Summary
Immune senescence as well as disturbed CD8+ T cell differentiation are a hallmark of chronic HIV infection. Here, we investigated to what extent immune senescence is reversible after initiation of anti‐retroviral treatment (ART). Peripheral blood mononuclear cells (PBMCs) from a cohort of HIV patients with different disease courses, including untreated viral controllers (n = 10), viral non‐controllers (n = 16) and patients on ART (n = 20), were analysed and compared to uninfected controls (n = 25) by flow cytometry on bulk and HIV‐specific major histocompatibility complex (MHC) class I tetramer+ CD8+ T cells for expression of the memory markers CCR7 and CD45RO, as well as the senescence marker CD57 and the differentiation and survival marker CD127. Furthermore, a subset of patients was analysed longitudinally before and after initiation of ART. Frequencies of CD57+CD8+ T cells decreased after initiation of ART in central memory (Tcm) but not in effector memory T cell populations (TemRO and TemRA). The frequency of CD127+CD8+ cells increased in Tcm and TemRO. We observed a reduction of CD127– T cells in Tcm, TemRO and partially in TemRA subsets after initiation of ART. Importantly, HIV‐specific CD8+ TemRO cells predominantly displayed a CD127–CD57+ phenotype in untreated HIV‐patients, whereas the CD127+CD57– phenotype was under‐represented in these patients. The frequency of the CD127+CD57–CD8+ T cell subpopulation correlated strongly with absolute CD4+ counts in HIV‐infected patients before and after initiation of ART. These findings can be interpreted as a phenotypical correlate of CD8+ memory T cell differentiation and the premature ‘ageing’ of the immune system, which was even observed in successfully virally suppressed HIV patients.
Keywords: AIDS, cytotoxic T cells, T cells, viral
Introduction
Immune senescence and disturbed CD8+ T cell differentiation have been associated with chronic HIV infection 1. Data from a recent clinical study show convincingly that initiation of anti‐retroviral treatment (ART) leads to a rapid improvement of several clinical outcomes 2. However, it has not been established to date to what extent these immunological signatures are restored during ART.
CD57 has been described as a marker for replicative senescence on T cells, and is associated with a shortening of telomeres after numerous cell divisions 3. This is not necessarily coupled to a functional impairment, as these cells are able to produce cytokines and cytotoxic granules 4, and have even been associated with reduced viral loads in HIV infection 5. The CD57‐defined T cell maturation has been described so far as unrelated to HIV epitope‐specific clonotype expansion but, rather, seems to be driven by T cell receptor (TCR)‐independent stimuli such as distinct cytokine profiles and/or immune activation 6. However, some studies challenge the concept of senescence of CD57+CD8+ T cells 7. Therefore, the clinical relevance of CD57 expression in HIV infection is investigated further in this study.
Conversely, CD127 [interleukin (IL)−7 Rα] expression is crucial for T cell differentiation and survival, as it forms part of the IL‐7 receptor and mediates signalling required for homeostatic proliferation, e.g. the expansion of naive T cells in lymphopenic hosts 8, 9. CD127 expression starts early during T cell development in the thymus but is down‐regulated in phases of expansion 10. Furthermore, CD127 has been discussed as a marker for long‐term memory cells 11, 12, as CD127+CD8+ T cells were shown to be able to survive without further antigenic stimulation 12. CD127 expression on CD8+ T cells is decreased in a variety of viral infections, including HIV infection 13. Moreover, CD127 down‐regulation has been associated with a loss of HIV‐specific CD8+ T cell function in terms of cytotoxic activity 14, as well as with a failing immune reconstitution under ART 15.
In the current study, we provide a detailed characterization of the alterations of CD8+ T cell memory differentiation 16 on bulk as well as HIV‐specific cells via flow cytometry in a large cohort, including viral controllers (VC) and viral non‐controllers (VNC), with focus on CD8+ T cell immune senescence (CD57) and differentiation (CD127). The main objective of this study was to understand whether ART leads to immune reconstitution of some or all central and effector memory populations and in how far the frequencies of CD127+ and CD57+CD8+ T cell populations are altered after initiation of ART. For the first time, to our knowledge, this combination of markers has been used to characterize functionally these clinically relevant subsets within a longitudinal patient cohort.
Materials and methods
Study subjects
All samples from HIV patients and healthy controls were recruited at the HIV out‐patient clinic at Hannover Medical School after written informed consent was obtained (Ethics vote no. 3150). The gender distribution within the healthy control group was similar to that within the HIV‐infected cohort (healthy: 43·7% female to 56·3% male, HIV: 42·4% female to 57·6% male). HIV viral load was determined using cobas® amplicor assays with a limit of detection of 50 RNA copies/ml. VC were defined by plasma viral loads of fewer than 2000 copies/ml, VNC by plasma viral loads of more than 2000 copies/ml. HIV CDC status, ART and CD4+ T cell counts were determined via chart review. All clinical information is summarized for each patient cohort in the Supporting information, Tables S1–S3.
