and Syn1 (2nd) (69), Syn103–107 (1st) and Syn118–123 (2nd) (Covance #SIG-38974) (70) and Syn100 (1st) and Syn130 (2nd) (Invitrogen #KHB0061).
EXPERIMENTAL PROCEDURES
Reagents
Recombinant full-length αSyn (purity >95%) was purchased from AJ Roboscreen GmbH (Leipzig, Germany) and the exact protein concentration was determined by amino acid analysis (Alphalyse A/S, Odense, Denmark). Full-length βSyn, γSyn and 15N-labeled αSyn (all with purity >95%) were from rPeptide (Bogart, GA) and exact βSyn concentration was determined using αSyn MRM of common peptides. Synthetic peptides (see supplemental Table S1) were purchased from Thermo Fisher Scientific.
Trypsin/LysC Mix was from Promega GmbH, triethylammonium bicarbonate (TEAB), ammonium hydroxide solution (LC-MS grade) and human serum albumin (HSA, #A3782) from Sigma, solid phase cation extraction disks from 3M (#2251, St. Paul, MN) and artificial CSF (aCSF) from EcoCyte Bioscience (Austin, TX). All LC solvents were of LC-MS grade and purchased from Thermo Fisher Scientific (DMSO, formic acid, TFA) or Carl Roth GmbH, Karlsruhe, Germany (ACN, methanol (MeOH), water).
Stock Solutions, Calibration Standards, and Quality Control (QC) Samples
Proteins and peptides were dissolved in LC-MS water at concentrations of 100–500 μg/ml, aliquoted in protein low binding tubes and stored at −80 °C. Calibration standards and QC samples were prepared freshly in aCSF containing 200 μg/ml HSA for each analytical sequence using recombinant αSyn, βSyn, γSyn, and synthetic peptides for sequences with PTMs and splice variants (see supplemental Table S1). Concentrations of the calibration standards and low, medium and high QC samples covered a range of 1.5–1310 pm (αSyn, βSyn) and 40–2000 pm (γSyn) and are listed in detail in supplemental Table S1. In addition, an unspiked CSF QC sample was included in each sequence.
Sample Preparation
CSF samples were thawed on ice and 200 μl of CSF, calibration standard or QC sample were mixed with 40 μl of internal standard (IS) solution (containing labeled peptides and 15N-αSyn in 0.5 m TEAB, see supplemental Table S1) and 12 μl of Trypsin/LysC solution (0.1 μg/μl in 100 mm TEAB) in protein low binding tubes (Sarstedt, Nümbrecht, Germany). Samples were digested for 16h at 27 °C. After addition of 700 μl water and 100 μl 10% TFA, tryptic peptides were captured with STAGE-tips (15) containing solid phase cation extraction disks (activated with ACN), washed with 0.2% TFA and eluted into 24-Well PCR plates with increasing concentrations of ammonium acetate in 20% ACN/0.5% formic acid (75 mm →fraction 1, 125 mm →fraction 2, 200 mm →fraction 3) and finally with 5% ammonium hydroxide/80% ACN (fraction 4).
Fractions were vacuum dried and redissolved in 25 μl of 0.1% TFA/6% ACN (fraction 1 and 3), 0.5% TFA/6% ACN (fraction 2) and 0.1% TFA/4% ACN (fraction 4) by thorough mixing and sonication, centrifuged and stored in the autosampler at 4 °C.
Serum (20 μl) was diluted with 180 μl aCSF and prepared as described for CSF.
LC-MRM Analysis
Samples were analyzed using an Agilent 1260 HPLC pump (Santa Clara, CA), Eksigent microLC200, and AB Sciex QTRAP6500 mass spectrometer (both AB Sciex, Framingham, MA) in positive ionization mode. Twenty microliters of sample were loaded on a C18 PepMap100, 5 μm, 0.3 × 5 mm trap column (Thermo Fisher Scientific) with mobile phase A: 0.05% TFA, and mobile phase B: 0.05% TFA in MeOH. Afterward, peptides were separated on an Eksigent HALO Fused-core C18, 2.7 μm, 0.5 × 100 mm column at 40 °C with mobile phase A: 4% DMSO/0.1% formic acid, and mobile phase B: 4% DMSO/96% ACN/0.1% formic acid (see supplemental Table S2 for gradient settings). The analytical column was connected to the QTRAP6500 with a 25 μm electrode and data were acquired in scheduled MRM mode (retention time window 40–120s, scan time 0.2–0.4s, dwell weight: 0.2 for labeled peptides, 1.0 for others). The ion source settings were as follows: 4900–5500 V, 175 °C, curtain gas (CUR) 30psi, nebulizer gas (GS1) 20–40psi, GS2 30psi and CAD gas high. Transitions used and individual MS settings are described in Table I and supplemental Table S3. Two or three transitions per peptide were acquired and the correct transition pattern of each peptide was verified in all samples using Skyline software 3.1 (16).
Table I. MS parameters and chromatographic characteristics. CE: collision energy, RT: retention time, z: charge state.
| Peptide sequence (position) | Protein | Precursor mass (labeled peptide) | z | Product ion | CE (V) | Fraction | RT (min) |
|---|---|---|---|---|---|---|---|
| MDVFMK (1–6) | αSyn | 385.7 (389.2) | 2+ | y5, y3 | 19, 23 | 4 | 5.1 |
| (1–6) | βSyn | ||||||
| Ac-MDVFMK (1–6) | αSyn | 812.4 (820.4) | 1+ | b3, y3 | 49, 47 | 2 | 9.2 |
| (1–6) | βSyn | ||||||
| EGVVAAAEK (13–21) | αSyn | 437.2 (442.2) | 2+ | y5, y6, y3 | 18, 20, 18 | 2 | 3.5 |
| (13–21) | βSyn | ||||||
| QGVAEAAGK (24–32) | αSyn | 415.7 (421.2) | 2+ | y6, y4 | 21, 21 | 2 | 2.1 |
| EGVLYVGSK (35–43) | αSyn | 476.3 (481.3) | 2+ | y5, y3 | 20, 21 | 3 | 4.4 |
| (35–43) | βSyn | ||||||
| EGVVHGVATVAEK (46–58) | αSyn | 648.4 (656.3) | 2+ | y8, y9 | 35, 34 | 4 | 3.4 |
| EGVVHGVAT(Phospho)VAEK (46–58) | αSyn | 688.3 (692.3) | 2+ | b5, y8, y4 | 37, 35, 39 | 3 | 6.1 |
| EGVVHGVAT(O-GlcNAc)VAEK (46–58) | αSyn | 749.9 (753.9) | 2+ | b5, b7, y8, 204.1 (oxonium ion) | 38, 46, 35, 38 | 3 | 3.1 |
| EQVTNVGGAVVTGVTAVAQK (61–80) | αSyn | 964.5 (976.5) | 2+ | y10, y9, y11 | 44, 44, 44 | 2 | 7.9 |
| TVEGAGSIAAATGFVK (81–96) | αSyn | 739.9 (748.4) | 2+ | y8, y11, y7 | 34, 34, 34 | 3 | 6.7 |
| TVEGAGS(Phospho)IAAATGFVK (81–96) | αSyn | 779.9 (785.9) | 2+ | Y8, y7, y142+ | 34, 34, 29 | 1 | 7.8 |
| TVEGAGS(O-GlcNAc)IAAATGFVK (81–96) | αSyn | 841.4 (847.4) | 2+ | y8, y7, y6, 204.1 (oxonium ion) | 43, 46, 49, 27 | 3 | 6.3 |
| EGYQDYEPEA (103–112) | αSyn112 | 1200.5 (1206.5) | 1+ | b7, b6, y7 | 52, 52, 59 | 1 | 5.3 |
| EGVLYVVAEK (35–44) | αSyn126 | 553.8 (557.8) | 2+ | y6, y7, y4 | 23, 25, 28 | 3 | 6.0 |
| EGVVQGVASVAEK (46–58) | βSyn | 636.8 (640.9) | 2+ | y8, y9, y10 | 30, 28, 28 | 2 | 7.0 |
| EQASHLGGAVFSGAGNIAAATGLVK (61–85) | βSyn | 776.1 (778.8) | 3+ | Y8, b172+ | 31, 27 | 4 | 8.1 |
| ENVVQSVTSVAEK (46–58) | γSyn | 695.4 (699.4) | 2+ | y8, y10 | 34, 34 | 2 | 7.2 |
| TVEEAENIAVTSGVVR (81–96) | γSyn | 837.4 (842.4) | 2+ | y8, y7 | 46, 37 | 3 | 6.0 |
| VNVDEVGGEALGR (19–31) | Hbb | 657.8 (662.8) | 2+ | y7, y8 | 30, 31 | 2 | 6.9 |
Data Analysis and Quantification of MRM Data
In each analytical sequence, calibration standards, QC samples and a blank sample (aCSF+HSA) with and without IS were analyzed in duplicate (one at the beginning and one at the end of the sequence). The order of CSF samples (single measurement) was defined by systematic randomization.
