Abstract
Leptospirosis is recognized as the most widespread zoonosis with a global distribution. In this study, the antigenic variation in Leptospira interrogans and Leptospira borgpetersenii isolated from human urine and field rat kidney was preliminarily confirmed by microscopic agglutination test using monoclonal antibodies, and was further subjected to amplification and identification of outer membrane lipoproteins with structural gene variation. Sequence similarity analysis revealed that these protein sequences, namely OmpL1, LipL32 and LipL41, showed no more homologies to outer membrane lipoproteins of non-pathogenic Leptospira and other closely related Spirochetes, but showed a strong identity within L. interrogans, suggesting intra-specific phylogenetic lineages that might be originated from a common pathogenic leptospiral origin. Moreover, the ompL1 gene showed more antigenic variation than lipL32 and lipL41 due to less conservation in secondary structural evolution within closely related species. Phylogenetically, ompL1 and lipL41 of these strains gave a considerable proximity to L. weilii and L. santarosai. The ompL1 gene of L. interrogans clustered distinctly from other pathogenic and non-pathogenic leptospiral species. The diversity of ompL genes has been analyzed and it envisaged that sequence-specific variations at antigenic determinant sites would result in slow evolutionary changes along with new serovar origination within closely related species. Thus, a crucial work on effective recombinant vaccine development and engineered antibodies will hopefully meet to solve the therapeutic challenges.
Key words: Leptospira, ompL1, lipL32, lipL41, phylogeny, antigenic variation
Introduction
Leptospirosis is now recognized as the most widespread zoonosis with a global distribution, which is caused by Leptospira, a genus of spirochetal bacteria, either through direct contact of infected animals or through urine (1). An international survey of human leptospirosis conducted by World Health Organization reported that approximately 100,000 severe cases (requiring hospitalization) occur annually (2). Infection with host-adapted leptospiral serovars can result in lifelong renal carriage and urinary shedding. In humans, contact to infected host animals and contaminated water/soil results in potentially lethal disease. Although many organs could be affected, lung and kidney have been commonly identified as potential targets to be invaded by the leptospires. In obligate intracellular bacteria, the outer membrane proteins (OMPs) play a crucial role in the process of adaptation by facilitating interactions between bacterial cells and its host (3). Though 258 serovars in the genus Leptospira have been identified, they are antigenically very distinct due to the lipopolysaccharide (LPS) and spatial arrangement of outer membrane lipoproteins (OmpL), particularly OmpL1, LipL32 and LipL41. In addition, such extensive serovar diversity with distinct antigenic determinant has been attributed to the structural composition of LPS 4., 5., 6. and the genetics of LPS biosynthesis 7., 8.. Although leptospiral LPS can elicit protective immunity, this immunity is generally serovar specific. Consequently, the focus of research on protective antigens has been shifted towards the identification of conserved OMPs, which may be able to stimulate heterologous immunity.
OMP is considered to play a role in the structural integrity of the organism and is surface-exposed and glycosylated (9). The most abundant class comprises the lipoproteins designated according to their apparent molecular weights determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, including the major OMP (MOMP) and immunodominant protein antigen LipL32 10., 11., the in vivo down-regulated proteins LipL32 (10) and LipL36 (12), the surface-exposed proteins LipL41 13., 14. and LipL21 (15). Among these, OmpL1, LipL32 and LipL41 are major antigens in the humoral immune response to leptospirosis and immunology-based diagnostics 16., 17., 18..
Comparative analysis of ompL gene sequences can reveal insights into a novel mechanism of molecular evolution in pathogenic bacteria. The 16S rRNA genes are the most conserved, followed by the lipL32, lipL41 and ompL1 genes, listed in the order of increasing sequence variability. The differences in sequence variability persist when synonymous mutations are considered. However, relatively little is known about the mechanisms and molecular diversity extent of leptospiral ompL genes (11). Accordingly, an evolutionary examination of the ompL genes may enlighten the actual role of OMPs in the processes of infection, humoral immunity, and speciation of these organisms. The purpose of the present study is to conclude the phylogenetic relationships of ompL genes of some leptospiral strains isolated from human urine and rat kidney with Leptospiraceae homologous sequences in order to better realize their molecular diversity and evolution, which ultimately increases the scope of developing a broad-spectrum OMP-specific vaccine antigen for protective immunity.