Flow cytometry
All analysis was performed with frozen peripheral blood mononuclear cell (PBMC) samples. PBMCs were stained with unlabelled CCR7 antibody (150503; R&D Systems, Minneapolis, MN, USA) followed by a secondary antibody staining (goat anti‐mouse) and a blocking step (mouse serum). Then, fluorochrome‐conjugated antibodies directed against T cell surface markers were added directly: CD3 (SK7; BD Pharmingen, San Jose, CA, USA), CD4 (RPA‐T4; BD Pharmingen), CD8 (RPA‐T8; BD Horizon/BD Pharmingen), CD45RO (UCHL1; Beckman Coulter, Brea, CA, USA), CD57 (NK‐1; BD Pharmingen) and CD127 (hIL‐7R‐M21; BD Pharmingen). CD14 (MΦP9; BD Pharmingen) and CD19 (4G7; BD Pharmingen) were included for exclusion of monocytes and B cells and Via‐Probe (BD Biosciences) for exclusion of dead cells. The proliferation capacity was measured via intranuclear staining of Ki‐67 using the Fix & Perm® cell permeabilization kit (Invitrogen/Molecular Probes, Carlsbad, CA, USA) and anti‐Ki‐67 antibody (B56), according to the manufacturer's protocol. HIV‐specific cells were stained with human leucocyte antigen (HLA) B*57, B*27 and B*08 restricted Gag‐ and Nef‐derived tetramers containing the following peptides: KAFSPEVIPMF (Gag162–72), KRWIILGLNK (Gag 131–140), FLKEKGGL (Nef 90–97) and EIYKRWII (Gag 260–267). For each marker of interest, fluorescence‐minus‐one controls (FMO) were prepared. At least 1 million events per sample were acquired on an LSR II flow cytometer (BD Biosciences). Raw data were analysed with fluorescence activated cell sorter (FACS) Diva version 5 (BD Biosciences, Heidelberg, Germany) and FlowJo software (TreeStar Inc., Ashland, OR, USA).
Statistical analysis
All groups were tested for normal distribution with the Kolmogorov–Smirnov test and were compared by the adequate test. For normally distributed data, parametric tests were applied: for two paired groups, paired t‐tests; for more than two groups, analysis of variance (anova) followed by Tukey's multiple comparisons test. Data that were not distributed normally were tested by the Wilcoxon test for paired groups or the Kruskal–Wallis test followed by Dunn's multiple comparisons test for more than two groups, respectively. All statistical analysis was carried out using Graphpad Prism version 5 software. A P‐value of < 0·05 was considered significant. The following symbols were applied to denote statistically significant findings: ***P < 0·001, **P < 0·01 and *P < 0·05.
Results
Increased frequencies of CD57+ cell populations and decreased frequencies of CD127+ cell populations in CD8+ T cell subsets in chronic HIV‐1 infection
Disturbances of CD8+ T cell memory populations in chronic HIV infection are associated with increased frequencies of CD57+ and decreased frequencies of CD127+ memory populations. In order to investigate the impact of chronic HIV infection on CD8+ T cell memory populations, we performed a cross‐sectional analysis of CD8+ T cell populations in chronic HIV‐infected patients in comparison to healthy controls. Patients with chronic HIV infection were stratified into VC, VNC and anti‐retrovirally treated patients. HIV‐infected groups experienced a substantial reduction of naive CD8+ T cell numbers and frequencies and a reduction of Tcm frequencies with a concomitant increase of CD8+ TemRO cells (Supporting information, Fig. S1). Interestingly, in untreated HIV‐infected patients these frequencies correlated with HIV viral loads (Supporting information, Fig. S2).
In the naive population, frequencies but not absolute numbers of CD57+ cells were elevated in patients within the VNC group compared to healthy controls (Fig. 1a). VNC subjects and patients under ART show a higher frequency of CD57+ cells compared to healthy controls in the Tcm population in our cross‐sectional study (Fig. 1b). In the TemRO population, higher frequencies of CD57+ cells were detected in all HIV‐infected groups compared to healthy individuals, although this was only statistically significant for the VC group, and the absolute CD57+ TemRO cells were significantly more abundant in the VNC group compared to healthy controls (Fig. 1c), reflecting the high absolute numbers of total TemRO cells in this group. Absolute numbers of CD57+ TemRA cells were increased in VNC subjects compared to healthy controls (Fig. 1d).
Figure 1.
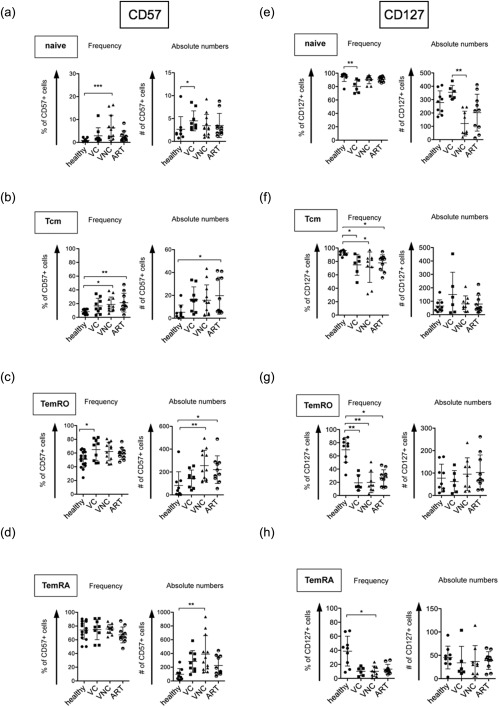
Elevated senescence and reduced survival capacity in naive, central memory and effector memory CD8+ T cells in HIV infection. Cumulative data from cross‐sectional flow cytometry analyses of CD57 expression (a–d) (21 (left panel) or 9 (right panel)) healthy donors, 9 viral controllers, 12 viral non‐controllers and 10 treated patients). Each symbol represents CD57+ cell numbers in percent of the CD8+ T cell subpopulation (frequency) or per μl of blood (absolute numbers). Cumulative data from cross‐sectional flow cytometry analyses of CD127 expression (E–H) (9 healthy donors, 9 viral controllers, 12 viral non‐controllers and 10 treated patients). Each symbol represents CD127+ cell numbers in percent of the CD8+ T cell subpopulation (frequency) or per μl of blood (absolute numbers).
