Abstract
Abnormal expression of annexin A2 contributes to metastasis and infiltration of cancer cells. To elucidate the cause of abnormal expression of annexin A2, Western blotting, immunoproteomics and immunohistochemical staining were performed to analyze differentially ubiquitinated proteins between fresh breast cancer tissue and its adjacent normal breast tissue from five female volunteers. We detected an ubiquitinated protein that was up-regulated in the cancer tissue, which was further identified as annexin A2 by mass spectrometry. These results suggest that abnormal ubiquitination and/or degradation of annexin A2 may lead to presence of annexin A2 at high level, which may further promote metastasis and infiltration of the breast cancer cells.
Keywords: Annexin A2, Breast cancer, Immunoproteomics, Ubiquitination
Introduction
Annexin A2 is a 36 kDa peripheral membrane protein that belongs to the annexin family of Ca2+ and phospholipid-binding proteins, which plays a role in membrane organization and dynamics in particular along the endocytic pathway [1]. Up-regulation of annexin A2 has been observed in cancer cells, along with high expression of plasminogen that is activated by annexin A2, which might contribute to hemorrhage in the tissue and metastasis of cancer cells [2]. Abnormal expression of annexin A2 was found in a variety of tumors such as pancreatic cancer, prostate carcinoma, hepatocellular carcinoma, oral squamous cell carcinoma and breast cancer [3], [4], [5], [6], [7], [8]. Over-expression of annexin A2 is associated with tumor invasion and progression [9].
Ubiquitin-dependent proteolysis mediates selective degradation of important proteins such as various cell cycle regulators, transcription factors, and tumor suppressors [10], [11], [12]. It has been reported that regulation of annexin A2 is associated with ubiquitination in endothelial cells [13]. In breast cancer tissue, we previously observed abnormal expression of some components of the ubiquitin proteasome system [14], and the results also indicated that the general ubiquitination profiling was changed in breast cancer. To understand whether abnormal expression of annexin A2 is correlated to abnormal ubiquitination in breast cancer tissue, we investigated differential protein ubiquitination between breast cancer and the adjacent normal tissue, using two-dimensional gel electrophoresis (2-DE), immunoblotting, mass spectrometry (MALDI-TOF-TOF MS) and immunohistochemistry.
Results
To examine the protein ubiquitination in cancer tissue and the adjacent normal tissue, we prepared protein samples from the two tissues and performed Western blotting using an anti-ubiquitin antibody after SDS–PAGE. As shown in Figure 1, different patterns were revealed for these two tissues. For example, protein of 66 kDa (serum albumin) was heavily ubiquitinated in cancer tissue, compared to the normal tissue. Interestingly, a band with molecular weight approximately 45 kDa was easily detected in cancer tissue sample by immunoblotting and protein staining with Coomassie Brilliant Blue R-250, while it was barely seen in sample from adjacent normal tissue.
Figure 1.
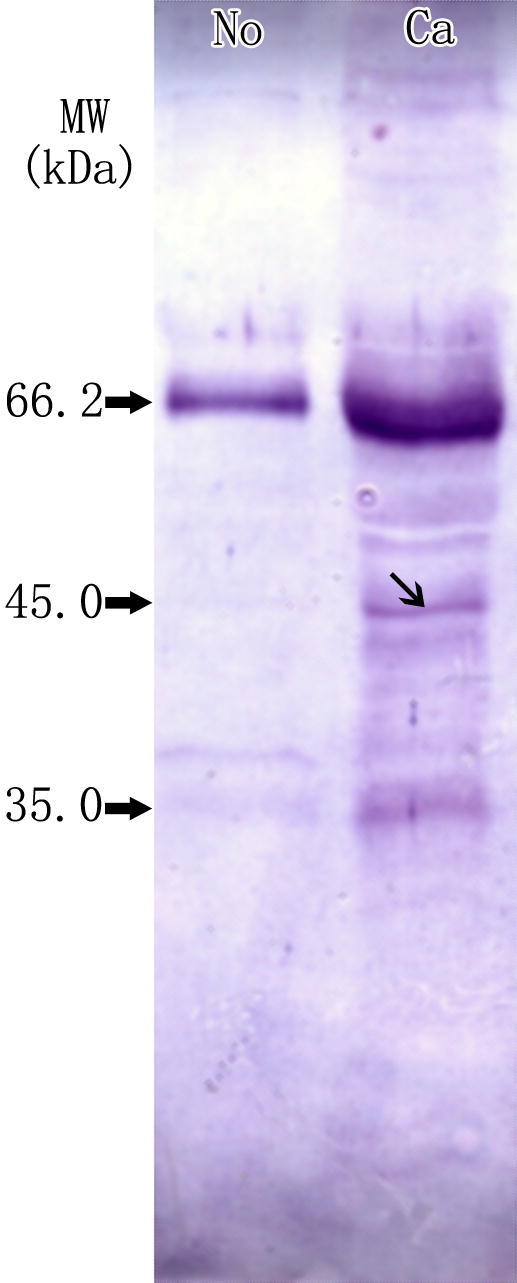
Differential ubiquitination between breast cancer tissue and the adjacent normal tissue using SDS–PAGE Western blotting was performed on protein samples from breast cancer tissue (Ca) and adjacent normal tissue (No) using anti-ubiquitin antibody. The molecular weight of the band indicated by ↘ is around 45 kDa.
