INTRODUCTION
In the setting of highly active antiretroviral therapy (ART), cardiovascular disease (CVD), particularly coronary artery disease, is among the leading causes of mortality among human immunodeficiency virus (HIV)-infected subjects.1 Several studies suggest that adults and children with HIV have an increased risk of CVD.2–4 The full details of the pathogenesis of atherogenesis in HIV infection remain to be elucidated. Traditional risk prediction models to estimate cardiovascular risk do not include emerging cardiovascular risk factors such as inflammation, coagulation disorders, immune activation, kidney disease, and HIV-1 RNA levels.4–6 Understanding the pathophysiology of increased CVD in HIV infection will help us develop strategies to prevent and treat this leading cause of morbidity and mortality in HIV-infected subjects.
The prevalence of several traditional risk factors for CVD is higher in HIV-infected individuals than among age-matched controls.2 Lipid changes may promote atherogenesis and may contribute to increased risk of CVD in HIV-infected subjects.7 The patterns of dyslipidemia change during the course of HIV disease. In untreated disease, elevations in triglycerides and low high-density lipoprotein cholesterol (HDL-c) predominate. Dyslipidemia that occurs during treatment for HIV disease is characterized by a range of values of serum concentrations of total cholesterol (TC); triglycerides, depending on the ART used; very low-density lipoprotein (VLDL); low-density lipoprotein cholesterol (LDL-c); apolipoprotein B (apoB); and low levels of HDL-c.7 In view of the high prevalence of dyslipidemia and the increased risk for CVD among patients with HIV, which is concerning for public health, this review aims to describe the changes in the lipid profile of HIV-infected patients and how these changes directly or indirectly contribute to the pathogenesis of atherosclerosis in HIV-infected subjects.8 Although the exact mechanisms are incompletely understood,9 we describe how host factors, HIV per se and ART, may contribute to lipid changes and how these atherogenic lipids may have a role in the development of atherosclerosis in HIV-infected patients.
FACTORS OTHER THAN DYSLIPIDEMIA MAY CONTRIBUTE TO ACCELERATED ATHEROSCLEROSIS IN HIV INFECTION
Cardiovascular risk factors have a major role in development of CVD disease. HIV-infected subjects have higher prevalence of established CVD risk factors, such as smoking, hypertension, insulin resistance, and dyslipidemia, compared with age-matched individuals.9 Cocaine use, which is relatively common among some groups of HIV-infected patients, renal function, and albuminuria have also been associated with the risk for coronary artery disease in HIV-infected patients.9,10 All of these risk factors are synergistic, and it is difficult to analyze the specific role of each. Recently, the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group developed a risk assessment tool tailored to HIV-infected patients.11
HIV replication can directly promote atherogenesis. HIV replication increases chronic inflammation as a part of the immune response to the virus. These changes may, in turn, contribute to an increased risk for death.4 HIV replication is associated with increased biomarkers of inflammation, including C-reactive protein (CRP). Elevated levels of CRP have been found to independently be associated with the risk of risk of myocardial infarction (MI) in adults, including those with HIV.4 In HIV infection, high CRP levels predict HIV disease progression.4 Increased concentrations of CRP, interleukin 6, and d-dimer have also been independently associated with CVD events in patients with HIV.12 Identifying biomarkers of inflammation and cardiovascular disease in HIV-infected subjects on ART with suppressed viremia may help us develop new targets for therapeutic interventions.13 The HIV virus can also cause increased endothelial injury caused by adhesion molecules and HIV Tat protein and may stimulate proliferation of vascular smooth muscle cells and induce coagulation disorders.14 Collectively, these HIV-induced effects may directly increase atherogenesis.
Immune activation may promote atherosclerosis in the absence of residual viral replication. Several studies suggest that increased activation of innate immunity is associated with the presence of subclinical atherosclerosis in patients with HIV.15–18 One potential mechanism that might trigger monocyte activation in HIV infection is microbial translocation across the gastrointestinal tract, which has been found to persist in treated HIV infection.4,19 Markers of monocyte activation, such as high soluble CD14 and CD163, and bacterial translocation, such as endotoxin and soluble CD14, were independently associated with a faster rate of progression of subclinical atherosclerosis in several independent studies.15–18 Collectively, these studies suggest that chronic monocyte activation could be an important marker of or target for future interventions to reduce CVD risk in treated patients with HIV. Further work is needed to determine contributing factors to immune activation and CVD and, importantly, whether atherogenic lipids may drive both immune activation and CVD in HIV infection.
DYSLIPIDEMIA AND CVD IN HIV INFECTION
Host Factors in HIV-Infected Subjects May Contribute to Dyslipidemia Development
HIV-infected subjects have increased prevalence of dyslipidemia; however, it is unclear to what extent this is associated with specific host factors. In the D:A:D study, 33.8% and 22.2% of a group of treated HIV-infected individuals had elevated levels of triglyceride and TC, respectively.20 Longitudinal and cross-sectional studies have assessed the role of single-nucleotide polymorphisms on the incidence of dyslipidemia in HIV patients.21 The Multicenter AIDS Cohort Study showed that biogeographical ancestry may contribute to development of ART-induced lipid changes.22 In a study of the metabolome in HIV-infected patients on suppressive ART, the observed patterns of metabolites suggested decreased lipolysis and dysregulation of receptors controlling inflammation and lipid metabolism.23 Overall, these data suggest that genetic and nongenetic host factors may contribute to the dyslipidemia in HIV-infected subjects.
The HIV Virus May Directly Induce Dyslipidemia
Mechanisms such as altered cytokine profile, decreased lipid clearance, and increased hepatic synthesis of VLDL, may explain how HIV infection, per se, might induce dyslipidemia and accelerate atherosclerosis based on data from in vitro, animal, and clinical studies.6,9,24,25 The SMART (Strategies for Management of Antiretroviral Therapy) study compared the outcomes of HIV-infected patients who were randomly assigned to receive continuous or intermittent ART. This study confirmed an increased risk of CVD among patients who discontinued ART6 and allowed comparison of lipid profiles between the treatment-interruption group and the continuous treatment group.8 These findings suggested that HIV viremia may have a role in accelerated atherogenesis. More recently, data from a large cohort study of 27,000 HIV-infected adults in care suggested that immunodeficiency and ongoing viral replication both independently contributed to the risk of MI, further confirming the putative role of HIV in the pathogenesis of CVD.26
HIV viremia is associated with quantitative lipid abnormalities, including elevated serum concentrations of triglycerides and low levels of cholesterol. Several studies found the associations between uncontrolled HIV viremia and dyslipidemia and increased CVD risk. The impact of HIV infection on lipids was studied within the Multi-center AIDS Cohort Study, in which a significant reduction in TC, LDL, and HDL was found in a group of 50 HIV seroconverters comparing pre-HIV with post-HIV infection lipid levels.27 In other studies, the levels of triglycerides were higher, and the levels of TC, LDL, and HDL were lower in HIV-infected patients receiving no ART when compared with uninfected controls.28 Elevations in triglyceride levels during untreated HIV infection are thought to be caused by an increase in the levels of inflammatory cytokines (tumor necrosis factor–α, interleukins, interferon-α)24 and steroid hormones.28 HDL levels are also found to be low in both untreated and treated HIV-infected patients, regardless of the CD4+ T cell count.27 The SMART study found that declines in HDL levels after stopping nonnucleoside reverse transcriptase inhibitor (NNRTI) treatment were associated with an increased risk of CVD, suggesting that the HDL-raising effects of this therapy had been cardioprotective.29 Enkhmaa and colleagues30 found that allele-specific apolipoprotein A (apoA) levels, which determine the amount of atherogenic small apoA related to a defined apoA allele size, were higher in individuals with low HIV viremia and high CD4 cell counts, indicating that HIV replication reduced allele-specific apoA levels. Therefore, HIV-infected individuals with immune reconstitution may have higher allele-specific apoA levels, which are related to progression of atherosclerosis.30 Overall, these data suggest mechanisms that explain how the HIV virus, per se, may induce dyslipidemia.