When we measured the expression of CD127, we observed an inverse expression pattern compared to CD57 with lower CD127 expression on CD8+ effector memory subsets (Fig. 1e–h). In addition, the VNC group showed a substantial reduction in absolute numbers of CD127+ CD8+ T cells in the naive cell pool compared to healthy controls and to the VC group without showing reduced frequencies (Fig. 1e), mirroring the reduced total number of naive CD8+ T cells (Supporting information, Fig. S1). In contrast, VC subjects showed a significant reduction in the frequency but not in absolute numbers of CD127+ cells within the naive CD8+ T cell population. The frequency of CD127+ Tcm cells was reduced in the VNC group compared to healthy controls (Fig. 1f). In the TemRO subset, frequencies (Fig. 1g) but not absolute numbers of CD127+ cells were reduced substantially in all HIV‐infected patients compared to healthy controls. Similar effects were observed in the TemRA subset, showing a significant decrease in frequencies of CD127+ cells for all HIV‐infected groups compared to healthy controls but no differences in absolute numbers (Fig. 1f).
In summary, our cross‐sectional data demonstrate increased frequencies of CD57+ cell populations and decreased frequencies of CD127+ cell populations in most CD8+ T cell subsets in chronic HIV infection.
HIV‐1‐specific cells for Gag‐ and Nef‐ epitopes in the CD8+ TemRO population of untreated patients preferentially display a CD127–CD57+ phenotype
In order to determine whether the observed expansions of CD8+ T cell subpopulations also occur in HIV‐specific cells, we used tetramer staining within a subset of HLA‐typed, untreated patients.
PBMC samples of four HLA‐B*27‐positive, three HLA‐B*57‐positive and two HLA‐B*08‐positive patients were stained with HIV major histocompatibility complex (MHC) class I tetramers containing the Gag‐ and Nef‐derived peptides KK10, KF11, EI8 and FL8, respectively. Tetramer‐positive cells showed mainly a TemRO phenotype (Fig. 2a) and were therefore compared to tetramer‐negative TemRO cells. HIV Gag‐ and Nef‐specific early effector memory CD8+ T cells preferentially displayed a CD127–CD57+ phenotype (Fig. 2b–e). In contrast, the CD127+CD57– phenotype was under‐represented significantly in those cells (Fig. 2e). The CD127+CD57+ phenotype was slightly less frequent within tetramer‐positive cells, although not statistically significant. The CD127–CD57– subset was equally frequent within tetramer‐positive and ‐negative cells.
Figure 2.

Elevated CD57 and reduced CD127 expression on HIV‐1‐specific CD8+ T cells. (a) Flow cytometry plot of HIV‐1 tetramer staining (EI8, Gag derived) (left). Flow cytometry plot overlay depicting tetramer positive cells in the CD8+ T cell population (centre). Flow cytometry plot depicting gating strategy for tetramer‐negative versus tetramer‐positive cells within the CD8+ TemRO population (right). (b) Histogram [depicting a human leucocyte antigen (HLA)‐B*08‐positive patient] and cumulative display of CD57 expression‐analysis and (c) CD127 expression analysis. Compared were tetramer‐positive and ‐negative CD8+ TemRO cells from nine untreated patients. Tetramers contained HLA B*08, B*57 and B*27 restricted Gag‐ and Nef‐ derived peptides (KF11, KK10, FL8 and EI8). Statistical significance comparing tetramer‐negative and ‐positive cells was determined via paired t‐test and defined as P < 0·05. (d) Cumulative display of combined CD127 and CD57 expression analysis on tetramer‐positive versus tetramer‐negative CD8+ TemRO cells from eight untreated HIV‐1 infected patients. Tetramers contained HLA B*08, B*57 and B*27 restricted Gag‐ and Nef‐derived peptides (KF11, KK10, Fl8 and EI8). (e) Pie charts depicting mean percentages of each CD127/CD57 subset for tetramer‐positive and ‐negative cells.
In summary, the phenotype of CD127– and CD57+ cells was even more prominent in the HIV‐specific CD8+ T cell subset. This finding suggests that this subset is possibly presenting a more effector‐like phenotype driven by antigen‐specific expansion.