Results
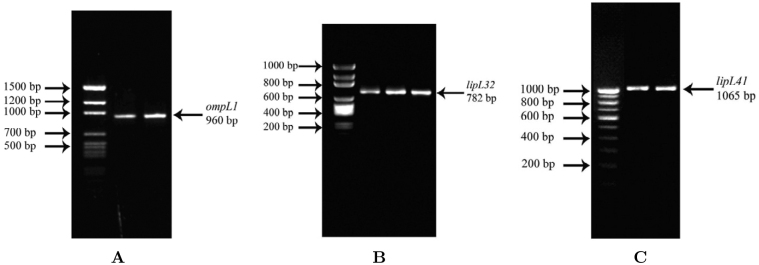
The amplified ompL1, lipL32 and lipL41 genes were viewed at 1% agarose gel and obtained in 960 bp, 782 bp and 1,065 bp, respectively (Figure 1); these products were then used for sequencing. A preliminary phylogenetic analysis of similar gene and protein sequences collected from an NCBI-BLAST search indicated that a substantial number of gene and protein sequences were identified as OmpL1, LipL32 and LipL41-like proteins, but they were widely distributed in different serovars of Leptospira interrogans, and secondly in Leptospira borgpetersenii. Gene and protein sequence similarity analysis (on the basis of e-value and identity score) implied that 96%–99% gene and 90%–95% protein sequence identities have been obtained to ompL sequences of L. interrogans and L. borgpetersenii, on which most of the sequences showed identity within L. interrogans. Similarity search tool BLASTn was used to identify similar sequences and the retrieved information is comprehensively summarized in Table 1, Table 2, Table 3. These data clearly evidenced that there is strong sequence conservation in these genes, which belongs to L. interrogans specific, but not serovar specific. Only a few sequence substitutions including transition or transversion could result in such close variation among ompL genes to bring a new serovar of the same species.
Figure 1.
Agarose gel electrophoresis of ompL genes. A. ompL1 genes of L. interrogans Grippotyphosa CH31 and L. interrogans Autumnalis N2. B. lipL32 genes of L. borgpetersenii Javanica R1R, R1L and L. interrogans Autumnalis N2. C. lipL41 genes of L. interrogans Grippotyphosa CH31 and L. interrogans Autumnalis N2.
Table 1.
BLAST search results of ompL1 genes of Leptospira used for phylogenetic analysis
| Organism | Serovar | Strain | Accession No. | Country |
|---|---|---|---|---|
| L. interrogans | Grippotyphosa | CH31 | EU091293 | India |
| L. interrogans | Autumnalis | N2 | EU091294 | India |
| L. interrogans | Lai | 017 | AF255308 | China |
| L. interrogans | Lai | 56601 | AF250318 | China |
| L. interrogans | Copenhageni | L1-130 | AY461983 | USA |
| L. interrogans | Lai | 56601 | AY461984 | USA |
| L. interrogans | Icterohaemorrhagiae | RGA | AY461985 | USA |
| L. interrogans | Lai | Lai | AY622661 | China |
| L. interrogans | Hebdomadis | P 7 | AY622665 | China |
| L. interrogans | Australis | 65-9 | AY622664 | China |
| L. interrogans | Autumnalis | Lin 4 | AY622658 | China |
| L. interrogans | Canicola | Lin | AY622662 | China |
| L. interrogans | Grippotyphosa | Lin 6 | AY622659 | China |
| L. interrogans | Wolffi | L 183 | AY622660 | China |
| L. interrogans | Pyrogens | Tian | AY622663 | China |
| L. interrogans | Paidjan | L 37 | AY622666 | China |
| L. kirschnerii | Grippotyphosa | RM52 | AY461991 | USA |
| L. nochugii | Pomona | Luo | AY622667 | China |
| L. weilii | Manhao II | L 105 | AY622668 | China |
Table 2.
BLAST search results of lipL32 genes of Leptospira used for phylogenetic analysis
| Organism | Serovar | Strain | Accession No. | Country |
|---|---|---|---|---|
| L. borgpetersenii | Javanica | R1L | EU293441 | India |
| L. borgpetersenii | Javanica | R1R | EU293442 | India |
| L. interrogans | Autumnalis | N2 | EU293443 | India |
| L. interrogans | Hardjo | – | AY442332 | India |
| L. interrogans | Wolffi | L 183 | AY609332 | China |
| L. interrogans | Paidjan | L 37 | AY609329 | China |
| L. interrogans | Canicola | Lin | AY609321 | China |
| L. interrogans | Autumnalis | Lin 4 | AY609324 | China |
| L. interrogans | Australis | 65-9 | AY609325 | China |
| L. interrogans | Grippotyphosa | Lin 6 | AY609327 | China |
| L. interrogans | Hebdomadis | P 7 | AY609328 | China |
| L. noguchii | Pomona | Luo | AY609326 | China |
| L. borgpetersenii | Mini | Nan 10 | AY609333 | China |
| L. interrogans | Pyrogenes | Tian | AY609323 | China |
| L. borgpetersenii | Tarassovi | 55-52 | AY609330 | China |
| L. borgpetersenii | Ballum | Pishu | AY609322 | China |
| L. borgpetersenii | – | M 10 | AY568680 | China |
| L. interrogans | Canicola | Hond Utrecht | AJ580493 | India |
| L. interrogans | Copenhageni | L1-130 | AF245281 | USA |
| L. weilii | Manhao II | L 105 | AY609331 | China |
Table 3.