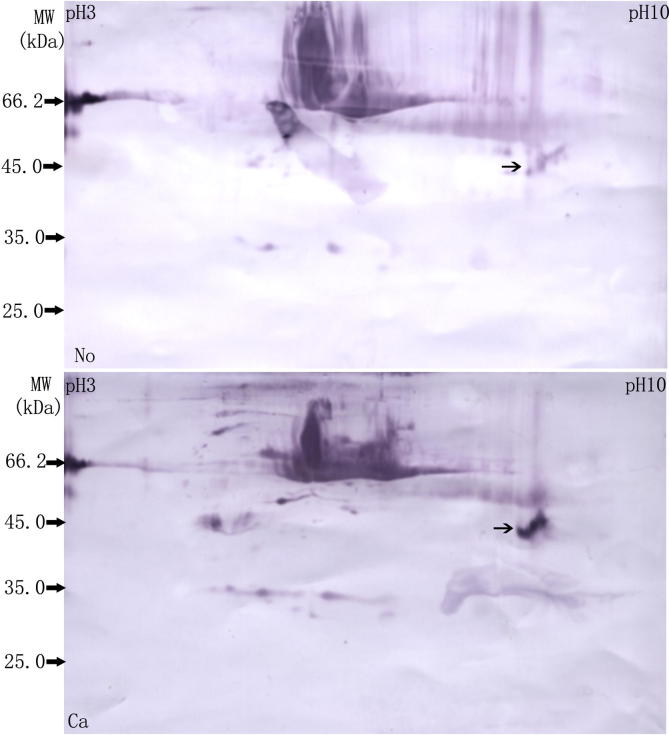
We next ran 2-D electrophoresis (2-DE) of these two samples and performed immunoblotting with an anti-ubiquitin antibody. As shown in Figure 2, we detected a broad dense spot of molecular weight approximately 45kD in protein samples from cancer tissues. The intensity of this spot was more than three fold as high as that from adjacent normal tissues according to PDQuest software 7.1, indicating a significant difference.
Figure 2.
Differentially-ubiquitinated spots between breast cancer tissue and the adjacent normal tissue using 2-DE Protein from breast cancer tissue (Ca) and adjacent normal tissue (No) was separated using 2-DE (IPG 7 cm, pH3–10, nonlinear). Western blotting was performed using anti-ubiquitin antibody. The molecular weight of the spot indicated by → is around 45 kDa.
The two spots (a and b), which corresponded to the single spot with differential ubiquitination in Figure 2, were excised from the duplicate gel and subjected to MALDI-TOF-TOF mass spectrometry (MS) (Figure 3). To avoid bias of position from membrane to gel, an adjacent spot (c) was also excised for MS analysis from the duplicate gel stained with Coomassie Brilliant Blue R-250. The results showed that all of these three spot were identified as annexin A2 (Table 1).
Figure 3.
2-DE gel spots subjected to MALDI-TOF-TOF MS Two spots (a and b) identified as differentially-ubiquitinated according to Figure 2 and an adjacent spot (c) were cut from 2-DE gels stained with Coomassie Brilliant Blue R-250 and subjected to MALDI-TOF-TOF MS. All of them were identified as annexin A2.