Intra-assay precision (%CV) was determined by analysis of four CSF-QC samples in a single run and inter-assay precision by analysis of duplicate CSF-QC samples in four independent runs. The lower limit of quantification (LLOQ) was defined as the lowest concentration with a CV and deviation of ≤20% and the limit of detection (LOD) with a signal-to-noise ratio of 3.
Stability of synucleins in CSF was tested by incubation of CSF at bench-top conditions (RT), on ice and with different freeze-thaw cycles (thawing for at least 2 h, freezing for at least 12 h). Dilution stability was determined by dilution of CSF with aCSF up to fourfold.
The IS-normalized peak area was used for quantification (synucleins) and all transitions from a single peptide were summed up. Peptide concentrations were calculated based on the calibration curve (weighting 1/x2) using Analyst software 1.6.2 (AB Sciex). Total αSyn and γSyn concentration was calculated as the mean concentration of all proteotypic peptides (see Fig. 1). Concentration of the Hbb (hemoglobin beta subunit) peptide was determined using the IS peak area for one-point calibration and total hemoglobin (Hb) concentration was calculated assuming a 1:1 ratio of the Hb alpha and beta subunit. The Hbb IS was added to samples in a final concentration of 200 ng/ml to increase accuracy at this cut-off. CSF samples with a hemoglobin concentration >200 ng/ml were ruled out for αSyn and γSyn analysis as recommended (17).
ELISAs
CSF and serum samples for αSyn ELISA (Covance #SIG-38974) measurements were diluted 1:20 and 1:200, respectively, and tau concentration in CSF was determined with a commercial ELISA (Fujirebio). All measurements were performed according to the manufacturer's instructions.
Patients and CSF Collection
Patients were enrolled at the Ulm University Hospital, Department of Neurology. Characteristics of patients are depicted in supplemental Table S4. Control patients had no neurodegenerative disease and CSF was collected to rule out acute or chronic inflammation of the brain. PD patients were diagnosed according to accepted criteria (18), PDD and LBD according to (19, 20). Diagnosis of PSP and CBS followed the criteria of (21, 22). AD patients fulfilled the NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association) criteria and CJD was diagnosed according to the WHO consensus criteria (23). All patients or their relatives gave written informed consent to participate in the study and the collection and analysis of CSF and serum samples was approved by the Ethics Committee of Ulm University.
CSF was collected by lumbar puncture at the Ulm University Hospital, Department of Neurology. Samples were centrifuged and stored at −80 °C within 2 h according to local SOPs and standard CSF parameters were determined (24).
Statistics
Statistical analysis was performed using GraphPad Prism 5.0. Disease groups were compared by Kruskal-Wallis test and Dunn′s post hoc test. Correlation analysis was performed using Spearman′s rank correlation coefficient.
RESULTS
Establishment of Method
All theoretical tryptic peptides of αSyn except the C-terminal peptide (see discussion section) were selected for the method to cover the largest part possible (about 70%) of the αSyn sequence. Proteotypic peptides for βSyn and γSyn and the optimal charge state of all peptides were selected based on the observed sensitivity and selectivity. MS parameters were optimized by direct infusion of tryptic peptides and are shown in supplemental Table S3. Fig. 1 gives an overview of selected peptides and their relation to the amino acid sequence and other synuclein variants. To optimize LC conditions we tested different column temperatures, flow rates and compositions of the mobile phases using ACN, MeOH, formic acid, and TFA. The settings were optimized for each sample fraction and are given in supplemental Table S2. The high dynamic range of protein concentrations in CSF significantly hampers the detection of low abundance proteins such as αSyn by LC-MS/MS and we tested several approaches to modify the digestion protocol in favor of synucleins. The synuclein proteins do not contain Cys-residues and a reduction and alkylation step is not necessary. Omitting reduction and alkylation increased sensitivity for αSyn peptides and markedly decreased intensity of selected HSA peptides (10–1000x, label-free estimation). Using a digestion temperature of 27 °C instead of 37 °C and using trypsin/LysC instead of trypsin alone also improved the sensitivity for synucleins. Heating or addition of small amount of ACN (5–10%) to samples before digestion reduced sensitivity for synucleins but increased the intensity of HSA peptides. Transitions of peptides for detection/quantification were selected based on their sensitivity and selectivity examined by spike-in experiments and comparison of the transition profile with the stable-labeled peptides in CSF. Representative chromatograms are shown in supplemental Fig. S1.
Precision, Sensitivity, Stability
We observed an intra-assay precision of 1.9–8.2% for αSyn peptides, 4.1–12.2% for βSyn peptides, 9.8–14.2% for γSyn peptides, and 13.1% for N-terminal acetylated α/βSyn1–6. The inter-assay precision was 5.9–11.5% for αSyn peptides, 5.9–11.6% for βSyn peptides, 15.7–16.8% for γSyn peptides, and 18.1% for N-terminal acetylated α/βSyn1–6.
The LOD and LLOQ were in the range of 0.2–2.0 pm (equivalent to 3–30pg/ml full-length protein) and 5.0–6.5 pm (75–97.5pg/ml) for αSyn peptides, 0.2–0.5 pm (3–7pg/ml) and 1.5–6.5 pm (21–91pg/ml) for βSyn peptides, 10–32 pm (133–427pg/ml) and 40 pm (533pg/ml) for γSyn peptides and 1.5–325 pm for αSyn PTMs and splice variants (see supplemental Table S1).
All synuclein proteins were stable for at least 4h on ice and five freeze/thaw cycles. Not all peptides were stable at room temperature. Dilution stability could be shown for up to fourfold dilution for all peptides except βSyn46–58. Therefore, this peptide was excluded from further analyses.
Characterization of Synucleins in CSF
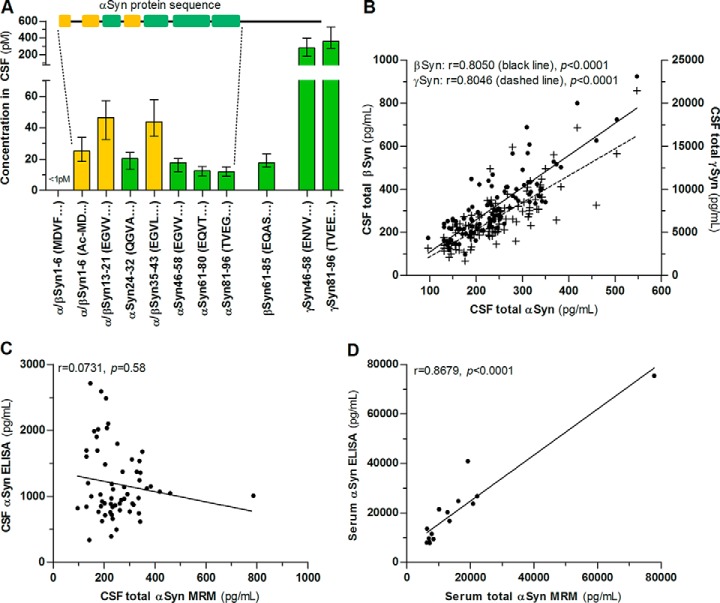
The concentration of total αSyn and βSyn in CSF of control patients was comparable and in the range of 9.57–31.8 pm (138–459pg/ml) for αSyn and 6.87–43.9 pm (98.2–627pg/ml) for βSyn. γSyn concentration was higher and ranged from 128–774 pm (1.70–10.3 ng/ml).
We quantified seven tryptic peptides across the αSyn protein with and without PTMs covering 70% of the αSyn sequence (Fig. 1). A large part of CSF αSyn is N-terminally acetylated (Fig. 2A) whereas the unmodified N-terminal peptide was below the LOD (1 pm) and thus less than 10% of all αSyn. The proteotypic αSyn peptides spanning the amyloidogenic NAC (non-Abeta component) region (αSyn61–80 and αSyn81–96) have a 38 and 40% lower concentration in CSF than the more N-terminal, proteotypic peptide αSyn24–32 (Fig. 2A), which carries only few (i.e. Lys32) potentially PTM sites. However, the phosphorylated and O-GlcNAcylated form of αSyn81–96 measured here (Fig. 1) were below the LOD (i.e. 325 pm and 2.5 pm) and do not allow a conclusion whether these concentration differences originate from PTMs. The concentration of αSyn46–58 is 13% lower than αSyn24–32 but the phosphorylated and O-GlcNAcylated forms measured here were below the LOD (320 pm and 38 pm). The concentrations of proteotypic peptides of αSyn splice variants αSyn112 and αSyn126 were also below the LOD (i.e. 13 pm and 1.5 pm).
