Summary
Interleukin (IL)‐12 family cytokines play critical roles in autoimmune diseases. Our previous study has shown that IL‐23p19 and Epstein–Barr virus‐induced 3 (Ebi3) form a new IL‐12 family heterodimer, IL‐23p19/Ebi3, termed IL‐39, and knock‐down of p19 or Ebi3 reduced diseases by transferred GL7+ B cells in lupus‐prone mice. In the present study, we explore further the possible effect of IL‐39 on murine lupus. We found that IL‐39 in vitro and in vivo induces differentiation and/or expansion of neutrophils. GL7+ B cells up‐regulated neutrophils by secreting IL‐39, whereas IL‐39‐deficient GL7+ B cells lost the capacity to up‐regulate neutrophils in lupus‐prone mice and homozygous CD19cre (CD19‐deficient) mice. Finally, we found that IL‐39‐induced neutrophils had a positive feedback on IL‐39 expression in activated B cells by secreting B cell activation factor (BAFF). Taken together, our results suggest that IL‐39 induces differentiation and/or expansion of neutrophils in lupus‐prone mice.
Keywords: autoimmune diseases, BAFF, IL‐39, lupus, neutrophils
Introduction
Systemic lupus erythematosus (SLE) has been considered widely as a prototype of systemic autoimmune disease with a wide spectrum of clinical manifestations 1. There are numerous murine models, including Murphy Roths large lymphoproliferation (MRL/lpr) mice, that have long been employed in an effort to understand the cellular and genetic requirements for SLE induction 2, 3. Various mechanisms have been suggested as drivers of SLE. These include dysregulation of the innate immune system, over‐production of inflammatory cytokines, including a critical B cell activation factor (BAFF), and autoantibody 4. It has been proposed that these mechanisms may be linked, although this needs further detailed investigation.
The interleukin (IL)‐12 family is currently comprised of five members 5, 6 that regulate both pro‐ and anti‐inflammatory responses, in part, by influencing the developmental fates of naive T and B cell lymphocytes 6, 7. IL‐12 and IL‐23 play crucial roles in the pathogenesis of autoimmune diseases by inducing the differentiation of T helper type 1 (Th1) and Th17 lymphocytes, while IL‐27 and IL‐35 suppress inflammatory responses and limit tissue injury by promoting the expansion of regulatory B and T cell subsets 8, 9. An increased level of IL‐12, IL‐23, IL‐27 and IL‐35 has been reported in the plasma of SLE patients compared with healthy controls 10, 11, 12, 13, 14. Currently, the role of IL‐12 family members in pathogenesis of SLE is not well understood. As such, understanding the immunobiology of IL‐12 family cytokines would undoubtedly provide valuable knowledge that can be exploited therapeutically.
IL‐12, IL‐23, IL‐27 and IL‐35 are comprised of heterodimers p35/p40, p19/p40, p28/Epstein–Barr virus‐induced 3 (Ebi3) and p35/Ebi3, respectively. As such, the IL‐12 family cytokine consists of one α subunit (p19, p28, p35) and one β chain (p40, Ebi3) 15, 16. Based on the pairing rules in this family, we and other researchers have proposed that p19/Ebi3 pairing may be possible 5, 15, 16, 17, 18, 19. We termed IL‐23p19/Ebi3 heterodimer as IL‐39 and found that knock‐down of p19 or Ebi3 reduced diseases by transferred GL7+ B cells in lupus‐prone mice 6.
In the present study, we explore further the possible effect of IL‐39 on murine lupus. We found that IL‐39, secreted by activated B cells, induces differentiation and/or expansion of neutrophils in lupus‐prone mice. In addition, IL‐39‐induced neutrophils had a positive feedback on IL‐39 expression in activated B cells by secreting BAFF. Taken together, our results suggest that IL‐39 induces differentiation and/or expansion of neutrophils in lupus‐prone mice.
Materials and methods
Mice
Seven‐to‐nine‐week‐old C57BL/6, Balb/c (Huafukang Corp., Beijing, China), 8‐week‐old or 6‐month‐old female lupus‐prone MRL/MpJ/lpr/lpr (MRL/lpr) mice, age‐matched MRL/MpJ/+/+ (MRL/+) and homozygous CD19cre (Nanjing Biomedical Research Institute of Nanjing University, Nanjing, China) were bred in our animal facilities under specific pathogen‐free conditions. Care, use and treatment of mice in this study were in strict agreement with international guidelines for the care and use of laboratory animals. This study was approved by the Animal Ethics Committee of the Beijing Institute of Basic Medical Sciences.
Treatment of C57BL/6 mice with IL‐39
Production and characterization of p19/Ebi3 were performed as described previously 6. The expression constructs pEZ‐Lv122/mouse p19 and pReceiver‐Lv18/mouse Ebi3 (GeneCopoeia, Rockville, MD, USA) were transduced/co‐transduced into Chinese hamster ovary (CHO) cells and stable transfectants were identified by drug selection (10 μg/ml puromycin and neomycin; Invitrogen, Carlsbad, CA, USA). The recombinant protein(s) secreted by the CHO cells was purified sequentially by the Ni‐NTA purification system (Invitrogen), size‐exclusion centricon filtration and two consecutive cycles of fast protein liquid chromatography (FPLC) gel filtration chromatography. Four hundred ng/mouse p19, Ebi3 and IL‐39 were injected intravenously (i.v.) into 8‐week‐old C57BL/6 mice. On day 7 after injection, splenocytes were analysed by fluorescence activated cell sorter (FACS).
Cell sorting
B cells from the spleen were sorted using B220 microbeads. B cells from lupus‐prone mice were stimulated for 3 days with 1 µg/ml lipopolysaccharide (LPS) and was performed by gating on GL7 and B220 on the surface of activated B cells and used to sort GL7+B220+ cells directly by multi‐colour flow cytometry. In some experiments, neutrophils from 8‐week‐old C57BL/6 mice were sorted on the basis of CD11b and Gr‐1 by FACS. All flow cytometry data were acquired with FACSCanto, FACSCantoI or FACSAria (BD Biosciences, San Jose, CA, USA). Live lymphocyte‐sized cells for T and B cell analysis on all live cells, including large granule cells for neutrophil analysis, were gated on the basis of forward‐ and side‐scatter, and analysed using FlowJo software (Tree Star, Ashland, OR, USA).
Control, p19‐, Ebi3‐, p28‐, p35‐ and p40‐specific shRNA‐infected GL7+B220+ B cells were transferred into lupus‐prone mice
GL7+B220+ cells were described as above and infected with p19, p28, p35, p40, Ebi3 (Santa Cruz Biotech, Santa Cruz, CA, USA; sc‐60028‐v, sc‐72185‐v, sc‐39639‐v, sc‐39641‐v, sc‐39411‐v, respectively)‐specific shRNA; 5 × 106 control, p19, p28, p35, p40, or Ebi3 ‐specific shRNA‐transfected GL7+ B220+ B cells per mouse were injected i.v. into 8‐week‐old female lupus‐prone MRL/lpr or CD19cre mice.
