Abstract
The association between alcohol intake and cutaneous squamous cell carcinoma (cSCC) is unclear. We studied the association between alcohol intake and incident invasive cSCC in three cohorts of women and men with repeated assessments of alcohol intake in the US. Information on alcohol intake was collected repeatedly during follow-up. Cumulative average of alcohol intakes was used. Multivariable Cox proportional hazards models with time-dependent exposure were used to estimate relative risks (RR) and 95% confidence intervals, followed by a meta-analysis. During a follow-up of 4,234,416 person-years, 2,938 cSCC were identified. Alcohol intake was associated with an increased risk of cSCC with a dose-response relationship. Each additional drink (12.8 gram of alcohol) per day was associated with a 22% increased risk of cSCC (RR 1.22, 95% confidence interval: 1.13 to 1.31). White wine consumption of ≥5 times/wk was associated with an increased risk of cSCC (RR 1.31, 95% confidence interval: 1.09 to 1.59). We found no increased risk of cSCC with other alcoholic beverages. The population attributable risk associated with alcohol intake of ≥20 grams/d was 3% of cSCCs. In conclusion, alcohol intake was associated with an elevated risk of cSCC. Among alcoholic beverages, white wine was associated with cSCC.
Keywords: skin cancer, ethanol, alcohol, squamous cell carcinoma
INTRODUCTION
Cutaneous squamous cell carcinoma (cSCC) is the second most common form of skin cancer and its incidence is increasing rapidly. (1, 2) The most important risk factor for cSCC is high level of cumulative ultraviolet light (UV) exposure. (3, 4) Other risk factors include age, male gender, skin phototype, light or red hair colour, and immunosuppressant use. (2, 5, 6) Although seldom fatal with a metastatic rate up to 5% of the cases (7), cSCC is associated with considerable burden of illness. (8, 9) It is therefore important to identify patients with a high risk of developing cSCC in order to minimize the treatment costs and improve primary and secondary prevention of cSCC.
The role of alcohol intake on the occurrence of cSCC is controversial (8) and has not been investigated in large epidemiological studies. Alcohol use has been associated with an increased risk of basal cell carcinoma (BCC) (10-12), the most common form of skin cancer. However, the cell of origin and molecular pathways of BCC and cSCC are different (13, 14) and the extent to which alcohol contributes to non-melanoma skin cancer risk may therefore also differ. We therefore evaluated the association between alcohol intake and risk of cSCC in three large cohorts of US women and men with repeated assessments of alcohol intake.
MATERIAL AND METHODS
Study population
The Nurses’ Health Study (NHS) is a prospective cohort study in which 121,700 married, registered, female nurses of 30-55 years old were enrolled in 1976. The Nurses’ Health Study II (NHS II) was established in 1989 when 116,686 female nurses 25-42 years old were enrolled. The Health Professionals’ Follow-up Study (HPFS) was established in 1986 with 51,529 men 40-75 years employed in a health profession. In all cohorts, participants completed self-administered, mailed questionnaires biennially inquiring about disease history and lifestyle habits with response rates generally exceeding 90%. All studies were approved by the Institutional Review Board of Brigham and Women's Hospital (Boston, MA, USA).
Assessment of alcohol intake
To assess dietary intake including alcohol intake, a food-frequency questionnaire that inquired about the average use of food and beverages during the past year was used. The dietary questionnaires including alcohol intake were completed in 1980, 1984, 1986, 1990, 1994, 1998, 2002 and 2006 for NHS, in 1991, 1995, 1999, 2003, 2007 for NHS II, and in 1986, 1990, 1994, 1998, 2002 and 2006 for HPFS. On the questionnaires, participants were asked how many times on average during the previous year they had consumed each alcoholic beverage: light beer, non-light beer, white wine, red wine, and liquor. The participants could choose from 9 frequency responses (never/almost never, 1-3 per month, 1 per week, 2-4 per week, 5-6 per week, 1 per day, 2-3 per day, 4-5 per day and ≥6 per day). For our analyses of individual alcoholic beverages, the categories were merged as follows: none, 1-3 per month, 1 per week, 2-4 per week and ≥5 per week. The total alcohol intake was assessed by summing up the alcohol content for a specific type of each beverage. Total amount of alcohol consumed was estimated at 12.8 g for a glass, bottle, or a can of beer (12 fl oz or 360 ml), 11 g for a glass of wine (4 fl oz or 120 ml), and 14 g for a shot of liquor (1.5 fl oz or 45 ml). Cumulative average of alcohol intakes was calculated. For example, alcohol intake in 1980 was used for analyses of cSCC diagnosed from 1980 through 1984, and the average alcohol intake through 1980 to 1984 was used for analyses of colon cancer diagnosed from 1984 through 1986 and so on in the NHS.The total average alcohol intake was divided into the following categories of grams per day: None, 0-4.9, 5-9.9, 10-19.9, and ≥20 as well as analysed as a continuous variable. When translating the total average alcohol intake back into daily drinks, one drink was defined as 12.8 g of alcohol (median amount of alcohol in beer, wine, and liquor). The dietary questionnaire has been validated previously against 1-week diet records. (15, 16) The observed correlation between the questionnaire and the diet record was ≥0.8 for different alcoholic beverages.
Identification of cases of cutaneous squamous cell carcinoma
Participants in the cohorts reported new cases of invasive cSCC and cSCC in situ biennially through 2008 in NHS, 2009 in NHS II, and 2008 in HPFS. All events were verified from primary histopathology reports by study physicians. Only the incident (first) cSCC was included in this analysis. Invasive and in situ cSCC were analysed as separate outcomes because we had no a priori knowledge on whether the carcinogenicity of ethanol and its metabolites differ between invasive and in situ cSCC. cSCC of more sun exposed sites included tumors of the scalp, forehead, eyes, cheeks, nose, mouth, face, ears, neck, upper arms, elbows, foreams, hands, and fingers. cSCC of less sun exposed sites included tumors of the trunk, shoulders, thighs, legs, ankles, and feet.