Fig. 2.
Synuclein peptide pattern in CSF and comparison with ELISA. A, Concentration of tryptic synuclein peptides in CSF of control patients measured by MRM. Green indicates proteotypic peptides and yellow are peptides common to αSyn and βSyn. The αSyn protein sequence is shown to visualize the position of αSyn peptides. B, Synuclein concentrations in CSF show a strong correlation with each other (black dots: βSyn, n = 113, crosses: γSyn, n = 116). The concentration of total αSyn in (C) CSF (n = 60) and (D) serum (n = 15) was determined by MRM and a commercially available ELISA. Columns and bars are median and interquartile range. Correlation was calculated using linear regression analysis and Spearman′s rank correlation coefficient.
We observed a strong correlation of synuclein concentrations in CSF (Fig. 2B).
Comparison of MRM and ELISA Measurements
In a subset of patients, we compared MRM and ELISA measurements of αSyn. αSyn values from MRM and ELISA in CSF did not correlate (Fig. 2C) and αSyn concentrations were on average 550% higher with the ELISA. In contrast, serum values showed a high correlation between MRM and ELISA measurements (Fig. 2C). We observed no correlation of serum αSyn and hemoglobin (r = 0.38, p = 0.16) in the investigated range (135–23,800 ng hemoglobin/ml) and αSyn concentrations in serum and CSF (r = 0.10, p = 0.73).
Investigation of Synuclein Origin and Hemolysis as Confounding Factor
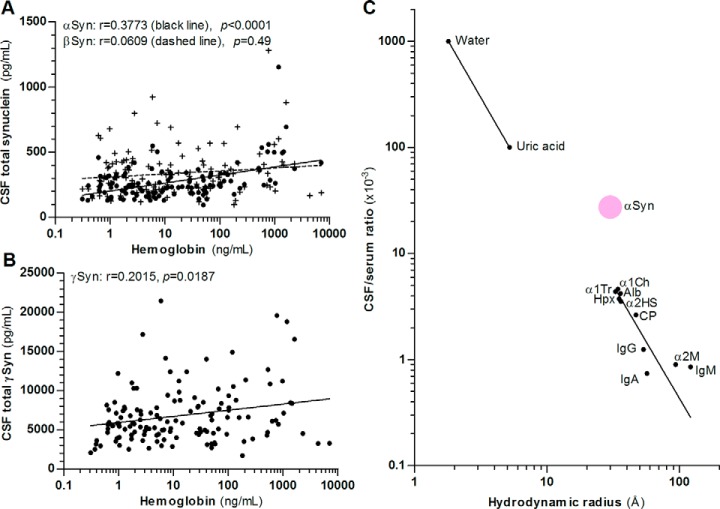
The value of a CSF biomarker is given by its origin in the brain and resistance to low blood contamination/hemolysis during lumbar puncture. We investigated the correlation of CSF synuclein concentrations with CSF hemoglobin concentration as a marker of hemolysis. CSF αSyn showed a correlation with CSF hemoglobin concentration whereas βSyn did not (Fig. 3A). γSyn also slightly correlated with CSF hemoglobin concentration (Fig. 3B). We tested how blood contamination (1 μl whole blood to 200 μl CSF) affects synuclein concentrations in CSF. αSyn peptides increased about 250–5200% (hemoglobin increase: 1409 ng/ml). βSyn was unaffected and γSyn showed a slight increase (6–30%).
Fig. 3.
Correlation of synucleins with hemoglobin and estimation of blood-derived αSyn in CSF. A, CSF βSyn concentration (crosses, n = 133) does not correlate with CSF hemoglobin concentration but αSyn (black dots, n = 123) and (B) γSyn (n = 136) show a correlation. Correlation was calculated using nonlinear regression analysis and Spearman′s rank correlation coefficient. C, Correlation of hydrodynamic radii and CSF/serum concentration ratio of serum compounds passively transferred into CSF according to Felgenhauer (25, 71) and of αSyn (mean MRM value from control patients with an albumin CSF/serum quotient <5, n = 5). Correlation of passively transferred serum compounds was calculated using nonlinear regression analysis and the blood-derived portion of αSyn in CSF was calculated using the received equation no. 1: Y = 10(m * logX + b) with m = −2.132, X = hydrodynamic radius αSyn (29.9Å), b = 3.896. Alb: serum albumin, α1Ch: α1-antichymotrypsin, α1Tr: α1-antitrypsin, α2HS: α2-HS-glycoprotein, α2M: α2-macroglobulin, CP: ceruloplasmin, Hpx: hemopexin, Ig: immunoglobulin.
The CSF concentration of αSyn and γSyn also correlates with the albumin CSF/serum quotient, a measure of the blood-CSF-barrier integrity (r = 0.23, p = 0.02, n = 105 for αSyn and r = 0.40, p < 0.0001, n = 116 for γSyn) but βSyn shows no correlation (r = 0.15, p = 0.10, n = 113), which is characteristic of solely brain-derived proteins. Because serum αSyn concentration is higher than CSF concentration, we calculated an estimate of the blood-derived αSyn portion in CSF based on the diffusion dynamics of blood-derived proteins at the blood-CSF-barrier (25). We used the mean of published hydrodynamic radii of αSyn (i.e. 29.9Å) for our calculation (26–29) and the blood-derived αSyn in CSF was 30 ± 19.6% (Fig. 3C).
CSF Synuclein Concentration in Neurodegenerative Diseases
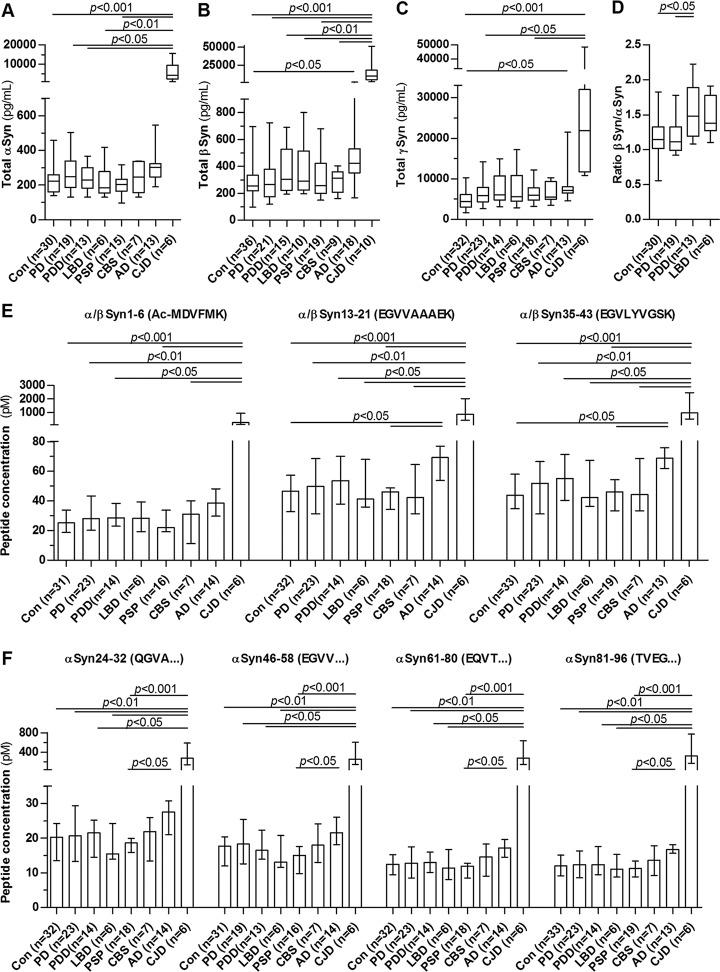
CSF synucleins are potential biomarker candidates forsynucleinopathies, especially PD, and we compared synuclein concentrations in different synucleinopathies (PD, PDD, LBD), atypical parkinsonian syndromes (PSP, CBS) and dementias (AD, CJD). All synucleins are markedly increased in CJD up to 40x (Fig. 4A–4C). βSyn and γSyn are also increased in AD whereas αSyn did not reach the significance level in AD. The synucleinopathies including PD and atypical parkinsonian syndromes did not differ significantly from the control patients. Because βSyn tended to be higher in dementia syndromes, we calculated the ratio of βSyn versus αSyn which was significantly higher in PDD compared with PD and controls (Fig. 4D). The concentrations of αSyn peptides (Fig. 4E–4F) showed the same behavior as total αSyn (i.e. increased in CJD and AD) but we could not identify a disease-specific pattern of the peptides. We observed no correlation of the CSF synuclein concentrations with disease severity (i.e. the Hoehn and Yahr score) in the group of synucleinopathies (PD, PDD, LBD) (αSyn: r = 0.01, p = 0.94, n = 37; βSyn: r = −0.11, p = 0.52, n = 39; γSyn: r = 0.08, p = 0.60, n = 42).
