Abstract
High blood pressure, or “hypertension,” is associated with high levels of oxidative stress in the paraventricular nucleus of the hypothalamus. While pomegranate extract is a known antioxidant that is thought to have antihypertensive effects, the mechanism whereby pomegranate extract lowers blood pressure and the tissue that mediates its antihypertensive effects are currently unknown. We have used a spontaneously hypertensive rat model to investigate the antihypertensive properties of pomegranate extract. We found that chronic treatment of hypertensive rats with pomegranate extract significantly reduced blood pressure and cardiac hypertrophy. Furthermore, pomegranate extract reduced oxidative stress, increased the antioxidant defense system, and decreased inflammation in the paraventricular nucleus of hypertensive rats. We determined that pomegranate extract reduced mitochondrial superoxide anion levels and increased mitochondrial function in the paraventricular nucleus of hypertensive rats by promoting mitochondrial biogenesis and improving mitochondrial dynamics and clearance. We went on to identify the AMPK-nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) pathway as a mechanism whereby pomegranate extract reduces oxidative stress in the paraventricular nucleus to relieve hypertension. Our findings demonstrate that pomegranate extract alleviates hypertension by reducing oxidative stress and improving mitochondrial function in the paraventricular nucleus, and reveal multiple novel targets for therapeutic treatment of hypertension.
Many studies have focused on dietary and lifestyle interventions to manage hypertension1,2, a chronic disease in which arterial blood pressure is persistently elevated3. Consuming food with high antioxidant activity is known to mitigate the symptoms of hypertension4, and has been the subject of clinical trials5. The paraventricular nucleus region of the hypothalamus is an endocrine-autonomic control area of the brain that regulates sympathetic output and salt appetite6, and has been implicated in hypertension. Specifically, neuronal hyperactivity and oxidative stress within the paraventricular nucleus are thought to promote hypertension7.
The spontaneously hypertensive rat is a well-established model of hypertension and cardiovascular disease. Spontaneously hypertensive adult rats have systolic blood pressures ranging from 180 to 200 mmHg, and have significantly higher levels of oxidative stress and inflammation within the paraventricular nucleus8,9. Reducing oxidative stress and inflammation in the hypothalamus by antioxidant treatment has been shown to effectively lower blood pressure in an angiotensin II-induced rat model of hypertension10, suggesting that antioxidant-based reduction of oxidative stress and inflammation in the paraventricular nucleus may be an effective strategy for lowering blood pressure.
The peel, seeds and juice of the pomegranate fruit are abundantly rich in antioxidants. Punicalagin, a molecule known to have anti-inflammatory, anti-tumor and anti-diabetic effects11,12,13, is a major bioactive constituent of pomegranates14,15. The health benefits of punicalagin are thought to derive from its antioxidant role as a scavenger and ferrous chelator of hydrogen peroxide13. We have previously reported that pomegranate extract-enriched punicalagin promotes mitochondrial function by decreasing oxidative stress in a rat model of obesity-associated nonalcoholic fatty liver disease16. In addition, pomegranate extract reduces systolic and diastolic blood pressure and increases antioxidant activity in hemodialysis patients17, and protects the liver against acetaminophen-induced toxicity11.
Despite the fact that pomegranate extract/punicaligin has promising roles as an antioxidant, anti-inflammatory, and antihypertensive, the mechanism whereby pomegranate extract exerts it effects is unknown4,5. Understanding how pomegranate extract effects the central nervous system is critical for the rational design of hypertension therapy. Our data suggest that pomegranate extract reduces hypertension by lowering oxidative stress and improving mitochondrial function in the paraventricular nucleus, and that pomegranate extract-induced reduction of oxidative stress is mediated by the AMPK-Nrf2 pathway.
Results
Pomegranate extract reduces blood pressure and heart hypertrophy in spontaneously hypertensive rats
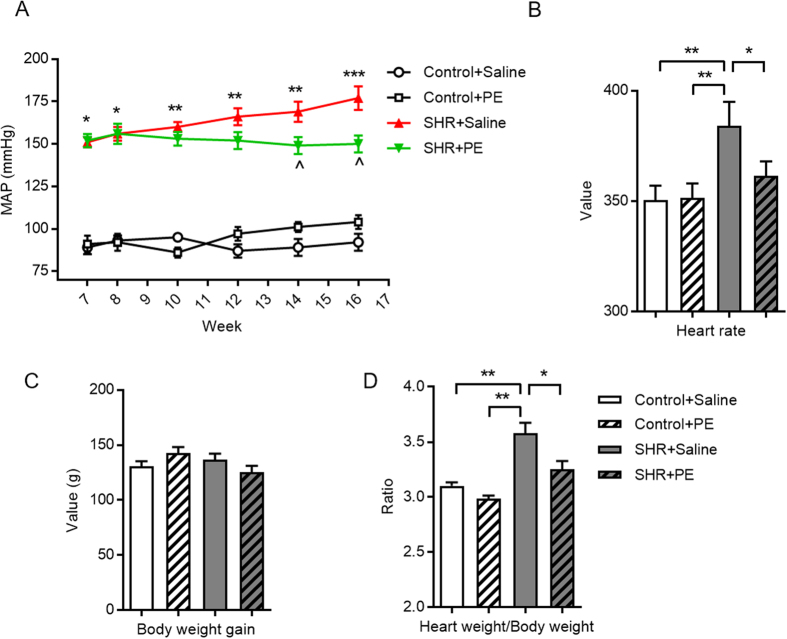
Throughout our studies, spontaneously hypertensive rats and controls (Wistar-Kyoto, referred to hereafter as “control”) were treated with pomegranate extract or saline, respectively, for eight weeks. To determine if pomegranate extract reduces hypertension in this model, we first recorded systolic and diastolic blood pressures using a rat tail artery blood pressure test system, and calculated mean arterial pressure. Mean arterial pressure of hypertensive rats treated with saline increased over time compared to controls, but remained at a stable, lower level in the pomegranate extract treated group (Fig. 1A). Heart rates of pomegranate extract-treated hypertensive rats were also significantly lower at the end of treatment than the saline-treated group (Fig. 1B). While total body weight gain before or after pomegranate extract treatment did not differ between the groups (Fig. 1C), pomegranate extract reduced the higher heart weight to body weight ratio, an indicator of cardiac hypertrophy, in hypertensive rats, indicating that pomegranate extract treatment decreases hypertension-induced cardiac hypertrophy (Fig. 1D).