Substantial reconstitution of CD127+ cells but no loss of CD57 after short‐term ART in HIV‐1 infected patients
In order to understand the impact of ART on immune reconstitution and immune senescence, we performed a longitudinal analysis of CD127 and CD57 expression on CD8+ T cell subsets after initiation of ART for at least 9 months.
ART led to an increase of naive CD8+ T cells and CD8+ Tcm as well as a decrease of CD8+ TemRO populations (Supporting information, Fig. S3). When we analysed the frequencies of CD57+ cells of different CD8+ populations, we observed a reduction in the frequency of CD57+ cells on naive CD8+ T cells in patients receiving ART compared to the pre‐ART situation (Fig. 3a, left panel). Absolute numbers of CD57– cells increased and repopulated the naive pool upon ART (Fig. 3a, right panel). The longitudinal follow‐up of Tcm also showed a reduced CD57+ cell frequency, which was due in part to reduced CD57+ cell numbers (Fig. 3b, middle panel), but also to an increase in CD57– cell numbers (Fig. 3b, right panel).
Figure 3.
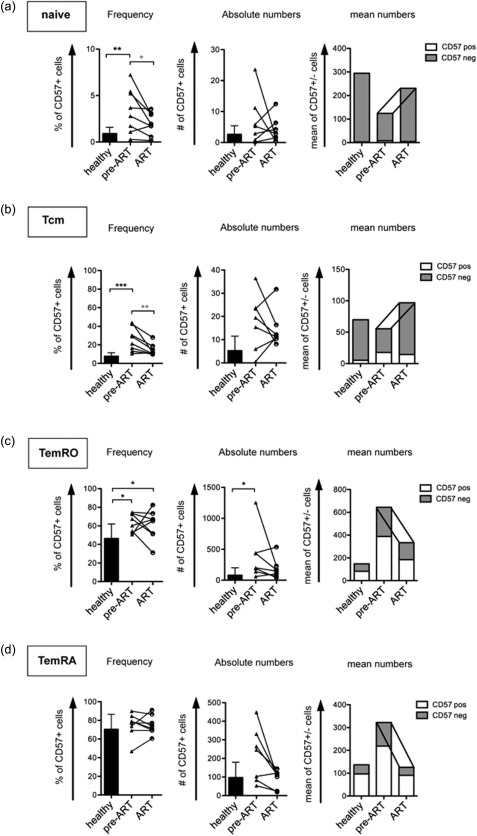
Elevated senescence is not reduced after 9 months of anti‐retroviral treatment (ART) within the early effector memory population. Cumulative data of longitudinal flow cytometry analyses of eight patients before and after at least 9 months of ART compared to healthy controls. Bar graphs depict mean numbers of CD57+ (white) and CD57– (grey) cells per μl of blood. Connecting lines indicate longitudinal analysis.
In marked contrast, the ratio and absolute numbers of CD57+ cells were not reduced significantly in the TemRO population after initiation of ART compared to the untreated state pre‐ART (Fig. 3c, middle panel), showing a non‐reversible accumulation of CD57+ cells in this subset without repopulation of CD57– cells.
However, CD57– TemRA cells were increased proportionally in viraemic patients pre‐ART and reduced after 9 months of ART (Fig. 3d, right panel) leading to similar frequencies of CD57+ cells in the TemRA population, even in healthy controls (Fig. 3d, left panel).
The frequency of CD127+ Tcm cells increased significantly after initiation of ART (Fig. 4b) compared to pre‐ART, reaching almost the level of healthy controls. For the TemRO population a partial restoration regarding frequencies of CD127+ cells was observed (Fig. 4c). Similarly to the observations we made in the central memory subset, CD127+ cells were not diminished in absolute numbers in untreated individuals compared to healthy controls, but increased under ART. Furthermore, frequencies of CD127+ TemRA cells were restored under ART, due to a reduction of CD127– cells (Fig. 4d).
Figure 4.
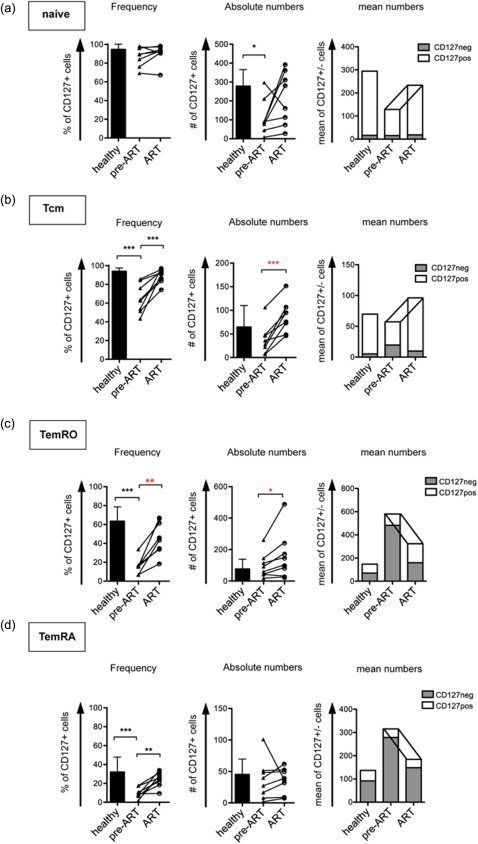
Restored survival capacity after 9 months of anti‐retroviral treatment (ART) within the early effector memory population. Cumulative data of longitudinal flow cytometry analyses of eight patients before and after at least 9 months of ART compared to healthy controls. Bar graphs depict mean numbers of CD127+ (white) and CD127– (grey) cells per μl of blood. Connecting lines indicate longitudinal analysis.