HIV may induce qualitative changes in lipids such as HDL through effects on metabolism and function that lead to increased atherogenesis. HDL is generally accepted to have anti-inflammatory/antioxidant effects.31 HIV may directly affect HDL metabolism by up-regulating the cholesteryl ester transfer protein activity, which enhances transfer of cholesterol to apoB lipoproteins that promote atherogenesis.32 These effects on HDL metabolism in combination with HIV-related hypertriglyceridemia,25 lead to an increased delivery of cholesterol to the arterial wall, where it is then taken up by macrophages, and atherogenic foam cells are formed. The capacity of HDL to increase cholesterol efflux from macrophages is an important function of HDL and may predict development of atherosclerosis.33 The HIV Nef protein (which is abundant during untreated HIV) inhibits transporters important to cholesterol efflux in macrophages, and this may initiate atherogenesis in the arterial wall.34 Intracellular cholesterol in monocytes in HIV-infected subjects is inversely associated with HDL-c levels; in contrast, in HIV-negative controls, cholesterol content in macrophages is correlated with LDL-c levels rather than with HDL-c.35 In the SMART study, HDL-c, lipoprotein particle concentrations, and the apolipoproteins were better indices of CVD risk than LDL-c levels.29 Consistent with these data, reduction in large lipoprotein particle concentrations after treatment with ART may indicate increased efflux of cholesterol from macrophages into smaller HDL particles.8 Thus, HIV induces effects on HDL function and cholesterol transport that may contribute to increased rates of CVD in HIV- infected patients.
HIV replication may also modify HDL indirectly through increases in systemic inflammation. The inflammatory response observed during HIV infection may reduce HDL levels and compromise cholesterol efflux from macrophages.31 Infections may induce nonspecific systemic inflammation that may at least partially modify HDL.36 In the SMART trial, levels of biomarkers of inflammation were associated with changes in HDL levels independently of HIV RNA levels.5,8 Finally, cytokines such as tumor necrosis factor-α and interleukin-6 appear to promote lipid peroxidation, and the production of reactive oxygen species,37 and this may further contribute to formation of oxidized, modified lipoproteins such as oxidized HDL. However, it is unclear whether HIV-infected subjects have increased levels of oxidized LDL, a marker of oxidative stress associated with lipoproteins and an emerging CVD risk factor,38 compared with uninfected subjects. The role of modified lipoproteins in CVD in HIV-infected subjects remains incompletely understood.
ART and Dyslipidemia
The introduction of ART led to substantial improvement in the prognosis of HIV patients,39 but several of the drugs in the first generation of effective combination ART were associated with changes in lipid metabolism, abnormalities in fat (both lipohypertrophy and subcutaneous fat loss), insulin resistance, dyslipidemia, osteopenia, and lactic acidosis.39 ART-associated dyslipidemia usually occurs within 3 months of starting treatment9 and was first described in patients who used first-generation protease inhibitors (PIs) but was also observed in patients who received regimens consisting of nucleoside reverse-transcriptase inhibitors (NRTI) and NNRTIs. Studies with HIV-infected children and adolescents and HIV-infected older adults receiving effective ART found high rates of fat changes and dyslipidemia, therefore, high risk for cardiovascular diseases in all age groups of HIV-infected subjects.9,39 A component of the initial changes in lipids has been ascribed to a return to health among patients with a chronic untreated illness who are undergoing effective treatment.27
Several studies investigated the potential effects of ART on risk of CVD and dyslipidemia. The specific effects of ART on dyslipidemia vary both within and across drug classes. Several randomized clinical trials have characterized changes in lipids after the initiation of ART. The AIDS Clinical Trials Group (ACTG) 5142 trial found important differences in metabolic outcomes in treatment-naive patients after the initiation of an NNRTI-sparing regimen (the boosted PI, lopinavir/ritonavir plus 2 NRTIs), PI-sparing regimen (NNRTI efavirenz [EFV] plus 2 NRTIs), or an NRTI-sparing regimen (lopinavir/ritonavir plus EFV).40 Although the NRTI-sparing regimen (a combination that included lopinavir/ritonavir and EFV) had the lowest risk of lipoatrophy, it also had the greatest likelihood of lipid elevations and subsequent use of lipid-lowering agents. The SMART study helped put these changes into perspective by showing that interrupting therapy through a structured treatment interruption was associated with worse outcomes than remaining on treatment.6 The D:A:D study, one of the most comprehensive surveys of CVD adverse events associated with ART, found a strong association between dyslipidemia and ART.11,20 These studies highlighted common (owing to viral suppression) and differential (owing to ART) lipid effects on starting ART in ART-naive HIV-infected patients.
HIV-infected patients on ART have low levels HDL and modified lipoproteins compared with normolipemic subjects. HIV patients with dyslipidemia on ART have impaired plasma lipolytic activity that may lead to low HDL-c plasma concentration and triglyceride-rich LDL and HDL, which become less stable than HDL particles in normolipemic patients.34 In addition, systemic inflammation may contribute to modification of HDL to a dysfunctional form that may increase the risk of CVD.41 We previously found that HIV-infected subjects with suppressed viremia on ART have dysfunctional HDL.42,43 In small study of HIV patients with low CVD risk profile, HDL function changed over time and was independently associated with obesity but not with subclinical atherosclerosis.44 In another study, HIV-infected subjects had dysfunctional HDL compared with matched uninfected subjects with comparable HDL levels, and this modified HDL was associated with macrophage activation and with presence of noncalcified coronary plaque.45 The role of HDL function in CVD in HIV-infected subjects with suppressed viremia remains to be determined.
Dyslipidemic effects of PIs
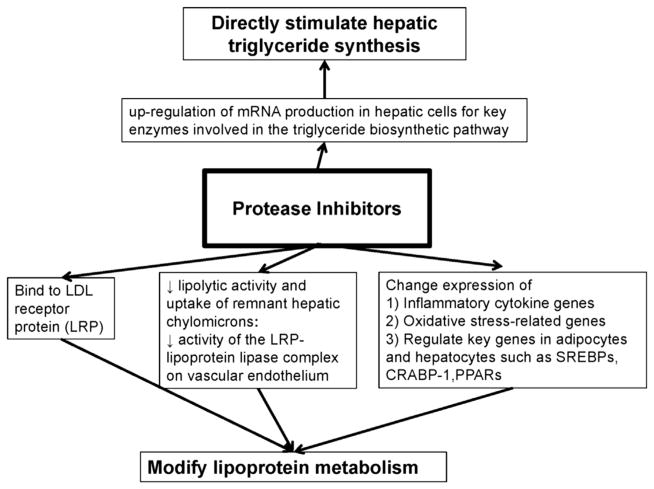
Patients, including children and pregnant women, with prolonged use of PIs often have hypertriglyceridemia, low levels of HDL-c and high levels of LDL-c, and apolipoproteins E and CIII; however, the effects vary by drugs within this class.9,39 Fig. 1 summarizes the mechanisms through which PIs may cause dyslipidemia.46 Table 1 summarizes the lipid effects of different drugs within the PI class.20,47–51 Newer agents have less significant effects on lipids than the first drugs to be available within this class.9,39,46 In the Data Collection on Adverse Events of Anti-HIV Drugs studies, within the PI class only cumulative exposure to lopinavir/ritonavir and indinavir were associated with increased risk of CVD, independently of lipid concentrations.52 Overall, ritonavir-boosted atazanavir and darunavir have more favorable lipid effects and tolerability compared with other PIs (see Table 1). In view of the differences in metabolic effects of drugs within the PI class, future epidemiologic studies examining CVD risk in HIV need to consider the effects of individual PIs.