Figure 1. Effects of pomegranate extract supplementation on mean arterial pressure, body weight gain, and heart weight in hypertensive and control rats.
Spontaneously hypertensive and control rats were administered either saline or pomegranate extract (150 mg/kg/day) for eight weeks. (A) The mean arterial pressure in control and hypertensive rats. *p < 0.05, **p < 0.01 and ***p < 0.001, ^p < 0.05. Asterisks indicate significantly elevated blood pressure of hypertensive rats versus controls. Arrows indicate significantly reduced blood pressure in hypertensive rats treated with pomegranate extract versus hypertensive rats treated with saline. (B) Heart rate in control and hypertensive rats. (C) Body weight gain in control and hypertensive rats. (D) Heart weight/body weight ratio. Values are the mean ± SEM from 10 animals. *p < 0.05, **p < 0.01. SHR, spontaneously hypertensive rats; PE, pomegranate extract.
Pomegranate extract inhibits superoxide anion and neuronal hyperactivity in the paraventricular nucleus of hypertensive rats
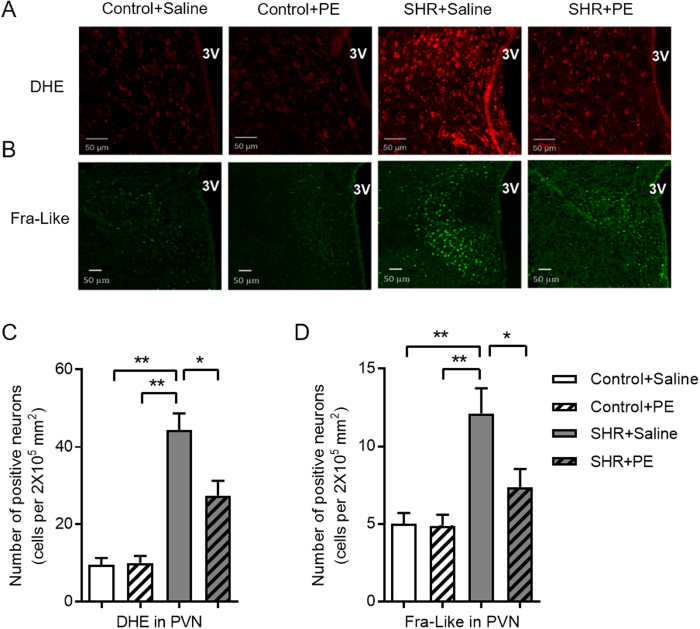
We next determined if pomegranate extract reduces hypertension-induced oxidative stress in the paraventricular nucleus. Based on dihydroethidium staining, a dye widely used for measuring superoxide, we observed that pomegranate extract significantly reduced superoxide generation in the paraventricular nucleus of hypertensive rats (Fig. 2A,C), but had no effect on oxidative stress in controls.
Figure 2. Effects of pomegranate extract supplementation on superoxide anion and chronic neuronal activation in the hypothalamic paraventricular nucleus of control and spontaneously hypertensive rats.
Spontaneously hypertensive rats and controls were administered either saline or pomegranate extract (150 mg/kg/day) for eight weeks, sacrificed, and frozen sections were prepared from the paraventricular nucleus. (A) A representative immunofluorescence image showing superoxide production as detected with dihydroethidium staining (DHE, red fluorescence). (B) A representative immunofluorescence image showing Fra-Like expression (green fluorescence). Statistical analysis of dihydroethidium (C) and Fra-Like (D) positive neurons in the paraventricular nucleus of hypertensive and control rats, with or without pomegranate extract treatment. Values are the mean ± SEM from 10 animals. *p < 0.05, **p < 0.01. 3V, third ventricle; SHR, spontaneously hypertensive rats; PE, pomegranate extract, PVN, paraventricular nucleus.
Having established that pomegranate extract reduces levels of hypertension-induced superoxide in rats, we next investigated sympathetic nerve hyperactivity, a known consequence of oxidative stress in the hypothalamic paraventricular nucleus, and stimulator of hypertension18. We assessed neuronal activation based on Fra-like immunoreactivity, an indicator of chronic neuronal activation. We observed significantly higher levels of neuronal activity in hypertensive rats versus controls, which was significantly reduced by pomegranate extract treatment (Fig. 2B,D). Taken together, our data suggest that pomegranate extract decreases oxidative stress and chronic neuronal activation in the hypothalamic paraventricular nucleus of hypertensive rats.
Pomegranate extract inhibits angiotensin converting enzyme expression in the paraventricular nucleus of hypertensive rats
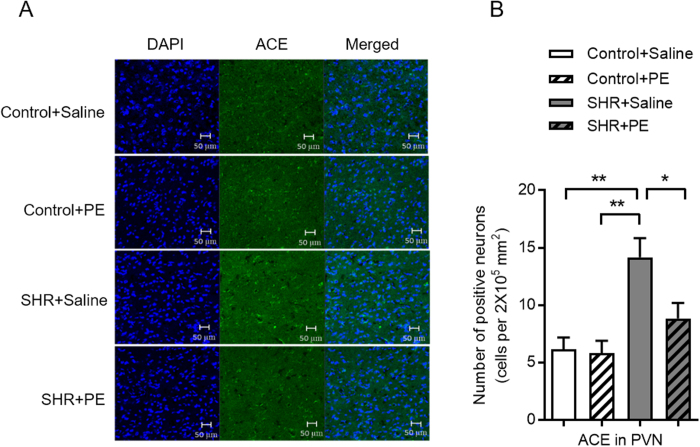
We next investigated the effects of pomegranate extract on the renin-angiotensin system, a known regulator of blood pressure. Components of the renin-angiotensin system, including angiotensin converting enzyme, are increased in the hypothalamic paraventricular nucleus of many animal models of hypertension19,20. We found significantly higher levels of angiotensin converting enzyme in the paraventricular nucleus of hypertensive rats compared to controls, and lower levels of angiotensin converting enzyme in hypertensive rats treated with pomegranate extract (Fig. 3A,B). These results suggest that pomegranate extract is effective in modulating the renin-angiotensin system in the paraventricular nucleus to relieve hypertension.
Figure 3. Effects of pomegranate extract supplementation on angiotensin converting enzyme expression in the hypothalamic paraventricular nucleus of control and hypertensive rats.