In summary, we observed a substantial increase of the frequency of CD127+ cells in the Tcm and TemRO populations in patients receiving ART. However, the elevated ratio of CD57+CD8+ T cells was only reversible in the naive and the central memory CD8+ T cell population. In the early effector memory population, which is the compartment where senescent cells accumulated, we did not see a similar reversal of CD57 expression.
Frequencies of CD57– and CD127+ cells correlate with total CD4+ T cell counts in treated and untreated HIV‐1 infected patients
Having shown that CD127+ memory populations reconstitute rapidly after initiation of ART, we were next interested in whether those populations correlate with established clinical surrogate markers of HIV infection. Thus, we performed a longitudinal analysis of the CD57+ and CD127+ subpopulations within the CD8+ TemRO subset and correlated those with the actual total CD4+ T cell counts.
We observed a strong inverse correlation in the total CD8+ T cell population between frequencies of CD127+ and CD57+ cells (Supporting information, Fig. S4). In the TemRO subset, the inverse correlation was observed in healthy individuals (left), but was completely undetectable in HIV infected individuals (centre) and was not restored in ART‐treated patients. The expression of CD127 and CD57 was not mutually exclusive. There were CD127+CD57+ double‐positive TemRO cells detectable in every patient group. The frequency of the CD127–CD57+ subset was increased in untreated patients compared to healthy individuals (Fig. 5a,b). A possible explanation for this finding is expansion due to proliferative activity in this subset, as the cells showed a significantly elevated Ki‐67 expression level (Fig. 5c). The frequency of the CD127–CD57+CD8+ T cell subset was reduced under ART with concomitant reduced proliferation. Similarly, the frequency of double‐negative CD127–CD57– cells was increased in untreated patients and reduced again under ART. This subset already had the overall highest expression of Ki‐67 in healthy controls, with a slight elevation in untreated HIV patients. Frequencies of CD127+CD57+CD8+ T cells were similar between healthy controls and untreated patients, but increased under ART without any measurable changes in proliferation (Fig. 5c). Frequencies of CD127+CD57– cells were reduced in untreated HIV patients, although showing augmented proliferation, and increased again after initiation of ART (Fig. 5b,c).
Figure 5.

Skewed association between CD57 and CD127 expression within early effector memory CD8+ T cells in HIV infection reveals expansion of CD127– subpopulations in HIV that are reduced under anti‐retroviral treatment (ART). (a) Representative flow cytometry plots depicting the subset definition within the CD8+ TemRO population according to their CD127 and CD57 expression in healthy controls and HIV patients before and upon the start of ART. (b) Longitudinal analysis of differentially CD127 and CD57‐expressig subsets in eight HIV infected individuals. Left panels: frequency of each subset out of CD8+ TemRO compared to nine healthy individuals. Statistical significance comparing all groups was determined via one‐way analysis of variance (anova) and Tukey's multiple comparison test and defined as P < 0·05 (black asterisk) and comparing longitudinal patient groups via paired t‐test (red asterisk). (c) Longitudinal analysis of Ki‐67 expression in different CD127/ CD57 subsets of CD8+ TemRO cells of six HIV‐1 infected individuals before and after the start of ART compared to six healthy controls. Statistical significance comparing all groups was determined via one‐way anova and Tukey's multiple comparison test and defined as P < 0·05. (d) Correlation analysis of relative CD127–CD57+ subset size of CD8+ TemRO cells with absolute CD4+ T cell count. Linear regressions and correlations only including untreated (pre‐ART) or treated (ART) patients, respectively. Parameters tested for correlation, statistical significance was defined as P < 0·05. R 2 is a fraction between 0·0 and 1·0 in which 1 presents a perfect fit of the linear regression.
To evaluate a possible relationship between these subsets and HIV‐induced disease, their relative numbers were correlated with the absolute CD4+ T cell count before and after treatment (Fig. 5d). This revealed a significant direct correlation between the CD127+CD57–CD8+ T cell subset and CD4+ T cell count in untreated (pre‐ART) and treated patients (ART), respectively, pointing towards a clinical importance of CD8+ T cell subsets with a high potential for renewal in HIV infection.
Discussion
Here, we present a study describing the efficacy of ART inducing the capacity for renewal within the CD8+ T cell population in HIV infection represented by partial reduction of senescence measured by CD57 expression, as well as elevated relative survival potential measured by CD127 expression.
We and others could show a significant reduction in the frequency of CD127+ cells 13, 17 in HIV infection, caused mainly by a massive increase of CD127– cells. These data are supported by a study by Paiardini et al. 18. In our current study, this frequency was restored rapidly upon initiation of ART in CD127+CD8+ central memory and the effector memory T cell population. In our study the analysis of cell populations was performed on cryopreserved PBMCs comparing a slightly younger healthy control cohort to HIV patients before and after treatment. These points are certainly a limitation to this study; however, viability of thawed cells was not below 90% and the main focus of this study is the longitudinal comparison of HIV patients upon treatment. Naive and memory CD8+ T cell subsets were defined by expression of CD45RO and CCR7. This gating does not exclude a certain contamination with stem cell‐like memory cells within the naive population; however, this population is very small and does not exceed 2–3% of total T cells in healthy individuals 19.