Assessment of covariates
Data on known skin cancer risk factors were obtained from cohort questionnaires for all cohorts in the 1980s and 1990s. These risk factors include age, natural hair colour, mole count, average UVB flux at residence (17), skin reaction to sun exposure during childhood/adolescence, the number of lifetime severe or blistering sunburns, family history of melanoma, smoking status and pack-years smoked (combined), body-mass index (kg/m2), caffeine intake and physical activity measured in metabolic equivalent hours per week. The time-dependent covariate information was updated with each questionnaire cycle or as available. In case of missing covariate information, information was carried over from previous questionnaire cycle.
Statistical analysis
Each participant contributed person-time from the date of the first questionnaire including information on alcohol intake to the date of diagnosis of first confirmed cSCC, report of any other cancer including other skin cancers or the end of follow-up. Those who were lost to follow-up were censored at the return date of the last questionnaire. Individuals with a prior history of any cancer were excluded from analysis. Additionally, because the cohorts were all greater than 95% Caucasian, non-white participants were excluded from the analysis. These included Asians, African-Americans, and Hispanics.
We used Cox proportional hazards regression models with time-dependent covariates stratified by age and 2-year time intervals to estimate the relative risks (RR) and 95% confidence intervals of developing invasive or in situ cSCC across alcohol intake categories. Multivariable analyses were performed by adjusting for age (continuous variable), smoking (categorical: see text below), family history of melanoma (categorical), number of moles (categorical), natural hair colour (categorical), childhood/adolescent reaction to sun exposure (categorical), number of sunburns (categorical), body-mass index (categorical), physical activity measured by metabolic equivalent hours per week (categorical), UVB flux at residence (categorical) and caffeine intake (categorical). Multivariable analyses for type of alcoholic beverage were additionally adjusted for total alcohol intake (categorical) because we were interested in knowing whether other ingredients than ethanol in different beverages would influence the skin cancer risk. In order to examine possible confounding by smoking in detail, we constructed a categorical variable combining smoking status and the number of pack-years (pyrs) of smoking. The categories were as follows: no smoking, past smoking < 10 pyrs, past smoking 10-19 pyrs, past smoking 20-39 pyrs, past smoking 40+ pyrs, past smoking unknown pyrs, current smoking <25 pyrs, current smoking 25-44 pyrs and current smoking 45+ pyrs/unknown. The categorization was determined a priori and based on number of pack-years with a reasonable number of participants in each category.
The meta-analyses of total average alcohol intake were performed using the data from multivariable models in all three cohorts during the follow-up. We tested the heterogeneity between studies and estimated the overall association from random effects. We tested the linear trend for one drink (12.8 gram alcohol) per day and estimated the dose-response trend between daily alcohol intake (grams per day) and cSCC in women and men separately.
We also evaluated the dose-response association between daily alcohol intake (grams per day) and cSCC in women and men separately using restricted cubic spline with knots at 5, 10, 20, and 30 grams of alcohol per day.
We statistically tested the interaction between alcohol intake and the following variables: age, gender, smoking, BMI, number of severe sunburns, and caffeine intake.
Population attributable risk (PAR; %) was calculated based on the RR obtained from meta-analyses using the following formula: 100*(Px*(RR-1))/(1+(Px*(RR-1))) where Px is the prevalence of alcohol use in the population at baseline.
All statistical analyses were conducted using Statistical Analysis System software (SAS, version 9.2; SAS Institute Inc, Cary, NC) and all P-values were calculated from 2-sided tests and considered significant if P < 0.05.
RESULTS
Among the 223,138 persons (174,998 women and 48,140 men) in the study cohorts, 2,938 incident invasive cSCC and 1,590 incident cSCC in situ were identified during a follow-up of 4,234,416 person-years. The duration of follow-up was 19 years on average. A total of 66,777 participants were excluded from the inception cohorts due to missing information on alcohol intake or birth date, death before first dietary questionnaire, history of cancer, or because they were non-white.
Baseline characteristics of the participants in each study
At the baseline, 68% of the participants in NHS, 58% of the participants in NHS II, and 77% of the participants in HPFS (66% of the total study population) reported to have consumed alcohol in the past year (Table 1). Current and past smoking were positively correlated with alcohol intake and heavier drinkers were more likely to smoke than those consuming smaller amounts of alcohol. Physical activity, caffeine intake, and number of severe burns were also positively correlated with alcohol intake in all cohorts. Relative to their total alcohol intake, men were more likely to consume liquor and less likely to consume white wine than women (Supplementary Table 1). Younger women (NHSII) were more likely to consume beer and light beer and less likely to consume liquor than older women(NHS).
Table 1.