Control and hypertensive rats were administered saline or pomegranate extract (150 mg/kg/day) for eight weeks, sacrificed, and frozen sections were prepared from the paraventricular nucleus. (A) Immunofluorescence of angiotensin converting enzyme-positive neurons (green fluorescence) in coronal sections of the paraventricular nucleus. (B) Statistical analysis of neurons that were positive for angiotensin converting enzyme expression. Values are the mean ± SEM from 8 animals. *p < 0.05, **p < 0.01. SHR, spontaneously hypertensive rats; PE, pomegranate extract; ACE, Angiotensin Converting Enzyme; PVN, paraventricular nucleus.
Pomegranate extract elevates the antioxidant defense system in the paraventricular nucleus of hypertensive rats
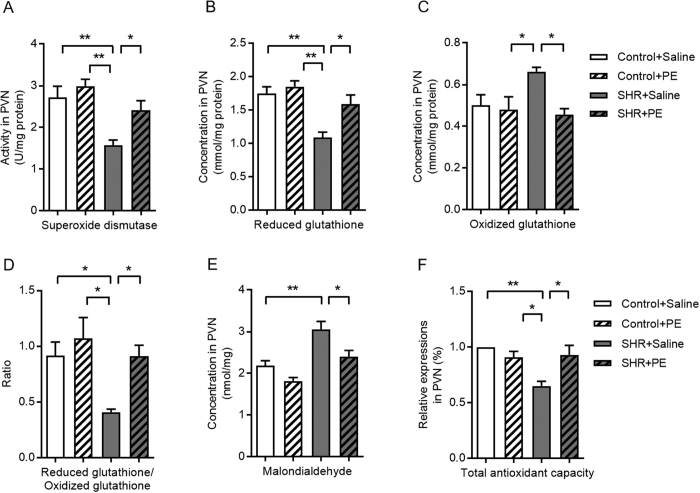
Since we found that pomegranate extract reduces total levels of reactive oxygen species in hypertensive rats, and lack of antioxidant protection is known to increase formation of reactive oxygen species21,22, we next determined if pomegranate extract affects the antioxidant defense system in the context of hypertension. We measured both enzymatic and nonenzymatic antioxidant levels in the paraventricular nucleus of pomegranate extract-treated hypertensive rats and controls based on individual antioxidant proteins, malondialdehyde, an indicator of lipid oxidation, and total antioxidant capacity, an assay that detects a panel of antioxidant enzymes. Hypertensive rats had significantly more oxidative stress in the paraventricular nucleus based on lower levels of superoxide dismutase (Fig. 4A) and reduced glutathione (Fig. 4B), higher levels of oxidized glutathione (Fig. 4C), a reduced ratio of reduced glutathione to oxidized glutathione (Fig. 4D), increased malondialdehyde (Fig. 4E) and reduced total antioxidant capacity (Fig. 4F) compared to controls, clearly demonstrating that hypertension significantly increases oxidative stress in the paraventricular nucleus. All of these indicators of oxidative stress were significantly reversed by pomegranate extract treatment of hypertensive rats (Fig. 4A–F), suggesting that pomegranate extract elevates the antioxidant defense system to protect the paraventricular nucleus from oxidative stress.
Figure 4. Effects of pomegranate extract supplementation on antioxidant capacity in the hypothalamic paraventricular nucleus of control and hypertensive rats.
Control and hypertensive rats were administered either saline or pomegranate extract (150 mg/kg/day) for eight weeks, sacrificed, and homogenates were prepared from the paraventricular nucleus. (A) Superoxide dismutase activity, (B) reduced glutathione level, (C) oxidized glutathione level, (D) the ratio of reduced glutathione/oxidized glutathione, (E) lipid peroxidation malondialdehyde concentration, and (F) total antioxidant capacities were calculated using commercial kits. Values are the mean ± SEM from 8 animals. *p < 0.05, **p < 0.01. SHR, spontaneously hypertensive rats; PE, pomegranate extract; PVN, paraventricular nucleus.
Pomegranate extract reduces inflammation in hypertensive rats
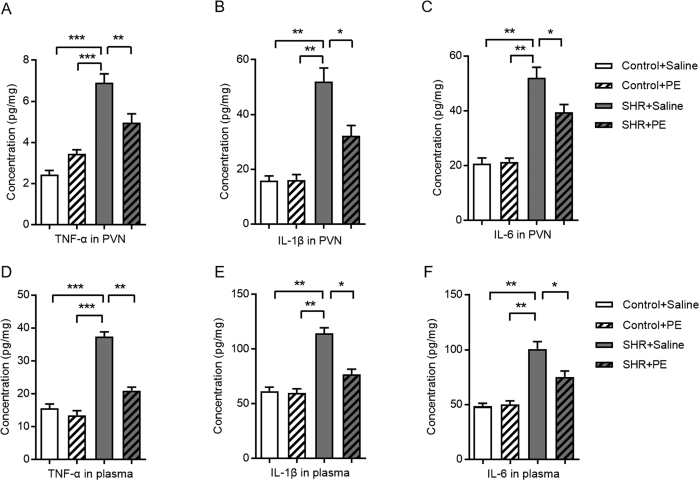
Since oxidative stress is a known mediator of inflammation23, we next investigated inflammation in the context of pomegranate extract-mediated reduction of oxidative stress in hypertension. We detected significantly increased levels of pro-inflammatory cytokines, including TNF-α (Fig. 5A), IL-1β (Fig. 5B) and IL-6 (Fig. 5C), in the paraventricular nucleus of hypertensive rats versus controls based on ELISA. After pomegranate extract treatment, however, IL-1β, TNF-α and IL-6 were significantly decreased in the paraventricular nucleus of hypertensive rats (Fig. 5A–C). We observed similar results in plasma from pomegranate extract-treated hypertensive rats and controls (Fig. 5D–F). These data indicate that pomegranate extract effectively reduces hypertension-induced inflammation.
Figure 5. Effects of pomegranate extract supplementation on paraventricular nucleus and plasma inflammatory cytokines of control and hypertensive rats.
Control and hypertensive rats were administered either saline or pomegranate extract (150 mg/kg/day) for eight weeks, sacrificed, tissue homogenates were prepared from the paraventricular nucleus, and plasma was collected. Pro-inflammatory cytokines (A) TNF-α, (B) IL-1β, and (C) IL-6 were measured in the paraventricular nucleus with commercial kits. (D) TNF-α, (E) IL-1β, and (F) IL-6 were measured in the plasma with commercial kits. Values are the mean ± SEM from 8 animals. *p < 0.05, **p < 0.01, ***p < 0.001. SHR, spontaneously hypertensive rats; PE, pomegranate extract; PVN, paraventricular nucleus.