Table 1.
Identification of protein spots by MALDI-TOF-TOF MS analysis
| Protein spot | Protein name | Accession No. | MW (Da) | Protein PI | No. of unique peptides matched |
|---|---|---|---|---|---|
| a | ANXA2 protein | gi|73909156 | 40502.8 | 8.41 | 13 |
| b | Chain A, Annexin A2 | gi|56966699 | 38637.8 | 6.92 | 13 |
| c | Annexin A2 | gi|16306978 | 38593.8 | 7.57 | 14 |
To further validate the expression of annexin A2 in cancer tissue, we did immunohistochemical staining using annexin A2 antibodies. It was shown that the cytoplasm of cancer cells from all patients was positive for annexin A2 staining while that of cells from adjacent normal tissue was negative (Figure 4).
Figure 4.
Immunohistochemical staining of annexin A2 in tissue sections Paraffin sections of breast cancer tissue (Ca) and the adjacent normal tissue (No) were staining by immunohistochemical method with anti-annexin A2 antibody (DAB coloring, 200 folds).
Discussion
The ubiquitin proteasome pathway degrades many growth factors, oncogene proteins (c-myc, c-fos, c-jun and src) and cancer suppressor proteins (p53 and p27), and is therefore associated with cancer transformation, tumor progress, immune monitoring escape and tumor drug resistance [16], [17]. Metastasis and infiltration are important biological characteristics of cancer cells, and overexpression of annexin A2 contributes to these processes in breast cancer cells [9].
In this study, we examined ubiquitination of total proteins from human fresh breast cancer tissue and its adjacent normal tissue with western blotting using anti-ubiquitin antibody, and observed that there was a differentially-ubiquitinated protein of approximately 45kD. 2-DE accompanied by western blotting and MALDI-TOF-TOF MS was used to identify differential spots.
We previously reported overexpression of genes and proteins of ubiquitin specific peptidases and proteasome subunits in breast cancer tissue, which suggested that functions of the ubiquitin proteasome pathway might be altered [14]. Here, by immunoproteomics and MALDI-TOF-TOF MS, we find that annexin A2 is ubiquitinated in human breast cancer tissue, suggesting that regulation of annexin A2 might involve the ubiquitin proteasome pathway in this tissue. By immunohistochemistry, we verified overexpression of annexin A2 in breast cancer tissue. The results indicated that annexin A2 in breast cancer was overexpressed and abnormally ubiquitinated.
Regulation of annexin A2 is associated with ubiquitination in endothelial cells [13]. Polyubiquitinated proteins are generally degraded by 26S proteasome, while monoubiquitination is a modulating signal which causes the target protein to alter its activity, positioning or structure, and thus regulating the endocytosis pathway, histone modification, gene transcription and protein nuclear localization [18]. Annexin A1, another member of annexin family, was reported to be modified by ubiquitin and SUMO (small ubiquitin-like modifier) [19]. Mono-ubiquitinated Annexin A1 has higher affinity for damaged DNA, while SUMOylated annexin A1 functioned in proliferation-differentiation [19]. Annexin A2 was ubiquitinated in the breast cancer tissue, which might be explained in two ways: (i) Annexin A2 is attached with poly ubiquitination (a and b spots, Figure 3), so annexin A2 might be degraded by 26S proteasome, which may explain much higher spot intensity detected in cancer sample (Figure 2). (ii) Annexin A2 was regulated by monoubiquitination (c spot, Figure 3) with its non-degradation function, such as ubiquitination-regulated genetic transcription and signal transduction [20], [21], and the results could explain that this position of c spot was not obviously detected in immunoblotting by ubiquitin antibody (Figure 2). Therefore, there could be two kinds of ubiquitination, including poly and mono ubiquitination. Further investigations are needed to figure out the status of ubiquitination for annexin A2 and their association with its upregulated expression in breast cancer.
Ubiquitination of proteins is a very complicated protein modification. Many proteins in human are regulated to a high or low level by ubiquitination. Abnormal protein levels caused by altered protein ubiquitination lead to occurrence of disease [22]. It has been reported that the ubiquitin proteasome system was not activated in a well-characterized cell culture based breast cancer model system [10]. To avoid controversy, we used fresh human breast cancer tissue to study ubiquitination of annexin A2, as ubiquitin activity is detected in human breast cancer cells.