Age-adjusted baseline characteristics of study population according to average alcohol intake categories
| Characteristic | Average alcohol intake, g per day | ||||
|---|---|---|---|---|---|
| None | 0.1-4.9 | 5.0-9.9 | 10.0-19.9 | 20.0+ | |
| NHS (female) n = 86,635 at baseline in 1980 | n=27,580 (32%) | n=29,063 (34%) | n=9,434 (11%) | n=13,245 (15%) | n=7,313 (8%) |
| Alcohol intake, gram per day | 0.0 (0.0) | 1.9 (1.2) | 7.0 (1.2) | 13.4 (2.5) | 35.1 (12.3) |
| Age1, years | 46.4 (7.3) | 45..5 (7..3) | 46.0 (7.2) | 46.8 (7.0) | 47.4 (6.8) |
| Body-mass index, kg/m2 | 25.3 (5.1) | 24.5 (4.4) | 23.7 (3.8) | 23.3 (3.6) | 23.3 (3.5) |
| Physical activity, metabolic equivalent hours per week | 12.1 (18.3) | 14.2 (20.7) | 15.8 (21.1) | 16.0 (22.5) | 14.6 (19.0) |
| 6+ sunburns that blistered in lifetime | 6 | 7 | 9 | 9 | 10 |
| 6+ moles on left arm | 5 | 5 | 5 | 5 | 4 |
| Red or blond hair colour at age 21 | 15 | 16 | 16 | 17 | 17 |
| Family history of melanoma | 3 | 3 | 3 | 3 | 3 |
| Childhood skin reaction after 2 hours in sun: painful burn, peel | 16 | 14 | 14 | 13 | 14 |
| Current smoker | 23 | 27 | 30 | 35 | 47 |
| Past smoker | 20 | 29 | 33 | 35 | 32 |
| Pack-years among ever-smokers | 21.3 (17.0) | 18.8 (15.5) | 19.4 (15.8) | 20.4 (16.4) | 25.3 (18.6) |
| UVB flux at residence in 1986, erg/cm2/year | 122 (25) | 120 (23) | 121 (24) | 122 (25) | 125 (26) |
| Caffeine intake, milligram per day | 357 (278) | 397 (267) | 417 (264) | 421 (259) | 432 (259) |
| NHS II (female) n = 88,363 at baseline in 1991 | n=37,134 (42%) | n=34,593 (39%) | n=8,789 (10%) | n=5,854 (7%) | n=1,993 (2%) |
| Alcohol intake, gram per day | 0.0 (0.0) | 2.1 (1.2) | 7.1 (1.4) | 13.2 (2.4) | 32.5 (12.4) |
| Age1, years | 36.2 (4.7) | 35.9 (4.7) | 35.9 (4.7) | 36.5 (4.7) | 37.4 (4.4) |
| Body-mass index, kg/m2 | 25.5 (5.9) | 24.3 (5.1) | 23.3 (4.0) | 23.3 (4.0) | 23.5 (4.1) |
| Physical activity, metabolic equivalent hours per week | 18.4 (25.1) | 21.6 (27.1) | 24.0 (29.9) | 25.0 (29.2) | 25.0 (32.0) |
| 5+ sunburns that blistered in lifetime | 9 | 10 | 11 | 11 | 12 |
| 5+ moles on lower legs | 21 | 22 | 23 | 22 | 21 |
| Red or blond hair colour at age 18 | 20 | 20 | 22 | 23 | 24 |
| Family history of melanoma | 4 | 4 | 5 | 5 | 5 |
| Adolescent/childhood skin reaction after 2 hours in sun: painful burn, peel | 26 | 24 | 22 | 20 | 22 |
| Current smoker | 9 | 13 | 15 | 20 | 31 |
| Past smoker | 16 | 24 | 31 | 33 | 34 |
| Pack-years among ever-smokers | 12.9 (9.2) | 11.4 (8.4) | 10.9 (7.9) | 11.6 (8.4) | 13.1 (9.0) |
| UVB flux at residence in 1991, erg/cm2/year | 125 (24) | 123 (24) | 125 (25) | 127 (25) | 130 (26) |
| Caffeine intake, milligram per day | 198 (209) | 254 (215) | 295 (213) | 317 (214) | 350 (225) |
| HPFS (male) n = 48,140 at baseline in 1986 | n=11,237 (23%) | n=11,581 (24%) | n=6,987 (15%) | n=9,864 (20%) | n=8,471 (18%) |
| Alcohol intake, gram per day | 0.0 (0.0) | 2.5 (1.2) | 7.3 (1.4) | 14.2 (2.6) | 39.1 (16.7) |
| Age1, years | 54.7 (10.0) | 53.6 (10.0) | 53.4 (9.8) | 54.3 (9.6) | 55.1 (9.6) |
| Body-mass index, kg/m2 | 25.7 (3.6) | 25.6 (3.4) | 25.4 (3.3) | 25.4 (3.2) | 25.5 (3.2) |
| Physical activity, metabolic equivalent hours per week | 18.4 (26.7) | 20.1 (27.7) | 22.3 (31.5) | 22.8 (32.0) | 22.0 (30.2) |
| 6+ sunburns that blistered in lifetime | 35 | 34 | 34 | 36 | 39 |
| 6+ moles on forearms | 6 | 5 | 5 | 5 | 5 |
| Red or blond hair colour at age 18 | 14 | 13 | 13 | 14 | 16 |
| Family history of melanoma | 3 | 3 | 3 | 3 | 3 |
| Adolescent skin reaction after 1 hours in sun: painful burn, peel | 27 | 26 | 24 | 24 | 23 |
| Current smoker | 7 | 8 | 9 | 10 | 17 |
| Past smoker | 33 | 41 | 45 | 50 | 54 |
| Pack-years among ever-smokers | 27.3 (20.8) | 24.1 (18.7) | 24.1 (19.0) | 24.1 (18.0) | 28.8 (20.5) |
| UVB flux at residence in 1988, erg/cm2/year | 133 (28) | 128 (27) | 128 (27) | 129 (28) | 131 (28) |
| Caffeine intake, milligram per day | 190 (227) | 210 (220) | 226 (217) | 244 (223) | 290 (238) |
Values are means (SD) or percentages and are standardized to the age distribution of the study population.
Value is not age adjusted
Total alcohol intake
After adjustment for other known risk factors for cSCC in a multivariable model, there was an increased risk for invasive cSCC in women and men who drank alcohol compared with abstainers (Table 2) and a highly significant dose-response relationship was observed across increasing alcohol intake categories in each cohort (all P trend ≤0.01) . When alcohol use was analysed as a continuous variable of 1 drink per day, the RRs were 1.27(95% CI 1.14 to 1.40) for NHS, 1.31 (95% CI 1.07 to 1.61) for NHS II and 1.15 (95% CI 1.04 to 1.27) for HPFS. When the association was evaluated by body location of cSCC based on sun exposure level, the positive association was consistent across cSCC of more sun exposed sites (scalp, forehead, eyes, cheeks, nose, mouth, face, ears, neck, upper arms, elbows, foreams, hands, and fingers) vs. less sun exposed sites (trunk, shoulders, thighs, legs, ankles, feet); the RRs for 1 drink per day was 1.16 (1.07-1.26) for more sun- exposed sites and 1.33 (1.16-1.54) for less sun-exposed sites. For cSCC in situ, the positive trend was only significant in men (P trend=0.023, Supplementary Table 2).