Fig. 1.
PIs may modify lipoprotein metabolism through multiple mechanisms. PIs directly stimulate the biosynthesis of triglycerides in hepatic cells and may also directly modify the metabolism of lipoproteins by binding to cellular receptors, reducing lipolysis and by regulating expression of key genes involved in the regulation of metabolic pathways in adipocytes and hepatocytes. CRABP-1, cellular retinoic acid-binding protein 1; LRP, low-density lipoprotein receptor protein; PPARs, peroxisome proliferator-activated receptors; SREBPs, sterol regulatory element-binding proteins.
Table 1.
Main studies investigating the effects of PIs on lipids
| PIs | Main Findings |
|---|---|
| Lopinavir/ritonavir and indinavir | D:A:D study: increased risk of MI with longer duration of treatment compared with other treatments.20 |
| Lopinavir/ritonavir and ritonavir-boosted fosamprenavir | The French Hospital Database: increased risk of MI with longer duration of treatment compared with other treatments. |
| PI-treated patients switched to atazanavir-containing regimens | Several randomized trials: improvement of lipid parameters, while the immunologic and virologic efficacy of the regimen was maintained.47 |
| Ritonavir-boosted atazanavir and darunavir | Different studies: pIs recommended for the initial treatment of HIV infection because each has shown better lipid effects and overall tolerability than ritonavir-boosted lopinavir.50,51 |
| Darunavir/ritonavir or atazanavir/ritonavir plus tenofovir-emtricitabine | Pilot study, Aberg and colleagues48: similar 48-wk lipid changes between darunavir and atazanavir. |
| Darunavir/ritonavir or atazanavir/ritonavir compared with raltegravir | Ofotokun and colleagues49: 96-wk trial found no difference in lipid profiles with atazanavir/ritonavir and darunavir/ritonavir. Raltegravir had more favorable lipid profile than both PIs. |
Dyslipidemic effects of NRTIs
Antiretroviral treatment regimens containing NRTIs have also been associated with metabolic alterations, particularly changes in serum triglyceride concentrations (Table 2).9,39,52–61 Replacement of NRTIs such as stavudine with tenofovir is a strategy to reduce the cardiovascular risk and improve the lipid profile of patients with dyslipidemia.9,39 Currently, the association between abacavir and excess CVD risk remains controversial. Several studies have found a consistent association52,57; however, others have not,55,56 and the mechanism underlying this association remains unclear.58 Switching from multidrug class-suppressive regimens to triple therapy containing 2 NRTIs showed increases in plasma lipids.62 Overall, within the NRTI class, tenofovir and lamivudine/emtricitabine seem to be the drugs that are not associated with dyslipidemia.
Table 2.
Studies investigating the effects of different NRTIs on lipids
| NRTIs | Comments |
|---|---|
| Stavudine | Stavudine is still used in some developing countries and at full doses induces significant metabolic abnormalities compared with other ART such as tenofovir.53 No association between stavudine use and risk of MI was found in the D:A:D study.57 |
| Tenofovir | Regimens containing tenofovir are associated with lower serum concentrations of LDL-c, TC, and triglycerides compared with regimens using other NRTIs, suggesting a lipid-lowering action of tenofovir, which differs from that of other NRTIs.54 No association between tenofovir use and risk of MI was found in the D:A:D study.52 Several studies100 found maintained virologic suppression and improved cholesterol concentrations in patients with increased lipid concentrations on ritonavir-boosted, PI-based regimens that included abacavir who were switched to tenofovir. |
| Abacavir and didanosine | The use of the NRTIs abacavir and didanosine was found to be an independent risk factor for myocardial infarction in the D:A:D study.52,57 Several analyses with conflicting results have been performed in an attempt to better understand the association between abacavir and, to a lesser extent, didanosine and CVD events.55,56 In a meta-analysis based on 52 clinical trials and a total 14,174 HIV-infected adults who received abacavir (n = 9502) or not (n = 4672), baseline demographics and HIV disease characteristics, including lipids values, MI rates were similar.58 Further data are needed to evaluate any association between abacavir and increased risk of MI. |
| Tenofovir-emtricitabine vs abacavir-lamivudine | In the ACTG 5202 study, changes in lipid concentrations were generally greater with abacavir-lamivudine than tenofovir-emtricitabine (when combined with either efavirenz or atazanavir/ritonavir); however, researchers found no differences in the TC:HDL-c ratios.59 In a study examining lipid subfractions, a more atherogenic LDL profile was noted in patients switched to abacavir-lamivudine compared with tenofovir-emtricitabine, including a decrease in LDL level in the abacavir group.60 Of note, in the SPIRAL study,61 no significant differences in lipid concentrations were identified between the tenofovir and abacavir recipients who switched from a ritonavir-boosted PI to raltegravir, suggesting that the combination of a ritonavir-boosted PI and abacavir might have distinct lipid effects. |
Dyslipidemic effects of NNRTIs
In patients who have initiated NNRTIs as first-line therapy, increases in the serum concentrations of TC, HDL, LDL, and triglycerides have been observed (Table 3).11,63–70 Many studies have reported that NNRTI may induce greater increases in HDL levels compared with PIs, hence, balancing out the overall lipid risk profile.70 Patients treated with efavirenz had increases of TC (at least 3% mean relative increase in levels) and triglyceride (at least 10% mean relative increase in levels) concentrations.63 Switching from a PI to efavirenz may improve the lipid profile, depending on the specific PI used.71 With regard to other agents, the newer NNRTIs, rilpivirine and etravirine, have more favorable lipid profiles than efavirenz.72
Table 3.
Studies investigating the effects of different NNRTIs on lipids
| NNRTIs | Comments |
|---|---|
| Efavirenz | Patients treated with efavirenz presented a significant increase of TC and triglyceride concentrations63 compared with baseline. In the ACTG study 520264 participants randomly assigned to efavirenz had statistically significantly greater increases in TC and LDL-c concentrations but not in TC:HDL-c ratios compared with participants receiving atazanavir/ritonavir (each in combination with abacavir-lamivudine or tenofovir-emtricitabine). A recent meta-analysis compared the effects of the NNRTI, efavirenz, and various ritonavir-boosted PIs (including darunavir/ritonavir) on lipid levels using data from 15 clinical trials of first-line antiretroviral therapy in which standardized 48-wk lipids data were reported (n = 6368).65 In this study, efavirenz and the more recently introduced PIs, such as DRV and atazanavir, had only a modest impact on serum lipids and their pattern of effect differed. In a substudy of a trial in 91 antiretroviral-naïve patients randomly assigned to tenofovir + emtricitabine + atazanavir/ritonavir or EFV (patients assigned to EFV had greater increases in TC, LDL-c, and HDL-c and in large HDL particles, but not in TC:HDL-c ratio or indication for lipid-lowering interventions relative to patients assigned to atazanavir and ritonavir.66 |
| Nevirapine | ART regimens containing nevirapine are associated with a favorable lipid profile, mainly because they provide higher serum concentrations of HDL-c.63 |
| Etravirine | Switching from efavirenz or ritonavir-boosted PIs to etravirine led to a significant improvement of lipids irrespective of the presence of previous hyperlipidemia and type of ART.67 |
| Rilpivirine | Two phase 3 trials (ECHO68 and THRIVE69) of similar design, with the exception of the background NRTI regimen, compared rilpivirine with efavirenz in ART-naive patients with HIV. After 48 wk, TC, HDL-c, LDL-c and triglyceride concentrations were significantly greater in the patients randomly assigned to efavirenz than those receiving rilpivirine; however, the TC:HDL-c ratio did not change significantly between the treatment groups because of a greater HDL increase in the patients given efavirenz. |
Dyslipidemic effects of integrase inhibitors and C-C chemokine receptor type 5 antagonists
The integrase inhibitors, raltegravir, elvitegravir, and dolutegravir, and the C-C chemokine receptor type 5 (CCR5) receptor antagonist, maraviroc, appear to have little or no impact on lipid parameters, even in long-term use.73,74 Switching to these agents in patients who are well suppressed on first-line therapy may benefit many HIV-infected patients by improving their lipid profiles (Table 4).74–85 Preliminary studies support the beneficial lipid profile of unboosted integrase inhibitors.79 Recent data suggest that elvitegravir-cobicistat-tenofovir-emtricitabine induced similar changes in lipids compared with atazanavir/ritonavir and had less prominent effects on total and LDL cholesterol compared with efavirenz.83,84 Data from 2 independent studies confirms that the boosted PIs, atazanavir and darunavir, are associated with greater increases in TC and triglycerides compared with raltegravir.48,49
Table 4.