Overexpression of annexin A2 may be associated with abnormal ubiquitination, but the mechanism is much more complicated, including its degradation and/or non-degradation function. Abnormal ubiquitination might be associated with higher level of annexin A2, and contribute to metastasis and infiltration of breast cancer cells. Therefore, annexin A2 could be a candidate marker for treatment of breast cancer.
Materials and methods
Tissue samples
Fresh breast cancer tissue and its adjacent normal tissue were obtained after surgical operation (mastectomy) from five female patients, aged 31–58 years, hospitalized in the Affiliated Hospital of North Sichuan Medical College, who suffered from breast cancer (T1N1M0 for 2 patients, T1N2M0 for 1 patient and T2N2M0 for 2 patients). Some samples were immediately frozen at –150 °C (Super Low Temperature Icebox, REVCO, CT, USA) and used for protein extraction, and some were used for pathological and immunohistochemical analysis. Cancer tissue for protein extraction was non-necrotic, non-purulent and non-hemorrhagic. The type of cancer from all of 5 patients was later verified with pathology as infiltrating ductal carcinoma (14). The research was approved by the Ethics Committee of North Sichuan Medical College and all patients signed the written informed consent to participate in the study.
Protein extraction
Protein was extracted using ReadyPreTM Protein Extraction Kit (total protein) (Bio-Rad, CA, USA), and protein samples were stored in aliquots of 200 μL at −80 °C. Concentration of total proteins was measured with Bio-Rad RC DC Protein Assay.
Western blotting analysis
Firstly, 35 μg of total protein sample of breast cancer or normal tissue was used for electrophoresis in 12% SDS–PAGE gel. Then the protein was transferred to nitrocellulose (0.45 μm; Bio-Rad) in transfer buffer (39 mM glycine, 48 mM Tris, 0.037% SDS, 20% methanol) with electroblotting (using Mini-Trans-Blot electrophoretic transfer cell by Bio-Rad) at 30 V overnight. Immunodetection was carried out as previously described [15] using the anti-ubiquitin antibody (Chemicon, CA, USA) as the primary antibody. The goat anti-rabbit second antibody conjugated with alkaline phosphatase was detected colorimetrically with NBT/BCIP (KPL, USA).
Immunoproteomics
Protein samples of breast cancer and adjacent normal tissues from each patient were separately used for 2-DE. Duplicate gels were run for different read-outs. Since the protein sample contained substances such as salts, nucleic acids and lipids that are known to interfere with isoelectric focusing (IEF), such substances were removed using the ReadyPreTM Cleanup Kit (Bio-Rad). With this procedure, the concentration of total proteins is increased. So the concentration was measured again prior to IEF.
The first-dimension isoelectric focusing (IEF) was performed with precast IPG strips (pH 3.0–10.0, nonlinear pH IPG, 7 cm, Bio-Rad) using a focusing tray (Bio-Rad). The loading volume of protein sample was 120 μL (the loading content of protein sample of 300 μg stained with Coomassie Brilliant Blue R-250 in every gel), and IEF was carried out at 20 °C using 50 μA/strip. The strips were equilibrated after IEF. The equilibrated strips were placed on top of a 12% SDS–PAGE gel prepared and sealed in place with 0.5% low-melt agarose (Sigma, CA, USA). The SDS–PAGE was performed with MINI PROTEAN IITM (Bio-Rad).
After 2-DE, 1 pair of gels (one for normal tissue and one for cancer tissue) were analyzed by Western blotting using anti-ubiquitin antibody (Chemicon) as the primary antibody. The other pair were stained with Coomassie Brilliant Blue R-250 (Amresco, NY, USA) and scanned with UMAXpowerlook 1120 (UMAX, Taipei, Taiwan) to obtain differentially-ubiquitinated protein spots using PDQuest software (7.1). The pilot experiments for repeatability test from ten Coomassie staining gels showed that the spot positional deviation was 1.469 ± 0.238 mm for IEF and 1.016 ± 0.197 mm for SDS–PAGE while the spot volume deviation was 19.6 ± 11.1% with PDQuest software 7.