Table 2.
Age-adjusted (“crude”) and multivariable relative risks (RRs) and pooled estimates for incident invasive cSCC by amount of average alcohol intake
| Invasive SCC | |||
|---|---|---|---|
| Alcohol intake, gram per day | No. of incident cases | Age-adjusted RR (95% CI) | Multivariable RR (95% CI) |
| NHS (female) | |||
| 0 | 470 | Reference | Reference |
| 0.1-4.9 | 378 | 1.20 (1.04 to 1.37) | 1.17 (1.01 to 1.34) |
| 5-9.9 | 163 | 1.49 (1.25 to 1.78) | 1.35 (1.13 to 1.62) |
| 10-19.9 | 223 | 1.63 (1.39 to 1.91) | 1.43 (1.21 to 1.69) |
| 20+ | 124 | 1.62 (1.33 to 1.98) | 1.41 (1.14 to 1.73) |
| P trend <0.0001 | |||
| 1 drink (12.8 g)/day (cont.) | 1358 | 1.41 (1.27 to 1.55) | 1.27 (1.14 to 1.40) |
| NHS II (female) | |||
| 0 | 127 | Reference | Reference |
| 0.1-4.9 | 148 | 1.31 (1.03 to 1.66) | 1.26 (0.99 to 1.60) |
| 5-9.9 | 57 | 1.51 (1.10 to 2.06) | 1.33 (0.97 to 1.84) |
| 10-19.9 | 60 | 1.79 (1.31 to 2.43) | 1.49 (1.08 to 2.06) |
| 20+ | 28 | 1.98 (1.31 to 2.98) | 1.58 (1.03 to 2.42) |
| P trend = 0.010 | |||
| 1 drink (12.8 g)/day (cont.) | 420 | 1.53 (1.26 to 1.85) | 1.31 (1.07 to 1.61) |
| HPFS (male) | |||
| 0 | 254 | Reference | Reference |
| 0.1-4.9 | 248 | 1.08 (0.90 to 1.28) | 1.11 (0.93 to 1.32) |
| 5-9.9 | 155 | 1.11 (0.91 to 1.36) | 1.13 (0.93 to 1.39 |
| 10-19.9 | 252 | 1.16 (0.97 to 1.38) | 1.14 (0.96 to 1.37) |
| 20+ | 251 | 1.35 (1.13 to 1.61) | 1.33 (1.11 to 1.60) |
| P trend = 0.006 | |||
| 1 drink (12.8 g)/day (cont.) | 1160 | 1.17 (1.07 to 1.29) | 1.15 (1.04 to 1.27) |
| Meta-analysis NHS, NHS II and HPFS | Age-adjusted RR (95% CI) | Multivariable RR (95% CI) | |
|---|---|---|---|
| 0 | 851 | Reference | Reference |
| 0.1-4.9 | 774 | 1.17 (1.06 to 1.30) | 1.16 (1.05 to 1.28) |
| 5-9.9 | 375 | 1.34 (1.09 to 1.66) | 1.26 (1.11 to 1.43) |
| 10-19.9 | 535 | 1.48 (1.13 to 1.93) | 1.32 (1.12 to 1.56) |
| 20+ | 403 | 1.54 (1.28 to 1.86) | 1.38 (1.21 to 1.57) |
| P trend < 0.0001 | |||
| 1 drink (12.8 g)/day (cont.) | 2938 | 1.34 (1.15 to 1.56) | 1.22 (1.13 to 1.31) |
Multivariable-adjusted model: Adjusted for age, BMI, smoking status and pack-years smoked, physical activity, caffeine intake, family history of melanoma, tanning ability, lifetime number of severe sunburns, number of moles, natural hair colour, and average annual UV-B flux at place of residence.
RR = relative risk, CI = confidence interval, cont. = continuous variable
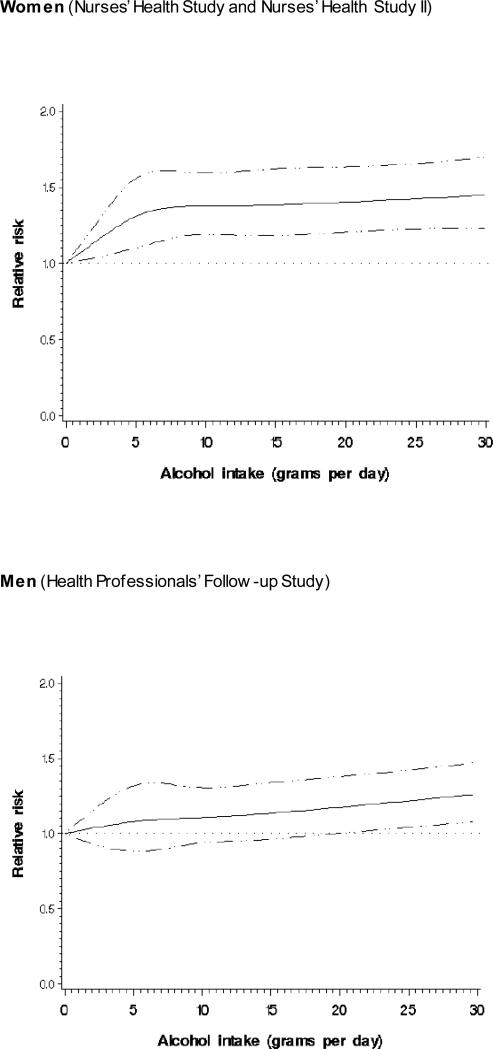
The risk estimates for women appeared higher than those for men at the same alcohol intake category. For example, in men, the increased risk for invasive cSCC associated with alcohol intake was statistically significant only at the highest category of alcohol intake (RR 1.33; 95% CI 1.11 to 1.60 for ≥20 grams per day) while a similar and statistically significant risk estimate was observed for women from the alcohol intake of 5.0-9.9 grams per day (RR 1.35, 95% CI 1.15 to 1.58) with the RR for the highest intake category of ≥20 grams per day of 1.44 (95% CI1.19 to 1.73) (Supplementary Table 3). Our regression analyses with restricted cubic spline suggested a similar dose-response association for women and men (Figure 1). In women, there was a steep increase in risk on lower intake levels of up to 5 grams per day after which the risk continued to increase slowly (non-linear relationship). In men, the risk increased in a linear manner as the daily alcohol dose increased.