In‐vitro cell culture
Splenocytes were collected from 8‐week‐old female C57BL/6 mice. Red blood cells were lysed by adding 1 × lysis buffer (BD# 349202) into splenocytes suspension. Cells were washed and cultured for 3 days in RPMI‐1640 medium containing 10% fetal bovine serum (FBS), 2 mM glutamine, penicillin (100 IU/ml), streptomycin (100 µg/ml) and 50 mM 2‐mercapthoethanol with 50 ng/ml p19, Ebi3 and IL‐39. Primary B cells from 8‐week‐old female C57BL/6 mice were sorted by B220 microbeads and stimulated for 3 days in RPMI‐1640 medium containing 10% FBS, 2mM glutamine, penicillin (100 IU/ml), streptomycin (100 µg/ml) and 50 mM 2‐mercapthoethanol with 50 ng/ml BAFF (PeproTech, Rocky Hill, NJ, USA). In some experiments different doses, such as 1, 5 and 10 μg/ml Bcl‐6 inhibitor (79‐6, cat no. 197345‐50MG; Calbiochem, EMD Millipore, Billerica, MA, USAUSA), were added into the culture of BAFF‐stimulated B cells.
Cytometric analysis and intracellular cytokine staining
All cell experiments were strictly prepared on ice, unless stated otherwise in other specific procedures. Cells (1 × 106 cells/sample) were washed with FACS staining buffer [phosphate‐buffered saline (PBS), 2% fetal bovine serum (FBS) or 1% BSA, 0.1% sodium azide]. All samples were incubated with anti‐Fc receptor antibody (clone 2.4G2; BD Biosciences) prior to incubation with other antibodies diluted in FACS buffer supplemented with 2% anti‐Fc receptor antibody. For intracellular cytokine staining, 50 ng/ml phorbol myristate acetate (PMA) and 1 μg/ml ionomycin (Sigma‐Aldrich, St Louis, MO, USA) were added and then 10 μg/ml brefeldin A and 2 μM monensin were added 3 h later. After 3 h, cells were collected and fixed for 50 min with 1 ml fixation buffer (IC fixation and permeabilization kit; eBioscience, San Diego, CA, USA). After washing, the fixed cells were stained. The samples were filtered immediately before analysis or cell sorting to remove any clumps. The following antibodies were used: fluorescence‐conjugated anti‐mouse p19 (eBioscience Corp., cat. no.50‐7023‐82), Ebi3 (R&D systems, cat. no. IC18341C), IL‐12Rβ1 (BD Pharmingen, San Diego, CA, USA; 551974), IL‐12Rβ2 (Miltenyi Biotech, San Diego, CA, USA; 130‐105‐018), IL‐23R (BD Pharmingen; 551974), IL‐27Ra (R&D Systems, Minneapolis, MA, USA; 263503), gp130 (eBioscience;17‐1302), B220 (eBioscience; RA3‐6B2), CD19 (eBioscience; MB19‐1), GL7 (eBioscience; GL‐7), CD138 (eBioscience; DL‐101), IL‐10 (eBioscience; JES5‐16E3), CD3 (eBioscience; 145‐2C11), CD4 (eBioscience; GK1.5), CD11b (eBioscience; M1/70), CD11c (eBioscience; N418), IL‐4 (eBioscience; 11B11), IL‐17A (eBioscience; 17F3), forkhead box protein 3 (FoxP3) (eBioscience; NRRF‐30), interferon (IFN)‐γ (eBioscience; XMG1.2), Gr‐1 (eBioscience; RB6‐8C5), BAFF (Pierce, MA, USA; 125955), phosphor signal transducer and activator of transcription‐1 (pSTAT‐1) (Santa Cruz Biotech; sc‐8394) and pSTAT‐3 (Santa Cruz Biotech; sc‐8059) antibodies. Data collection and analyses were performed on a FACSCalibur flow cytometer using CellQuest software.
Differentiation of neutrophils was induced in vitro
ScaI+ bone marrow (BM) cells were selected using ScaI MultiSort microbeads and midi‐magnetic‐activated cell sorting (MACS) separation columns (Miltenyi Biotec, Auburn, CA, USA). ScaI+ cells were lineage‐depleted by labelling with fluorescein isothiocyanate (FITC)‐conjugated anti‐CD4, anti‐CD8, anti‐CD11b, anti‐Gr‐1 and anti‐B220 (BD PharMingen), binding to anti‐FITC microbeads (Miltenyi Biotec) and passing them through miniMACS separation columns (purity, 95 ± 2% Lin– ScaI+). Lin– ScaI+ cells were grown as suspension cultures for 5 days in Teflon jars (37°C, CO2) with Iscove's modified Dulbecco's medium (IMDM) (Life Technologies, Grand Island, NY, USA), supplemented with 10% FBS (HyClone Laboratories, Logan, UT, USA), 1 ng/ml granulocyte–macrophage colony‐stimulating factor (GM‐CSF) (Peprotech) and 50 ng/ml p19, Ebi3 or IL‐39.
Statistics
Statistics were analysed using GraphPad Prism version 5.0 (GraphPad Software Inc., San Diego, CA, USA). The data were shown as mean ± standard error of the mean (s.e.m.). Student's t‐test was employed to determine significance between two groups (paired or unpaired) and one‐way analysis of variance (anova) analysis was used to determine significance among several groups. Differences were considered statistically significant when P < 0·05.
Results
IL‐39‐expanded CD11b+ cells
Our previous study has shown that knock‐down of IL‐39 subunit p19 or Ebi3 reduced diseases by transferred GL7+ B cells in lupus‐prone mice 6. To explore further the possible role of IL‐39 in lupus‐prone mice, we first investigated whether IL‐39 might influence the development and/or expansion of various lymphoid and haematopoietic cell types. To study the physiological role of IL‐39 on various haematopoietic and lymphoid cell types, we used the purified IL‐39 to treat mouse splenocytes in vitro and in vivo. Surprisingly, we found that IL‐39 could induce the expansion of CD11b+ cells (Fig. 1a,b) but not B cell subpopulations (GL7+ B cells, CD138+ plasma cells and IL‐10+ regulatory B cells) (Fig. 1c–e) or T lymphocyte subsets [CD4+ and CD8+ T cells, Th1, Th2, Th17 or regulatory T cells (Tregs)] (Fig. 1f–j), whereas IL‐39 subunit p19 or Ebi3 alone could not affect CD11b+ cells (Fig. 1a,b). These results suggest that IL‐39 mainly expands CD11b+ cells.
Figure 1.