BLAST search results of lipL41 genes of Leptospira used for phylogenetic analysis
| Organism | Serovar | Strain | Accession No. | Country |
|---|---|---|---|---|
| L. interrogans | Autumnalis | N2 | EU091295 | India |
| L. interrogans | Grippotyphosa | CH31 | EU091296 | India |
| L. interrogans | Wolffi | L 183 | AY622686 | China |
| L. weilii | Manhao II | L 105 | AY622685 | China |
| L. borgpetersenii | Tarassovi | 55-52 | AY622684 | China |
| L. interrogans | Paidjan | L 37 | AY622683 | China |
| L. interrogans | Hebdomadis | P 7 | AY622682 | China |
| L. interrogans | Grippotyphosa | Lin 6 | AY622681 | China |
| L. noguchii | Pomona | Luo | AY622680 | China |
| L. interrogans | Australis | 65-9 | AY622679 | China |
| L. interrogans | Autumnalis | Lin 4 | AY622678 | China |
| L. interrogans | Pyrogens | Tian | AY622677 | China |
| L. borgpetersenii | Ballum | Pishu | AY622676 | China |
| L. interrogans | Canicola | Lin | AY622675 | China |
| L. borgpetersenii | Javanica | M 10 | AY622674 | China |
| L. interrogans | Lai | Lai | AY622673 | China |
| L. interrogans | Canicola | – | AY642287 | India |
| L. interrogans | Hardjo | – | AY642286 | India |
| L. interrogans | Hebdomadis | Akiyami B | AB240675 | Japan |
| L. interrogans | Australis | Akiyami C | AB240676 | Japan |
| L. interrogans | Icterohaemorrhagiae | RGA | AB240677 | Japan |
| L. interrogans | Manilae | UP-MMC | AB240678 | Japan |
| L. interrogans | Autumnalis | Akiyami A | AB240674 | Japan |
Multiple sequence alignments (MSAs) have been proved to be of high quality, with few insertions or deletions. Results obtained from MSAs of selected leptospiral strains indicated that there were short ranges of sequence variation within the conserved domain of these genes, including deletion or insertion, which brought a major diversification in this family. Moreover, unlike other ompL genes of L. interrogans, L. kirschneri and L. weilii, a variation in stretch of nucleotides of these genes has highly influenced on the phylogenetic relationship of our strains. Therefore, it resulted in a separate cluster in the phylogenetic trees as shown in Figure 2, Figure 3, Figure 4. Even in the regions with gaps, the alignment showed high regions of similarity, and most of the large gaps occurred due to only a few sequences that had long insertions relative to others. We also found greater numbers of indels and a high level of dissimilarity when comparing the alignment of these genes, confirming the appropriateness of these sequences as our groups. Comparative sequence analysis of OMPs has provided information about the variability of ompL-like genes found in Leptospira, suggesting the probability of slow evolutionary changes among serotypes of even the same species that can elicit immunogenicity and host infection (Figure 5).
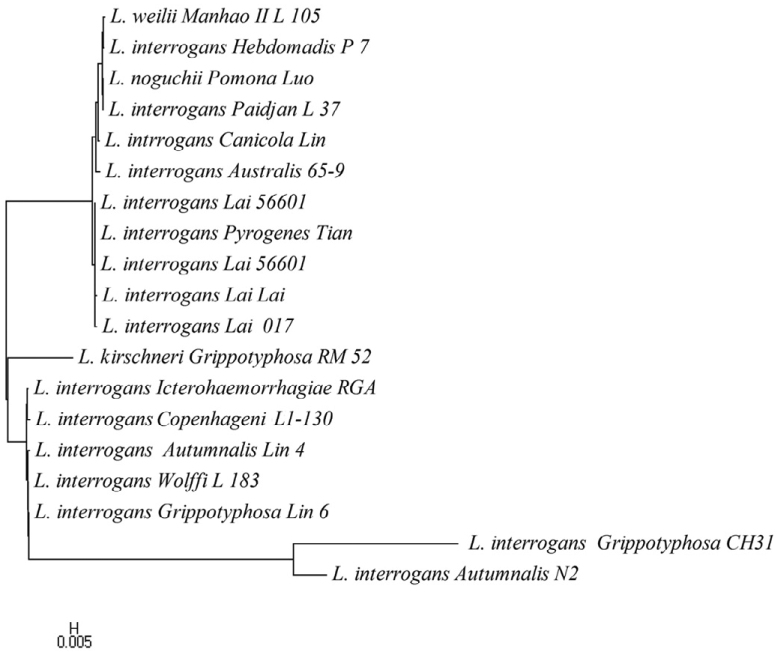
Figure 2.
Phylogenetic tree based on ompL1 nucleotide sequences of Leptospira using NJ method.
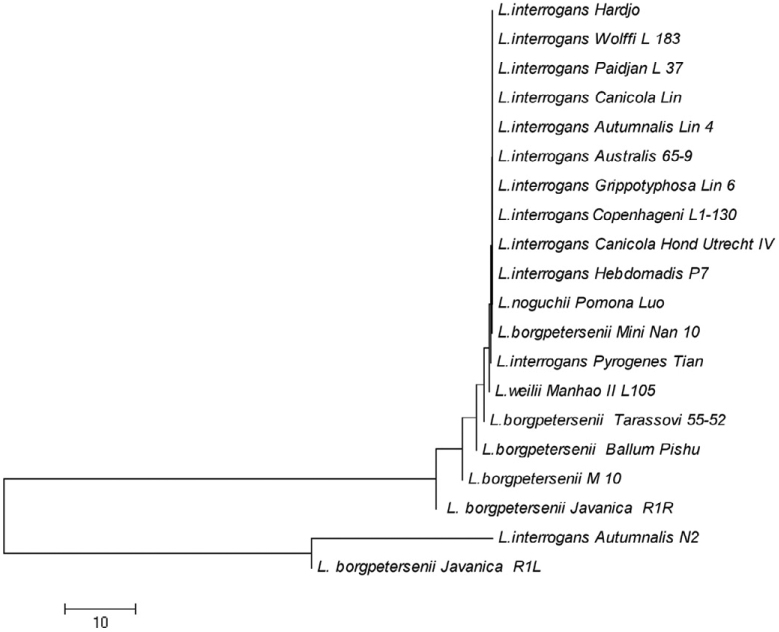
Figure 3.