Fig 1.
Dose-response relative risk (solid line) and 95% confidence interval (dotted lines) of invasive cutaneous squamous cell carcinoma by alcohol intake (grams per day) for women and men. Model was adjusted for age, BMI, smoking status and pack-years smoked, physical activity, caffeine intake, family history of melanoma, tanning ability, lifetime number of severe sunburns, number of moles, natural hair colour, and average annual UV-B flux at place of residence.
In a meta-analysis of all three cohorts (women and men, Table 2 and Supplementary Table 2), total alcohol intake was significantly associated with a higher risk for invasive cSCC and cSCC in situ. There was a significant dose-response relationship for both invasive cSCC and in situ cSCC. Each additional drink (12.8 gram of alcohol) per day was associated with 22% increased risk of invasive cSCC (RR 1.22; 95% CI 1.13 to 1.31, P trend <0.0001) and 14% increased risk for cSCC in situ (RR 1.14, 95% CI 1.04 to 1.26, P trend=0.007). The pooled multivariable adjusted RR for those who drank more than 20 grams of alcohol per day was 1.38 (1.21 to 1.57) for invasive cSCC and 1.28 (1.05 to 1.54) for cSCC in situ compared with abstainers. The PAR for the highest alcohol intake category of ≥20 grams per day was 3.0% for invasive cSCC and 2.2% for cSCC in situ.
We statistically tested the interaction between alcohol intake and the following variables: age, gender, smoking, BMI, number of severe sunburns, and caffeine intake and found no significant interaction (data not shown).
Type of Alcoholic Beverage
The association between beverage type and invasive cSCC was analyzed in alcohol-adjusted multivariable models, thereby aiming to evaluate individual beverages independent of total alcohol intake. Consuming ≥ 5 drinks of white wine per week was significantly associated with a higher risk of invasive cSCC (RR 1.31, 1.09 to 1.59) in the meta-analysis of beverage type (Table 3) independent of its ethanol content. No other alcoholic beverages were statistically significantly associated with cSCC in a meta-analysis although intakes of light beer in NHS and NHSII and liquor in NHS were associated with elevated risk of cSCC. Individual alcoholic beverages were not associated with cSCC in situ (Supplementary Table 4)
Table 3.
Alcohol-adjusted multivariable relative risks (RRs) for invasive cSCC according to beverage-specific alcohol intake
| Invasive cSCC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Light beer | Non-light beer | Red wine | White wine | Liquor | ||||||
| Drinks consumed | No. of Incident cases | RR (95% CI) | No. of Incident cases | RR (95% CI) | No. of Incident cases | RR (95% CI) | No. of Incident cases | RR (95% CI) | No. of Incident cases | RR (95% CI) |
| NHS (female) | ||||||||||
| None | 837 | Reference | 862 | Reference | 876 | Reference | 653 | Reference | 875 | Reference |
| 1-3 per month | 83 | 1.09 (0.86 to 1.39) | 70 | 1.04 (0.80 to 1.35) | 178 | 0.88 (0.74 to 1.05) | 273 | 1.08 (0.90 to 1.30) | 163 | 1.17 (0.97 to 1.40) |
| 1 per week | 29 | 1.14 (0.77 to 1.70) | 28 | 1.11 (0.74 to 1.67) | 90 | 1.11 (0.87 to 1.42) | 121 | 1.10 (0.87 to 1.39) | 91 | 1.46 (1.16 to 1.84) |
| 2-4 per week | 15 | 0.97 (0.58 to 1.65) | 14 | 1.01 (0.59 to 1.75) | 95 | 1.19 (0.93 to 1.53) | 121 | 1.04 (0.82 to 1.33) | 228 | 1.48 (1.17 to 1.86) |
| ≥5 per week | 16 | 1.94 (1.14 to 3.29) | 5 | 0.65 (0.26 to 1.59) | 47 | 0.91 (0.65 to 1.28) | 118 | 1.48 (1.12 to 1.97) | ||
| 1 drink per day (cont.) | 1.27 (1.02 to 1.58) | 0.84 (0.60 to 1.19) | 0.97 (0.85 to 1.12) | 1.16 (1.04 to 1.30) | 1.20 (1.05 to 1.36) | |||||
| NHS II (female) | ||||||||||
| None | 306 | Reference | 332 | Reference | 254 | Reference | 218 | Reference | 303 | Reference |
| 1 -3 per month | 56 | 1.13 (0.83 to 1.54) | 52 | 1.00 (0.73 to 1.37) | 83 | 1.19 (0.90 to 1.58) | 92 | 0.81 (0.60 to 1.09) | 66 | 1.02 (0.77 to 1.35) |
| 1 per week | 18 | 0.96 (0.58 to 1.60) | 16 | 0.74 (0.44 to 1.26) | 34 | 0.97 (0.64 to 1.46) | 43 | 0.97 (0.66 to 1.42) | 30 | 1.33 (0.89 to 1.98) |
| 2 -4 per week | 24 | 1.66 (1.04 to 2.65) | 15 | 1.12 (0.63 to 1.97) | 27 | 0.78 (0.48 to 1.27) | 40 | 1.11 (0.72 to 1.71) | 21 | 0.90 (0.53 to 1.52) |