After Western blotting, we aligned the membrane with the duplicate gels stained with Coomassie Brilliant Blue R-250. The identified differentially-ubiquitinated protein spots with high repeatability and one adjacent spot were located and excised from the duplicate gels and then identified using MALDI-TOF-TOF MS. MS analysis of peptides was performed on an ABI 4700 TOF-TOF Proteomics (Applied Biosystems). The UV laser was operated at a 200 Hz repetition rate with wavelength of 355 nm. The accelerated voltage was operated at 20 kV, and mass resolution was maximized at 1500 Da. Myoglobin digested with trypsin was used to calibrate the mass instrument with an internal calibration mode. All spectra of samples acquired were processed using 4700 ExploreTM Software (Applied Biosystems) in a default mode. The data were searched by GPS Explorer V3.6 with the search engine MASCOT 2.1. The search parameters were as follows: the database NCBInr (NCBI2009: 9874297 sequences, 3367796496 residues), taxonomy Homo Sapiens [human], protein molecular mass ranged from 700 to 3000 Da, trypsin digest with one missing cleavage, MS tolerance was set at 0.3 Da, and MS/MS tolerance at 0.4 Da. Data were processed and analyzed in Fudan University [14]. Proteins with scores >59 or best ion scores (MS/MS) >30 were considered as significant (P < 0.05).
Immunohistochemical staining
Tissue samples were cut into small blocks (4–6 mm in length) and fixed in 10% formalin solution. After dehydration in ethanol and clearing in xylene, tissue blocks were embedded in paraffin and sectioned at 5 μm in thickness. Immunohistochemical staining was performed using an UltrasensitiveTM SP Kit (MAIXIN-BIO, Fuzhou, China) with the primary antibody (dilution 1:50) of anti-annexin A2 (Abcam, Cambridge, UK). The rabbit anti-rat second antibody conjugated with horse radish peroxidase (HRP) was detected by DAB coloring (AR1022, MAIXIN-BIO).
Authors’ contributions
SD carried out the immunoproteomics studies and immunohistochemical staining, and participated in the design of the study. BJ participated in the immunoproteomics studies. TX obtained tissue samples. ZY participated in the design of the study and performed the PDQest analysis. All authors read and approved the final manuscript.
Competing interests
The authors have declared that no competing interests exist.
Acknowledgments
This study was supported by grants from the Sichuan Science and Technology Support Program (Grant No. 2009SZ0116), Natural Science Foundation of China (Grant No. 81172496) and Sichuan Department of Education (Grant No. 10ZA075).
References
- 1.Morel E., Gruenberg J. The p11/S100A10 light chain of annexin A2 is dispensable for annexin A2 association to endosomes and functions in endosomal transport. PLoS One. 2007;2:e1118. doi: 10.1371/journal.pone.0001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ween M.P., Oehler M.K., Ricciardelli C. The role of annexin A2 in tumorigenesis and cancer progression. Cancer Microenviron. 2011;4:199–208. doi: 10.1007/s12307-011-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng L., Foley K., Huang L., Leubner A., Mo G., Olino K. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PLoS One. 2011;6:e19390. doi: 10.1371/journal.pone.0019390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammad H.S., Kurokohchi K., Yoneyama H., Tokuda M., Morishita A., Jian G. Annexin A2 expression and phosphorylation are up-regulated in hepatocellular carcinoma. Int J Oncol. 2008;33:1157–1163. [PubMed] [Google Scholar]
- 5.Inokuchi J., Narula N., Yee D.S., Skarecky D.W., Lau A., Ornstein D.K. Annexin A2 positively contributes to the malignant phenotype and secretion of IL-6 in DU145 prostate cancer cells. Int J Cancer. 2009;124:68–74. doi: 10.1002/ijc.23928. [DOI] [PubMed] [Google Scholar]