Studies investigating the effects of different NNRTIs on lipids
| New Antivirals | Comments |
|---|---|
| Maraviroc | In a mouse model of genetic dyslipidemia, maraviroc reduced atherosclerotic progression by interfering with inflammatory cell recruitment into plaques and by reversing the proinflammatory profile.75 Switching from PIs or NNRTIs to maraviroc, decreased TC and triglycerides in a small, randomized, clinical trial.76 MacInnes and colleagues74 investigated treatment-naive patients randomly assigned to receive either maraviroc or efavirenz in combination with zidovudine-lamivudine for up to 96 wk. The investigators reported that of the patients with baseline TC and LDL-c less than the National Cholesterol Education Programme treatment thresholds, more patients receiving efavirenz than those treated with maraviroc exceeded the thresholds for TC and LDL-c. Additionally, among participants with baseline lipid concentrations exceeding National Cholesterol Education Programme thresholds, 84% of patients on efavirenz vs 50% of those on maraviroc still exceeded the thresholds at 96 wk. |
| Raltegravir | In the STARTMRK trial, Rockstroh and colleagues77 compared raltegravir with efavirenz in treatment-naive adults, and identified that 240-wk increases in fasting triglycerides and TC, LDL-c, and HDL-c were significantly greater in those receiving efavirenz than raltegravir. Additionally, 9% of adults given raltegravir vs 34% given efavirenz needed initiation of lipid-lowering treatment during follow-up. Switching from different class-suppressive regimens to raltegravir and tenofovir and emtricitabine or abacavir and lamivudine led to improvements in plasma lipids after 48 wk.78 In virologically suppressed aging HIV-positive patients, there are promising results from small, short-term studies assessing dual therapy with raltegravir and a nonnucleoside reverse inhibitor such as etravirine or nevirapine.79,80 |
| Dolutegravir | Dolutegravir is a once-daily integrase inhibitor that does not need a boosting drug. In the SPRING-2 trial,81 a 96-wk, phase 3, randomized, double-blind, noninferiority study comparing dolutegravir with raltegravir in treatment-naive patients, no significant changes in lipid measures were shown in either treatment group. |
| Elvitegravir-cobicistat-emtricitabine-tenofovir DF | The fixed-dose combination of elvitegravir-cobicistat-emtricitabine-tenofovir DF, has been compared with efavirenz-tenofovir-emtricitabine and ritonavir plus atazanavir plus emtricitabine-tenofovir DF in treatment-naive adults, and statistically similar overall changes in lipid profiles among the 3 regimens were found.82 Rockstroh and colleagues83 compared elvitegravir-cobicistat with atazanavir/ritonavir in treatment-naive patients and reported that after 96 wk, changes in TC were greater with elvitegravir-cobicistat, but triglyceride increases were greater with atazanavir/ritonavir, and no difference in the TC:HDL-c ratio was noted between the 2 treatment groups. Sax and colleagues84 compared treatment of efavirenz-tenofovir DF-emtricitabine with elvitegravir-cobicistat-tenofovir DF-emtricitabine and showed that mean changes in TC, HDL-c, and LDL-c were greater in the efavirenz than elvitegravir-cobicistat group, whereas the TC:HDL-c ratio was the same in both groups. In another study, Elion and colleagues85 randomly assigned treated patients to either elvitegravir or raltegravir combination with a ritonavir-boosted PI and a third active drug. No differences in lipid concentrations were reported between the 2 treatment groups. Collectively, these results suggest that elvitegravir-cobicistat-tenofovir DF-emtricitabine has a similar lipid profile to ritonavir-atazanavir, and has less severe TC and LDL-c perturbations (but also less HDL improvement) than does efavirenz. |
LIPID CHANGES DURING TREATED HIV DISEASE MAY CONTRIBUTE TO IMMUNE ACTIVATION IN HIV INFECTION
A hallmark of HIV infection is activation of the immune system, which persists to some degree even after the initiation of effective ART.19 Although the exact mechanisms that drive this immune activation are unclear, residual HIV replication, microbial translocation, and inflammatory lipids are potential contributors. Modified lipoproteins such as oxidized LDL carry oxidized lipids and may regulate immunity.86 We recently showed that HIV-infected subjects have dysfunctional HDL that is associated with biomarkers of T-cell activation.87 We also showed that among HIV-infected subjects but not controls that dysfunctional HDL was significantly associated with circulating levels of the macrophage activation marker, soluble CD163.45 The temporal relationship between these observations remains unclear. Among HIV-infected patients, studies suggest that after the initiation of ART, salutary changes to HDL structure occur, possibly caused by improvements in immune activation.8 Data suggesting that oxidized forms of LDL are present in atherosclerotic lesions and constitute major epitopes for natural antibodies show that these lipids may be a stimulus for monocytes from HIV-infected subjects.38,88,89 Modified lipoproteins may also directly activate immune cells such as macrophages and T cells.90 Further studies are needed to elucidate the interplay between immune activation, ART, HDL structure/function of modified lipoproteins, and CVD risk in HIV.
MANAGEMENT OF LIPID DISORDERS
Diagnosis of Lipid Disorders in the Context of CVD
Current HIV treatment recommendations emphasize the importance of CVD risk screening in all patients starting ART and throughout the course of treatment.91,92 Fasting lipid levels should be obtained at initiation of care for HIV-infected subjects and before and within 1 to 3 months after starting ART.91 In addition, screening for other metabolic abnormalities should also be performed. For example, fasting blood glucose or hemoglobin A1c should be obtained before and within 1 to 3 months after starting ART.91,92 When triglycerides are greater than 500 mg/dL, the measurements of non–HDL-c, apoB, or both may be useful because measurement of LDL-c may underestimate the CVD risk.9,41 However, although fasting lipid levels determine well-established quantitative lipid abnormalities, there are no established diagnostic methods to determine qualitative abnormalities of lipids and lipoproteins.
Treatment of Dyslipidemia in HIV Infection
Management of dyslipidemia in patients with HIV follows recommendations for the general population according to the National Cholesterol Education Program Guidelines (Table 5).9,39,91–94
Table 5.