| ≥5 per week | 16 | 1.91 (1.00 to 3.64) | 5 | 0.83 (0.32 to 2.16) | 22 | 1.24 (0.69 to 2.25) | 27 | 1.12 (0.62 to 2.03) | ||
| 1 drink per day (cont.) | 1.28 (0.99 to 1.65) | 0.94 (0.65 to 1.37) | 1.10 (0.87 to 1.39) | 1.06 (0.85 to 1.34) | 0.92 (0.69 to 1.24) | |||||
| HPFS (male) | ||||||||||
| None | 563 | Reference | 441 | Reference | 616 | Reference | 531 | Reference | 550 | Reference |
| 1-3 per month | 131 | 0.97 (0.79 to 1.19) | 171 | 1.08 (0.89 to 1.32) | 212 | 0.84 (0.70 to 1.00) | 292 | 1.12 (0.94 to 1.33) | 165 | 1.13 (0.94 to 1.37) |
| 1 per week | 56 | 0.98 (0.73 to 1.31) | 78 | 0.93 (0.72 to 1.22) | 112 | 0.86 (0.68 to 1.09) | 134 | 1.04 (0.83 to 1.31) | 90 | 1.12 (0.88 to 1.42) |
| 2-4 per week | 44 | 1.00 (0.73 to 1.38) | 73 | 0.91 (0.70 to 1.19) | 131 | 0.92 (0.73 to 1.16) | 120 | 1.03 (0.81 to 1.31) | 352 | 1.14 (0.93 to 1.39) |
| ≥5 per week | 29 | 0.95 (0.64 to 1.41) | 62 | 1.07 (0.79 to 1.45) | 81 | 0.74 (0.56 to 0.97) | 74 | 1.21 (0.91 to 1.60) | ||
| 1 drink per day (cont.) | 0.95 (0.81 to 1.12) | 1.00 (0.88 to 1.14) | 0.91 (0.82 to 1.01) | 1.06 (0.95 to 1.18) | 1.04 (0.94 to 1.16) | |||||
| Meta-analysis | ||||||||||
| None | 1706 | Reference | 1635 | Reference | 1746 | Reference | 1402 | Reference | 1728 | Reference |
| 1 -3 per month | 270 | 1.04 (0.90 to 1.20) | 293 | 1.05 (0.91 to 1.21) | 473 | 0.93 (0.78 to 1.12) | 657 | 1.03 (0.88 to 1.21) | 394 | 1.12 (1.00 to 1.27) |
| 1 per week | 103 | 1.02 (0.82 to 1.26) | 122 | 0.94 (0.77 to 1.16) | 236 | 0.98 (0.83 to 1.15) | 298 | 1.05 (0.90 to 1.22) | 211 | 1.29 (1.08 to 1.54) |
| 2 -4 per week | 83 | 1.16 (0.83 to 1.61) | 102 | 0.96 (0.77 to 1.19) | 253 | 0.99 (0.80 to 1.24) | 281 | 1.05 (0.89 to 1.23) | 601 | 1.21 (0.95 to 1.55) |
| ≥5 per week | 58 | 1.46 (0.87 to 2.44) | 72 | 1.00 (0.76 to 1.31) | 150 | 0.86 (0.67 to 1.11) | 219 | 1.31 (1.09 to 1.59) | ||
| 1 drink per day (cont.) | 1.14 (0.93 to 1.40) | 0.98 (0.87 to 1.09) | 0.95 (0.87 to 1.04) | 1.10 (1.03 to 1.19) | 1.08 (0.96 to 1.23) | |||||
Alcohol-adjusted multivariable model: Adjusted for age, BMI, smoking status and pack-years smoked, physical activity, caffeine intake, family history of melanoma, tanning ability, lifetime number of severe sunburns, number of moles, natural hair colour, and average annual UV-B flux at place of residence as well as for total average alcohol intake
RR = relative risk, CI = confidence interval, cont. = continuous variable
DISCUSSION
We conducted a large epidemiological study in three cohorts of US women and men and observed that alcohol intake was associated with an increased risk of invasive and in situ cSCC. There was a significant dose-response relationship between total alcohol intake and risk of both malignancies. These associations were independent of other known risk factors for cSCC, such as age, gender, skin phototype, and number of severe burns and consistent across body location of tumors based on sun exposure level. When examining the individual alcohol containing beverages, correcting for the total alcohol intake, only white wine consumption was significantly associated with an additional risk of cSCC.
A summary of the three previous studies on alcohol intake and cSCC is presented as a Supplementary Table 5. A recent Danish prospective cohort study with 198 cSCC cases found no convincing association between alcohol intake and cSCC. (12) Although the RR for alcohol use was above 1, the study lacked statistical power and the risk estimate was not statistically significant. An earlier study in transplant recipients from five European countries with 224 cSCC cases reported an inconsistent association between alcohol intake and cSCC. (18) The prevalence of alcohol consumption in controls in this study varied from 22% (Germany) to 91% (France), which may explain the inconsistency as the power to study alcohol exposure might have been too low in some study populations. In an Australian study (n=127 cases), no association was observed between alcohol intake and cSCC except that the risk of cSCC was higher for patients with a history of skin cancer consuming sherry or port wine, which was possibly a chance finding. (19) In all these studies, alcohol intake was assessed only at baseline. We also had much larger sample size than these studies.