Fig. 4.
CSF synuclein concentration and αSyn peptide pattern in neurodegenerative diseases. A, αSyn, (B) βSyn and (C) γSyn concentration was determined in CSF of control patients (Con) and patients with Parkinson′s disease (PD), PD dementia (PDD), Lewy body dementia (LBD), progressive supranuclear palsy (PSP), corticobasal syndrome (CBS), Alzheimer′s disease (AD), and Creutzfeldt-Jakob disease (CJD) using MRM. D, Ratio of CSF βSyn-to-αSyn in synucleinopathies. Boxes are median and interquartile range, whiskers are minimum and maximum. E and F, show concentrations of the measured αSyn peptides. Values are median and interquartile range. All data were analyzed using Kruskal-Wallis test and Dunn′s post hoc test.
CSF tau protein was increased in AD and CJD and showed a high correlation with βSyn (Fig. 5).
Fig. 5.
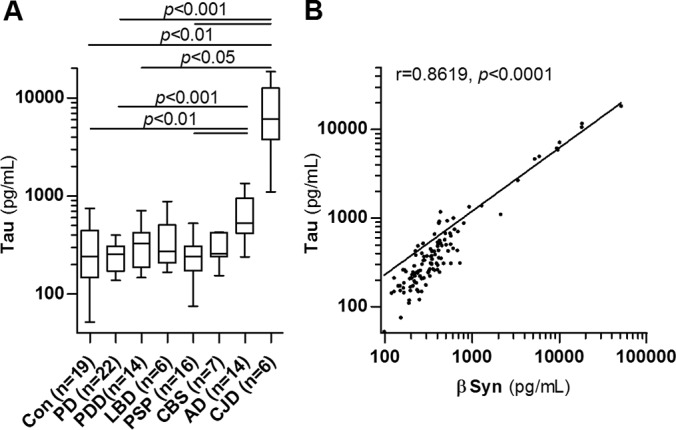
CSF tau concentration and correlation with βSyn. A, Tau protein concentration in CSF was determined by ELISA in control patients (Con) and patients with Parkinson′s disease (PD), PD dementia (PDD), dementia with Lewy bodies (LBD), progressive supranuclear palsy (PSP), corticobasal syndrome (CBS), Alzheimer′s disease (AD) and Creutzfeldt-Jakob disease (CJD). Boxes are median and interquartile range, whiskers are minimum and maximum. B, Correlation analysis of CSF tau (ELISA) and βSyn (MRM) in control and diseased patients (n = 117) using Spearman′s rank correlation coefficient.
DISCUSSION
We here describe for the first time a mass spectrometric method for the quantification of synucleins in CSF and serum. CSF αSyn shows high N-terminal acetylation and the concentration of unmodified peptides in the NAC region is about 40% lower compared with the N-terminal region. We could not confirm reduced αSyn concentrations in PD described in previous studies with immunoassays. βSyn and γSyn are increased in AD and CJD and because of the strong correlation of βSyn with tau protein, βSyn might be used as an alternative biomarker to or in combination with tau.
Because of the inconsistent results of CSF αSyn concentrations in studies with immunoassays (30), a mass spectrometric method as an alternative approach with high selectivity is demanded in the field for several years. However, the low concentration of αSyn in CSF has hindered the establishment of such a method so far. Normally, the sensitivity of MRM assays is in the ng/ml range or requires the use of large sample volumes (>500 μl) or time-consuming sample preparation methods (31) which is both difficult to implement in the collection and analysis of a large CSF sample cohort. By optimizing our sample preparation and digestion protocol in favor of synucleins e.g. by omitting reduction and alkylation or small-scale fractionation in STAGE-Tips, we could also renounce depletion of high-abundance proteins further reducing sample loss and variability. With our MRM method we could push the limit of detection to the low pg/ml range, i.e. 3–30pg/ml αSyn (depending on the peptide combination used for quantification), in a CSF volume of 200 μl and thus enabling for the first time the measurement of CSF αSyn by MRM in a reasonable amount of patients with high analytical accuracy and precision as required for clinical bioanalysis (see “Tier 1” in (32)). The sample preparation can be automatized and performed in 96-well plates and is applicable to the analysis of large sample amounts.
We could confirm previous observations of increased CSF αSyn concentrations in CJD (33–36) and observed also a tendency to elevated concentrations in AD (37–39). αSyn has been suggested as a biomarker for the differential diagnosis of AD and CJD (33) which is supported by our data. However, αSyn determination has some drawbacks including susceptibility to blood contamination. Because it correlates strong with other synucleins, we suggest to replacing it with βSyn (see discussion below).
Although most studies about CSF αSyn in PD show reduced concentrations, there are also inconsistent results (5) and there is a debate about the usefulness of CSF αSyn as a biomarker in PD. Most studies used immunoassays but antibodies (see Fig. 1) and concentration ranges vary considerably which raises questions about the selectivity of the assays. High batch-to-batch variability and interlaboratory variation has been described for ELISAs (40, 41). To overcome this issue and to validate the previous observations we established an alternative method using mass spectrometry, i.e. the highly selective MRM. The use of 15N-labeled, recombinant αSyn as internal standard allows accurate and precise quantification of αSyn in CSF and serum and we validated the method following in large parts recent recommendations (42). We could not confirm altered αSyn concentrations in PD and related disorders (PDD, LBD) with our MRM method. The MRM values did not correlate with values measured with a common ELISA and ELISA values in CSF were also substantially higher. Serum concentrations of MRM and ELISA correlated very well. This discrepancy could indicate different αSyn species in serum and CSF. Our MRM method detects only the unmodified αSyn peptides and differences in the PTM spectrum of αSyn between serum and CSF would be an explanation for the results, assuming the ELISA detects all αSyn species. In fact, it is not known what αSyn species are indeed recognized by the ELISA antibodies. On the other hand, the higher dilution of serum than CSF samples in the ELISA method could also indicate matrix effects in CSF. A major advantage of our MRM is the exact knowledge of the measured αSyn species and a detailed characterization of the recognized αSyn species by the ELISA antibodies would be helpful to clarify the difference between the MRM and ELISA measurements in CSF.
We cannot rule out that the discrepancy of our MRM results with other studies using immunoassays originates in the different parts of αSyn that are recognized by the methods. Most immunoassays use antibodies against the C-terminal part of αSyn (including the ELISA used here) whereas the peptides in our MRM are from the N-terminal part (see Fig. 1). We did not include the C-terminal part of αSyn in our MRM because C-terminal peptides released by proteolytic digestion of αSyn (with trypsin or other enzymes) carry a high amount of acidic residues and show poor ionization efficiency during electrospray ionization. These properties did not allow a highly sensitive determination of the C-terminal peptides at that time which is necessary for their detection in CSF. This issue should be worked on in future MRM assays because the C-terminal part of αSyn carries several important PTMs (see discussion below) and their quantification by mass spectrometry would be helpful to validate observations with immunoassays.
CSF is produced by ultrafiltration of the blood in the choroid plexus (ChP) in the brain′s ventricular system. The ependymal cells in the ChP form the blood-CSF-barrier. Although proteins cannot pass the cell membrane of ependymal cells (except there is a transcellular transport mechanism), the composition of the tight junctions (different to the blood-brain-barrier) allows a low diffusion of molecules into the CSF and the extent of the diffusion directly correlates with the hydrodynamic radius of the molecules (see Fig. 3C) (25, 43). Thus, 80% of CSF protein is blood-derived (44). For a possible clinical application of a CSF biomarker it is important to know whether it is brain-derived or originates (in part) in the blood. In addition, αSyn is also expressed in erythrocytes and hemolysis is a confounding factor that should be investigated (45). We used the CSF/serum concentration ratio and published hydrodynamic radii to estimate the blood-derived αSyn portion in CSF assuming passive transfer of blood-derived αSyn into CSF. In contrast to earlier assumptions (46), our results with mass spectrometry point to a substantial portion (30%) of blood-derived αSyn in CSF which is supported by the correlation of CSF αSyn concentration with the albumin quotient. Albumin is a blood-derived protein and the CSF/serum concentration ratio (albumin quotient) will increase when the blood-CSF-barrier is impaired. Thus, it is used as a measure of the blood-CSF-barrier integrity (44). Correlation of a proteins CSF concentration with the albumin quotient indicates that at least a part of the protein comes from the blood as shown for αSyn here. Recent evidence shows that αSyn can pass the blood-CSF-barrier also via a transcellular transport (47) indicating that the blood-derived portion calculated in our study might still underestimate the real value. This could be another reason for the inconsistency of study results because of differences in the blood-CSF-barrier integrity between patient cohorts.