Treatment of dyslipidemia in the context of CVD in HIV infection
| Therapeutic Intervention | Comments |
|---|---|
| Lifestyle modifications | Diet, exercise, and smoking cessation. |
| Lipid-lowering therapy | |
| Statins that have minimal interactions with ART | PIs and ritonavir mainly inhibit cytochrome P and could increase the toxicity of some statins. NNRTIs (eg, efavirenz) are inducers of cytochrome P and could reduce statin efficacy. In the HIV-infected patient taking a PI, statins with a low risk for interaction with PIs should be preferred, such as pravastatin, fluvastatin, low-dose atorvastatin, or low-dose rosuvastatin.9,91,92 |
| Fibrates | Fibrates should be prescribed when the triglyceride concentration is >500 mg/d.9 |
| Fish oil | Limited evidence on the role of omega-3 fatty acids on the management and prevention of the metabolic abnormalities in HIV-infected patients but randomized, controlled clinical trials have not shown a clear benefit.93 |
| Switch strategies | |
| Switch PI to an NNRTI or to a new drug such as integrase or CCR5 inhibitors | See evidence presented in Tables 1–4. |
| Switch first-generation NRTIs (eg, stavudine) to second-generation NRTIs (eg, tenofovir) | See evidence presented in Table 2. |
| Switch first-generation NNRTIs (eg, efavirenz) to new NNRTI (eg, etravirine, rilpivirine) | See evidence presented in Table 3. |
| Switch first-generation NNRTIs (eg, efavirenz) to a new drug such as integrase inhibitors (eg, raltegravir) | See evidence presented in Tables 2 and 4. |
| Manage other comorbidities that may contribute to CVD | Regarding hypertension, blockers of the rennin-angiotensin system should be the first therapy because of their protective effects on the vasculature, kidney function, and favorable metabolic effects.9 Telmisartan is being evaluated for favorable effects on visceral adiposity in HIV-infected subjects.39 Antiplatelet drugs such as aspirin, clopidogrel, prasugrel, and ticagrelor should be given according to the guidelines for the general population.9 Diabetes and insulin resistance should be managed according to the guidelines for the general population and HIV-infected subjects.91,92 |
Lifestyle changes are the initial step in management of dyslipidemia and CVD risk in HIV-infected subjects. Lifestyle modifications including diet, exercise, and smoking cessation should be the first step in management, whereas lipid-lowering therapy and ART changes should be considered for patients at high risk of CVD.91 Diet and exercise improved lipid profile in patients on ART with hypertriglyceridemia95; however, a recent meta-analysis of dietary intervention studies in patients with HIV reported only slight effects on triglyceride concentrations.96
Lipid-lowering therapy should be prescribed with caution in HIV-infected patients. There are significant drug interactions between lipid-lowering agents and PIs most notably among the statin drugs simvastatin and lovastatin.9 HIV guidelines recommend the use of statins that have fewer interactions with ART, such as pravastatin and atorvastatin,91 whereas use of newer statins such as pitavastatin may further reduce these interactions.91 Treating hypertriglyceridemia may be challenging in HIV-infected patients. In a recent study, fibrates were more effective than fish oil or atorvastatin at lowering plasma triglycerides in HIV-infected patients with hypertrigly-ceridemia,93 suggesting that fibrates should be the first choice.
Switch strategies that deploy newer antiretrovirals with more favorable lipid profile (integrase inhibitors, second-generation NNRTIs, and newer PIs) are increasingly used as an intervention for ART-related dyslipidemia. Improvements in lipids have been seen when patients with dyslipidemia on ritonavir-boosted PIs were switched from abacavir to tenofovir97 or when the PI was switched to another agent.98 The SWITCHMRK study98 found that switch from a lopinavir/ritonavir-based regimen to a raltegravir-based regimen had favorable effects on the lipid profile in patients. However, clinicians should consider prior treatment history before switching antiretrovirals, as higher rates of virologic failure have been noted among those with prior failure who were switched from a boosted PI to a raltegravir based regimen.
Strategies to increase HDL cholesterol levels and HDL function in HIV-infected individuals should be investigated. Although there are available therapies for elevated LDL-c levels, therapeutic strategies to increase HDL levels are limited and of unclear clinical significance.99 In general, treatment with NNRTI-based regimens appears to increase HDL levels more so than therapy with other classes of drugs. The clinical significance of this effect is unclear. Thus, therapies that may also improve HDL function in HIV infection need further study as a CVD prevention strategy.
SUMMARY
As the HIV population ages, it is important to prevent development of long-term comorbidities such as CVD. The mechanisms of atherosclerosis in HIV remain to be fully elucidated. Host, virus, immune deficiency, and ART factors have a major role in the increased risk for CVD in HIV and lipid changes may both be a consequence and a driver of these interactions (Fig. 2). During untreated HIV infection, lipid alterations are associated with the virus and its effects on the immune system. These are characterized by a decrease of TC, LDL-c, and HDL-c and by an increase of triglyceride levels. In contrast, ART regimens promoted distinct alterations in the lipid metabolism of these patients and vary by individual agents. Thus, it is critical to address traditional risk factors for CVD, such as dyslipidemia, in the HIV-infected population. Clinicians need to focus on improved methods for screening and treatment of lipid disorders while taking into consideration potential drug-drug interactions, particularly with statins and ART. Newer HIV drugs, such as etravirine, rilpivirine, raltegravir, dolutegravir, and elvitegravir, are metabolically well-tolerated drug options and may be particularly useful for aging HIV-infected patients. As our understanding of genetic predisposition to dyslipidemia in HIV-infected patients improves, these findings should translate from research to clinical care. Further research is needed to fully elucidate the pathophysiology of dyslipidemia in HIV-infected patients, with particular emphasis on defining the roles of lipids in inflammation, immune activation, and CVD.
Fig. 2.
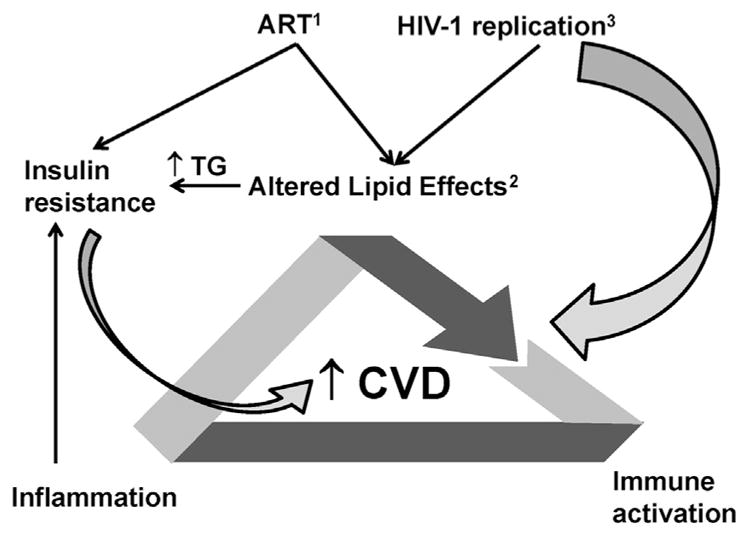
Factoring influencing dyslipidemia and CVD risk in HIV. ART directly alters lipid metabolism and may increase the risk of insulin resistance (1). The lipid effects of ART include elevated triglycerides and for some agents elevations in TC. The elevations in triglycerides may also contribute to insulin resistance and to CVD risk (2). HIV replication per se increases immune activation and may have a direct effect on CVD risk (3). In addition HIV replication may indirectly alter HDL function via indirect effects on inflammation. These altered lipids may have direct and indirect immunoregulatory effects and induce further activation of immune cells (T cells and monocytes/macrophages), which may further increase systemic inflammation. Inflammation also contributes to insulin resistance, which, in turn, may increase CVD risk. Thus, there are complex interactions between these pathophysiologic processes that are at least partially driven by altered lipids that are formed during HIV infection and that may directly or indirectly contribute to increased CVD in HIV-infected subjects. TG, triglycerides.
KEY POINTS.