- 6.Zhong L.P., Wei K.J., Yang X., Zhang L., Zhou X.J., Pan H.Y. Increased expression of Annexin A2 in oral squamous cell carcinoma. Arch Oral Biol. 2009;54:17–25. doi: 10.1016/j.archoralbio.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Takano S., Togawa A., Yoshitomi H., Shida T., Kimura F., Shimizu H. Annexin II overexpression predicts rapid recurrence after surgery in pancreatic cancer patients undergoing gemcitabine-adjuvant chemotherapy. Ann Surg Oncol. 2008;15:3157–3168. doi: 10.1245/s10434-008-0061-5. [DOI] [PubMed] [Google Scholar]
- 8.Chuthapisith S., Bean B.E., Cowley G., Eremin J.M., Samphao S., Layfield R. Annexins in human breast cancer: possible predictors of pathological response to neoadjuvant chemotherapy. Eur J Cancer. 2009;45:1274–1281. doi: 10.1016/j.ejca.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F., Zhang L., Zhang B., Wei X., Yang Y., Robert Z.Q. Anxa2 plays a critical role in enhanced invasiveness of the multidrug resistant human breast cancer cells. J Proteome Res. 2009;8:5041–5047. doi: 10.1021/pr900461c. [DOI] [PubMed] [Google Scholar]
- 10.Chen L., Madura K. Increased proteasome activity, ubiquitin-conjugating enzymes, and eEF1A translation factor detected in breast cancer tissue. Cancer Res. 2005;65:5599–5606. doi: 10.1158/0008-5472.CAN-05-0201. [DOI] [PubMed] [Google Scholar]
- 11.Urano T., Saito T., Tsukui T., Fujita M., Hosoi T., Muramatsu M. Efp targets 14-3-3 sigma for proteolysis and promotes breast tumour growth. Nature. 2002;417:871–875. doi: 10.1038/nature00826. [DOI] [PubMed] [Google Scholar]
- 12.Verma R., Aravind L., Oania R., McDonald W.H., Yates J.R., Koonin E.V. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science. 2002;298:611–615. doi: 10.1126/science.1075898. [DOI] [PubMed] [Google Scholar]
- 13.He K.L., Deora A.B., Xiong H., Ling Q., Weksler B.B., Niesvizky R. Endothelial cell annexin A2 regulates polyubiquitination and degradation of its binding partner S100A10/p11. J Biol Chem. 2008;283:19192–19200. doi: 10.1074/jbc.M800100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng S., Zhou H., Xiong R., Lu Y., Yan D., Xing T. Over-expression of genes and proteins of ubiquitin specific peptidases (USPs) and proteasome subunits (PSs) in breast cancer tissue observed by the methods of RFDD-PCR and proteomics. Breast Cancer Res Treat. 2007;104:21–30. doi: 10.1007/s10549-006-9393-7. [DOI] [PubMed] [Google Scholar]
- 15.Upritchard H.G., Cordwell S.J., Lamont I.L. Immunoproteomics to examine cystic fibrosis host interactions with extracellular Pseudomonas aeruginosa proteins. Infect Immun. 2008;76:4624–4632. doi: 10.1128/IAI.01707-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doucet C., Gutierrez G.J., Lindon C., Lorca T., Lledo G., Pinset C. Multiple phosphorylation events control mitotic degradation of the muscle transcription factor Myf5. BMC Biochem. 2005;6:27. doi: 10.1186/1471-2091-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asher G., Tsvetkov P., Kahana C., Shaul Y. A mechanism of ubiquitin-independent proteasomal degradation of the tumor suppressors p53 and p73. Genes Dev. 2005;19:316–321. doi: 10.1101/gad.319905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadowski M., Suryadinata R., Tan A.R., Roesley S.N., Sarcevic B. Protein monoubiquitination and polyubiquitination generate structural diversity to control distinct biological processes. IUBMB Life. 2012;64:136–142. doi: 10.1002/iub.589. [DOI] [PubMed] [Google Scholar]
- 19.Hirata F., Thibodeau L.M., Hirata A. Ubiquitination and SUMOylation of annexin A1 and helicase activity. Biochim Biophys Acta. 2010;1800:899–905. doi: 10.1016/j.bbagen.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Sun Z.W., Allis C.D. Ubiquitination of histone H2B regulates 1-13 methylation and gene silencing in yeast. Nature. 2002;418:104–108. doi: 10.1038/nature00883. [DOI] [PubMed] [Google Scholar]
- 21.Xirodimas D.P., Savilh M.K., Bourdon J.C., Hay R.T., Lane D.P. Mdm2 mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell. 2004;118:83–97. doi: 10.1016/j.cell.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Petroski M.D. The ubiquitin system, disease, and drug discovery. BMC Biochem. 2008;9(Suppl. 1):S7. doi: 10.1186/1471-2091-9-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]