Phylogenetic tree based on lipL32 nucleotide sequences of Leptospira using NJ method.
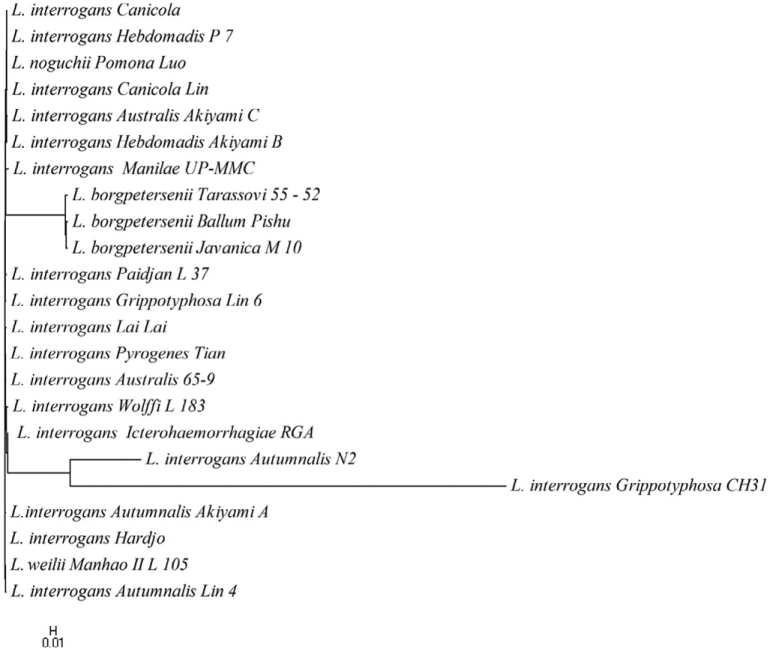
Figure 4.
Phylogenetic tree based on lipL41 nucleotide sequences of Leptospira using NJ method.
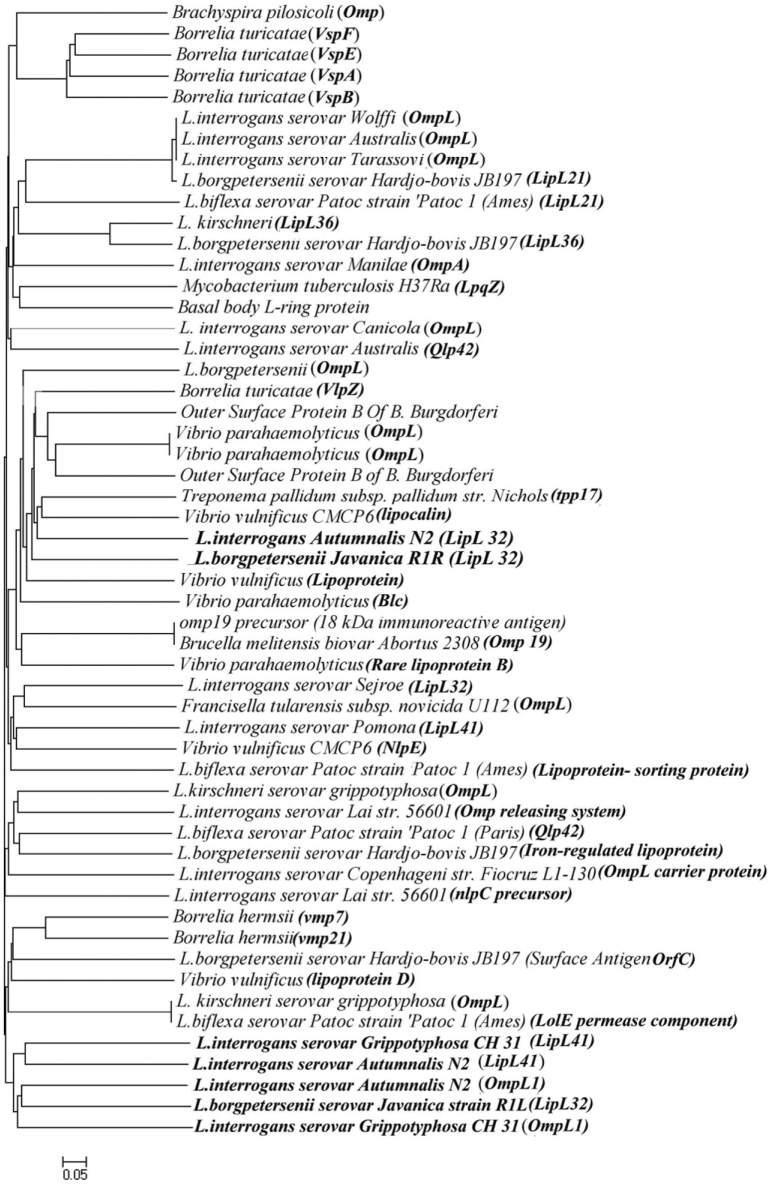
Figure 5.