Since the advent of effective antiretroviral therapy, cardiovascular disease has become a major cause of morbidity and mortality in the population with human immunodeficiency virus.
The pathogenesis of atherosclerosis in human immunodeficiency virus–infected individuals is complex, and proatherogenic quantitative and qualitative changes in lipids have a major role in this process.
HIV replication, chronic inflammation and immune activation, and exposure to antiretroviral drugs (either directly or through metabolic abnormalities) may contribute to development of dyslipidemia in human immunodeficiency virus infection.
As we gain a better understanding of lipid abnormalities in human immunodeficiency virus–infected patients and their role in immune activation and cardiovascular disease, these findings must translate into interventions for clinical care.
Footnotes
Disclosures: J.S. Currier: Received grant funds to UCLA from Merck. T. Kelesidis: None.
References
- 1.Sackoff JE, Hanna DB, Pfeiffer MR, et al. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145(6):397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 2.Currier JS, Lundgren JD, Carr A, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–35. doi: 10.1161/CIRCULATIONAHA.107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51(3):268–73. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker JV, Neuhaus J, Duprez D, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011;56(1):36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Sadr WM, Lundgren J, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 7.Grunfeld C, Delaney JA, Wanke C, et al. Preclinical atherosclerosis due to HIV infection: carotid intimamedial thickness measurements from the FRAM study. AIDS. 2009;23(14):1841–9. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker JV, Neuhaus J, Duprez D, et al. Inflammation predicts changes in high-density lipoprotein particles and apolipoprotein A1 following initiation of antiretroviral therapy. AIDS. 2011;25(17):2133–42. doi: 10.1097/QAD.0b013e32834be088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boccara F, Lang S, Meuleman C, et al. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol. 2013;61(5):511–23. doi: 10.1016/j.jacc.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 10.Choi AI, Li Y, Deeks SG, et al. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121(5):651–8. doi: 10.1161/CIRCULATIONAHA.109.898585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friis-Moller N, Thiebaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17(5):491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 12.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. Important reference for key points. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan RC, Landay AL, Hodis HN, et al. Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr. 2012;60(4):359–68. doi: 10.1097/QAI.0b013e31825b03be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gresele P, Falcinelli E, Sebastiano M, et al. Endothelial and platelet function alterations in HIV-infected patients. Thromb Res. 2012;9(3):301–8. doi: 10.1016/j.thromres.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Kelesidis T, Kendall MA, Yang OO, et al. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206(10):1558–67. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204(8):1227–36. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308(4):379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blodget E, Shen C, Aldrovandi G, et al. Relationship between microbial translocation and endothelial function in HIV infected patients. PLoS One. 2012;7(8):e42624. doi: 10.1371/journal.pone.0042624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 20.Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients–association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17(8):1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 21.Egana-Gorrono L, Martinez E, Cormand B, et al. Impact of genetic factors on dyslipidemia in HIV-infected patients starting antiretroviral therapy. AIDS. 2013;27(4):529–38. doi: 10.1097/QAD.0b013e32835d0da1. [DOI] [PubMed] [Google Scholar]
- 22.Nicholaou MJ, Martinson JJ, Abraham AG, et al. HAART-associated dyslipidemia varies by biogeographical ancestry in the multicenter AIDS cohort study. AIDS Res Hum Retroviruses. 2013;29(6):871–9. doi: 10.1089/aid.2012.0169. Important reference for key points. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassol E, Misra V, Holman A, et al. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis. 2013;13:203. doi: 10.1186/1471-2334-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grunfeld C, Feingold KR. The role of the cytokines, interferon alpha and tumor necrosis factor in the hypertriglyceridemia and wasting of AIDs. J Nutr. 1992;122(3 Suppl):749–53. doi: 10.1093/jn/122.suppl_3.749. [DOI] [PubMed] [Google Scholar]
- 25.Grunfeld C, Pang M, Doerrler W, et al. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74(5):1045–52. doi: 10.1210/jcem.74.5.1373735. Important reference for key points. [DOI] [PubMed] [Google Scholar]
- 26.Drozd DR, Nance RM, Delaney JA, et al. Lower CD4 Count and Higher Viral Load Are Associated With Increased Risk of Myocardial Infarction. Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) Cohort. 21st Conference on Retroviruses and Opportunistic Infections; Boston (MA). March 3–6, 2014; abstract: 739. [Google Scholar]
- 27.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289(22):2978–82. doi: 10.1001/jama.289.22.2978. Important reference for key points. [DOI] [PubMed] [Google Scholar]
- 28.Grunfeld C, Kotler DP, Hamadeh R, et al. Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1989;86(1):27–31. doi: 10.1016/0002-9343(89)90225-8. [DOI] [PubMed] [Google Scholar]
- 29.Duprez DA, Kuller LH, Tracy R, et al. Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis. 2009;207(2):524–9. doi: 10.1016/j.atherosclerosis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enkhmaa B, Anuurad E, Zhang W, et al. HIV disease activity as a modulator of lipoprotein(a) and allele-specific apolipoprotein(a) levels. Arterioscler Thromb Vasc Biol. 2013;33(2):387–92. doi: 10.1161/ATVBAHA.112.300125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khovidhunkit W, Memon RA, Feingold KR, et al. Infection and inflammation-induced proatherogenic changes of lipoproteins. J Infect Dis. 2000;181(Suppl 3):S462–72. doi: 10.1086/315611. [DOI] [PubMed] [Google Scholar]
- 32.Rose H, Hoy J, Woolley I, et al. HIV infection and high density lipoprotein metabolism. Atherosclerosis. 2008;199(1):79–86. doi: 10.1016/j.atherosclerosis.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khera AV, Cuchel M, Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–35. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mujawar Z, Rose H, Morrow MP, et al. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 2006;4(11):e365. doi: 10.1371/journal.pbio.0040365. Important reference for key points. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feeney ER, McAuley N, O’Halloran JA, et al. The expression of cholesterol metabolism genes in monocytes from HIV-infected subjects suggests intracellular cholesterol accumulation. J Infect Dis. 2013;207(4):628–37. doi: 10.1093/infdis/jis723. [DOI] [PubMed] [Google Scholar]
- 36.Van Lenten BJ, Hama SY, de Beer FC, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995;96(6):2758–67. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 38.Holvoet P, Lee DH, Steffes M, et al. Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299(19):2287–93. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lake JE, Currier JS. Metabolic disease in HIV infection. Lancet Infect Dis. 2013;13(11):964–75. doi: 10.1016/S1473-3099(13)70271-8. [DOI] [PubMed] [Google Scholar]