Pomegranate extract improves mitochondrial function in the paraventricular nucleus of hypertensive rats
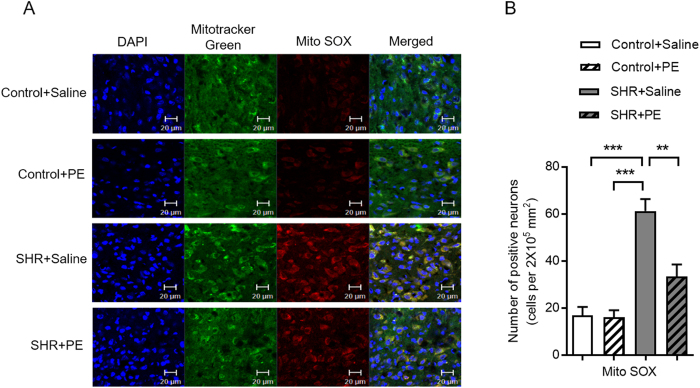
Since oxidative stress is known to impair mitochondrial function24,25, we investigated mitochondrial function in the paraventricular nucleus of hypertensive rats. We first assessed superoxide levels in mitochondria of the hypothalamic paraventricular nucleus in rat brain slices using MitoTracker Green, a fluorescent dye that localizes to mitochondria, combined with MitoSOX Red, a fluorescent dye that detects mitochondrial superoxide. We found significantly higher levels of mitochondrial superoxide anion in the hypertensive rats compared to controls, which was reduced by pomegranate extract (Fig. 6A,B). Reduction of mitochondrial superoxide anion in hypertensive rats suggested to us that mitochondrial function may improve upon pomegranate extract treatment.
Figure 6. Effects of pomegranate extract supplementation on mitochondrial superoxide in the hypothalamic paraventricular nucleus of control and hypertensive rats.
Control and hypertensive rats were administered either saline or pomegranate extract (150 mg/kg/day) for eight weeks, sacrificed, and frozen sections were prepared from the paraventricular nucleus. (A) Mitochondrial superoxide (red fluorescence) was measured using the fluorescent probe Mito SOX, which was detected with confocal microscopy. Mitotracker green was used to localize mitochondria. (B) Statistical analysis of the number of mitochondrial superoxide-positive neurons in control and hypertensive rats. Values are the mean ± SEM from 8 animals. **p < 0.01, ***p < 0.001. SHR, spontaneously hypertensive rats; PE, pomegranate extract.
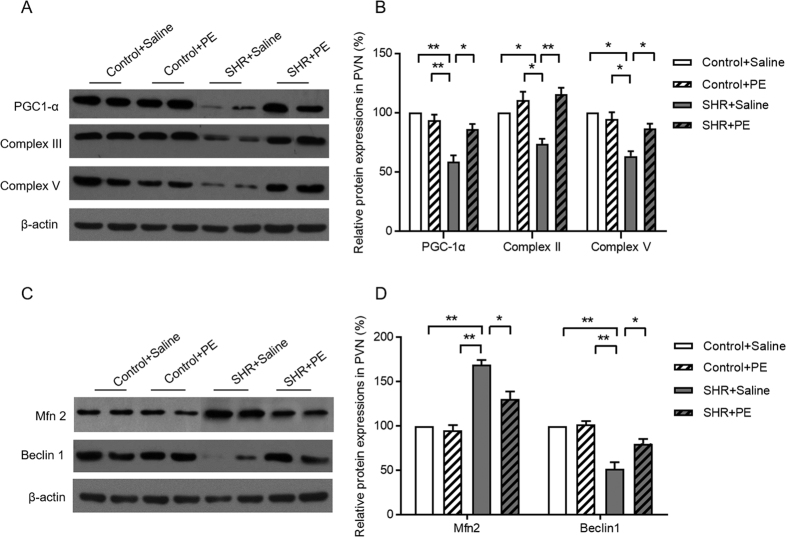
We next investigated mitochondrial biogenesis, a strong indicator of mitochondrial function26, in the context of hypertension. Compared to controls, hypertensive rats had significantly lower levels of proteins that are critical for mitochondrial biogenesis, including proliferator-activated receptor gamma co-activator (PGC-1α) and mitochondrial respiratory chain Complex III and Complex V (Fig. 7A,B). Pomegranate extract treatment increased levels of PGC-1α, Complex III and Complex V in hypertensive rats, suggesting that pomegranate extract alleviates hypertension-induced reduction of mitochondrial biogenesis (Fig. 7A,B).
Figure 7. Effects of pomegranate extract supplementation on the expression of mitochondrial related proteins in the hypothalamic paraventricular nucleus of control and hypertensive rats.
Control and hypertensive rats were administered either saline or pomegranate extract (150 mg/kg/day) for eight weeks, sacrificed, and lysates were prepared from the paraventricular nucleus for Western blotting. (A,B) Expression levels are shown for the mitochondrial biogenesis proteins PGC-1α, Complex III and V subunits (A, Western blotting image; B, statistical analysis). (C,D) Expression levels are shown for the mitochondrial dynamics proteins, Mfn2 and Beclin1 (C, Western blotting image; D, statistical analysis). Values are the mean ± SEM from 8 animals. *p < 0.05, **p < 0.01. SHR, spontaneously hypertensive rats; PE, pomegranate extract; PVN, paraventricular nucleus; PGC-1α, proliferator-activated receptor gamma co-activator.
A balance between mitochondrial fission and fusion, or “mitochondrial dynamics”, is required for proper mitochondrial function. To determine if hypertension alters mitochondrial dynamics, we measured levels of Mfn2, a mitochondrial membrane protein that promotes mitochondrial fusion and contributes to maintenance and operation of the mitochondrial network27, in hypertensive rats. We found that Mfn2 levels were increased in the paraventricular nucleus of hypertensive rats compared to controls, indicating that hypertension promotes mitochondrial fusion. Treatment of hypertensive rats with pomegranate extract significantly decreased Mfn2 levels (Fig. 7C,D), suggesting that pomegranate extract improves mitochondrial dynamics.