Phylogenetic tree based on deduced nucleotide sequences of OMPs and OMP-related proteins retrieved from different bacterial species using NJ method.
We used a neighbor-joining (NJ) analysis and the underlying pairwise distance matrix to remove redundant sequences and to select a set of ompL representing the greatest diversity in terms of both sequence and genome diversity for phylogenetic analysis. The ompL1, lipL32 and lipL41 sequences formed monophyletic groups with posterior probabilities of 1.0, 0.9 and 0.79, respectively, and high NJ bootstrap support of 90%–95%. Both minimum evolution (ME) and NJ analyses produced similar trees, with some discrepancy in branching order of lipL32 gene. These analyses produced trees that segregated the serovars of L. interrogans into four clusters consistent with different serotypes. The phylogenetic analyses also provided some limited insights into the relationships among the ompL clusters in L. interrogans because a separate cluster with a long diversification period has been attributed. Nevertheless, we found reasonable levels of relationship within species, but not to the serotypes of this genus.
The thirteen serovars of Leptospira in ompL1 pattern were grouped into three major clusters (Figure 2). In the first cluster, serovars Manhao, Hebdomadis, Pomona, Paidjan, Canicola, Australis, Lai and Pyrogenes formed a monophyletic group and the relatedness exhibited a simple pattern with little distinction. The serovars Grippotyphosa, Icterohaemorrhagiae, Copenhageni, Autumnalis and Wolffi in cluster 2 have some distance with each other, which showed a low similarity with clusters 1 and 3. In cluster 3, serovars Grippotyphosa and Autumanalis clustered together and formed a monophyletic group, but it was closely related to cluster 2. L. interrogans with different serovars clustered in a separate clade, suggesting the close evolutionary relationship among them. The NJ tree of lipL41 gene sequence in Figure 4 showed that serovar Grippotyphosa of L. interrogans was distantly related with serovar Autumnalis and then formed a cluster similar to that of ompL1. However, lipL41 of L. borgpetersenii group formed a separate cluster consisting Ballum, Javanica and Tarassovi serovars. As in the ompL1 tree, the L. interrogans group doesn’t emerge from the L. kirschneri branch. This indicates a greater degree of separation of the L. interrogans sequences from the L. kirschneri sequences. In Figure 3, it was found that lipL32 genes of the L. borgpetersenii group could not produce a separate cluster as resulted in ompL1 and lipL41 trees, but the rest of the other groups almost clustered as L. interrogans and L. borgpetersenii, L. weilii, and L. noguchii. This finding indicates that there is a strong sero-specific variation in lipL41 and lipL32 gene sequences, which determines the specific antigenic properties of proteins encoded by these genes. Our three gene sequences formed a cluster with members of the L. kirschneri, L. noguchii and L. borgpetersenii species as a separate branch. This is consistent with the finding that the ompL1, lipL32 and lipL41 sequences of L. interrogans strains are not identical to other leptospiral strains presently analyzed in this work.
Among 56 OMP-related genes (deduced amino acids) of different bacterial strains examined, 10 major clusters were formed in the phylogenetic tree as shown in Figure 5, on which our gene sequences have been related together to a great extent. lipL32 sequence of L. borgpetersenii showed similarity to ompL1 of L. interrogans, confirming evolutionary relationship of these strains. Although the relatedness of the ompL gene family was not too diversified, most of them belonged to the same group of organisms but different serovars.
Discussion
Although different strains of the same serovars clustered together, there are some genetic dissimilarities that appear to be accountable for such strain variability in the clinical presentations (19). Similarly, the strong dissimilarities within conserved regions of ompL genes were found in L. interrogans, which could bring some genetic variations during evolutionary process. Bacterial porin proteins are highly conserved, allowing for the selective movement of hydrophilic solutes through the outer membrane of Gram-negative bacteria (20). Nevertheless, only the difference in sequence similarity could not determine the evolutionary relationship and gene diversity of these ompL genes. Some of the homologous characters (orthologs and paralogs) were assigned to reveal how these sequences were diversified from other closely and distantly related organisms. Determination of orthology or paralogy is critically important because paralogous genes often have distinct functional roles in organisms.
The BLAST tool is arguably the most powerful and useful tool in bioinformatics and has been used to functionally annotate millions of genes, providing remarkable insights into biological systems. Although this algorithm is both deceptively simple and remarkably powerful, researchers have recognized that the BLAST algorithm cannot reliably distinguish between orthologous and paralogous genes 21., 22.. Therefore, high similarity sequences obtained from BLAST search were subjected to evaluating their phylogeny to find orthologous features. Phylogenetic analyses, on the other hand, easily distinguish orthologs from paralogs given sufficient sampling of related sequences, and these methods have often been used to characterize new functional groups of proteins (23). The sequence identities of ompL gene family apart from our ompL gene sequences reasonably showed less variation in all aligned sequences; therefore, only closely related leptospiral strains have been used to infer evolutionary significance. We observed that there are strong sequence dissimilarities between ompL of L. interrogans isolated in this study and ompL of the same species used in phylogenetic tree. It may be resulted from key modification of amino acid residues located in antigenic determinant sites of OMPs that are conferring antigenicity of these strains, leading to serovar diversification but not to species evolution. In earlier studies, host environment and geographical regions also contributed for sero-specific (serovar evolution) variation of L. interrogans 19., 24.. In pathogenic leptospiral strains, ompL1 and lipL41 are highly conserved sequences that are expressed both in cultivated organisms and during infection in mammals (13).