Although epidemiological evidence on alcohol intake and cSCC is scarce, based on animal studies and studies on other cancers several mechanisms have been proposed to increase skin cancer risk in alcohol users. (20-23) The ethanol molecule is not carcinogenic (24) but International Agency for Research on Cancer (IARC) has classified ethanol in alcoholic beverages and its main metabolite acetaldehyde as Group 1 carcinogens, meaning that they are carcinogenic to humans. (8) Acetaldehyde is also a by-product of the fermentation process and present as such in many alcoholic beverages. (25) Ingested alcohol is also metabolized into acetaldehyde in the body. Acetaldehyde has direct carcinogenic effects in cells through e.g. the formation of DNA adducts which can interfere with normal DNA replication and mitosis in different tissues (22, 26) and by acting as a photosensitizer. (21) Importantly for our study, alcohol intake may increase skin cancer risk through its immunosuppressive effects. (23, 27) The suggested immunosuppressive mechanism may be especially relevant for cSCC as it is generally known that the suppression of the immune system increases the risk of cSCC up to 250-fold. (2, 28)
We observed an increased risk associated with consumption of white wine in our study when adjusted for the total average alcohol intake. White wine contains higher concentrations of acetaldehyde and sulfites than red wine. (25) However, it is unclear whether ingested acetaldehyde or sulfites have any carcinogenic activities in the skin. On the other hand, it has been suggested that the polyphenols, known antioxidants, in red wine may protect against DNA damage. (29, 30) It is also possible that the association is a result of residual confounding by other life-style factors or confounding by other unknown and unmeasured factors. Finally, these results should be interpreted with caution due to multiple testing.
There are several factors that may influence the relationship between alcohol use and skin cancer in observational studies. We tried to account for the confounders in our analyses and examine the effect modification.
In this study, the information on alcohol intake was collected before diagnosis of skin cancer minimizing recall bias. Furthermore, our study was conducted in three cohorts of health professionals who may be better aware of the harmful effects of high alcohol intake and therefore consume less alcohol than the general population. This would mean that the PAR calculated in this study may be lower than that of a general population.
We excluded participants with a history of cancer from our analyses because they are expected to have a different baseline risk of cSCC due to possible high-dose immunosuppression or radiation. We adjusted for smoking as a combined variable of (time-dependent) smoking status and the number of pack-years.
The different dose-response curves for women and men may be explained by the fact that blood alcohol levels increase 10-15% in women compare to men because of the relatively high body fat percentage. (8) To further control for these differences in body size, we also adjusted for BMI in our analyses.
To our knowledge, our study was the largest prospective study of alcohol intake and cSCC. It is also the first longitudinal study using updated information on alcohol intake during follow-up (time-dependent covariates). The proportion of participants consuming alcohol intake in our study was 66% at baseline, allowing meaningful comparisons between exposed and non-exposed to alcohol. All skin tumours were histopathologically validated and alcohol intake was assessed using a validated food frequency questionnaire. The participants were followed for on average of 19 years and detailed information was available for smoking and other covariates enabling us to capture changes in lifestyle during follow-up.
Our study has limitations. Although our study was the largest of its kind, the statistical power may still have limited to study some alcoholic beverages, especially beer in women. Furthermore, we cannot rule out residual confounding due to unmeasured factors.
In summary, the results of this large epidemiological study indicate that consumption of alcohol is associated with an increased risk of developing invasive and in situ cSCC - approximately 2-3% of cSCC are due to alcohol intake of 20 grams per day or more. Alcohol intake is a potentially modifiable life-style risk factor and, although our results are still to be replicated, physicians may consider counselling their patients about the association between alcohol consumption and risk for cSCC.
Supplementary Material
Novelty and impact of the work.
Ethanol in alcoholic beverages and its main metabolite acetaldehyde are carcinogenic. Previous observational studies on the association between alcohol intake and cutaneous squamous cell carcinoma (cSCC) have been small and inconclusive. This study in three large cohorts suggest that each additional daily alcoholic drink is associated with 22% increased risk of invasive cSCC and 14% increased risk for cSCC in situ. Alcohol intake of ≥20 grams per day is responsible for 2-3% of all cSCC.
ACKNOWLEDGEMENTS
We are indebted to the participants in the NHS and HPFS for their dedication to this study. We thank the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming. This work was supported by Departmental Funding, NIH CA186107, CA176726, CA167551, and CA137365 and The Netherlands Organisation for Health Research and Development (ZonMW) grant 91711315.
Abbreviations
- cSCC
cutaneous squamous cell carcinoma
- NHS
Nurses’ Health Study
- NHS II
Nurses’ Health Study II
- HPFS
Health Professionals Follow-up Study
- RR
relative risk
- CI
confidence interval
Footnotes
Conflicts of interest: The authors declared no conflicts of interest.