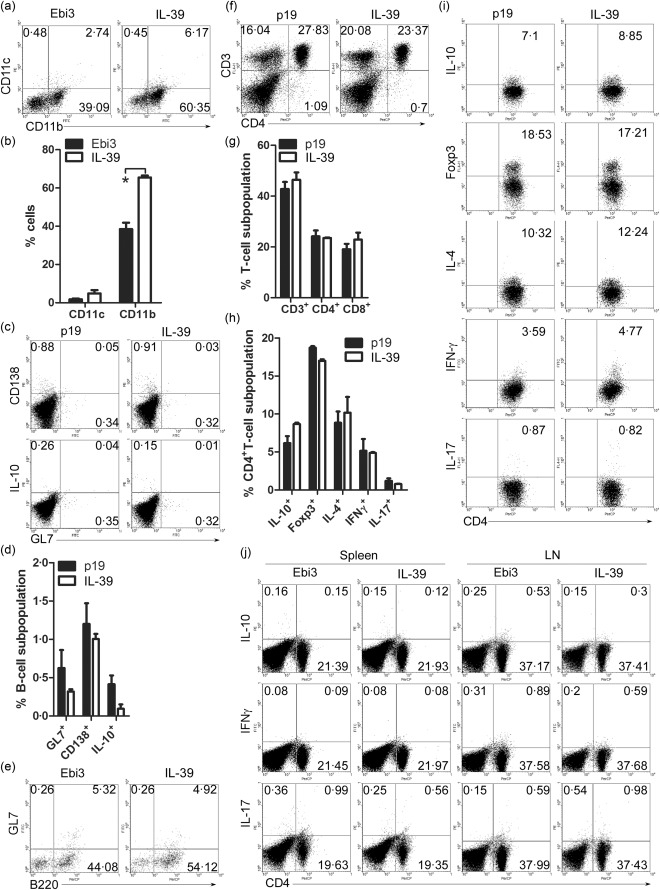
Interleukin (IL)‐39 [IL‐23p19/Epstein–Barr virus‐induced 3 (Ebi3) expanded CD11b+ cells. (a,b) Splenocytes were separated from 8‐week‐old C57BL/6 mice and cultured in vitro for 3 days in the presence of 50 ng/ml Ebi3 and IL‐39. All live cells, including large granule cells, were gated on the basis of forward‐ and side‐scatter and analysed by fluorescence activated cell sorter (FACS). The percentages of CD11c+ and CD11b+ cells (a) and statistical analysis of the percentage (b) are shown; (c–e) 400 ng/mouse p19, Ebi3 and IL‐39 were injected intravenously (i.v.) into 8‐week‐old C57BL/6 mice (six mice per group). On day 7 after injection, live lymphocyte‐sized cells were gated on the basis of forward‐ and side‐scatter and analysed by FACS. The percentages of CD138, IL‐10 or GL7‐expressing B220+ B cells (c,e) and statistical analysis of the percentage (d) are shown; (f–i) 400 ng/mouse p19 and IL‐39 were injected i.v. into 8‐week‐old C57BL/6 mice (six mice per group). On day 7 after injection, live lymphocyte‐sized cells were gated on the basis of forward‐ and side‐scatter and analysed by FACS. The percentages of CD3+ and CD4+ T cells (f) and statistical analysis of the percentage (g), IL‐10, forkhead box protein 3 (FoxP3), IL‐4, interferon (IFN)‐γ, IL‐17A‐expressing CD4+ T cells (i) and statistical analysis of the percentage (h) are shown. (j) Splenocytes were separated from 8‐week‐old C57BL/6 mice and cultured in vitro for 3 days in the presence of 50 ng/ml Ebi3 or IL‐39. Live lymphocyte‐sized cells were gated on the basis of forward‐ and side‐scatter and analysed by FACS. The percentages of IL‐10, IFN‐γ, IL‐17A‐expressing CD4 + T cells are shown. Results represent at least three independent experiments. *P < 0·05 (two‐tailed Student's t‐test). Error bars, standard error of the mean.
IL‐39 in vivo‐ and in vitro‐induced differentiation and/or expansion of neutrophils
CD11b is expressed on the surface of neutrophils, monocytes, macrophages and a subset of B cells, etc. To determine further which population of cells is affected by IL‐39, we first used a Gr‐1 marker to gate neutrophils (Gr‐1+ CD11b+) from CD11b+ cells. We found that IL‐39 up‐regulated Gr‐1+CD11b+ neutrophils significantly but not Gr‐1–CD11b+ cells in cultured splenocytes (Figs 2a–c and Supporting information, Fig. S1) and in C57BL/6 mice (Fig. 2d,e). These results suggest that IL‐39 in vivo and in vitro induces expansion of neutrophils. To test the effect of IL‐39 on the differentiation of neutrophils, we developed an in‐vitro neutrophil differentiation cultured system and used it to establish that IL‐39 can induce the differentiation of neutrophils (Fig. 2f,g). Together, these results suggest that IL‐39 in vivo and in vitro induces differentiation and/or expansion of neutrophils.
Figure 2.
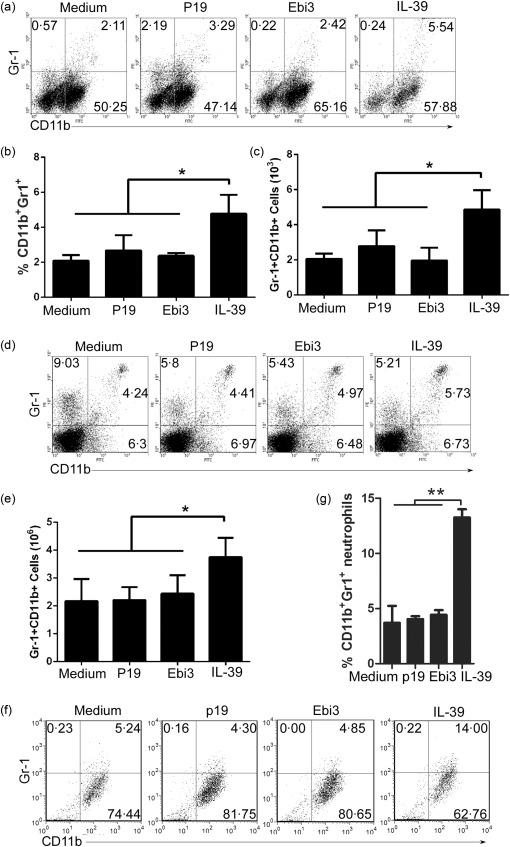
Interleukin (IL)‐39 expanded and/or induced neutrophils. (a–c) Splenocytes were separated from 8‐week‐old C57BL/6 mice, cultured for 3 days in the presence of medium, 50 ng/ml p19, Epstein–Barr virus‐induced 3 (Ebi3) or IL‐39 and analysed by fluorescence activated cell sorter (FACS). The percentages of Gr‐1+CD11b+ neutrophils (a), statistical analysis of the percentage (b) and cultured cell numbers (c) are shown; (d,e) 400 ng/mouse IL‐39 were injected intravenously (i.v.) into 8‐week‐old C57BL/6 mice (six mice per group). On day 7 after IL‐39 injection, splenocytes were analysed by FACS. The percentages of Gr‐1+CD11b+ neutrophils (d) and cultured cell numbers (e) are shown. (f,g) Lin– ScaI+ cells were sorted from 8‐week‐old C57BL/6 mice by microbeads and stimulated for 5 days with 1 ng/ml granulocyte–macrophage colony‐stimulating factor (GM‐CSF) in the presence of 50 ng/ml IL‐39. Cells were analysed by FACS and the percentages of Gr‐1+CD11b+ neutrophils (f) and cultured cell numbers (g) are shown. Results represent at least three independent experiments. *P < 0·05; **P < 0·01. One‐way analysis of variance (anova) plus Dunnett's multiple comparison test: compare all columns versus control column. Error bars, standard error of the mean.