A variation in a particular stretch of amino acids or single amino acid in OMP sequences could bring some specific antigenic variants in these organisms. However, we found only a minor change in ompL gene sequences of L. interrogans other than the sequences of non-leptospiral strains used in our study. Thus, we assumed that these strains could act as outgroup organisms due to a separate cluster with distinguished evolutionary distance generated. Most of these insertions or deletions are in the variable extracellular regions of the porin regions, which are known to undergo relatively rapid evolutionary change (20). Moreover, there is a lot of evidence supporting that the antigenic variation is resulted due to genetic alternation in highly variable ompL genes in Leptospira, suggesting the use of an adaptive mode of evolution to escape immune pressures (10).
Changes in the antigenic composition of LPS are also thought to account for serovar diversity (25). Accordingly, serovar diversity of these strains would have been resulted on the basis of diversification process of ompL gene family during infection. Sometimes, the entire lipL41 sequence of L. interrogans can also be acquired to L. borgpetersenii strain HB10 during the putative transformation event as reported by Haake et al. (11). Horizontal transfer of DNA appears to involve both entire lipL41 gene and portions of ompL1 gene. Three different mosaic patterns of ompL1 gene recombination were observed, varying from two to four sites of interspecies recombination involving DNA derived from two to three different leptospiral species origins per mosaic pattern (11). The investigators also noted that LipL41 amino acid sequence of L. interrogans serovar Pomana is identical (99%) to that of L. kirschneri serovar Grippotyphosa (13). In our study, lipL32 of L. interrogans is highly identical to that of L. borgpetersenii, suggesting a close phylogenetic relationship between them, which is more distinct than other pathogenic leptospiral strains.
The strains MG569, MG633, D22 and ALC10 of serovar Ratnapura showed close relation to L. interrogans associated with pulmonary hemorrhages in Andaman Islands, whereas the strain ALC10, though belonging to the same serovar, showed close relation to L. kirschneri and was responsible for hepatorenal failure in south India (19). As the amino acid OmpL1 is highly conserved among pathogenic Leptospira species (13), this work also suggested evidence on the unique nature of OmpL1, LipL41 and Lip32 in L. interrogans isolated from human urine. Nonetheless, our study on OMPs suggests that implementing an automated phylogenomic approach, combined with gene position analyses and BLAST searches, could significantly enhance the accuracy of gene sequence annotations with special reference to antigenic determinants.
The patterns of substitution in OMPs at both the nucleic acid level and the amino acid level to identify residues may be correlated with serovar-specific tissue tropism or pathogenic potential by virtue of shared evolutionary divergence. The immunological relevance of the increased amino acid sequence variation in OMPs such as ompL1, lipL32 and lipL41 can now be assessed. The significance of our study is that antigenic and pathogenic characteristics of these strains, notably on serovar-specific ones, are thought to be determined by some sequence variations occurred in ompL of L. interrogans during evolutionary process. Moreover, such sequence variation will also provide guidance for the development of OMP-specific vaccines for leptospirosis in India and other places.
Materials and Methods
Isolation of leptospiral strains
Human urine samples were processed as per standard procedures from which 1–2 drops of urine was inoculated into Ellinghausen-McCullough-Johnson-Harris (EMJH) semisolid medium (Difco, Sparks, USA) in MaCartney bottles with a hole cut in the aluminum cap and a rubber lining underneath 26., 27.. The clinical samples in this study were collected according to ethical guidelines governed by ethical committee of Regional Medical Research Centre (ICMR), Port Blair and perception of individual patients. The rat (Rattus norvegicus) kidney was aseptically dislocated from field rats and a piece of fleshy tissue was immediately inoculated in EMJH medium (28).
Growth and maintenance of leptospiral strains
The isolated leptospiral strains were cultivated in EMJH medium with additional supplementation of 0.2% agarose (Sigma, USA), 1% bovine serum albumin (Sigma), 2% rabbit serum, 0.1% sodium pyruvate, and 100 μg/mL 5-flurouracil (as a selective agent). Every sample in triplicates was inoculated in tubes containing the above medium, incubated at 30°C in dark and the presence of leptospires was examined at weekly intervals using a dark field microscope. The positive growth of these strains was sub-cultured in vials having fresh EMJH medium and further incubated at 30°C for 7 days. All strains were identified up to serovar level and maintained in the leptospiral repository in RMRC Port Blair with a periodic regeneration in EMJH medium. The N2 strain of urine isolate, CH31 strain of human blood isolate, R1R and R1L strains of rat isolates (unpublished) were obtained and utilized for this study (24).