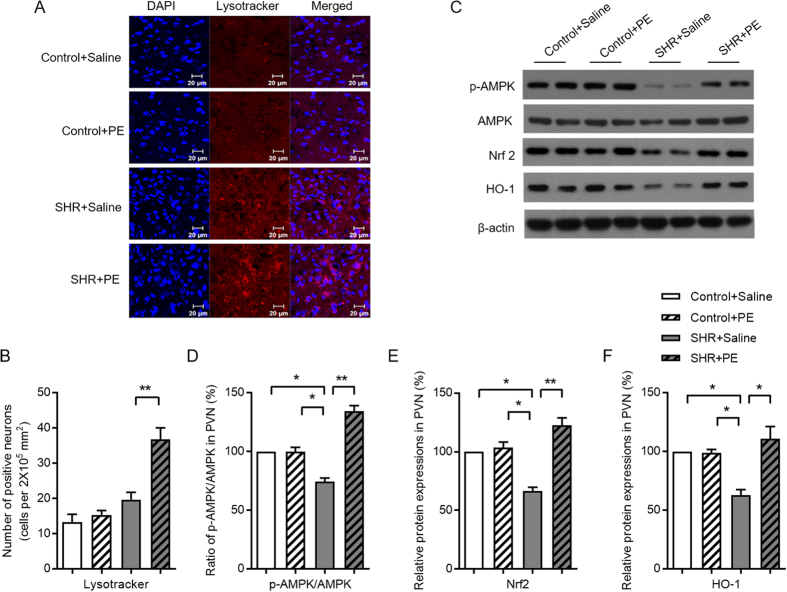
As a final indicator of mitochondrial function, we determined how pomegranate extract effects mitophagy, the process of removing impaired or abnormal mitochondria from the cell, in hypertensive rats. We found that levels of Beclin1, a key regulator of mitophagy, were reduced in the paraventricular nucleus of hypertensive rats, but were restored upon pomegranate extract treatment, suggesting that pomegranate extract promotes the clearance of dysfunctional mitochondria in the context of hypertension (Fig. 7C,D). We also found a significant increase in Lysotracker Red, an additional indicator of mitophagy, in hypertensive rats treated with pomegranate extract (Fig. 8A,B). Taken together, our data suggest that hypertension impairs mitochondrial function and clearance in the paraventricular nucleus, and that pomegranate extract alleviates hypertension-induced mitochondrial dysfunction and clearance.
Figure 8. Effects of pomegranate extract supplementation on mitophagy and the expression of AMPK-Nrf2 pathway components in the hypothalamic paraventricular nucleus of control and hypertensive rats.
(A) Mitophagy (red fluorescence) was measured using the fluorescent probe Lysotracker, which was detected with confocal microscopy. (B) Statistical analysis of the number of the mitophagy-positive neurons. (C,D,E,F) The protein expression levels of AMPK, p-AMPK, Nrf2 and hemeoxygenase were analyzed by Western blotting (B, Western blotting image; C,D,E, statistical analysis). Values are the mean ± SEM from 8 animals. **p < 0.01, ***p < 0.001. SHR, spontaneously hypertensive rats; PE, pomegranate extract; PVN, paraventricular nucleus; Nrf2, nuclear factor-erythroid 2 p45-related factor 2; HO-1, hemeoxygenase.
Pomegranate extract acts via the AMPK-Nrf2 pathway to reduce oxidative stress in the paraventricular nucleus of hypertensive rats
We next determined the mechanism whereby pomegranate extract reduces oxidative stress and improves mitochondrial function in the paraventricular nucleus of hypertensive rats. AMPK is a phosphorylation-dependent metabolic master switch that controls mitochondrial biogenesis28. Phosphorylated AMPK activates Nrf2, which promotes expression of antioxidant proteins, including hemeoxygenase, that protect against oxidative damage triggered by injury and inflammation29. We found significantly lower levels of p-AMPK in the paraventricular nucleus of hypertensive rats, which was reversed after pomegranate extract treatment (Fig. 8C,D). Likewise, Nrf2 and hemeoxygenase protein levels increased in response to pomegranate extract treatment of hypertensive rats (Fig. 8C,E,F). These data suggest that pomegranate extract-induced reduction in oxidative stress and improvement of mitochondrial function in the paraventricular nucleus of hypertensive rats is mediated by AMPK phosphorylation and downstream Nrf2/hemeoxygenase signaling.
Discussion
In this study, we have investigated the mechanism of action of pomegranate extract-mediated alleviation of hypertension. Using a spontaneously hypertensive rat model, we have found that pomegranate extract activates AMPK in the paraventricular nucleus of the hypothalamus, which stimulates Nrf2/hemeoxyganse signaling, causing scavenging of free radicals and reduction of oxidative stress, which improves mitochondrial function. In addition to lowering hypertension-induced oxidative stress, we found that pomegranate extract decreases chronic sympathetic activity in the paraventricular nucleus. Hypertension-related sympathetic overdrive in the hypothalamic paraventricular nucleus is thought to result from high levels of oxidative stress6, and clearance of free superoxide radicals in the paraventricular nucleus lowers excessive sympathetic activity and arterial blood pressure in hypertensive rats30. We thus propose that pomegranate extract-induced reduction of sympathetic overdrive in hypertensive rats is due to the effects of pomegranate extract as an antioxidant.
Oxidative stress in the hypothalamic paraventricular nucleus is also associated with the production of pro-inflammatory cytokines31, and reducing hypertension in rats is known to decrease pro-inflammatory cytokine levels in plasma32. Furthermore, activation of NF-κB, a pro-inflammatory signaling protein, in the paraventricular nucleus increases oxidative stress and excess sympathetic excitation, while inhibition of NF-κB reduces both pro-inflammatory cytokine levels and oxidative stress, and alleviates hypertension9. In our study, pomegranate extract treatment decreased pro-inflammatory cytokines in hypertensive rats, suggesting that an imbalance between pro- and anti-inflammatory cytokines in the paraventricular nucleus could contribute to hypertension-induced sympathetic excitation.
Impaired mitochondrial function increases oxidative stress, which causes organ damage in hypertension. Antioxidants provide promising strategies to decrease hypertension-mediated mitochondrial dysfunction and organ damage. In our current study, chronic pomegranate extract administration significantly reduced production of mitochondrial superoxide anion in hypertensive rats. Furthermore, pomegranate extract significantly improved mitochondrial function in hypertensive rats based on assays measuring mitochondrial biogenesis (PGC-1α, Complex III and Complex V), mitochondrial dynamics (fusion-related protein Mfn2), and mitochondrial clearance (mitophagy-related protein Beclin1).