IL‐39‐induced STAT‐3 activation in neutrophils
The IL‐12 family of cytokines mediate their biological activities through activation of homodimeric or heterodimeric IL‐12 cytokine receptor subunits (IL‐12Rβ1, IL‐12Rβ2, IL‐23R, gp130 or IL‐27Rα) and Janus kinase (JAK/STAT) signalling pathways 20. Our previous study has shown that IL‐39 activates STAT‐1 and STAT‐3, but not STAT‐4 or STAT‐5 in B cells via its receptor IL‐23R/gp130 6. We first established that neutrophils express all IL‐12 cytokine receptor subunits, including IL‐39 receptor IL‐23R/gp130 (Fig. 3a,b). Next, we examined whether IL‐39 could induce STAT‐1 and STAT‐3 activation. We found that IL‐39 activates STAT‐3 but not STAT‐1 in neutrophils (Fig. 3c,d). These results reveal that IL‐39 induces STAT‐3 activation in neutrophils.
Figure 3.
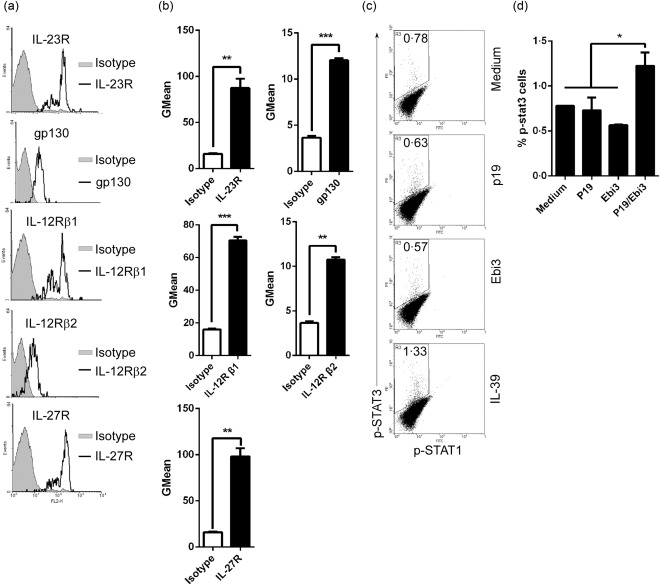
Interleukin (IL)‐39 activated signal transducer and activator of transcription‐3 (STAT‐3) pathways in neutrophils. (a) The expression of IL‐12Rβ1, IL‐12Rβ2, IL‐23R, gp130 or IL‐27R on the surface of neutrophils from the spleen of 8‐week‐old C57BL/6 mice was analysed by fluorescence activated cell sorter (FACS). Isotype antibody was used as the staining control. (b) GMean (mean fluorescence intensity) of IL‐12Rβ1, IL‐12Rβ2, IL‐23R, gp130 or IL‐27R staining from (a). (c,d) CD11b+Gr‐1+ neutrophils were sorted from the spleen of 8‐week‐old female C57BL/6 mice by FACS. The cells were cultured for 30 min in the presence of 50 ng/ml p19, Epstein–Barr virus‐induced 3 (Ebi3) and IL‐39 and analysed by FACS. The percentages (c) and the statistical analysis of the percentages (d) of phospho (p)‐STAT‐3‐expressing neutrophils are shown. (b,d) Data are shown as mean ± standard error of the mean (s.e.m.) (n = 3) from three independent experiments. *P < 0·05; **P < 0·01; ***P < 0·001. (b) Two tailed Student's t‐test; (d) one‐way analysis of variance (anova) plus Dunnett's multiple comparison test: compare all columns versus control column. Error bars, s.e.m.
IL‐39 deficiency reduced the capacity of GL7+ B cells in up‐regulating neutrophils in lupus‐prone mice
The results above suggest that IL‐39 may affect differentiation and/or expansion of neutrophils to mediate inflammation in lupus‐prone mice. During the past decade, compelling evidence has emerged that implicates neutrophils in the initiation and perpetuation of SLE and also in the resultant organ damage observed frequently in patients with this disease 21, 22. Our data also demonstrated that neutrophils increased in the spleen of lupus‐prone mice (Fig. 4a,b). SLE and its murine model including MRL/lpr mice are characterized by B cell over‐activation 23, 24, 25, 26, 27, 28, 29. Our previous study has shown that GL7+ B cells induces inflammation by secreting IL‐39 in lupus‐prone mice 6. Of note, GL7 serves as a marker for germinal centres B cells and as an activation marker of LPS‐stimulated B cells 30. We isolated and sorted GL7+ B cells from lupus‐prone mice and used shRNA to deplete p19, Ebi3, p19, p28 or p40 (Supporting information, Fig. S2a) in GL7+ B cells, as described previously 6. Haematoxylin and eosin (H&E) staining of kidney sections demonstrated that, compared with untreated MRL/lpr, GL7+ B cells promoted inflammatory cells to infiltrate into the kidney and destroyed the structure of the glomerular region, whereas IL‐39‐deficiency reduced GL7 + B‐induced infiltrating inflammatory cells in the kidney of lupus‐prone MRL/lpr mice (Supporting information, Fig. S2b). These results suggest that IL‐39 may play an important role in inflammatory cell infiltration into the kidney in MRL/lpr mice. Of importance, we found that adoptive transfer of GL7+ B cells enhanced significantly the numbers of neutrophils in lupus‐prone mice (Fig. 4c,d), whereas IL‐39 subunit p19 or Ebi3 deficiency suppressed the effects mediated by GL7+ B cells (Fig. 4c,d). Moreover, compared with mice that received GL7+ B cells depleted of p28, p35 or p40, mice that received p19‐ or Ebi3‐deficient GL7+ B cells exhibited a phenotype characterized by a greatly reduced number of neutrophils (Fig. 4c,d). Together, our results suggest that IL‐39 deficiency reduced the capacity of GL7+ B cells in up‐regulating neutrophils in lupus‐prone mice.
Figure 4.
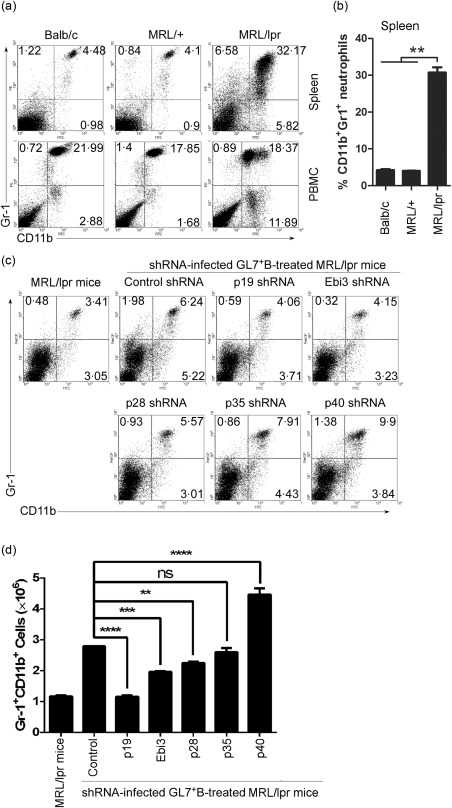
Interleukin (IL)‐39‐deficient GL7+ B cells could not induce neutrophils in lupus‐prone mice. (a,b) CD11b+Gr‐1+ neutrophils from spleen and peripheral blood mononuclear cells (PBMC) of 8‐month‐old female BALB/C, non‐lupus‐prone Murphy Roths large (MRL)+ and lupus‐prone MRL/lpr mice (six mice per group) were analysed by fluorescence activated cell sorter (FACS). The percentages of CD11b+Gr‐1+ neutrophils in the spleen and PBMC (a) and statistical analysis of the percentages in the spleen (b) are shown. (c,d) GL7+ B cells from 8‐month‐old female lupus‐prone MRL/lpr mice were sorted by FACS and infected with control shRNA or IL‐12 family subunits p28, p35 or p40, p19 or Epstein–Barr virus‐induced 3 (Ebi3)‐specific shRNA. On day 1 after infection, 5 × 106 control, p28, p35, p40, p19 and Ebi3‐specific shRNA‐infected GL7+B220+ B cells per mouse were injected intravenously (i.v.) into 8‐week‐old female lupus‐prone MRL/lpr mice (six mice per group). Age‐matched MRL/+ mice and control shRNA‐infected GL7+ B cells transferred group were used as non‐lupus‐prone mice and control shRNA, respectively. On day 14 after cell transfer, splenocytes are analysed by FACS. The percentages (c) and the absolute numbers (d) of CD11b+Gr‐1+ neutrophils per spleen are shown. We used two‐tailed Student's t‐test to analyse the difference between each of p28, p35 or p40, p19 or Ebi3‐specific shRNA‐infected GL7+ B cells transfer group and control shRNA‐infected GL7+ B cells transfer group. (b,d) Data are shown as mean ± standard error of the mean (s.e.m.) (n = 6) from one experiment representative of two other similar experiments. **P < 0·01; ***P < 0·001; ****P < 0·0001. One‐way analysis of variance (anova) plus Dunnett's multiple comparison test: compare all columns versus control column. Error bars, s.e.m.