DNA extraction, amplification and sequencing
DNA was extracted from seven-day-old broth cultures of Leptospira according to Boom et al. (29). The genes encoding for OmpL1, LipL41 and LipL32 proteins were amplified from the purified DNA by using PCR primers as listed in Table 4. Briefly, each 50 μL PCR reaction mixture contained approximately 50 ng of purified DNA, 0.1 μM primer, 250 μM of each dNTP (Fermentas, USA), 25 mM MgCl2, 0.5 U of Taq DNA Polymerase (Fermentas), 10 mM Tris-HCl (pH 9) and 50 mM KCl. Amplification was performed in thermal cycler (Eppendorf, Germany) at 94°C for 5 min, followed by 40 cycles of 94°C for 30 s, 51°C for 30 s, and 72°C for 60 s, with a final single extension of 72°C for 7 min. The annealing temperature of 51°C (lipL32), 58°C (lipL41) and 60°C (ompL1) were used. Amplified products were characterized by electrophoresis of 5 μL of each reaction on a 1.2% agarose gel for 30 min at 85 V. Sequencing reaction products were purified with magnetic carboxylate beads (Agencourt Bioscience, Beverly, USA) and sequenced on an AB 3100 sequencer (Applied Biosystems, USA).
Table 4.
PCR primer sequences for amplifying ompL genes studied in this work
| Primer* | Oligonucleotide sequence |
|---|---|
| ompL1 F | 5ʹ-TTGATTGAATTCTTAGAGTTCGTGTTTATA-3ʹ |
| ompL1 R | 5ʹ-AAGGAGAAGCTTATGATCCGTAACATAAGT-3ʹ |
| lipL32 F | 5ʹ-TTACCGCTCGAGGTGCTTTCGGTGGTCTGC-3ʹ |
| lipL32 R | 5ʹ-TGTTAACCCGGGTTACTTAGTCGCGTCAGA-3ʹ |
| lipL41 F | 5ʹ-AAAGGACTCGAGTTACTTTGCGTTGCTTTC-3ʹ |
| lipL41 R | 5ʹ-TGTTACCCATGGGGAGAAAATTATCTTCTCT-3ʹ |
F: forward primer; R: reverse primer.
Phylogenetic analysis
The highly identical sequences for ompL1, lipL32 and lipL41 used in this study were retrieved from NCBI database using BLASTn and PSI-BLAST tools with default parameters (30). Sequences with more significant identity were aligned with ClustalW algorithm implemented in MEGA 4.0 (31) using Smith-Waterman substitution matrix and trimmed to consensus, and NJ and ME algorithms were used to construct phylogenetic trees using MEGA 4.0 with 1,000 bootstraps at uniform divergence rates with Jukes and Cantor evolutionary model and 0.25 gamma distribution factor. Gaps in the aligned sequences were replaced by Ns in BioEdit 7.0.4.1 (32). Maximum likelihood (ML) with molecular clock v3.6a2.1 tree was constructed with DNAMLK 3.5c program at a constant rate variation with substation rate and 2 transition/transversion ratio. Posterior probability and conserved regions among closely related sequences were carried out by MEGA 4.0 and BioEdit 7.0.4.1, respectively.
GenBank accession numbers
Nucleotide sequences have been deposited in the GenBank of NCBI database with the accession numbers EU091293–EU091296 and EU293441–EU293443.
Authors’ contributions
KV carried out experiments throughout this study and prepared this manuscript. KN conceived the idea of using this approach, supervised the project and co wrote this manuscript. PC contributed in phylogenetic analysis and assisted with manuscript preparation. SGP and JS supported to isolate the strains and provided lab facilities. SS and PV helped to identify the strains. All authors read and approved the final manuscript.
Competing interests
The authors have declared that no competing interests exist.
Acknowledgements
This study was supported by grants from the Department of Science and Technology, Government of India (Sanction order No. SR/FT/L-47/2006). The authors would like to thank the Vice Chancellor, Bharathidasan University for the facilities provided in this study.