- 40.Haubrich RH, Riddler SA, DiRienzo AG, et al. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. AIDS. 2009;23(9):1109–18. doi: 10.1097/QAD.0b013e32832b4377. Important reference for key points. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navab M, Reddy ST, Van Lenten BJ, et al. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8(4):222–32. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 42.Kelesidis T, Currier JS, Huynh D, et al. A biochemical fluorometric method for assessing the oxidative properties of HDL. J Lipid Res. 2011;52(12):2341–51. doi: 10.1194/jlr.D018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelesidis T, Yang OO, Currier JS, et al. HIV-1 infected patients with suppressed plasma viremia on treatment have pro-inflammatory HDL. Lipids Health Dis. 2011;10:35. doi: 10.1186/1476-511X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelesidis T, Yang OO, Kendall MA, et al. Dysfunctional HDL and progression of atherosclerosis in HIV-1-infected and -uninfected adults. Lipids Health Dis. 2013;12:23. doi: 10.1186/1476-511X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zanni MV, Kelesidis T, Fitzgerald ML, et al. HDL redox activity is increased in HIV-infected men in association with macrophage activation and noncalcified coronary atherosclerotic plaque. Antivir Ther. doi: 10.3851/IMP2756. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carr A, Samaras K, Chisholm DJ, et al. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998;351(9119):1881–3. doi: 10.1016/S0140-6736(98)03391-1. Important reference for key points. [DOI] [PubMed] [Google Scholar]
- 47.Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24(8):1228–30. doi: 10.1097/QAD.0b013e328339192f. [DOI] [PubMed] [Google Scholar]
- 48.Aberg JA, Tebas P, Overton ET, et al. Metabolic effects of darunavir/ritonavir versus atazanavir/ritonavir in treatment-naive, HIV type 1-infected subjects over 48 weeks. AIDS Res Hum Retroviruses. 2012;28(10):1184–95. doi: 10.1089/aid.2011.0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ofotokun I, Ribaudo H, Na L, et al. Darunavir or atazanavir vs raltegravir lipid changes are unliked to ritonavir exposure: ACTG 5257. Presented at the 21st Conference on Retroviruses and Opportunistic Infections; Boston (MA). March 3–6, 2014; abstract: #746. [Google Scholar]
- 50.Mobius U, Lubach-Ruitman M, Castro-Frenzel B, et al. Switching to atazanavir improves metabolic disorders in antiretroviral-experienced patients with severe hyperlipidemia. J Acquir Immune Defic Syndr. 2005;39(2):174–80. [PubMed] [Google Scholar]
- 51.Mills AM, Nelson M, Jayaweera D, et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS. 2009;23(13):1679–88. doi: 10.1097/QAD.0b013e32832d7350. [DOI] [PubMed] [Google Scholar]
- 52.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D: A:D) study. J Infect Dis. 2010;201(3):318–30. doi: 10.1086/649897. Important reference for key points. [DOI] [PubMed] [Google Scholar]
- 53.Menezes CN, Crowther NJ, Duarte R, et al. A randomized clinical trial comparing metabolic parameters after 48 weeks of standard- and low-dose stavudine therapy and tenofovir disoproxil fumarate therapy in HIV-infected South African patients. HIV Med. 2014;15(1):3–12. doi: 10.1111/hiv.12074. [DOI] [PubMed] [Google Scholar]
- 54.Crane HM, Grunfeld C, Willig JH, et al. Impact of NRTIs on lipid levels among a large HIV-infected cohort initiating antiretroviral therapy in clinical care. AIDS. 2011;25(2):185–95. doi: 10.1097/QAD.0b013e328341f925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ribaudo HJ, Benson CA, Zheng Y, et al. No risk of myocardial infarction associated with initial antiretroviral treatment containing abacavir: short and long-term results from ACTG A5001/ALLRT. Clin Infect Dis. 2011;52(7):929–40. doi: 10.1093/cid/ciq244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lang S, Mary-Krause M, Cotte L, et al. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med. 2010;170(14):1228–38. doi: 10.1001/archinternmed.2010.197. [DOI] [PubMed] [Google Scholar]
- 57.Sabin CA, Worm SW, Weber R, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D: A:D study: a multi-cohort collaboration. Lancet. 2008;371(9622):1417–26. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brothers CH, Hernandez JE, Cutrell AG, et al. Risk of myocardial infarction and abacavir therapy: no increased risk across 52 GlaxoSmithKline-sponsored clinical trials in adult subjects. J Acquir Immune Defic Syndr. 2009;51(1):20–8. doi: 10.1097/QAI.0b013e31819ff0e6. [DOI] [PubMed] [Google Scholar]
- 59.Sax PE, Tierney C, Collier AC, et al. Abacavir/lamivudine versus tenofovir DF/emtricitabine as part of combination regimens for initial treatment of HIV: final results. J Infect Dis. 2011;204(8):1191–201. doi: 10.1093/infdis/jir505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saumoy M, Ordonez-Llanos J, Martinez E, et al. Low-density lipoprotein size and lipoprotein-associated phospholipase A2 in HIV-infected patients switching to abacavir or tenofovir. Antivir Ther. 2011;16(4):459–68. doi: 10.3851/IMP1785. [DOI] [PubMed] [Google Scholar]
- 61.Martinez E, d’Albuquerque PM, Perez I, et al. Abacavir/lamivudine versus tenofovir/emtricitabine in virologically suppressed patients switching from ritonavir-boosted protease inhibitors to raltegravir. AIDS Res Hum Retroviruses. 2013;29(2):235–41. doi: 10.1089/aid.2012.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guaraldi G, Zona S, Cossarizza A, et al. Randomized trial to evaluate cardiometabolic and endothelial function in patients with plasma HIV-1 RNA suppression switching to darunavir/ritonavir with or without nucleoside analogues. HIV Clin Trials. 2013;14(4):140–8. doi: 10.1310/hct1404-140. [DOI] [PubMed] [Google Scholar]
- 63.Williams P, Wu J, Cohn S, et al. Improvement in lipid profiles over 6 years of follow-up in adults with AIDS and immune reconstitution. HIV Med. 2009;10(5):290–301. doi: 10.1111/j.1468-1293.2008.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daar ES, Tierney C, Fischl MA, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med. 2011;154(7):445–56. doi: 10.1059/0003-4819-154-7-201104050-00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hill A, Sawyer W, Gazzard B. Effects of first-line use of nucleoside analogues, efavirenz, and ritonavir-boosted protease inhibitors on lipid levels. HIV Clin Trials. 2009;10(1):1–12. doi: 10.1310/hct1001-001. [DOI] [PubMed] [Google Scholar]
- 66.Gotti D, Cesana BM, Albini L, et al. Increase in standard cholesterol and large HDL particle subclasses in antiretroviral-naive patients prescribed efavirenz compared to atazanavir/ritonavir. HIV Clin Trials. 2012;13(5):245–55. doi: 10.1310/hct1305-245. [DOI] [PubMed] [Google Scholar]
- 67.Casado JL, de los Santos I, Del Palacio M, et al. Lipid-lowering effect and efficacy after switching to etravirine in HIV-infected patients with intolerance to suppressive HAART. HIV Clin Trials. 2013;14(1):1–9. doi: 10.1310/hct1401-1. [DOI] [PubMed] [Google Scholar]
- 68.Molina JM, Cahn P, Grinsztejn B, et al. Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): a phase 3 randomised double-blind active-controlled trial. Lancet. 2011;378(9787):238–46. doi: 10.1016/S0140-6736(11)60936-7. [DOI] [PubMed] [Google Scholar]
- 69.Cohen CJ, Andrade-Villanueva J, Clotet B, et al. Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV-1 (THRIVE): a phase 3, randomised, non-inferiority trial. Lancet. 2011;378(9787):229–37. doi: 10.1016/S0140-6736(11)60983-5. [DOI] [PubMed] [Google Scholar]
- 70.van der Valk M, Kastelein JJ, Murphy RL, et al. Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. AIDS. 2001;15(18):2407–14. doi: 10.1097/00002030-200112070-00008. [DOI] [PubMed] [Google Scholar]