In conclusion, the lack of concentration differences in synucleinopathies, the estimated high blood-derived αSyn portion in CSF in our study and the risk for interference by hemolysis/blood contamination supports the notion that total αSyn in CSF is not a useful diagnostic biomarker for synucleinopathies. However, this might not be true for monitoring of αSyn modulating effects in clinical trials and when using the MRM method presented here it might also be possible to identify off-target effects on βSyn and γSyn.
In addition to our aim to measure unmodified αSyn, we included a few PTMs that we thought might be interesting biomarker candidates. PTMs are normally present in substoichiometric quantities making their detection much more challenging, In addition, modified peptides frequently show lower ionization efficiency in the MS, require a special set-up of the instrument (e.g. removal of metallic surfaces for phosphopeptides) and ionization in the MS is suppressed by the much more abundant, unmodified peptides demanding pre-enrichment of the modified peptides (13). For a complete, quantitative characterization of the CSF PTM status, which would be of great scientific interest, these issues would require a large volume of CSF and the performance of different sample preparations in parallel. In addition, the number of possible, modified peptides will be tremendous because most tryptic αSyn peptides can carry more than one PTM (2). Regarding these technical issues (ionization efficiency, sensitivity), large CSF volume needed and number of possible modified peptides, it is beyond the practical design of a clinical CSF MRM assay, as described here, to monitor all possible PTMs why we focused on a selection of PTMs only.
Several PTMs and truncations have been reported for αSyn and there is evidence that these modifications represent better biomarker candidates than total αSyn (13). For instance, several studies observed increased Ser129P-αSyn concentrations in CSF of PD patients (48, 49). However, the measurement of the C-terminal peptide of αSyn (αSyn103–140) was not possible in the present MRM as discussed above. Ser87 is part of the NAC region (aa61–95), which is important for aggregation (50), and Ser87 phosphorylation has been demonstrated in several studies in vitro (51, 52) and in vivo (53). It is a known modifier of αSyn aggregation (53) and thus a promising biomarker candidate. There is also evidence for O-GlcNAcylation at Ser87 from a proteomic screen in human erythrocytes although not further verified (54). O-GlcNAcylation has been shown to influence protein aggregation as well (55) and we selected Ser87 O-GlcNAc as an interesting biomarker candidate and to confirm this O-GlcNAcylation site of αSyn. The selection of the Thr54 PTMs was based on a technical issue because the unmodified peptide αSyn46–58 showed one of the most intense ionization efficiencies of the αSyn peptides and we thought it is the best candidate to reach the sensitivity necessary to detect substoichiometric PTMs. Only two PTM sites in this peptide have been described so far. Lys58 acetylation described by Weinart et al. (56) and Thr54 O-GlcNAcylation in a proteomic screen of murine synaptosomes but with no additional verification (57). Here, we selected the Thr54 O-GlcNAcylation and we were also interested whether we can measure Thr54 phosphorylation (although not yet described). In addition, we investigated N-terminal acetylation of αSyn.
Here, the unmodified peptides of the NAC region showed a lower concentration in CSF compared with a more N-terminal peptide that contains only few potential PTM sites. This observation could indicate a high portion of αSyn to be post-translationally modified in the NAC region. We measured the aforementioned αSyn PTMs in our MRM (see Fig. 1) to test this hypothesis and to uncover alterations in neurodegenerative diseases. Except N-terminal acetylation, other peptides carrying PTMs were not detectable in CSF at present without enrichment. Thus, we could not prove high modification of the NAC region experimentally. Other factors might also be responsible for our observation. Conformational changes in the aggregation prone NAC region that are not reflected by the internal standard protein or PTMs itself could influence trypsin digestion efficiency and thus alter the ratio of the αSyn peptides. Different truncated forms of αSyn have been described including cleavages within the NAC region, e.g. αSyn1–78, αSyn1–83, αSyn1–91, or αSyn1–93 (2). Truncation within the NAC region would also result in a lower concentration of the intact, unmodified peptides measured here and give an explanation for our observation. We can also not rule out that the NAC peptides carry more than one PTM simultaneously which would deprive them from detection with our MRM for singly modified peptides. Beyond the evidence from our data, the portion of modified NAC region in αSyn needs further evaluation.
The N-terminally acetylated αSyn is the major form in brain tissue (58) and we could show that it is also a main form in CSF. N-terminal acetylation is important for membrane interaction of αSyn which itself influences αSyn conformation (59). In addition, it also influences aggregate formation (59) and alterations could be indicative of a different aggregation propensity in disease. Apart from the increase in CJD, we did, however, not observe concentration differences in synucleinopathies and atypical parkinsonian syndromes. For an indirect measure of PTM alterations we measured seven (unmodified) αSyn peptides covering 70% of the αSyn sequence. There was no specific pattern of the peptides for synucleinopathies and atypical parkinsonian syndromes. However, the determination of specific PTMs might be more sensitive as has been shown for Ser129P-αSyn (48, 49) and should be the focus of future mass spectrometric improvements for the characterizations of αSyn in CSF.
This is the first study presenting quantitative data on βSyn and γSyn in CSF. Both synucleins are increased in AD and especially in CJD and are new potential biomarker candidates for these diseases. The magnitude of the alteration is comparable to tau protein which is already used as a diagnostic biomarker (60) and we showed a high correlation of βSyn and tau in CSF. βSyn is a predominantly brain-derived protein which is indicated in our study by the failure to correlate with the albumin quotient and it is supported by protein expression data (61, 62). Furthermore, our data show that it is not affected by blood contamination which makes it an attractive biomarker candidate that might be used as an alternative to or in combination with tau. This could be especially interesting for measurements in blood which is currently established for several CNS biomarkers such as neurofilaments (63) because blood collection is more convenient for patients and clinicians.
In our study, βSyn concentrations tended to be higher in PDD and LBD patients in comparison with PD and controls. βSyn is not present in Lewy bodies, the major hallmark of synucleinopathies, but βSyn accumulation is found in these diseases in brain regions involved in memory formation such as the hippocampus (6). Furthermore, βSyn mutations are linked to certain types of LBD (64) and mutant βSyn leads to more profound memory deficits compared with impairments of the motoric system in transgenic animals (65). These observations link βSyn to dementia symptoms rather than motor impairment. We calculated a ratio of βSyn versus αSyn concentrations in CSF and the ratio was significantly increased in PDD. These results imply an imbalance of αSyn and βSyn in dementia and are in line with the observations in transgenic animals and histopathology mentioned above. However, because βSyn has been shown to inhibit αSyn aggregation (7), an increase of the ratio βSyn/αSyn in disease is unexpected but may indicate a secondary increase of βSyn expression in response to αSyn aggregation as a protective mechanism of the cell. Further studies are needed to confirm whether the βSyn/αSyn ratio is a suitable biomarker to distinguish PDD from PD patients or identify PD patients at risk for the development of a dementia.
In conclusion, our mass spectrometric quantification of αSyn in CSF provides an important contribution to the long-lasting discussion about the usefulness of total αSyn concentration as a biomarker in PD and does not support alterations observed previously with immunoassays. Furthermore, the common increase in AD and CJD points to synucleins as general markers of synaptic degeneration instead of being specific for synucleinopathies. βsyn is an attractive biomarker candidate that might be used as an alternative to or in combination with tau in AD and CJD diagnosis and in combination with αSyn it is a biomarker candidate for PDD.
Supplementary Material
Acknowledgments
We thank Stephen Meier, Mehtap Bulut-Karac, Sandra Hübsch and Dagmar Schattauer for their excellent technical assistance and we thank our patients for participating in this study.
Footnotes
Author contributions: P.O., F.M., and M.O. designed research; P.O. performed research; P.O., F.M., M.N., C.A.v., S.H., P.S., A.C.L., and M.O. analyzed data; P.O., F.M., M.N., C.A.v., S.H., P.S., A.C.L., and M.O. wrote the paper.
* This study was supported by the JPND networks SOPHIA (01ED1202A), Prefrontal (01ED1512) and BiomarkAPD (01ED1203F), the German Federal Ministry of Education and Research (FTLDc O1GI1007A, KKMS, MND-Net 01GM1103A), the EU (NADINE 246513, FAIR-PARK II 633190), the foundation of the state Baden-Württemberg (D.3830, www.bwstiftung.de) and BIU (D.5009, http://fakultaet.medizin.uni-ulm.de/en/research/projects/biu/).