In an in‐vitro study by Alves et al. it was shown that CD127 down‐regulation can be restored on a single‐cell level upon withdrawal of the inducing stimulus. In their study, CD127 was down‐regulated upon IL‐7‐ and TCR/CD28‐mediated signalling, and the level of re‐expression was lower when the previous stimulus was TCR‐mediated 20.
In contrast, frequencies and absolute numbers of CD57+ cells were increased in every CD8+ T cell subpopulation in HIV‐infected individuals. VNC showed an absolute increase of CD57+ TemRO cell numbers, with a concomitant increase of CD57– TemRO cells, whereas in VC fewer CD57+ TemRO cells expanded without simultaneous expansion of CD57– TemRO cells. During ART only the naive and central memory populations were replenished with less differentiated CD57– cells, but this renewal under ART did not occur within the TemRO subset, together leading to an irreversible accumulation of terminally differentiated cells in this subset. The irreversible increase of CD57 on bulk CD8+ T cells is in accordance with results from another recent study 21.
As a matter of debate, a longer duration of ART would eventually lead to a rebalanced CD57+/CD57–ratio in effector memory subsets. The increasing age of the treated patients would then lead to increasing frequencies of CD57+CD8+ T cells and simultaneously to a lower thymic output of cells 22.
Even though no direct correlation between CD57 expression and HIV viral load could be observed in our current study (data not shown), VNC showed the highest frequencies and numbers of CD57+ T cells for most of the CD8+ subpopulations. The association of CD57 expression of CD8+ T cells and viral load has been discussed controversially in the past 5, 21, 23. Brenchley et al. also reported no correlation, but suggested that CD57+ cells are extremely sensitive to activation‐induced apoptosis and therefore a correlation could be masked 3. Furthermore, replicative senescence reflects the total antigenic experience or state of immune activation of a CD8+ T cell pool 24, and might therefore depend not only on the HIV load but also on the overall encounter with various pathogens such as cytomegalovirus (CMV) 25.
The majority of HIV Gag‐ and Nef‐specific CD8+ T cells exhibited a CD57+CD127– TemRO phenotype. In other studies, no difference of the expression of senescence markers between HIV‐specific and tetramer‐negative CD8+ TemRO cells was detected 6. However, frequencies of CD57+ TemRO cells were similar to those detected in our study (approximately 60%). It has to be taken into account that this is probably due also to the particular composition of TCR specificities within the tetramer‐negative TemRO population 26, as CD8+ T cells specific for CMV or other concomitant pathogens show even higher expression of CD57 27. Frequencies of CD127‐expressing cells were uniformly lower within HIV Gag‐ and Nef‐specific CD8+ TemRO cells, which is in corroboration with results from others 28, 29.
We have described a strong under‐representation of CD127+CD57– cells in HIV Gag‐ and Nef‐specific CD8+ TemRO cells. The size of this subset of the total TemRO cells was correlated with the CD4+ T cell counts in the untreated state and after initiation of ART. This indicates that, within the effector memory population, the balance between the cells that are still able to undergo further divisions and differentiation versus those cells that have reached their terminal differentiation after expansion is associated with disease outcome.
The strong inverse correlation between CD57 and CD127 seen in healthy individuals seems to be uncoupled in HIV infection in favour of a higher differentiation and lower survival capacity, and this perturbation could not be restored under ART. The expansion of CD127–CD8+ T cell subsets during untreated HIV infection occurred in conjunction with high cellular turnover and decreased again after initiation of ART. In contrast, CD127+ cells showed very low levels of proliferation and were elevated slightly in numbers upon initiation of ART. We observed an elevated proliferative potential in CD127‐negative subsets even under concomitant CD57 expression, which was unexpected, as the absence of CD127 and the presence of CD57 were described to be associated with reduced or lacking proliferation 3, 11. However, these results were obtained after in‐vitro restimulation and therefore differ from our ex‐vivo Ki‐67 measurements, which might capture a moment of proliferation that has led to down‐regulation of CD127 and up‐regulation of CD57. Together, these findings present more effector‐like qualities for CD127– TemRO cells with elevated proliferation in untreated HIV infection leading to high numbers of these cells, which are not maintained in the setting of reduced antigen presence under ART. This is in accordance with the reported reduced proliferation upon restimulation. In contrast, CD127+ cells showed very low levels of proliferation and were elevated slightly in numbers upon ART, which highlights their suggested role as real long‐term memory cells 12.
Subsequent studies will need to address the question of whether these phenotypical abnormalities are reversible after rapid initiation of ART during the acute primary infection or after long‐term treatment with ART for several years. Unfortunately, our current cohort was too small to stratify our ART‐treated patients for their CD4+ T cell nadir.