IL‐39‐deficient GL7+ B cells did not up‐regulate neutrophils in homozygous CD19cre mice
To eliminate the effect of endogenous IL‐39‐expressing GL7+ B cells on neutrophils regulated by exogenous IL‐39‐deficient GL7+ B cells, we used GL7+ B cell‐reduced mice. Our previous study has shown that GL7+ B cells are reduced significantly in homozygous CD19cre mice 25, 29, 31. Thus, we transferred IL‐39‐deficient GL7+ B cells into CD19cre mice. We found that compared with mice that received control GL7+ B cells, mice that received p19‐ or Ebi3‐deficient GL7+ B cells exhibited a phenotype characterized by a greatly reduced number of neutrophils (Fig. 5a,b). Together, our results suggest that IL‐39‐deficient GL7+ B cells could not up‐regulate neutrophils in GL7+ B cell‐reduced mice.
Figure 5.
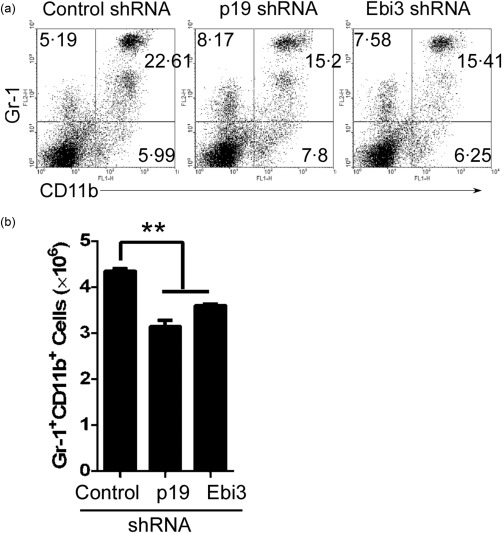
Interleukin (IL)‐39‐deficient GL7+ B cells could not induce neutrophils in homozygous CD19cre mice. GL7+ B cells were sorted from 8‐month‐old female lupus‐prone Murphy Roths large (MRL)/lpr mice by fluorescence activated cell sorter (FACS) and infected with control shRNA or IL‐39 subunits p19 or Epstein–Barr virus‐induced 3 (Ebi3)‐specific shRNA. On day 1 after infection, 5 × 106 control, p19 and Ebi3‐specific shRNA‐infected GL7+B220+ B cells per mouse were injected intravenously (i.v.) into 8‐week‐old female CD19cre mice (six mice per group). On day 14 after cell transfer, splenocytes were analysed by FACS. The percentages (a) and absolute numbers (b) of CD11b+Gr‐1+ neutrophils per spleen are shown. Data are shown as mean ± standard error of the mean (s.e.m.) (n = 6) from one experiment representative of two other similar experiments. *P < 0·05; **P < 0·01. One‐way analysis of variance (anova) plus Dunnett's multiple comparison test: compare all columns versus control column. Error bars, s.e.m.
Neutrophils promoted IL‐39 expression in activated B cells by secreting BAFF
Previous studies have shown that neutrophils can express BAFF, which plays a critical pathogenic role in SLE 32, 33, 34. Our data here demonstrated that the percentages and absolute numbers of BAFF‐expressing neutrophils were up‐regulated significantly in lupus‐prone MRL/lpr mice (Fig. 6a–c). Further, we found that the percentages of BAFF+ in CD11b+Gr‐1low and CD11b+ Gr‐1hi cells are similar (Supporting information, Fig. S3a). Critically, we found that during IL‐39‐induced neutrophils, the BAFF level in cultured supernatant was up‐regulated significantly (Fig. 6d). The data suggest that IL‐39 may result in the secretion of BAFF by neutrophils. It became clear that BAFF is a positive regulator of B cell function, with effects on cell survival, activation and differentiation 24, 35. Our previous study has shown that B cells were activated to secrete IL‐39 6. Thus, we tested the effect of BAFF on IL‐39 expression. As expected, BAFF up‐regulated IL‐39 (p19/Ebi3) expression significantly in B cells (Fig. 6e,f). In addition, the inhibitor of B cell proliferation‐inducing Bcl‐6 transcription factor 36, 37 IL‐39 (p19+Ebi3+) expression reduced dose‐dependently in BAFF‐activated B cells (Fig. 6g). Together, our data suggest that IL‐39‐induced neutrophils have a positive feedback on IL‐39 expression in B cells by secreting BAFF, which may mediate a pathogenic role in lupus‐prone mice.
Figure 6.