REFERENCES
- 1.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012 May;166(5):1069–80. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 2.Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet. 2010 Feb 20;375(9715):673–85. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 3.Ramos J, Villa J, Ruiz A, Armstrong R, Matta J. UV dose determines key characteristics of nonmelanoma skin cancer. Cancer Epidemiol Biomarkers Prev. 2004 Dec;13(12):2006–11. [PubMed] [Google Scholar]
- 4.Rigel DS. Cutaneous ultraviolet exposure and its relationship to the development of skin cancer. J Am Acad Dermatol. 2008 May;58(5 Suppl 2):S129–32. doi: 10.1016/j.jaad.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 5.Nan H, Kraft P, Hunter DJ, Han J. Genetic variants in pigmentation genes, pigmentary phenotypes, and risk of skin cancer in Caucasians. Int J Cancer. 2009 Aug 15;125(4):909–17. doi: 10.1002/ijc.24327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welsh MM, Karagas MR, Kuriger JK, Houseman A, Spencer SK, Perry AE, Nelson HH. Genetic determinants of UV-susceptibility in non-melanoma skin cancer. PLoS One. 2011;6(7):e20019. doi: 10.1371/journal.pone.0020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brantsch KD, Meisner C, Schonfisch B, Trilling B, Wehner-Caroli J, Rocken M, Breuninger H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008 Aug;9(8):713–20. doi: 10.1016/S1470-2045(08)70178-5. [DOI] [PubMed] [Google Scholar]
- 8.IARC. Consumption of alcoholic beverages. IARC Monographs on the evaluation of carcinogenic risk to humans. 2012;100E:373–498. [Google Scholar]
- 9.Kyle JW, Hammitt JK, Lim HW, Geller AC, Hall-Jordan LH, Maibach EW, De Fabo EC, Wagner MC. Economic evaluation of the US Environmental Protection Agency's SunWise program: sun protection education for young children. Pediatrics. 2008 May;121(5):e1074–84. doi: 10.1542/peds.2007-1400. [DOI] [PubMed] [Google Scholar]
- 10.Freedman DM, Sigurdson A, Doody MM, Mabuchi K, Linet MS. Risk of basal cell carcinoma in relation to alcohol intake and smoking. Cancer Epidemiol Biomarkers Prev. 2003 Dec;12(12):1540–3. [PubMed] [Google Scholar]
- 11.Fung TT, Hunter DJ, Spiegelman D, Colditz GA, Rimm EB, Willett WC. Intake of alcohol and alcoholic beverages and the risk of basal cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev. 2002 Oct;11(10 Pt 1):1119–22. [PubMed] [Google Scholar]
- 12.Jensen A, Birch-Johansen F, Olesen AB, Christensen J, Tjonneland A, Kjaer SK. Intake of Alcohol May Modify the Risk for Non-Melanoma Skin Cancer: Results of a Large Danish Prospective Cohort Study. J Invest Dermatol. 2012 Dec;132(12):2718–26. doi: 10.1038/jid.2012.198. [DOI] [PubMed] [Google Scholar]
- 13.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008 Oct;8(10):743–54. doi: 10.1038/nrc2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratushny V, Gober MD, Hick R, Ridky TW, Seykora JT. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Invest. 2012 Feb 1;122(2):464–72. doi: 10.1172/JCI57415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993 Jul;93(7):790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991 Apr 15;133(8):810–7. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 17.Bertrand KA, Chang ET, Abel GA, Zhang SM, Spiegelman D, Qureshi AA, Laden F. Sunlight exposure, vitamin D, and risk of non-Hodgkin lymphoma in the Nurses' Health Study. Cancer Causes Control. 2011 Dec;22(12):1731–41. doi: 10.1007/s10552-011-9849-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouwes Bavinck JN, Euvrard S, Naldi L, Nindl I, Proby CM, Neale R, Abeni D, Tessari GP, Feltkamp MC, Claudy A, Stockfleth E, Harwood CA. Keratotic skin lesions and other risk factors are associated with skin cancer in organ-transplant recipients: a case-control study in The Netherlands, United Kingdom, Germany, France, and Italy. J Invest Dermatol. 2007 Jul;127(7):1647–56. doi: 10.1038/sj.jid.5700776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansems TM, van der Pols JC, Hughes MC, Ibiebele T, Marks GC, Green AC. Alcohol intake and risk of skin cancer: a prospective study. Eur J Clin Nutr. 2008 Feb;62(2):162–70. doi: 10.1038/sj.ejcn.1602717. [DOI] [PubMed] [Google Scholar]
- 20.Agnew KL, Bunker CB. Multiple cutaneous squamous carcinoma in a psoriatic associated with ciclosporin, alcohol abuse and ultraviolet radiation exposure which were suppressed by acitretin. J Eur Acad Dermatol Venereol. 2003 Jan;17(1):113–4. doi: 10.1046/j.1468-3083.2003.00519_12.x. [DOI] [PubMed] [Google Scholar]
- 21.Saladi RN, Nektalova T, Fox JL. Induction of skin carcinogenicity by alcohol and ultraviolet light. Clin Exp Dermatol. 2009 Jan;35(1):7–11. doi: 10.1111/j.1365-2230.2009.03465.x. [DOI] [PubMed] [Google Scholar]
- 22.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007 Aug;7(8):599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 23.Waldschmidt TJ, Cook RT, Kovacs EJ. Alcohol and inflammation and immune responses: summary of the 2005 Alcohol and Immunology Research Interest Group (AIRIG) meeting. Alcohol. 2006 Feb;38(2):121–5. doi: 10.1016/j.alcohol.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Salaspuro M. Acetaldehyde: a cumulative carcinogen in humans. Addiction. 2009 Apr;104(4):551–3. doi: 10.1111/j.1360-0443.2009.02546.x. [DOI] [PubMed] [Google Scholar]
- 25.Liu S-QaP, G. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. International Journal of Food Science & Technology. 2000;35:49–61. [Google Scholar]
- 26.Fang JL, Vaca CE. Detection of DNA adducts of acetaldehyde in peripheral white blood cells of alcohol abusers. Carcinogenesis. 1997 Apr;18(4):627–32. doi: 10.1093/carcin/18.4.627. [DOI] [PubMed] [Google Scholar]
- 27.Merimsky O, Inbar M. Alcohol intake-associated skin and mucosal cancer. Clin Dermatol. 1999 Jul-Aug;17(4):447–55. doi: 10.1016/s0738-081x(99)00031-0. [DOI] [PubMed] [Google Scholar]
- 28.Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part I. Epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011 Aug;65(2):253–61. doi: 10.1016/j.jaad.2010.11.062. [DOI] [PubMed] [Google Scholar]
- 29.Afaq F, Katiyar SK. Polyphenols: skin photoprotection and inhibition of photocarcinogenesis. Mini Rev Med Chem. 2011 Dec;11(14):1200–15. doi: 10.2174/13895575111091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moehrle M, Dietrich H, Patz CD, Hafner HM. Sun protection by red wine? J Dtsch Dermatol Ges. 2009 Jan;7(1):29–32. doi: 10.1111/j.1610-0387.2008.06793.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.