In summary, we observe a restoration of CD127 expression on all CD8+ T cell memory subpopulations, whereas the increased expression of the senescence marker CD57 on CD8+ effector memory T cell subsets in chronic HIV infection is not reversible after initiation of ART. Importantly, the frequencies of CD127+CD57–CD8+ TemRO cells correlated with the absolute CD4+ T cell counts, thus supporting the hypothesis that this population might be a correlate for successful immune reconstitution. Our findings can be interpreted as a phenotypical correlate of CD8+ memory T cell differentiation and the premature ‘ageing’ of the immune system, which can still be observed despite successful viral suppression.
Disclosure
The authors declare no competing interests.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Figure S1. Skewed CD8+ naive and memory T cell populations in HIV‐1 infection. Summary data depicting flow cytometry analyses of the relative distribution (left panels) or absolute cell counts (right panels) of CD8+ T cell populations including 9 viral controllers, 12 viral non‐controllers and 9 treated HIV‐1 patients compared to healthy controls.
Figure S2. Viral load correlates with relative CD8+ T cell population sizes. Linear regressions and correlation analyses of frequencies of naive and memory populations from total CD8+ T cells vs. logarithms of viral load from HIV‐1 infected viral controllers and viral non‐controllers. Statistical significance was defined as P < 0·05. R 2 is a fraction between 0·0 and 1·0 in which 1 presents a perfect fit of the linear regression.
Figure S3. Influence of ART on CD8+ T cell memory formation. Longitudinal flow cytometry analysis of CD8+ T cell distribution in naive and memory populations of 8 HIV‐1 infected individuals before and after start of ART compared to healthy controls. The size of each T cell subpopulations is given as a percentage of total CD8+ T cells (left panels) or absolute cell numbers given per μl of blood (right panels).
Figure S4. Loss of correlation between CD57 and CD127 expression on CD8+ TemRO cells in HIV infection. Linear regressions and correlation analysis of CD57 and CD127 expression on total and TemRO CD8+ T cells for healthy controls, untreated (pre‐ART) and treated HIV‐1 patients (ART). The parameters were tested for correlation; statistical significance was defined as P < 0·05. R 2 is a fraction between 0·0 and 1·0 in which 1 presents a perfect fit of the linear regression.
Table S1. Cross‐sectional patient cohort.
Table S2. Longitudinal patient cohort.
Table S3. Human leucocyte antigen (HLA)‐typed patient cohort for analysis of HIV‐1‐specific CD8+ T cells.
Acknowledgements
We thank the patients who participated in this study. This work was supported by the DZIF (German Center for Infection Research; JME, JSzW, RES, DMO). JME and JSzW are part of the Epistem, EHVA and ECHAM (Hivera) consortium.
References
- 1. Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strategies for Management of Antiretroviral Therapy Study Group , El‐Sadr WM, Lundgren J. et al CD4+ count‐guided interruption of antiretroviral treatment. N Engl J Med 2006; 355:2283–96. [DOI] [PubMed] [Google Scholar]
- 3. Brenchley JM, Karandikar NJ, Betts MR. et al Expression of CD57 defines replicative senescence and antigen‐induced apoptotic death of CD8+ T cells. Blood 2003; 101:2711–20. [DOI] [PubMed] [Google Scholar]
- 4. Chattopadhyay PK, Betts MR, Price DA. et al The cytolytic enzymes granyzme A, granzyme B, and perforin: expression patterns, cell distribution, and their relationship to cell maturity and bright CD57 expression. J Leukoc Biol 2009; 85:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lieberman J, Trimble LA, Friedman RS. et al Expansion of CD57 and CD62L–CD45RA+ CD8 T lymphocytes correlates with reduced viral plasma RNA after primary HIV infection. AIDS 1999; 13:891–9. [DOI] [PubMed] [Google Scholar]
- 6. Meyer‐Olson D, Simons BC, Conrad JA. et al Clonal expansion and TCR‐independent differentiation shape the HIV‐specific CD8+ effector‐memory T‐cell repertoire in vivo . Blood 2010; 116:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chong LK, Aicheler RJ, Llewellyn‐Lacey S, Tomasec P, Brennan P, Wang EC. Proliferation and interleukin 5 production by CD8hi CD57+ T cells. Eur J Immunol 2008; 38:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin‐7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 2000; 1:426–32. [DOI] [PubMed] [Google Scholar]
- 9. Tan JT, Dudl E, LeRoy E. et al IL‐7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA 2001; 98:8732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mazzucchelli R, Durum SK. Interleukin‐7 receptor expression: intelligent design. Nat Rev Immunol 2007; 7:144–54. [DOI] [PubMed] [Google Scholar]
- 11. Huster KM, Busch V, Schiemann M. et al Selective expression of IL‐7 receptor on memory T cells identifies early CD40L‐dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci USA 2004; 101:5610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long‐lived memory cells. Nat Immunol 2003; 4:1191–8. [DOI] [PubMed] [Google Scholar]
- 13. Boutboul F, Puthier D, Appay V. et al Modulation of interleukin‐7 receptor expression characterizes differentiation of CD8 T cells specific for HIV, EBV and CMV. AIDS 2005; 19:1981–6. [DOI] [PubMed] [Google Scholar]