We then investigated the AMPK-Nrf2 pathway, a known sensor of metabolic stress, as a mechanism whereby pomegranate extract decreases oxidative stress in the context of hypertension. Oxidative stress causes phosphorylation of AMPK29, which activates Nrf2, which increases hemeoxygenase, a potent antioxidant enzyme29. The spontaneously hypertensive rats are associated with impaired Nrf2 signaling, inflammation, and oxidative stress, which can be restored by administration of resveratrol33. Impaired Nrf-2-regulated mitochondrial biogenesis in rostral ventrolateral medulla contributes to hypertension under systemic inflammation34. Hemeoxygenase is a known target of punicalagin, and punicalagin-induced hemeoxygenase expression is dramatically blocked after specific inhibition of Nrf235. We found that hypertensive rats treated with pomegranate extract had increased levels of phosphorylated AMPK, Nrf2 and hemeoxygenase protein compared to controls, suggesting that pomegranate extract acts through the AMPK-Nrf2 pathway to reduce hypertension-induced oxidative stress and downstream mitochondrial dysfunction.
Punicaligin is the main antioxidant compound in pomegranate peels and has been the topic of many studies14. Punicalagin has a neuroprotective effect in ischemia-induced brain injury, where it decreases both malondialdehyde and mitochondria-generated reactive oxygen species, and increases enzymes that protect against oxidative stress12. We have previously shown that punicalagin promotes Nrf2 protein expression and nuclear translocation followed by induction of antioxidative enzymes including hemeoxygenas36. Of the polyphenols found in pomegranates, punicalagin is the most abundant ellagitannin, a class of proteins with known antioxidant properties. We have previously shown that pomegranate extract gavage causes a time-dependent increase in serum punicalagin levels, suggesting that punicalagin circulates in the body to exert its beneficial effects. We have previously shown that pomegranate extract and exercise have an additive effect in improving immune function in high-fat-diet fed rats by inhibiting inflammatory cytokine secretion and decreasing oxidative stress37. As in the previous study of our group16,36,38, we used pomegranate extract containing 40% punicalagin and showed that punicalagin has been identified as the major active component. While we did not test pure punicaligin directly in the present study, our data are consistent with punicalagin as the primary active component in pomegranate extract that acts to decrease oxidative stress in the paraventricular nucleus of hypertensive rats. Future experiments will be carried out with the pure punicalagin in our system. Additionally, another possible mechanism, the regulating effect on mitophagy by pomegranaate metabolite Urolithin A, should be further studies39.
In conclusion, these results obtained, together with previous studies of our group16,36,38,40,41,42, may suggest that pomegranate extract alleviates symptoms of hypertension by reducing oxidative stress, improving mitochondrial function, and decreasing chronic activation of the sympathetic nervous system in the paraventricular nucleus, and have identified the AMPK-Nrf2 pathway as a mechanistic link between pomegranate extract-induced reduction of oxidative stress. Our studies thus reveal novel insights into the mechanism of action of pomegranate extract as an antihypertensive therapy, and identify additional new targets for the therapeutic treatment of hypertension.
Materials and Methods
Experimental design
Ten-week-old male spontaneously hypertensive rats and Wistar-Kyoto rats (used as the control of spontaneously hypertensive rats) were purchased from Charles River Laboratory Animal, Ltd. (Beijing, China). Wistar-Kyoto control rats were administered pomegranate extracts or normal saline; spontaneously hypertensive rats were administered pomegranate extracts or normal saline. The rats were housed in individual standard cages on a 12 hour light/12 hour dark cycle in a temperature-controlled (21 ± 2.0 °C) environment for a week of acclimatization, with ad libitum access to water and a commercial diet. After adapting to the environment for one week, the rats were administered pomegranate extracts (purchased from Tianjing Jianfeng Natural Product R&D Co. Ltd., China, containing 40% punicalagin, at a dose of 150 mg/kg/day, diluted into 2 ml of saline) or an equal volume of saline by gavage for eight weeks. The growing area of pomegranate is in Shaanxi province of China. The main composition are polyphenol and punicalagin. The extract preparation method was mainly column chromatography. The animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication No. 85-23, revised 1996) and were approved by the Institutional Animal Care and Use Committees of Xi’an Jiaotong University.
At the end of the eight weeks, all rats were weighed and intraperitoneally injected with ketamine (80 mg/kg) and acepromazine (10 mg/kg) as anesthesia. Blood was sampled from the carotid artery. The brain was removed and fixed in formaldehyde solution for one week, deposited in 30% sucrose solution for 2–3 days, and then thin-sectioned for use in further experiments. Another portion of rats were sacrificed, and the paraventricular nucleus of the hypothalamus was rapidly dissected from fresh brain and stored in a tube at −80 °C.
Blood pressure measurement
Blood pressure and heart rate were measured each week at 8:00–10:00 am in conscious rats using a noninvasive tail-cuff system (NIBP, AD Instruments, Australia). The temperature of the incubator was adjusted to 36 °C with floor heating. The blocker and sensor were successively set on the tail of awake rats in the incubator. Rats were allowed to calm, at which time the cuff pressure was increased for blood pressure measurement. The blocker deflates and decompresses until there is a systolic blood pressure pulse, which was recorded as the systolic blood pressure. The pressure continues to decrease, and there is a peak, which was recorded as the diastolic blood pressure. The mean arterial pressure was calculated as diastolic blood pressure+(systolic blood pressure-diastolic blood pressure)/3, and the final value was the mean value of five measurements.
Microscopic anatomy of the hypothalamic paraventricular nucleus
Palkovits’ micro-dissection procedure was employed to isolate the paraventricular nucleus from rat brains. Briefly, the brain was serially sectioned into 300 mm slices, from the bregma to lambda, with a cryostat. The sections were transferred to coverslips and placed on a cooling stage maintained at −80 °C. The paraventricular nucleus of the hypothalamus was punched with the help of a stereotaxic atlas. The micro-dissected paraventricular nuclei were then stored at −80 °C until future analysis.
Fluorescence staining
Preparation of the paraventricular nucleus slice
The frozen paraventricular nucleus slice was equilibrated for 10 min at room temperature and permeated for 30 min with 0.3% Triton x-100 at 37 °C. The slice was then washed with PBS three times for 5 min.