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- αSyn
- α-synuclein
- Ac
- N-terminal acetylation
- aCSF
- artificial CSF
- AD
- Alzheimer′s disease
- Alb
- serum albumin
- α1Ch
- α1-antichymotrypsin
- α1Tr
- α1-antitrypsin
- α2HS
- α2-HS-glycoprotein
- α2M
- α2-macroglobulin
- βSyn
- β-synuclein
- CBS
- corticobasal syndrome
- CE
- collision energy
- ChP
- choroid plexus
- CJD
- Creutzfeldt-Jakob disease
- CP
- ceruloplasmin
- CSF
- cerebrospinal fluid
- CUR
- curtain gas
- IS
- internal standard
- γSyn
- γ-synuclein
- HSA
- human serum albumin
- Hb
- hemoglobin
- Hbb
- hemoglobin beta subunit
- Hpx
- hemopexin
- Ig
- immunoglobulin
- LBD
- Lewy body dementia
- LLOQ
- lower limit of quantification
- LOD
- limit of detection
- MeOH
- methanol
- MRM
- multiple reaction monitoring
- NAC
- non-Abeta component
- NINCDS-ADRDA
- National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association
- O-GlcNAc
- O-linked β-N-acetylglucosamine
- PD
- Parkinon′s disease
- PDD
- PD dementia
- PSAQ
- protein standard absolute quantification
- PSP
- progressive supranuclear palsy
- PTM
- post-translational modification
- QC sample
- quality control sample
- TEAB
- triethylammonium bicarbonate.
REFERENCES
- 1. McCann H., Stevens C. H., Cartwright H., and Halliday G. M. (2014) α-Synucleinopathy phenotypes. Parkinsonism Relat. Disord. 20, S62–S7 [DOI] [PubMed] [Google Scholar]
- 2. Beyer K., and Ariza A. (2013) α-Synuclein posttranslational modification and alternative splicing as a trigger for neurodegeneration. Mol. Neurobiol. 47, 509–524 [DOI] [PubMed] [Google Scholar]
- 3. Lashuel H. A., Overk C. R., Oueslati A., and Masliah E. (2013) The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 14, 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El-Agnaf O. M. A., Salem S. A., Paleologou K. E., Cooper L. J., Fullwood N. J., Gibson M. J., Curran M. D., Court J. A., Mann D. M. A., Ikeda S., Cookson M. R., Hardy J., and Allsop D. (2003) Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 17, 1945–1947 [DOI] [PubMed] [Google Scholar]
- 5. Parnetti L., Cicognola C., Eusebi P., and Chiasserini D. (2016) Value of cerebrospinal fluid α-synuclein species as biomarker in Parkinson's diagnosis and prognosis. Biomark. Med. 10, 35–49 [DOI] [PubMed] [Google Scholar]
- 6. Galvin J. E., Uryu K., Lee V. M., and Trojanowski J. Q. (1999) Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc. Natl. Acad. Sci. U.S.A. 96, 13450–13455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hashimoto M., Rockenstein E., Mante M., Mallory M., and Masliah E. (2001) beta-Synuclein inhibits alpha-synuclein aggregation: a possible role as an anti-parkinsonian factor. Neuron 32, 213–223 [DOI] [PubMed] [Google Scholar]
- 8. Ninkina N., Peters O., Millership S., Salem H., van der Putten H., and Buchman V. L. (2009) Gamma-synucleinopathy: neurodegeneration associated with overexpression of the mouse protein. Hum. Mol. Genet. 18, 1779–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guldbrandsen A., Vethe H., Farag Y., Oveland E., Garberg H., Berle M., Myhr K-M, Opsahl J. A., Barsnes H., and Berven F. S. (2014) In-depth characterization of the cerebrospinal fluid (CSF) proteome displayed through the CSF proteome resource (CSF-PR). Mol. Cell. Proteomics 13, 3152–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mukaetova-Ladinska E. B., Milne J., Andras A., Abdel-All Z., Cerejeira J., Greally E., Robson J., Jaros E., Perry R., McKeith I. G., Brayne C., Xuereb J., Cleghorn A., Doherty J., McIntosh G., and Milton I. (2008) Alpha- and gamma-synuclein proteins are present in cerebrospinal fluid and are increased in aged subjects with neurodegenerative and vascular changes. Dement. Geriatr. Cogn. Disord. 26, 32–42 [DOI] [PubMed] [Google Scholar]
- 11. Brun V., Dupuis A., Adrait A., Marcellin M., Thomas D., Court M., Vandenesch F., and Garin J. (2007) Isotope-labeled protein standards: toward absolute quantitative proteomics. Mol. Cell. Proteomics 6, 2139–2149 [DOI] [PubMed] [Google Scholar]
- 12. Oeckl P., Steinacker P., von Arnim C. A. F., Straub S., Nagl M., Feneberg E., Weishaupt J. H., Ludolph A. C., and Otto M. (2014) Intact protein analysis of ubiquitin in cerebrospinal fluid by multiple reaction monitoring reveals differences in Alzheimer′s disease and frontotemporal lobar degeneration. J. Proteome Res. 13, 4518–4525 [DOI] [PubMed] [Google Scholar]
- 13. Schmid A. W., Fauvet B., Moniatte M., and Lashuel H. A. (2013) Alpha-synuclein post-translational modifications as potential biomarkers for Parkinson disease and other synucleinopathies. Mol. Cell. Proteomics 12, 3543–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barthélemy N. R., Fenaille F., Hirtz C., Sergeant N., Schraen-Maschke S., Vialaret J., Buée L., Gabelle A., Junot C., Lehmann S., and Becher F. (2016) Tau Protein Quantification in Human Cerebrospinal Fluid by Targeted Mass Spectrometry at High Sequence Coverage Provides Insights into Its Primary Structure Heterogeneity. J. Proteome Res. 15, 667–676 [DOI] [PubMed] [Google Scholar]
- 15. Rappsilber J., Mann M., and Ishihama Y. (2007) Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896–1906 [DOI] [PubMed] [Google Scholar]
- 16. MacLean B., Tomazela D. M., Shulman N., Chambers M., Finney G. L., Frewen B., Kern R., Tabb D. L., Liebler D. C., and MacCoss M. J. (2010) Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26, 966–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong Z., Shi M., Chung K. A., Quinn J. F., Peskind E. R., Galasko D., Jankovic J., Zabetian C. P., Leverenz J. B., Baird G., Montine T. J., Hancock A. M., Hwang H., Pan C., Bradner J., Kang U. J., Jensen P. H., and Zhang J. (2010) DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain 133, 713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hughes A. J., Daniel S. E., Kilford L., and Lees A. J. (1992) Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Emre M., Aarsland D., Brown R., Burn D. J., Duyckaerts C., Mizuno Y., Broe G. A., Cummings J., Dickson D. W., Gauthier S., Goldman J., Goetz C., Korczyn A., Lees A., Levy R., Litvan I., McKeith I., Olanow W., Poewe W., Quinn N., Sampaio C., Tolosa E., and Dubois B. (2007) Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov. Disord. 22, 1689–1707; quiz 1837 [DOI] [PubMed] [Google Scholar]
- 20. McKeith I. G., Dickson D. W., Lowe J., Emre M., O'Brien J. T., Feldman H., Cummings J., Duda J. E., Lippa C., Perry E. K., Aarsland D., Arai H., Ballard C. G., Boeve B., Burn D. J., Costa D., Del Ser T., Dubois B., Galasko D., Gauthier S., Goetz C. G., Gomez-Tortosa E., Halliday G., Hansen L. A., Hardy J., Iwatsubo T., Kalaria R. N., Kaufer D., Kenny R. A., Korczyn A., Kosaka K., Lee V. M. Y., Lees A., Litvan I., Londos E., Lopez O. L., Minoshima S., Mizuno Y., Molina J. A., Mukaetova-Ladinska E. B., Pasquier F., Perry R. H., Schulz J. B., Trojanowski J. Q., and Yamada M. (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65, 1863–1872 [DOI] [PubMed] [Google Scholar]
- 21. Litvan I., Agid Y., Calne D., Campbell G., Dubois B., Duvoisin R. C., Goetz C. G., Golbe L. I., Grafman J., Growdon J. H., Hallett M., Jankovic J., Quinn N. P., Tolosa E., and Zee D. S. (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47, 1–9 [DOI] [PubMed] [Google Scholar]
- 22. Armstrong M. J., Litvan I., Lang A. E., Bak T. H., Bhatia K. P., Borroni B., Boxer A. L., Dickson D. W., Grossman M., Hallett M., Josephs K. A., Kertesz A., Lee S. E., Miller B. L., Reich S. G., Riley D. E., Tolosa E., Tröster A. I., Vidailhet M., and Weiner W. J. (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80, 496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization (1998) Consensus on criteria for sporadic CJD.