- 71.Vigano A, Aldrovandi GM, Giacomet V, et al. Improvement in dyslipidaemia after switching stavudine to tenofovir and replacing protease inhibitors with efavirenz in HIV-infected children. Antivir Ther. 2005;10(8):917–24. [PubMed] [Google Scholar]
- 72.Fatkenheuer G, Duvivier C, Rieger A, et al. Lipid profiles for etravirine versus efavirenz in treatment-naive patients in the randomized, double-blind SENSE trial. J Antimicrob Chemother. 2012;67(3):685–90. doi: 10.1093/jac/dkr533. [DOI] [PubMed] [Google Scholar]
- 73.Rockstroh JK, Lennox JL, DeJesus E, et al. Long-term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment-naive human immunodeficiency virus-1-infected patients: 156-week results from STARTMRK. Clin Infect Dis. 2011;53(8):807–16. doi: 10.1093/cid/cir510. [DOI] [PubMed] [Google Scholar]
- 74.MacInnes A, Lazzarin A, Di Perri G, et al. Maraviroc can improve lipid profiles in dyslipidemic patients with HIV: results from the MERIT trial. HIV Clin Trials. 2011;12(1):24–36. doi: 10.1310/hct1201-24. [DOI] [PubMed] [Google Scholar]
- 75.Cipriani S, Francisci D, Mencarelli A, et al. Efficacy of the CCR5 antagonist maraviroc in reducing early, ritonavir-induced atherogenesis and advanced plaque progression in mice. Circulation. 2013;127(21):2114–24. doi: 10.1161/CIRCULATIONAHA.113.001278. [DOI] [PubMed] [Google Scholar]
- 76.Bonjoch A, Pou C, Perez-Alvarez N, et al. Switching the third drug of antiretroviral therapy to maraviroc in aviraemic subjects: a pilot, prospective, randomized clinical trial. J Antimicrob Chemother. 2013;68(6):1382–7. doi: 10.1093/jac/dks539. [DOI] [PubMed] [Google Scholar]
- 77.Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63(1):77–85. doi: 10.1097/QAI.0b013e31828ace69. [DOI] [PubMed] [Google Scholar]
- 78.Fabbiani M, Mondi A, Colafigli M, et al. Safety and efficacy of treatment switch to raltegravir plus tenofovir/emtricitabine or abacavir/lamivudine in patients with optimal virological control: 48-week results from a randomized pilot study (Raltegravir Switch for Toxicity or Adverse Events, RASTA Study) Scand J Infect Dis. 2014;46(1):34–45. doi: 10.3109/00365548.2013.840920. [DOI] [PubMed] [Google Scholar]
- 79.Monteiro P, Perez I, Laguno M, et al. Dual therapy with etravirine plus raltegravir for virologically suppressed HIV-infected patients: a pilot study. J Antimicrob Chemother. 2014;69(3):742–8. doi: 10.1093/jac/dkt406. [DOI] [PubMed] [Google Scholar]
- 80.Reliquet V, Chirouze C, Allavena C, et al. Nevirapine-raltegravir combination, an NRTI and PI/r sparing regimen, as maintenance antiretroviral therapy in virologically suppressed HIV-1-infected patients. Antivir Ther. 2014;19(1):117–23. doi: 10.3851/IMP2691. [DOI] [PubMed] [Google Scholar]
- 81.Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–43. doi: 10.1016/S0140-6736(12)61853-4. [DOI] [PubMed] [Google Scholar]
- 82.FDA notifications. Ongoing safety review of abacavir, possible MI risk. AIDS Alert. 2011;26(5):58–9. [PubMed] [Google Scholar]
- 83.Rockstroh JK, DeJesus E, Henry K, et al. A randomized, double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir DF vs ritonavir-boosted atazanavir plus coformulated emtricitabine and tenofovir DF for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr. 2013;62(5):483–6. doi: 10.1097/QAI.0b013e318286415c. [DOI] [PubMed] [Google Scholar]
- 84.Sax PE, DeJesus E, Mills A, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379(9835):2439–48. doi: 10.1016/S0140-6736(12)60917-9. [DOI] [PubMed] [Google Scholar]
- 85.Elion R, Molina JM, Ramon Arribas LJ, et al. A randomized phase 3 study comparing once-daily elvitegravir with twice-daily raltegravir in treatment-experienced subjects with HIV-1 infection: 96-week results. J Acquir Immune Defic Syndr. 2013;63(4):494–7. doi: 10.1097/QAI.0b013e318298469c. [DOI] [PubMed] [Google Scholar]
- 86.Tsimikas S, Miller YI. Oxidative modification of lipoproteins: mechanisms, role in inflammation and potential clinical applications in cardiovascular disease. Curr Pharm Des. 2011;17(1):27–37. doi: 10.2174/138161211795049831. [DOI] [PubMed] [Google Scholar]
- 87.Kelesidis T, Flores M, Tseng CH, et al. HIV-infected adults with suppressed viremia on antiretroviral therapy have dysfunctional HDL that is associated with T cell activation. Presented at IDWeek 2012; San Diego (CA). October 17–21, 2012. Abstract 662. [Google Scholar]
- 88.Bjorkbacka H, Fredrikson GN, Nilsson J. Emerging biomarkers and intervention targets for immune-modulation of atherosclerosis - a review of the experimental evidence. Atherosclerosis. 2013;227(1):9–17. doi: 10.1016/j.atherosclerosis.2012.10.074. [DOI] [PubMed] [Google Scholar]
- 89.Yilmaz A, Jennbacken K, Fogelstrand L. Reduced IgM levels and elevated IgG levels against oxidized low-density lipoproteins in HIV-1 infection. BMC Infect Dis. 2014;14:143. doi: 10.1186/1471-2334-14-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Graham LS, Parhami F, Tintut Y, et al. Oxidized lipids enhance RANKL production by T lymphocytes: implications for lipid-induced bone loss. Clin Immunol. 2009;133(2):265–75. doi: 10.1016/j.clim.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(1):1–10. doi: 10.1093/cid/cit757. Important reference for key points. [DOI] [PubMed] [Google Scholar]
- 92.Lundgren JD, Battegay M, Behrens G, et al. European AIDS Clinical Society (EACS) guidelines on the prevention and management of metabolic diseases in HIV. HIV Med. 2008;9(2):72–81. doi: 10.1111/j.1468-1293.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 93.Munoz MA, Liu W, Delaney JA, et al. Comparative effectiveness of fish oil versus fenofibrate, gemfibrozil, and atorvastatin on lowering triglyceride levels among HIV-infected patients in routine clinical care. J Acquir Immune Defic Syndr. 2013;64(3):254–60. doi: 10.1097/QAI.0b013e3182a60e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grinspoon SK, Grunfeld C, Kotler DP, et al. State of the science conference: Initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS: executive summary. Circulation. 2008;118(2):198–210. doi: 10.1161/CIRCULATIONAHA.107.189622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wooten JS, Nambi P, Gillard BK, et al. Intensive lifestyle modification reduces Lp-PLA2 in dyslipidemic HIV/HAART patients. Med Sci Sports Exerc. 2013;45(6):1043–50. doi: 10.1249/MSS.0b013e3182843961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stradling C, Chen YF, Russell T, et al. The effects of dietary intervention on HIV dyslipidaemia: a systematic review and meta-analysis. PLoS One. 2012;7(6):e38121. doi: 10.1371/journal.pone.0038121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Campo R, DeJesus E, Bredeek UF, et al. SWIFT: prospective 48-week study to evaluate efficacy and safety of switching to emtricitabine/tenofovir from lamivudine/abacavir in virologically suppressed HIV-1 infected patients on a boosted protease inhibitor containing antiretroviral regimen. Clin Infect Dis. 2013;56(11):1637–45. doi: 10.1093/cid/cis1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eron JJ, Young B, Cooper DA, et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multi-centre, double-blind, randomised controlled trials. Lancet. 2010;375(9712):396–407. doi: 10.1016/S0140-6736(09)62041-9. Important reference for key points. [DOI] [PubMed] [Google Scholar]
- 99.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007;298(7):786–98. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 100.Behrens G, Maserati R, Rieger A, et al. Switching to tenofovir/emtricitabine from abacavir/lamivudine in HIV-infected adults with raised cholesterol: effect on lipid profiles. Antivir Ther. 2012;17(6):1011–20. doi: 10.3851/IMP2305. [DOI] [PubMed] [Google Scholar]