Detection of angiotensin-converting enzyme or superoxide anion
The prepared paraventricular nucleus slice was incubated with dihydroethidium (diluted to 1:200, Santa Cruz) for 20 min at 37 °C. After washing with PBS three times, 5 min each time, the slice was mounted and observed with a confocal laser scanning microscope (Zeiss 510, Germany).
Detection of the expression of Fra-Like or angiotensin-converting enzyme
The prepared paraventricular nucleus slice was incubated with primary antibody (anti-Fra-Like or anti-angiotensin converting enzyme, diluted to 1:200, Santa Cruz) overnight at 4 °C. The slice was washed three times with PBS, 5 min each time, and was then incubated with FITC-conjugated secondary antibody (diluted to 1:200, Santa Cruz) for 1 h at 37 °C. After washing, the slice was observed with a confocal laser scanning microscope.
Detection of mitochondrial superoxide anion
The prepared paraventricular nucleus slice was incubated with 50 nM MitoTracker Green and then 1 μM Mito SOX (Invitrogen, Molecular Probes, Carlsbad, CA) for 20 min. The slice was washed three times with PBS, 5 min each time. DAPI was applied to stain cell nuclei (diluted to 1:300, Invitrogen). After washing, the slice was observed with a confocal laser scanning microscope.
Antioxidant capacity detection
The paraventricular nucleus was homogenized, and the indicators for oxidative stress and antioxidant capacity in paraventricular nucleus homogenate were analyzed, including superoxide dismutase, reduced glutathione, oxidized glutathione, the ratio of reduced glutathione/oxidized glutathione, lipid peroxidation malonaldehyde, and total antioxidant capacity with commercial kits (Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the manufacturer’s instructions.
Western blotting
The paraventricular nucleus was lysed with IP lysis buffer (Beyotime, Nanjing, China) and then homogenized. The protein in the supernatant was quantified with a BCA assay kit (Pierce, Rockford, IL, USA), which was performed according to the manufacturer’s instructions. Equal amounts of protein were denatured and then analyzed with polyacrylamide gel electrophoresis. The separated proteins were transferred to a PVDF membrane. The membrane was blocked with plasma. Primary antibodies included anti-PGC-1α, anti-Mfn2, anti-Nrf2, anti-hemeoxygenase, anti-β-actin (Santa Cruz), anti-OxPhos Complex III or anti-Complex V (Invitrogen), anti-Beclin1, anti- AMPK, and anti-p-AMPK (Thr 172, Cell Signaling Technology, Beverly, MA). Primary antibodies were added and incubated with the membrane. After washing, the membrane was incubated with secondary antibody, and signals were observed by chemiluminescence. The result was photographed and analyzed with NIH Image J analysis software.
ELISA for cytokine assessment
The levels of IL-1β, TNF-α and IL-6 in the extracted tissue and plasma were measured with a commercially available enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The optical density of each well was determined using a microplate reader (Bio-Rad, Hercules, CA).
Statistical analysis
The values are presented as the mean ± SEM. The data were analyzed using a Student’s t-test for two-group comparisons or a one-way ANOVA followed by the Newman–Keuls correction for multiple comparisons. Blood pressure data were analyzed by repeated measures ANOVA. GraphPad Prism 5.0 statistical and graphing software was used for the statistical analyses. P values < 0.05 were considered statistically significant.
Additional Information
How to cite this article: Sun, W. et al. Pomegranate extract decreases oxidative stress and alleviates mitochondrial impairment by activating AMPK-Nrf2 in hypothalamic paraventricular nucleus of spontaneously hypertensive rats. Sci. Rep. 6, 34246; doi: 10.1038/srep34246 (2016).
Acknowledgments
This work was supported by the Major State Basic Research Development Program (2015CB856302), China Postdoctoral Science Foundation (No. 2015M582639), the Fundamental Research Funds for the Central Universities, National Basic Research Program of China (No. 2012CB517805) and National Natural Science Foundation of China (Nos 91439120, 81370356 and 81573104).
Footnotes
Author Contributions J.K.L., Y.M.K., W.Y.S. and C.H.Y. planned the experiments. W.Y.S., C.H.Y., X.W. and C.H. performed the experiments. W.Y.S., M.Q.Z., H.L.G. and B.F. analyzed the results and prepared the manuscript. All authors reviewed the manuscript.
References
- Lopez S. et al. Virgin olive oil and hypertension. Curr Vasc Pharmacol 14, 323–329 (2016). [DOI] [PubMed] [Google Scholar]
- Allison M. A. et al. A randomized trial of a low-fat diet intervention on blood pressure and hypertension: tertiary analysis of the WHI dietary modification trial. Am J Hypertens 29, 959–968 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- White W. B. et al. Detection, evaluation, and treatment of severe and resistant hypertension: proceedings from an American Society of Hypertension Interactive forum held in Bethesda, MD, USA, October 10th 2013. J Am Soc Hypertens 8, 743–757 (2014). [DOI] [PubMed] [Google Scholar]
- Huang W. Y., Davidge S. T. & Wu J. Bioactive natural constituents from food sources-potential use in hypertension prevention and treatment. Crit Rev Food Sci Nutr 53, 615–630 (2013). [DOI] [PubMed] [Google Scholar]
- Houston M. C. The role of nutrition, nutraceuticals, vitamins, antioxidants, and minerals in the prevention and treatment of hypertension. Altern Ther Health Med 19, 32–49 (2013). [PubMed] [Google Scholar]
- Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol 28, 95–99 (2001). [DOI] [PubMed] [Google Scholar]
- Goncharuk V. D., Van Heerikhuize J., Swaab D. F. & Buijs R. M. Paraventricular nucleus of the human hypothalamus in primary hypertension: activation of corticotropin-releasing hormone neurons. J Comp Neurol 443, 321–331 (2002). [DOI] [PubMed] [Google Scholar]
- Nishihara M., Hirooka Y., Kishi T. & Sunagawa K. Different role of oxidative stress in paraventricular nucleus and rostral ventrolateral medulla in cardiovascular regulation in awake spontaneously hypertensive rats. J Hypertens 30, 1758–1765 (2012). [DOI] [PubMed] [Google Scholar]
- Song X. A. et al. Inhibition of TNF-alpha in hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy by inhibiting neurohormonal excitation in spontaneously hypertensive rats. Toxicol Appl Pharmacol 281, 101–108 (2014). [DOI] [PubMed] [Google Scholar]
- Su Q. et al. Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicol Appl Pharmacol 276, 115–120 (2014). [DOI] [PubMed] [Google Scholar]