- 14. Carini C, McLane MF, Mayer KH, Essex M. Dysregulation of interleukin‐7 receptor may generate loss of cytotoxic T cell response in human immunodeficiency virus type 1 infection. Eur J Immunol 1994; 24:2927–34. [DOI] [PubMed] [Google Scholar]
- 15. Shive CL, Clagett B, McCausland MR. et al Inflammation perturbs the IL‐7 axis, promoting senescence and exhaustion that broadly characterize immune failure in treated HIV infection. J Acquir Immune Defic Syndr 2016; 71:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004; 22:745–63. [DOI] [PubMed] [Google Scholar]
- 17. MacPherson PA, Fex C, Sanchez‐Dardon J, Hawley‐Foss N, Angel JB. Interleukin‐7 receptor expression on CD8(+) T cells is reduced in HIV infection and partially restored with effective antiretroviral therapy. J Acquir Immune Defic Syndr 2001; 28:454–7. [DOI] [PubMed] [Google Scholar]
- 18. Paiardini M, Cervasi B, Albrecht H. et al Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV‐infected individuals. J Immunol 2005; 174:2900–9. [DOI] [PubMed] [Google Scholar]
- 19. Gattinoni L, Lugli E, Ji Y. et al A human memory T cell subset with stem cell‐like properties. Nat Med 2011; 17:1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alves NL, van Leeuwen EM, Derks IA, van Lier RA. Differential regulation of human IL‐7 receptor alpha expression by IL‐7 and TCR signaling. J Immunol 2008; 180:5201–10. [DOI] [PubMed] [Google Scholar]
- 21. Mojumdar K, Vajpayee M, Chauhan NK, Singh A, Singh R, Kurapati S. Loss of CD127 and increased immunosenescence of T cell subsets in HIV infected individuals. Indian J Med Res 2011; 134:972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferrando‐Martinez S, Ruiz‐Mateos E, Hernandez A. et al Age‐related deregulation of naive T cell homeostasis in elderly humans. Age (Dordr) 2011; 33:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burgers WA, Riou C, Mlotshwa M. et al Association of HIV‐specific and total CD8+ T memory phenotypes in subtype C HIV‐1 infection with viral set point. J Immunol 2009; 182:4751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Papagno L, Spina CA, Marchant A. et al Immune activation and CD8+ T‐cell differentiation towards senescence in HIV‐1 infection. PLOS Biol 2004; 2:E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res 2011; 157:175–9. [DOI] [PubMed] [Google Scholar]
- 26. Appay V, Dunbar PR, Callan M. et al Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 2002; 8:379–85. [DOI] [PubMed] [Google Scholar]
- 27. Hoji A, Connolly NC, Buchanan WG, Rinaldo CR, Jr . CD27 and CD57 expression reveals atypical differentiation of human immunodeficiency virus type 1‐specific memory CD8+ T cells. Clin Vaccine Immunol 2007; 14:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lecuroux C, Girault I, Boutboul F. et al Antiretroviral therapy initiation during primary HIV infection enhances both CD127 expression and the proliferative capacity of HIV‐specific CD8+ T cells. AIDS 2009; 23:1649–58. [DOI] [PubMed] [Google Scholar]
- 29. Conrad JA, Ramalingam RK, Smith RM. et al Dominant clonotypes within HIV‐specific T cell responses are programmed death‐1high and CD127low and display reduced variant cross‐reactivity. J Immunol 2011; 186:6871–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Figure S1. Skewed CD8+ naive and memory T cell populations in HIV‐1 infection. Summary data depicting flow cytometry analyses of the relative distribution (left panels) or absolute cell counts (right panels) of CD8+ T cell populations including 9 viral controllers, 12 viral non‐controllers and 9 treated HIV‐1 patients compared to healthy controls.
Figure S2. Viral load correlates with relative CD8+ T cell population sizes. Linear regressions and correlation analyses of frequencies of naive and memory populations from total CD8+ T cells vs. logarithms of viral load from HIV‐1 infected viral controllers and viral non‐controllers. Statistical significance was defined as P < 0·05. R 2 is a fraction between 0·0 and 1·0 in which 1 presents a perfect fit of the linear regression.
Figure S3. Influence of ART on CD8+ T cell memory formation. Longitudinal flow cytometry analysis of CD8+ T cell distribution in naive and memory populations of 8 HIV‐1 infected individuals before and after start of ART compared to healthy controls. The size of each T cell subpopulations is given as a percentage of total CD8+ T cells (left panels) or absolute cell numbers given per μl of blood (right panels).
Figure S4. Loss of correlation between CD57 and CD127 expression on CD8+ TemRO cells in HIV infection. Linear regressions and correlation analysis of CD57 and CD127 expression on total and TemRO CD8+ T cells for healthy controls, untreated (pre‐ART) and treated HIV‐1 patients (ART). The parameters were tested for correlation; statistical significance was defined as P < 0·05. R 2 is a fraction between 0·0 and 1·0 in which 1 presents a perfect fit of the linear regression.
Table S1. Cross‐sectional patient cohort.
Table S2. Longitudinal patient cohort.
Table S3. Human leucocyte antigen (HLA)‐typed patient cohort for analysis of HIV‐1‐specific CD8+ T cells.