References
- 1.Levett P.N. Leptospirosis. Clin. Microbiol. Rev. 2001;14:296–326. doi: 10.1128/CMR.14.2.296-326.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Leptospirosis worldwide, 1999. Wkly. Epidemiol. Rec. 1999;74:237–242. [PubMed] [Google Scholar]
- 3.Nguyen T.X. Phylogenetic analysis of general bacterial porins: a phylogenomic case study. J. Mol. Microbiol. Biotechnol. 2006;11:291–301. doi: 10.1159/000095631. [DOI] [PubMed] [Google Scholar]
- 4.Bulach D.M. Lipopolysaccharide biosynthesis in Leptospira. J. Mol. Microbiol. Biotechnol. 2000;2:375–380. [PubMed] [Google Scholar]
- 5.Farrelly H.E. Opsonic monoclonal antibodies against lipopolysaccharide antigens of Leptospira interrogans serovar hardjo. J. Med. Microbiol. 1987;23:1–7. doi: 10.1099/00222615-23-1-1. [DOI] [PubMed] [Google Scholar]
- 6.Jost B.H. A monoclonal antibody reacting with a determinant on leptospiral lipopolysaccharide protects guinea pigs against leptospirosis. J. Med. Microbiol. 1986;22:269–275. doi: 10.1099/00222615-22-3-269. [DOI] [PubMed] [Google Scholar]
- 7.de la Pena-Moctezuma A. Genetic differences among the LPS biosynthetic loci of serovars of Leptospira interrogans and Leptospira borgpetersenii. FEMS Immunol. Med. Microbiol. 2001;31:73–81. doi: 10.1111/j.1574-695X.2001.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 8.de la Pena-Moctezuma A. Comparative analysis of the LPS biosynthetic loci of the genetic subtypes of serovar Hardjo: Leptospira interrogans subtype Hardjoprajitno and Leptospira borgpetersenii subtype Hardjobovis. FEMS Microbiol. Lett. 1999;177:319–326. doi: 10.1111/j.1574-6968.1999.tb13749.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuo C. An N-linked high-mannose type oligosaccharide, expressed at the major outer membrane protein of Chlamydia trachomatis, mediates attachment and infectivity of the microorganism to HeLa cells. J. Clin. Invest. 1996;98:2813–2818. doi: 10.1172/JCI119109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haake D.A. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect. Immun. 2000;68:2276–2285. doi: 10.1128/iai.68.4.2276-2285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haake D.A. Molecular evolution and mosaicism of leptospiral outer membrane proteins involves horizontal DNA transfer. J. Bacteriol. 2004;186:2818–2828. doi: 10.1128/JB.186.9.2818-2828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haake D.A. Characterization of leptospiral outer membrane lipoprotein LipL36: down-regulation associated with late-log-phase growth and mammalian infection. Infect. Immun. 1998;66:1579–1587. doi: 10.1128/iai.66.4.1579-1587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haake D.A. Leptospiral outer membrane protein OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect. Immun. 1999;67:6572–6582. doi: 10.1128/iai.67.12.6572-6582.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang E.S. Molecular cloning and sequence analysis of the gene encoding LipL41, a surface-exposed lipoprotein of pathogenic Leptospira species. Infect. Immun. 1996;64:2322–2330. doi: 10.1128/iai.64.6.2322-2330.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cullen P.A. LipL21 is a novel surface-exposed lipoprotein of pathogenic Leptospira species. Infect. Immun. 2003;71:2414–2421. doi: 10.1128/IAI.71.5.2414-2421.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flannery B. Evaluation of recombinant Leptospira antigen-based enzyme-linked immunosorbent assays for the serodiagnosis of leptospirosis. J. Clin. Microbiol. 2001;39:3303–3310. doi: 10.1128/JCM.39.9.3303-3310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerreiro H. Leptospiral proteins recognized during the humoral immune response to leptospirosis in humans. Infect. Immun. 2001;69:4958–4968. doi: 10.1128/IAI.69.8.4958-4968.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Natarajaseenivasan K. Serodiagnosis of severe leptospirosis: evaluation of ELISA based on the recombinant OmpL1 or LipL41 antigens of Leptospira interrogans serovar autumnalis. Ann. Trop. Med. Parasitol. 2008;102:699–708. doi: 10.1179/136485908X355229. [DOI] [PubMed] [Google Scholar]
- 19.Natarajaseenivasan K. Phylogenetic relatedness among leptospiral strains belonging to same serovar recovered from patients with different clinical syndromes. Infect. Genet. Evol. 2005;5:185–191. doi: 10.1016/j.meegid.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiu J.C. OrthologID: automation of genome-scale ortholog identification within a parsimony framework. Bioinformatics. 2006;22:699–707. doi: 10.1093/bioinformatics/btk040. [DOI] [PubMed] [Google Scholar]
- 22.Daubin V. A phylogenomic approach to bacterial phylogeny: evidence of a core of genes sharing a common history. Genome Res. 2002;12:1080–1090. doi: 10.1101/gr.187002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley S.T., Thackeray V.G. Phylogenetic analyses reveal ancient duplication of estrogen receptor isoforms. J. Mol. Evol. 1999;49:609–614. doi: 10.1007/pl00006582. [DOI] [PubMed] [Google Scholar]
- 24.Roy S. Antigenic and genetic relatedness of Leptospira strains isolated from the Andaman Islands in 1929 and 2001. J. Med. Microbiol. 2003;52:909–911. doi: 10.1099/jmm.0.05101-0. [DOI] [PubMed] [Google Scholar]
- 25.Zuerner R. Technological advances in the molecular biology of Leptospira. J. Mol. Microbiol. Biotechnol. 2000;2:455–462. [PubMed] [Google Scholar]
- 26.Djordjevic S. Restriction-endonuclease analysis of Australian isolates of Leptospira interrogans serovar hardjo from cattle with agalactia and abortion. Aust. Vet. J. 1993;70:98–100. doi: 10.1111/j.1751-0813.1993.tb03285.x. [DOI] [PubMed] [Google Scholar]
- 27.Everard C.O. An investigation of some risk factors for severe leptospirosis on Barbados. J. Trop. Med. Hyg. 1992;95:13–22. [PubMed] [Google Scholar]
- 28.Faine S. second edition. MediSci; Melbourne, Australia: 1999. Leptospira and Leptospirosis. [Google Scholar]
- 29.Boom R. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altschul S.F. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura K. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 32.Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]