- Lin C. C., Hsu Y. F., Lin T. C. & Hsu H. Y. Antioxidant and hepatoprotective effects of punicalagin and punicalin on acetaminophen-induced liver damage in rats. Phytother Res 15, 206–212 (2001). [DOI] [PubMed] [Google Scholar]
- Yaidikar L., Byna B. & Thakur S. R. Neuroprotective effect of punicalagin against cerebral ischemia reperfusion-induced oxidative brain injury in rats. J Stroke Cerebrovasc Dis 23, 2869–2878 (2014). [DOI] [PubMed] [Google Scholar]
- Aqil F. et al. Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res Int 49, 345–353 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Les F., Prieto J. M., Arbones-Mainar J. M., Valero M. S. & Lopez V. Bioactive properties of commercialised pomegranate (Punica granatum) juice: antioxidant, antiproliferative and enzyme inhibiting activities. Food Funct 6, 2049–2057 (2015). [DOI] [PubMed] [Google Scholar]
- Chen B. et al. Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts. Am J Physiol Endocrinol Metab 302, E1142–E1152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X. et al. Mitochondrial dysfunction in obesity-associated nonalcoholic fatty liver disease: the protective effects of pomegranate with its active component punicalagin. Antioxid Redox Signal 21, 1557–1570 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. T. et al. Effects of pomegranate extract supplementation on cardiovascular risk factors and physical function in hemodialysis patients. J Med Food 18, 941–949 (2015). [DOI] [PubMed] [Google Scholar]
- Ding L. et al. Superoxide anions in paraventricular nucleus modulate adipose afferent reflex and sympathetic activity in rats. PLoS One 8, e83771, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Zhang Z. H., Wei S. G., Weiss R. M. & Felder R. B. Peroxisome proliferator-activated receptor-gamma regulates inflammation and renin-angiotensin system activity in the hypothalamic paraventricular nucleus and ameliorates peripheral manifestations of heart failure. Hypertension 59, 477–484 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. M. et al. Chronic infusion of enalaprilat into hypothalamic paraventricular nucleus attenuates angiotensin II-induced hypertension and cardiac hypertrophy by restoring neurotransmitters and cytokines. Toxicol Appl Pharmacol 274, 436–444 (2014). [DOI] [PubMed] [Google Scholar]
- Poljsak B., Suput D. & Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013, 956792 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahal A. et al. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int 2014, 761264 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal M., Siddiqui M. R., Tran K., Reddy S. P. & Malik A. B. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20, 1126–1167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyakhovich A. & Graifer D. Mitochondria-mediated oxidative stress: old target for new drugs. Curr Med Chem 22, 3040–3053 (2015). [DOI] [PubMed] [Google Scholar]
- Ahadpour M. et al. Mitochondrial oxidative stress and dysfunction induced by isoniazid: study on isolated rat liver and brain mitochondria. Drug Chem Toxicol 39, 224–232 (2016). [DOI] [PubMed] [Google Scholar]
- Rubattu S. et al. Pathogenesis of target organ damage in hypertension: role of mitochondrial oxidative stress. Int J Mol Sci 16, 823–839 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. et al. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160, 189–200, (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanli T., Steinberg G. R., Singh G. & Tsakiridis T. AMP-activated protein kinase (AMPK) beyond metabolism: a novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol Ther 15, 156–169 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo C. et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal 20, 574–588 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan N. et al. SOD1 gene transfer into paraventricular nucleus attenuates hypertension and sympathetic activity in spontaneously hypertensive rats. Pflugers Arch 465, 261–270 (2013). [DOI] [PubMed] [Google Scholar]
- Yu X. J. et al. Inhibition of NF-kappaB activity in the hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy by modulating cytokines and attenuating oxidative stress. Toxicol Appl Pharmacol 284, 315–322 (2015). [DOI] [PubMed] [Google Scholar]
- Jia L. L. et al. Exercise training attenuates hypertension and cardiac hypertrophy by modulating neurotransmitters and cytokines in hypothalamic paraventricular nucleus. PLoS One 9, e85481 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javkhedkar A. A. et al. Resveratrol restored Nrf2 function, reduced renal inflammation, and mitigated hypertension in spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 308, R840–R846 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K. L., Wu C. W., Chao Y. M., Hung C. Y. & Chan J. Y. Impaired Nrf2 regulation of mitochondrial biogenesis in rostral ventrolateral medulla on hypertension induced by systemic inflammation. Free Radic Biol Med 97, 58–74 (2016). [DOI] [PubMed] [Google Scholar]
- Xu X. et al. Punicalagin induces Nrf2/HO-1 expression via upregulation of PI3K/AKT pathway and inhibits LPS-induced oxidative stress in RAW264.7 macrophages. Mediators Inflamm 2015, 380218 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C. et al. Punicalagin attenuates palmitate-induced lipotoxicity in HepG2 cells by activating the Keap1-Nrf2 antioxidant defense system. Mol Nutr Food Res 60, 1139–1149 (2016). [DOI] [PubMed] [Google Scholar]
- Zhao F. et al. Pomegranate extract and exercise provide additive benefits on improvement of immune function by inhibiting inflammation and oxidative stress in high-fat-diet-induced obesity rats. J Nutr Biochem 32, 20–28 (2016). [DOI] [PubMed] [Google Scholar]
- Cao K. et al. Punicalagin, an active component in pomegranate, ameliorates cardiac mitochondrial impairment in obese rats via AMPK activation. Sci Rep 5, 14014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu D. et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med 22, 879–888 (2016). [DOI] [PubMed] [Google Scholar]
- Zheng A. et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: role of AMP-activated protein kinase activation. Br J Nutr 113, 1667–1676 (2015). [DOI] [PubMed] [Google Scholar]
- Zheng A. et al. Maternal hydroxytyrosol administration improves neurogenesis and cognitive function in prenatally stressed offspring. J Nutr Biochem 26, 190–199 (2015). [DOI] [PubMed] [Google Scholar]
- Cao K. et al. AMPK activation prevents prenatal stress-induced cognitive impairment: modulation of mitochondrial content and oxidative stress. Free Radic Biol Med 75, 156–166 (2014). [DOI] [PubMed] [Google Scholar]