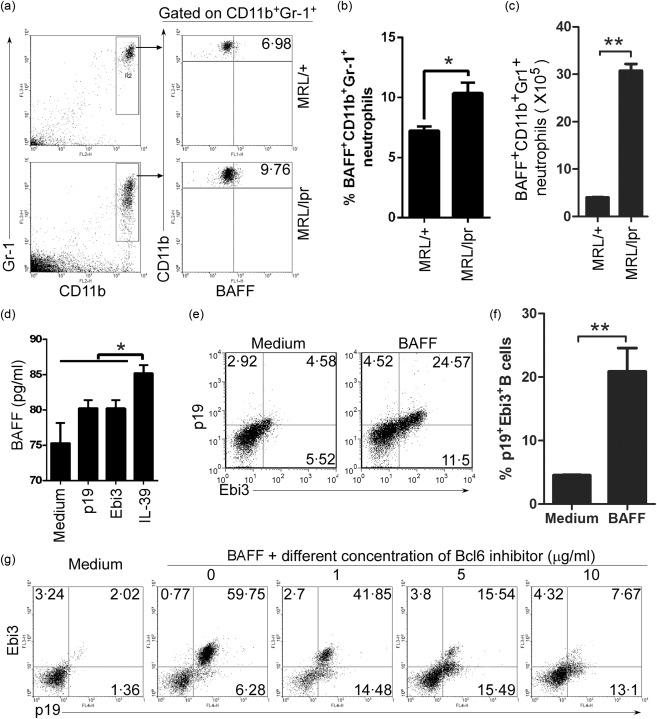
Coordinate expression of B cell activating factor (BAFF) in neutrophils and interleukin (IL)‐39 in B cells. (a–c) BAFF‐expressing CD11b+Gr‐1+ neutrophils from 8‐month‐old female non‐lupus‐prone Murphy Roths large (MRL)+ and lupus‐prone MRL/lpr mice (six mice per group) were analysed by fluorescence activated cell sorter (FACS). The percentages (a), the statistical analysis of the percentages (b) and absolute numbers (c) of BAFF‐expressing CD11b+Gr‐1+ neutrophils per spleen are shown. (d) The cultured supernatant was collected from an in‐vitro IL‐39‐induced neutrophil differentiation cultured system (Fig. 2f,g). BAFF level was determined by sandwich enzyme‐linked immunosorbent assay (ELISA) assay. (e,f) B cells from 8‐week‐old C57BL/6 mice were sorted by B220 microbeads, cultured for 3 days with 50 ng/ml BAFF and analysed by FACS. The percentages of IL‐39 [p19 and Epstein–Barr virus‐induced 3 (Ebi3)]‐expressing B cells (e) and statistical analysis of the percentage (f) are shown. (g) B cells indicated in (Fig. 6e,f) were cultured for 3 days in the presence of a different concentration of Bcl6 inhibitor, and analysed by FACS. The percentages of IL‐39‐expressing B cells are shown. Results represent at least three independent experiments. *P < 0·05; **P < 0·01. (b,c,f) Two‐tailed Student's t‐test; (d) One‐way analysis of variance (anova) plus Dunnett's multiple comparison test: compare all columns versus control column. Error bars, standard error of the mean.
Discussion
Our previous study has shown that knock‐down of the IL‐39 subunit p19 or Ebi3 reduced diseases by transfer of GL7+ B cells in lupus‐prone mice 6. In the present study, we found that IL‐39‐induced differentiation and/or expansion of neutrophils and IL‐39‐induced neutrophils to secrete BAFF in lupus‐prone mice.
EBI‐3 knock‐out mice are resistant to the induction of immunopathology associated with oxazolone‐induced colitis 38, develop deteriorated delayed‐type hypersensitivity responses 39 and have a pathological alteration of autoimmune glomerulonephritis and sialadenitis in MRL/lpr mice 40. These studies suggest that Ebi3 has a proinflammatory function that may result from the fact that, except for suppressive IL‐27 and IL‐35, Ebi3‐related IL‐39 is a proinflammatory cytokine. p19 knock‐out mice were highly deficient in the production of IFN‐γ, IL‐17A and tumour necrosis factor (TNF) 41. Thus, p19 facilitates the development of T cells towards both Th1 and Th17 pathways, suggesting that except for Th17‐favouring IL‐23, p19‐related IL‐39 may favour the Th1 pathway. These studies supported our previous and present studies, finding that IL‐39 may be a proinflammatory cytokine in lupus‐prone mice 6.
We also examined the physiological function of IL‐39 in vivo following i.v. injection of IL‐39 or adoptive transfer of GL7+ B cells depleted of p19 or Ebi3 into lupus‐prone mice. Adoptive transfer of IL‐39‐deficient GL7+ B cells into lupus‐prone mice induced a dramatic reduction in the numbers of neutrophils, a pathological feature of the disease in lupus‐prone mice. Conversely, expansion of IL‐39‐secreting GL7+ B cells correlated with the development of neutrophils in lupus‐prone mice, further underscoring the physiological relevance of IL‐39 in the production of neutrophils. In addition, we found that IL‐39 induces differentiation/expansion of neutrophils by activating STAT‐3 in lupus‐prone mice. The data are in line with a previous study suggesting that over‐expression of STAT‐3 in differentiating myeloid cells results in neutrophil expansion 42.
Many studies have shown the effect of the IL‐12 family on neutrophils. IL‐12 stimulated human neutrophils effectively to secrete IFN‐γ 43. IL‐23 activated neutrophils to secrete IL‐17A 44. IL‐27 is a negative regulator of human neutrophil function 45. Tumour‐derived IL‐35 promotes tumour growth by enhancing neutrophils 46. Of particular importance, we found that IL‐39 induced the differentiation and/or expansion of neutrophils and a significant number of the neutrophils expressed relatively high levels of BAFF, suggesting that IL‐39 might promote the expansion of BAFF‐expressing neutrophils that have been implicated in autoimmune diseases including SLE 21, 22, 47. In fact, BAFF concentration is higher in patients with various autoimmune conditions compared with normal subjects 35, 48, and BAFF is regarded as a potential therapeutic target in many autoimmune diseases 24, 32, 33, 34, 49. Together, our data suggest that IL‐39‐induced neutrophils have a positive feedback on IL‐39 expression in B cells by secreting BAFF, which may mediate a pathogenic role in lupus‐prone mice.
In conclusion, IL‐39 induces and/or expands neutrophils in lupus‐prone mice. In addition, IL‐39‐induced neutrophils had a positive feedback on IL‐39 expression in activated B cells by secreting BAFF. Thus, IL‐39 confers an important immunopathogenic effect in autoimmune diseases and could be used as a possible target for the treatment of autoimmune diseases such as SLE.
Disclosure
The authors declare no commercial or financial conflicts of interest.
Author contributions
X. W., X. L., Y. Z., Z. W., G. Z. and C. H. performed experiments; G. H., G. C., T. W., N. M., B. S., Y. L. and H. X. contributed essential reagents and materials for the experiments; R. W. conceived and designed the studies; all authors contributed to data analysis and manuscript preparation.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Gating strategy for interleukin (IL)‐39 expanded/induced neutrophils. Splenocytes were separated from 8‐week‐old C57BL/6 mice and red blood cells were lysed using 1 × lysis buffer. Cells were washed and cultured for 3 days in the presence of medium, 50 ng/ml p19, Epstein–Barr virus‐induced 3 (Ebi3) or IL‐39 and analysed by fluorescence activated cell sorter (FACS). All live cells (a) or large granule cells (b) were gated and the percentages of Gr‐1+CD11b+ neutrophils are shown. Unless stated otherwise in other specific procedures, neutrophils were analysed by gating all live cells (a).
Fig. S2. Interleukin (IL)‐39 deficiency reduced GL7+ B‐induced inflammatory cells infiltration into the kidney of lupus‐prone Murphy Roths large (MRL)/lpr mice. GL7+ B cells from 8‐month‐old female lupus‐prone MRL/lpr mice were sorted by fluorescence activated cell sorter (FACS) and infected with control shRNA or IL‐12 family subunits p28, p35 or p40, p19 or Epstein–Barr virus‐induced 3 (Ebi3)‐specific shRNA. On day 1 after infection, (a) p28, p35, p40, p19 and Ebi3 mRNA expression were analysed by quantitative polymerase chain reaction (qPCR); (b) 5 × 106 control, p28, p35, p40, p19 and Ebi3‐specific shRNA‐infected GL7+ B cells per mouse were injected intravenously (i.v.) into 8‐week‐old female lupus‐prone MRL/lpr mice (six mice per group). Two weeks after treatment, kidney sections were stained with haematoxylin and eosin (H&E). Red arrows show glomeruli; blue arrows show infiltrating inflammatory cells. Scale bars, 50 μM. (a) Data are shown as mean ± standard error of the mean (s.e.m.) (n = 6) and are representative of three independent experiments. (b) Data represent at least three independent experiments. *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001 (two‐tailed Student's t‐test).