- 24. Jesse S., Brettschneider J., Süssmuth S. D., Landwehrmeyer B. G., von Arnim C. A. F., Ludolph A. C., Tumani H., and Otto M. (2011) Summary of cerebrospinal fluid routine parameters in neurodegenerative diseases. J. Neurol. 258, 1034–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Felgenhauer K. (1995) in New Concepts of a Blood—Brain Barrier (Springer), pp 209–217, Science+Business Media, New York [Google Scholar]
- 26. Nath S., Meuvis J., Hendrix J., Carl S. A., and Engelborghs Y. (2010) Early aggregation steps in alpha-synuclein as measured by FCS and FRET: evidence for a contagious conformational change. Biophys. J. 98, 1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McNulty B. C., Tripathy A., Young G. B., Charlton L. M., Orans J., and Pielak G. J. (2006) Temperature-induced reversible conformational change in the first 100 residues of alpha-synuclein. Protein Sci. 15, 602–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morar A. S., Olteanu A., Young G. B., and Pielak G. J. (2001) Solvent-induced collapse of alpha-synuclein and acid-denatured cytochrome c. Protein Sci. 10, 2195–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weinreb P. H., Zhen W., Poon A. W., Conway K. A., and Lansbury P. T. (1996) NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry 35, 13709–13715 [DOI] [PubMed] [Google Scholar]
- 30. Halbgebauer S., Öckl P., Wirth K., Steinacker P., and Otto M. (2016) Protein biomarkers in Parkinson's disease: Focus on cerebrospinal fluid markers and synaptic proteins. Mov. Disord. 31, 848–860 [DOI] [PubMed] [Google Scholar]
- 31. Shi T., Song E., Nie S., Rodland K. D., Liu T., Qian W-J, and Smith R. D. (2016) Advances in targeted proteomics and applications to biomedical research. Proteomics, doi: 10.1002/pmic.201500449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carr S. A., Abbatiello S. E., Ackermann B. L., Borchers C., Domon B., Deutsch E. W., Grant R. P., Hoofnagle A. N., Hüttenhain R., Koomen J. M., Liebler D. C., Liu T., MacLean B., Mani D. R., Mansfield E., Neubert H., Paulovich A. G., Reiter L., Vitek O., Aebersold R., Anderson L., Bethem R., Blonder J., Boja E., Botelho J., Boyne M., Bradshaw R. A., Burlingame A. L., Chan D., Keshishian H., Kuhn E., Kinsinger C., Lee J. S. H., Lee S-W, Moritz R., Oses-Prieto J., Rifai N., Ritchie J., Rodriguez H., Srinivas P. R., Townsend R. R., Van Eyk J., Whiteley G., Wiita A., and Weintraub S. (2014) Targeted peptide measurements in biology and medicine: best practices for mass spectrometry-based assay development using a fit-for-purpose approach. Mol. Cell. Proteomics 13, 907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Llorens F., Kruse N., Schmitz M., Shafiq M., da Cunha J. E. G., Gotzman N., Zafar S., Thune K., de Oliveira J. R. M., Mollenhauer B., and Zerr I. (2015) Quantification of CSF biomarkers using an electrochemiluminescence-based detection system in the differential diagnosis of AD and sCJD. J. Neurol. 262, 2305–2311 [DOI] [PubMed] [Google Scholar]
- 34. Llorens F., Zafar S., Ansoleaga B., Shafiq M., Blanco R., Carmona M., Grau-Rivera O., Nos C., Gelpí E., Del Río J. A., Zerr I., and Ferrer I. (2015) Subtype and regional regulation of prion biomarkers in sporadic Creutzfeldt-Jakob disease. Neuropathol. Appl. Neurobiol. 41, 631–645 [DOI] [PubMed] [Google Scholar]
- 35. Kasai T., Tokuda T., Ishii R., Ishigami N., Tsuboi Y., Nakagawa M., Mizuno T., and El-Agnaf O. M. A. (2014) Increased α-synuclein levels in the cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. J. Neurol. 261, 1203–1209 [DOI] [PubMed] [Google Scholar]
- 36. Mollenhauer B., Cullen V., Kahn I., Krastins B., Outeiro T. F., Pepivani I., Ng J., Schulz-Schaeffer W., Kretzschmar H. A., McLean P. J., Trenkwalder C., Sarracino D. A., Vonsattel J-P, Locascio J. J., El-Agnaf O. M. A., and Schlossmacher M. G. (2008) Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp. Neurol. 213, 315–325 [DOI] [PubMed] [Google Scholar]
- 37. Hansson O., Hall S., Ohrfelt A., Zetterberg H., Blennow K., Minthon L., Nägga K., Londos E., Varghese S., Majbour N. K., Al-Hayani A., and El-Agnaf O. M. (2014) Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson's disease with dementia and dementia with Lewy bodies compared to Alzheimer's disease. Alzheimers. Res. Ther. 6, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slaets S., Vanmechelen E., Le Bastard N., Decraemer H., Vandijck M., Martin J-J, De Deyn P. P., and Engelborghs S. (2014) Increased CSF α-synuclein levels in Alzheimer's disease: correlation with tau levels. Alzheimers. Dement. 10, S290–S8 [DOI] [PubMed] [Google Scholar]
- 39. Hall S., Öhrfelt A., Constantinescu R., Andreasson U., Surova Y., Bostrom F., Nilsson C., Håkan W., Decraemer H., Någga K., Minthon L., Londos E., Vanmechelen E., Holmberg B., Zetterberg H., Blennow K., and Hansson O. (2012) Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch. Neurol. 69, 1445–1452 [DOI] [PubMed] [Google Scholar]
- 40. Kruse N., Persson S., Alcolea D., Bahl J. M. C., Baldeiras I., Capello E., Chiasserini D., Bocchio Chiavetto L., Emersic A., Engelborghs S., Eren E., Fladby T., Frisoni G., García-Ayllón M-S, Genc S., Gkatzima O., Heegaard N. H. H., Janeiro A. M., Kováčech B., Kuiperij H. B., Leitão M. J., Lleó A., Martins M., Matos M., Mollergard H. M., Nobili F., Öhrfelt A., Parnetti L., de Oliveira C. R., Rot U., Sáez-Valero J., Struyfs H., Tanassi J. T., Taylor P., Tsolaki M., Vanmechelen E., Verbeek M. M., Zilka N., Blennow K., Zetterberg H., and Mollenhauer B. (2015) Validation of a quantitative cerebrospinal fluid alpha-synuclein assay in a European-wide interlaboratory study. Neurobiol. Aging 36, 2587–2596 [DOI] [PubMed] [Google Scholar]
- 41. Kruse N., and Mollenhauer B. (2015) Validation of a commercially available enzyme-linked immunoabsorbent assay for the quantification of human α-Synuclein in cerebrospinal fluid. J. Immunol. Methods 426, 70–75 [DOI] [PubMed] [Google Scholar]
- 42. Andreasson U., Perret-Liaudet A., van Waalwijk van Doorn L. J. C., Blennow K., Chiasserini D., Engelborghs S., Fladby T., Genc S., Kruse N., Kuiperij H. B., Kulic L., Lewczuk P., Mollenhauer B., Mroczko B., Parnetti L., Vanmechelen E., Verbeek M. M., Winblad B., Zetterberg H., Koel-Simmelink M., and Teunissen C. E. (2015) A Practical Guide to Immunoassay Method Validation. Front. Neurol. 6, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Johanson C. E., Stopa E. G., and McMillan P. N. (2011) The blood-cerebrospinal fluid barrier: structure and functional significance. Methods Mol. Biol. 686, 101–131 [DOI] [PubMed] [Google Scholar]
- 44. Tumani H., Teunissen C., Süssmuth S., Otto M., Ludolph A. C., and Brettschneider J. (2008) Cerebrospinal fluid biomarkers of neurodegeneration in chronic neurological diseases. Expert Rev. Mol. Diagn. 8, 479–494 [DOI] [PubMed] [Google Scholar]
- 45. Barbour R., Kling K., Anderson J. P., Banducci K., Cole T., Diep L., Fox M., Goldstein J. M., Soriano F., Seubert P., and Chilcote T. J. (2008) Red blood cells are the major source of alpha-synuclein in blood. Neurodegener. Dis. 5, 55–59 [DOI] [PubMed] [Google Scholar]