Fig. S3. B cell activating factor (BAFF)‐expressing CD11b+Gr‐1high and CD11b+Gr‐1low cells are similar. BAFF‐expressing CD11b+Gr‐1high and CD11b+Gr‐1low cells from the splenocytes of 8‐month‐old female non‐lupus‐prone Murphy Roths large (MRL)+ and lupus‐prone MRL/lpr mice were analysed by fluorescence activated cell sorter (FACS). (a) The percentages of BAFF‐expressing CD11b+Gr‐1high and CD11b+Gr‐1low neutrophils are shown. (b) The statistical analysis of the percentages of BAFF‐expressing CD11b+Gr‐1high and CD11b+Gr‐1low cells from lupus‐prone MRL/lpr mice. Results represent at least three independent experiments; n.s. = no significance (two‐tailed Student's t‐test). Error bars, standard error of the mean.
Acknowledgements
This study was supported by National Basic Research Program 973 grants (2013CB530506, 2015CB553704), National Nature and Science Fund (81471529, 81401332, 81272320, 81471540 and 81472647), the Key Program of the Beijing Natural Science Foundation (7141007) and Service Industry Scientific Research of National Health and Family Planning Commission of China (2015SQ00192).
Contributor Information
X. Wang, Email: wang_renxi@yahoo.com
H. Xiao, Email: yinher2001@yahoo.com
References
- 1. Mills JA. Systemic lupus erythematosus. N Engl J Med 1994; 330:1871–9. [DOI] [PubMed] [Google Scholar]
- 2. Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J Biomed Biotechnol 2011; 2011:271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol 1985; 37:269–390. [DOI] [PubMed] [Google Scholar]
- 4. Gualtierotti R, Biggioggero M, Penatti AE, Meroni PL. Updating on the pathogenesis of systemic lupus erythematosus. Autoimmun Rev 2010; 10:3–7. [DOI] [PubMed] [Google Scholar]
- 5. Ramnath D, Tunny K, Hohenhaus DM et al TLR3 drives IRF6‐dependent IL‐23p19 expression and p19/EBI3 heterodimer formation in keratinocytes. Immunol Cell Biol 2015; 93:771. [DOI] [PubMed] [Google Scholar]
- 6. Wang X, Wei Y, Xiao H et al A novel IL‐23p19/Ebi3 (IL‐39) cytokine mediates inflammation in lupus‐like mice. Eur J Immunol 2016; 46:1343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun L, He C, Nair L, Yeung J, Egwuagu CE. Interleukin 12 (IL‐12) family cytokines: role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 2015; 75:249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen P, Roch T, Lampropoulou V et al IL‐35‐producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 2014; 507:366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang RX, Yu CR, Dambuza IM et al Interleukin‐35 induces regulatory B cells that suppress autoimmune disease. Nat Med 2014; 20:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Du J, Li Z, Shi J, Bi L. Associations between serum interleukin‐23 levels and clinical characteristics in patients with systemic lupus erythematosus. J Int Med Res 2014; 42:1123–30. [DOI] [PubMed] [Google Scholar]
- 11. Xia LP, Li BF, Shen H, Lu J. Interleukin‐27 and interleukin‐23 in patients with systemic lupus erythematosus: possible role in lupus nephritis. Scand J Rheumatol 2015; 44:200–5. [DOI] [PubMed] [Google Scholar]
- 12. Mok MY, Wu HJ, Lo Y, Lau CS. The relation of interleukin 17 (IL‐17) and IL‐23 to Th1/Th2 cytokines and disease activity in systemic lupus erythematosus. J Rheumatol 2010; 37:2046–52. [DOI] [PubMed] [Google Scholar]
- 13. Qiu F, Song L, Yang N, Li X. Glucocorticoid downregulates expression of IL‐12 family cytokines in systemic lupus erythematosus patients. Lupus 2013; 22:1011–6. [DOI] [PubMed] [Google Scholar]
- 14. Smith S, Gabhann JN, Higgs R et al Enhanced interferon regulatory factor 3 binding to the interleukin‐23p19 promoter correlates with enhanced interleukin‐23 expression in systemic lupus erythematosus. Arthritis Rheum 2012; 64:1601–9. [DOI] [PubMed] [Google Scholar]
- 15. Vignali DA, Kuchroo VK. IL‐12 family cytokines: immunological playmakers. Nat Immunol 2012; 13:722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL‐23 and IL‐27: related but functionally distinct regulators of inflammation. Annu Rev Immunol 2007; 25:242. [DOI] [PubMed] [Google Scholar]
- 17. Flores RR, Kim E, Zhou L et al IL‐Y, a synthetic member of the IL‐12 cytokine family, suppresses the development of type 1 diabetes in NOD mice. Eur J Immunol 2015; 45:3114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Egwuagu CE, Yu CR, Sun L, Wang R. Interleukin 35: critical regulator of immunity and lymphocyte‐mediated diseases. Cytokine Growth Factor Rev 2015; 26:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang RX, Yu CR, Mahdi RM, Egwuagu CE. Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune uveitis by inhibiting autoreactive Th1/Th17 cells and promoting expansion of regulatory T cells. J Biol Chem 2012; 287:36012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trinchieri G, Pflanz S, Kastelein RA. The IL‐12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 2003; 19:641–4. [DOI] [PubMed] [Google Scholar]
- 21. Kaplan MJ. Neutrophils in the pathogenesis and manifestations of SLE. Nat Rev Rheumatol 2011; 7:691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith CK, Kaplan MJ. The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Curr Opin Rheumatol 2015; 27:448–53. [DOI] [PubMed] [Google Scholar]
- 23. Furukawa F. Animal models of cutaneous lupus erythematosus and lupus erythematosus photosensitivity. Lupus 1997; 6:193–202. [DOI] [PubMed] [Google Scholar]
- 24. Vincent FB, Morand EF, Mackay F. BAFF and innate immunity: new therapeutic targets for systemic lupus erythematosus. Immunol Cell Biol 2012; 90:293–303. [DOI] [PubMed] [Google Scholar]
- 25. Xing C, Ma N, Xiao H et al Critical role for thymic CD19+CD5+CD1dhiIL‐10+ regulatory B cells in immune homeostasis. J Leukocyte Biol 2015; 97:547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma N, Liu X, Xing C et al Ligation of metabotropic glutamate receptor 3 (Grm3) ameliorates lupus‐like disease by reducing B cells. Clin Immunol 2015; 160:142–54. [DOI] [PubMed] [Google Scholar]
- 27. Ma N, Xing C, Xiao H et al BAFF suppresses IL‐15 expression in B cells. J Immunol 2014; 192:4192–201. [DOI] [PubMed] [Google Scholar]
- 28. Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol 1991; 9:243–69. [DOI] [PubMed] [Google Scholar]
- 29. Wang X, Wei Y, Xiao H et al Pre‐existing CD19‐independent GL7– Breg cells are expanded during inflammation and in mice with lupus‐like disease. Mol Immunol 2016; 71:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cervenak L, Magyar A, Boja R, Laszlo G. Differential expression of GL7 activation antigen on bone marrow B cell subpopulations and peripheral B cells. Immunol Lett 2001; 78:89–96. [DOI] [PubMed] [Google Scholar]
- 31. Zheng M, Xing C, Xiao H et al Interaction of CD5 and CD72 is involved in regulatory T and B cell homeostasis. Immunol Invest 2014; 43:705–16. [DOI] [PubMed] [Google Scholar]
- 32. Mackay F, Woodcock SA, Lawton P et al Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med 1999; 190:1697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khare SD, Sarosi I, Xia XZ et al Severe B cell hyperplasia and autoimmune disease in TALL‐1 transgenic mice. Proc Natl Acad Sci USA 2000; 97:3370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gross JA, Johnston J, Mudri S et al TACI and BCMA are receptors for a TNF homologue implicated in B‐cell autoimmune disease. Nature 2000; 404:995–9. [DOI] [PubMed] [Google Scholar]
- 35. Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol 2009; 9:491–502. [DOI] [PubMed] [Google Scholar]
- 36. Fairfax KA, Corcoran LM, Pridans C et al Different kinetics of Blimp‐1 induction in B cell subsets revealed by reporter gene. J Immunol 2007; 178:4104–11. [DOI] [PubMed] [Google Scholar]
- 37. Klein U, Dalla‐Favera R. Germinal centres: role in B‐cell physiology and malignancy. Nat Rev Immunol 2008; 8:22–33. [DOI] [PubMed] [Google Scholar]
- 38. Nieuwenhuis EE, Neurath MF, Corazza N et al Disruption of T helper 2‐immune responses in Epstein‐Barr virus‐induced gene 3‐deficient mice. Proc Natl Acad Sci USA 2002; 99:16951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tong H, Miyazaki Y, Yamazaki M et al Exacerbation of delayed‐type hypersensitivity responses in EBV‐induced gene‐3 (EBI‐3)‐deficient mice. Immunol Lett 2010; 128:108–15. [DOI] [PubMed] [Google Scholar]
- 40. Igawa T, Nakashima H, Sadanaga A et al Deficiency in EBV‐induced gene 3 (EBI3) in MRL/lpr mice results in pathological alteration of autoimmune glomerulonephritis and sialadenitis. Mod Rheumatol 2009; 19:33–41. [DOI] [PubMed] [Google Scholar]
- 41. Kim BJ, Lee S, Berg RE, Simecka JW, Jones HP. Interleukin‐23 (IL‐23) deficiency disrupts Th17 and Th1‐related defenses against Streptococcus pneumoniae infection. Cytokine 2013; 64:375–81. [DOI] [PubMed] [Google Scholar]
- 42. Redell MS, Tsimelzon A, Hilsenbeck SG, Tweardy DJ. Conditional overexpression of Stat3alpha in differentiating myeloid cells results in neutrophil expansion and induces a distinct, antiapoptotic and pro‐oncogenic gene expression pattern. J Leukoc Biol 2007; 82:975–85. [DOI] [PubMed] [Google Scholar]
- 43. Rodrigues DR, Fernandes RK, Balderramas Hde A et al Interferon‐gamma production by human neutrophils upon stimulation by IL‐12, IL‐15 and IL‐18 and challenge with Paracoccidioides brasiliensis . Cytokine 2014; 69:102–9. [DOI] [PubMed] [Google Scholar]
- 44. Taylor PR, Roy S, Leal SM Jr et al Activation of neutrophils by autocrine IL‐17A‐IL‐17RC interactions during fungal infection is regulated by IL‐6, IL‐23, RORγt and dectin‐2. Nat Immunol 2014; 15:143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li JP, Wu H, Xing W et al Interleukin‐27 as a negative regulator of human neutrophil function. Scand J Immunol 2010; 72:284–92. [DOI] [PubMed] [Google Scholar]
- 46. Wang Z, Liu JQ, Liu Z et al Tumor‐derived IL‐35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesis. J Immunol 2013; 190:2415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coquery CM, Wade NS, Loo WM et al Neutrophils contribute to excess serum BAFF levels and promote CD4+ T cell and B cell responses in lupus‐prone mice. PLOS ONE 2014; 9:e102284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yanaba K, Bouaziz ZD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B‐lymphocyte contributions to human autoimmune disease. Immunol Rev 2008; 223:284–99. [DOI] [PubMed] [Google Scholar]
- 49. De S, Barnes BJ. B cell transcription factors: potential new therapeutic targets for SLE. Clin Immunol 2014; 152:140–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Gating strategy for interleukin (IL)‐39 expanded/induced neutrophils. Splenocytes were separated from 8‐week‐old C57BL/6 mice and red blood cells were lysed using 1 × lysis buffer. Cells were washed and cultured for 3 days in the presence of medium, 50 ng/ml p19, Epstein–Barr virus‐induced 3 (Ebi3) or IL‐39 and analysed by fluorescence activated cell sorter (FACS). All live cells (a) or large granule cells (b) were gated and the percentages of Gr‐1+CD11b+ neutrophils are shown. Unless stated otherwise in other specific procedures, neutrophils were analysed by gating all live cells (a).
Fig. S2. Interleukin (IL)‐39 deficiency reduced GL7+ B‐induced inflammatory cells infiltration into the kidney of lupus‐prone Murphy Roths large (MRL)/lpr mice. GL7+ B cells from 8‐month‐old female lupus‐prone MRL/lpr mice were sorted by fluorescence activated cell sorter (FACS) and infected with control shRNA or IL‐12 family subunits p28, p35 or p40, p19 or Epstein–Barr virus‐induced 3 (Ebi3)‐specific shRNA. On day 1 after infection, (a) p28, p35, p40, p19 and Ebi3 mRNA expression were analysed by quantitative polymerase chain reaction (qPCR); (b) 5 × 106 control, p28, p35, p40, p19 and Ebi3‐specific shRNA‐infected GL7+ B cells per mouse were injected intravenously (i.v.) into 8‐week‐old female lupus‐prone MRL/lpr mice (six mice per group). Two weeks after treatment, kidney sections were stained with haematoxylin and eosin (H&E). Red arrows show glomeruli; blue arrows show infiltrating inflammatory cells. Scale bars, 50 μM. (a) Data are shown as mean ± standard error of the mean (s.e.m.) (n = 6) and are representative of three independent experiments. (b) Data represent at least three independent experiments. *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001 (two‐tailed Student's t‐test).
Fig. S3. B cell activating factor (BAFF)‐expressing CD11b+Gr‐1high and CD11b+Gr‐1low cells are similar. BAFF‐expressing CD11b+Gr‐1high and CD11b+Gr‐1low cells from the splenocytes of 8‐month‐old female non‐lupus‐prone Murphy Roths large (MRL)+ and lupus‐prone MRL/lpr mice were analysed by fluorescence activated cell sorter (FACS). (a) The percentages of BAFF‐expressing CD11b+Gr‐1high and CD11b+Gr‐1low neutrophils are shown. (b) The statistical analysis of the percentages of BAFF‐expressing CD11b+Gr‐1high and CD11b+Gr‐1low cells from lupus‐prone MRL/lpr mice. Results represent at least three independent experiments; n.s. = no significance (two‐tailed Student's t‐test). Error bars, standard error of the mean.