- 46. Mollenhauer B., Trautmann E., Otte B., Ng J., Spreer A., Lange P., Sixel-Döring F., Hakimi M., Vonsattel J-P, Nussbaum R., Trenkwalder C., and Schlossmacher M. G. (2012) α-Synuclein in human cerebrospinal fluid is principally derived from neurons of the central nervous system. J. Neural Transm. 119, 739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bates C. A., Fu S., Ysselstein D., Rochet J.-C., and Zheng W.. Expression and Transport of α-Synuclein at the Blood-Cerebrospinal Fluid Barrier and Effects of Manganese Exposure. ADMET DMPK 3, 15–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stewart T., Sossi V., Aasly J. O., Wszolek Z. K., Uitti R. J., Hasegawa K., Yokoyama T., Zabetian C. P., Leverenz J. B., Stoessl A. J., Wang Y., Ginghina C., Liu C., Cain K. C., Auinger P., Kang U. J., Jensen P. H., Shi M., and Zhang J. (2015) Phosphorylated α-synuclein in Parkinson's disease: correlation depends on disease severity. Acta Neuropathol. Commun. 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Y., Shi M., Chung K. A., Zabetian C. P., Leverenz J. B., Berg D., Srulijes K., Trojanowski J. Q., Lee VM-Y, Siderowf A. D., Hurtig H., Litvan I., Schiess M. C., Peskind E. R., Masuda M., Hasegawa M., Lin X., Pan C., Galasko D., Goldstein D. S., Jensen P. H., Yang H., Cain K. C., and Zhang J. (2012) Phosphorylated α-synuclein in Parkinson's disease. Sci. Transl. Med. 4, 121ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Giasson B. I., Murray I. V., Trojanowski J. Q., and Lee V. M. (2001) A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J. Biol. Chem. 276, 2380–2386 [DOI] [PubMed] [Google Scholar]
- 51. Okochi M., Walter J., Koyama A., Nakajo S., Baba M., Iwatsubo T., Meijer L., Kahle P. J., and Haass C. (2000) Constitutive phosphorylation of the Parkinson's disease associated alpha-synuclein. J. Biol. Chem. 275, 390–397 [DOI] [PubMed] [Google Scholar]
- 52. Kim E. J., Sung J. Y., Lee H. J., Rhim H., Hasegawa M., Iwatsubo T., Min D. S., Kim J., Paik S. R., and Chung K. C. (2006) Dyrk1A phosphorylates alpha-synuclein and enhances intracellular inclusion formation. J. Biol. Chem. 281, 33250–33257 [DOI] [PubMed] [Google Scholar]
- 53. Paleologou K. E., Oueslati A., Shakked G., Rospigliosi C. C., Kim H-Y, Lamberto G. R., Fernandez C. O., Schmid A., Chegini F., Gai W. P., Chiappe D., Moniatte M., Schneider B. L., Aebischer P., Eliezer D., Zweckstetter M., Masliah E., and Lashuel H. A. (2010) Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions. J. Neurosci. 30, 3184–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang Z., Park K., Comer F., Hsieh-Wilson L. C., Saudek C. D., and Hart G. W. (2009) Site-specific GlcNAcylation of human erythrocyte proteins: potential biomarker(s) for diabetes. Diabetes 58, 309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhu Y., Shan X., Yuzwa S. A., and Vocadlo D. J. (2014) The emerging link between O-GlcNAc and Alzheimer disease. J. Biol. Chem. 289, 34472–34481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weinert B. T., Schölz C., Wagner S. A., Iesmantavicius V., Su D., Daniel J. A., and Choudhary C. (2013) Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 4, 842–851 [DOI] [PubMed] [Google Scholar]
- 57. Trinidad J. C., Barkan D. T., Gulledge B. F., Thalhammer A., Sali A., Schoepfer R., and Burlingame A. L. (2012) Global identification and characterization of both O-GlcNAcylation and phosphorylation at the murine synapse. Mol. Cell. Proteomics 11, 215–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anderson J. P., Walker D. E., Goldstein J. M., de Laat R., Banducci K., Caccavello R. J., Barbour R., Huang J., Kling K., Lee M., Diep L., Keim P. S., Shen X., Chataway T., Schlossmacher M. G., Seubert P., Schenk D., Sinha S., Gai W. P., and Chilcote T. J. (2006) Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 281, 29739–29752 [DOI] [PubMed] [Google Scholar]
- 59. Bartels T., Kim N. C., Luth E. S., and Selkoe D. J. (2014) N-alpha-acetylation of α-synuclein increases its helical folding propensity, GM1 binding specificity and resistance to aggregation. PLoS ONE 9:e103727, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jack C. R., Albert M. S., Knopman D. S., McKhann G. M., Sperling R. A., Carrillo M. C., Thies B., and Phelps C. H. (2011) Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers. Dement. 7, 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wilhelm M., Schlegl J., Hahne H., Moghaddas Gholami A., Lieberenz M., Savitski M. M., Ziegler E., Butzmann L., Gessulat S., Marx H., Mathieson T., Lemeer S., Schnatbaum K., Reimer U., Wenschuh H., Mollenhauer M., Slotta-Huspenina J., Boese J-H, Bantscheff M., Gerstmair A., Faerber F., and Kuster B. (2014) Mass-spectrometry-based draft of the human proteome. Nature 509, 582–587 [DOI] [PubMed] [Google Scholar]
- 62. Kim M-S, Pinto S. M., Getnet D., Nirujogi R. S., Manda S. S., Chaerkady R., Madugundu A. K., Kelkar D. S., Isserlin R., Jain S., Thomas J. K., Muthusamy B., Leal-Rojas P., Kumar P., Sahasrabuddhe N. A., Balakrishnan L., Advani J., George B., Renuse S., Selvan L. D. N., Patil A. H., Nanjappa V., Radhakrishnan A., Prasad S., Subbannayya T., Raju R., Kumar M., Sreenivasamurthy S. K., Marimuthu A., Sathe G. J., Chavan S., Datta K. K., Subbannayya Y., Sahu A., Yelamanchi S. D., Jayaram S., Rajagopalan P., Sharma J., Murthy K. R., Syed N., Goel R., Khan A. A., Ahmad S., Dey G., Mudgal K., Chatterjee A., Huang T-C, Zhong J., Wu X., Shaw P. G., Freed D., Zahari M. S., Mukherjee K. K., Shankar S., Mahadevan A., Lam H., Mitchell C. J., Shankar S. K., Satishchandra P., Schroeder J. T., Sirdeshmukh R., Maitra A., Leach S. D., Drake C. G., Halushka M. K., Prasad T. S. K., Hruban R. H., Kerr C. L., Bader G. D., Iacobuzio-Donahue C. A., Gowda H., and Pandey A. (2014) A draft map of the human proteome. Nature 509, 575–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Weydt P., Oeckl P., Huss A., Müller K., Volk A. E., Kuhle J., Knehr A., Andersen P. M., Prudlo J., Steinacker P., Weishaupt J. H., Ludolph A. C., and Otto M. (2016) Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann. Neurol. 79, 152–158 [DOI] [PubMed] [Google Scholar]
- 64. Ohtake H., Limprasert P., Fan Y., Onodera O., Kakita A., Takahashi H., Bonner L. T., Tsuang D. W., Murray I. V. J., Lee VM-Y, Trojanowski J. Q., Ishikawa A., Idezuka J., Murata M., Toda T., Bird T. D., Leverenz J. B., Tsuji S., and La Spada A. R. (2004) Beta-synuclein gene alterations in dementia with Lewy bodies. Neurology 63, 805–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fujita M., Sugama S., Sekiyama K., Sekigawa A., Tsukui T., Nakai M., Waragai M., Takenouchi T., Takamatsu Y., Wei J., Rockenstein E., Laspada A. R., Masliah E., Inoue S., and Hashimoto M. (2010) A β-synuclein mutation linked to dementia produces neurodegeneration when expressed in mouse brain. Nat. Commun. 1, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tokuda T., Salem S. A., Allsop D., Mizuno T., Nakagawa M., Qureshi M. M., Locascio J. J., Schlossmacher M. G., and El-Agnaf O. M. A. (2006) Decreased alpha-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson's disease. Biochem. Biophys. Res. Commun. 349, 162–166 [DOI] [PubMed] [Google Scholar]
- 67. van Geel W. J. A., Abdo W. F., Melis R., Williams S., Bloem B. R., and Verbeek M. M. (2008) A more efficient enzyme-linked immunosorbent assay for measurement of alpha-synuclein in cerebrospinal fluid. J. Neurosci. Methods 168, 182–185 [DOI] [PubMed] [Google Scholar]
- 68. Emmanouilidou E., Elenis D., Papasilekas T., Stranjalis G., Gerozissis K., Ioannou P. C., and Vekrellis K. (2011) Assessment of α-synuclein secretion in mouse and human brain parenchyma. PLoS ONE 6, e22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kruse N., Schulz-Schaeffer W. J., Schlossmacher M. G., and Mollenhauer B. (2012) Development of electrochemiluminescence-based singleplex and multiplex assays for the quantification of α-synuclein and other proteins in cerebrospinal fluid. Methods 56, 514–518 [DOI] [PubMed] [Google Scholar]
- 70. Mollenhauer B., Trautmann E., Taylor P., Manninger P., Sixel-Döring F., Ebentheuer J., Trenkwalder C., and Schlossmacher M. G. (2013) Total CSF α-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci. Lett. 532, 44–48 [DOI] [PubMed] [Google Scholar]
- 71. Felgenhauer K. (1974) Protein size and cerebrospinal fluid composition. Klin. Wochenschr. 52, 1158–1164 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.